Abstract
Evidence suggests that patients with irritable bowel syndrome (IBS) are more vigilant to pain-associated stimuli. The aims of this study were to compare women with IBS (n = 20) to healthy control (HC, n = 20) women on pain sensitivity, conditioned pain modulation (CPM) efficiency and salivary cortisol levels before and after the CPM test; and examine the relationship of CPM efficiency with gastrointestinal, somatic pain, and psychological distress symptoms in each group. Women, ages 20–42, gave consent, completed questionnaires and kept a symptom diary for 2 weeks. CPM efficiency was tested with a heat test stimulus and cold water condition stimulus in a laboratory between 8 and 10 a.m. on a follicular phase day. Salivary cortisol samples were collected just before and after the experimental testing. Compared to the HC group, women with IBS reported more days with gastrointestinal and somatic pain/discomfort, psychological distress, fatigue, and feeling stressed. During the CPM baseline testing women with IBS reported greater pain sensitivity compared to the HC group. In the IBS group, CPM efficiency was associated with the pain impact (PROMIS) measure, daily abdominal pain/discomfort, psychological distress, in particular anxiety. There was no group difference in salivary cortisol levels. Overall, women with IBS exhibit an increased sensitivity to thermal stimuli. Impaired CPM was present in a subset of women with IBS.
Keywords: conditioned pain modulation, irritable bowel syndrome, cortisol, female
INTRODUCTION
Functional gastrointestinal (GI) disorders such as irritable bowel syndrome (IBS) are among the most common and costly health care problems in the United States (Faresjo et al., 2007; Nyrop et al., 2007; Sandler, 1990; Spiegel, Strickland, Naliboff, Mayer, & Chang, 2008). In terms of GI diseases and disorders, the annual direct and indirect cost of treating IBS only ranks second to gastroesophageal reflux disorders. In addition, the severity and frequency of IBS symptom are linked to poorer quality of life (Bond et al., 2009; Deechakawan, Cain, Jarrett, Burr, & Heitkemper, 2012; Spiegel, et al., 2008). IBS is typified by the presence of abdominal pain or discomfort associated with bowel pattern changes and/or relieved by bowel movement, with patients usually suffering from diarrhea and/or constipation. In the United States more women than men seek health care services for their symptoms (Lovell & Ford, 2012). A number of potential mechanisms have been identified as potential initiators, symptom triggers, or exacerbating factors contribute to the etiology and pathophysiology of IBS. These include peripheral mechanisms such as increased small bowel and colonic permeability, changes in the colonic microbiome (Camilleri, 2012). Central mechanisms include alterations in central processing as seen in brain imaging studies (e.g., greater activation in the prefrontal regions, anterior and mid-cingulate cortex compared to controls) (Elsenbruch, 2011; Hubbard et al., 2011; Naliboff et al., 2006; Tillisch, Mayer, & Labus, 2011). Heightened visceral sensitivity to intestinal stimulation may be the result of both peripheral and central mechanisms (Keszthelyi et al., 2012; Piche, Bouin, Arsenault, Poitras, & Rainville, 2011).
As defined by the Rome III criteria abdominal pain/discomfort is a key feature of IBS regardless of the bowel pattern disturbance. Visceral hypersensitivity, defined as the experience of pain/discomfort within the viscera that is more intense than normal, can be assessed by increasing distensions in the rectal or sigmoid area. With this paradigm approximately 40–60% of IBS patients demonstrate significantly lower pain tolerance than the comparison group (Lovell & Ford, 2012). Another way of examining pain processing is by activating the diffuse noxious inhibitory control (DNIC) system. The DNIC system involves the spinal-medullary-spinal pathway and includes both afferent and efferent limbs. It acts as a type of filter that determines the amount of focus given to a particular stimulus within the background of normal somesthetic and visceral input (van Wijk & Veldhuijzen, 2010). It has recently been recommended that the term Conditioned Pain Modulation (CPM) be used in place of DNIC when referring to the observed phenomenon that pain perception is reduced by a second, conditioning pain, as determined by an experimental procedures (Yarnitsky, 2010; Yarnitsky et al., 2010). We will follow that recommendation in this report.
Tests of CPM efficiency (Yarnitsky, 2010; Yarnitsky, et al., 2010) have been performed in healthy controls as well as patients with chronic pain conditions. The CPM is elicited by applying a painful stimulus to a remote site (known as conditioning stimulus) which then induces inhibition of a pain stimulus from another part of the body (test stimulus) (Heymen et al., 2010). This approach is used to determine whether deficits in the endogenous analgesic mechanism could portend greater overall sensory (cutaneous, musculoskeletal, visceral) sensitivity in patients with chronic pain conditions such as IBS (Rezaii, Hirschberg, Carlstrom, & Ernberg, 2012).
Three studies have shown that patients with IBS demonstrate differences in CPM efficiency when compared to healthy controls (Heymen, et al., 2010; King et al., 2009; Piche, et al., 2011). The CPM efficiency relies on painful conditioning stimulation of one part of the body to inhibit pain perception in another part. For example, in healthy controls a noxious stimulus applied on one body part results in increased pain thresholds elsewhere (Heymen, et al., 2010). There are both gender and ethnic differences in the CPM efficiency; men show greater CPM efficiency than women (Heymen, et al., 2010). Menstrual cycle phase may also influence CPM efficiency in healthy women (Tousignant-Laflamme & Marchand, 2009). For example, greater CPM efficiency occurred in the ovulatory phase as compared to the luteal and menstrual phases in two studies (Rezaii, et al., 2012; Tousignant-Laflamme & Marchand, 2009). However, Bartley and Rhudy failed to show menstrual cycle phase differences when women were studied in the follicular versus luteal phases (Bartley & Rhudy, 2012).
Studies of patients with other chronic pain-related conditions show that CPM efficiency is influenced by report of stress or the introduction of stress in a laboratory setting as well as psychological variables including anxiety and depression (Johannesson, de Boussard, Brodda Jansen, & Bohm-Starke, 2007; Normand et al., 2011) or the presence of other pain-related conditions (Arendt-Nielsen, Sluka, & Nie, 2008; Williams & Clauw, 2009). As a group, patients with IBS report higher levels of anxiety and depression (Goncalves de Medeiros et al., 2012; Jones, Koloski, Boyce, & Talley, 2011; Orr, Crowell, Lin, Harnish, & Chen, 1997), stress and more co-morbid conditions (Gulewitsch, Enck, Hautzinger, & Schlarb, 2011) suggesting that CPM efficiency may be reduced. In addition, several studies found that IBS patients have dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis as evidenced by either hyper or hyposecretion of cortisol or dysregulation between ACTH and cortisol levels (Chang et al., 2009; Heitkemper et al., 2012). However, the relationship between HPA markers and CPM efficiency remains unexplored in patients with IBS.
Thus the aims of this study were to compare women with IBS to healthy controls (HC) on pain sensitivity, CPM efficiency and salivary cortisol levels before and after the CPM test; and examine the relationship of CPM efficiency with GI, somatic pain, and psychological distress symptoms and salivary cortisol levels in each group. We hypothesize that the IBS group will have increased pain sensitivity and poorer CPM efficiency compared to HC group.
MATERIALS AND METHODS
Design and Participants
In this cross-sectional study, women (ages 18–45) with IBS and healthy women were recruited through the university research studies volunteer’s web site and flyers posted in the community. Women were screened for eligibility by telephone and during the initial laboratory visit where they gave written consent before being oriented to the study protocol. To be included in the IBS group participants had to have a prior diagnosis of IBS for at least 6 months made by a health care provider (e.g., internist, gastroenterologist). Over the preceding 3 months they had to have abdominal discomfort or pain more than 25% of the time that was associated with two of three features: 1) relieved with defecation, 2) onset associated with a change in frequency of stool, or 3) onset associated with a change in form (appearance) of stool. Participants were classified as IBS-constipation (IBS-C) if they had greater than or equal to 25% of stools that were hard and lumpy and less than 25% loose (mushy) or watery. Criteria for IBS-diarrhea (IBS-D) were greater than or equal to 25% of stools loose and watery, and less than 25% hard or lumpy. Mixed group (IBS-M) had greater than or equal to 25% of stools that meet the criteria for either hard and lumpy or loose and watery. The Rome III criteria were confirmed with the Rome III Diagnostic Questionnaire for Functional GI Disorders (Drossman et al., 2006). Women in the HC group could not have a functional GI disorder or serious health problems. In both groups, women were excluded if they: 1) had a history of an organic GI disease, cardiac arrhythmia, renal, or gynecological pathology; 2) were currently taking certain medications, e.g., prokinetic drugs, laxatives (but not fiber supplements), anti-diarrheals or antispasmodics for GI symptoms; 3) were currently taking other medications daily that would alter cortisol (e.g., phenytoin, synthetic glucocorticoids); 4) had a body mass index (BMI, kg/m2) > 35; 5) had moderate - severe co-morbid pain or psychiatric conditions. We balanced the enrollment to the HC group by the age, race and education of the women in the IBS group. University institutional review board approval was obtained prior to recruitment and renewed annually. Of the 140 women screened, three women in the control group withdrew after the initial visit (too busy, time conflict) and two women were withdrawn at the initial visit when an exclusion diagnosis was identified.
Procedures
At the time of enrollment the women signed informed consent and completed validated data collection tools (e.g., PROMIS measures, Rome III Diagnostic Questionnaire for the Adult Functional GI Disorders, Brief Symptom Inventory). Women started their daily diary at the onset of their next menses and completed it each evening for approximately 14 days.
Following the start of menses women were scheduled for the CPM testing in the laboratory (median = 6 days after start of menses; range, 4 – 12 days). Women were asked to maintain a clear liquid diet beginning 12 hours before their test visit and refrain from drinking liquids for at least 1½ hours prior to the testing procedure. The CPM test took place between 08:00 – 10:00 AM and lasted approximately 30 minutes. The two salivary samples for cortisol were obtained by having the participant passively drool through a straw into a 15ml plastic centrifuge tube. This was done immediately before starting and immediately following the CPM testing. Participants were told that the testing would be terminated if they wanted to stop the procedure at any time.
CPM procedure
The CPM testing procedure included four phases, described below. The Pain & Sensory Evaluation System (Pathway model ATS, Israel) was used to generate a noxious heat test stimulus. A 12°C water bath was used as the noxious conditioning stimulus. The CPM testing was performed by a trained research nurse in our psychological testing laboratory.
Familiarization phase
The familiarization phase introduced the participant to the procedure. The baseline temperature for the thermode was 32°C with increasing or decreasing (back to baseline) temperature rates of 8°C/second. The first step was to place the thermode (30 × 30 mm) on the volar surface of the dominant arm, secured with a Velcro strap. A script was used to guide the participant through the testing procedure. The participant was exposed to a heat stimulus of 43°C and 44°C with a 2 second inter-stimuli interval at 32°C. At each temperature, the participant verbally rated pain intensity on a visual numerical pain score ranging from 0 (no pain) to 10 (worst pain imaginable). This was followed by a 5-minute break without the thermode.
Pain sensitivity and pain-6 temperature determination phase
The second step was to determine a temperature at which the participant consistently rated pain as a ‘6’ on the 0 – 10 scale (the pain-6 temperature). A series of three temperatures (45, 46 and 47°C) were administered through the thermode in random order for 7 seconds each with 2 seconds inter-interval duration, and the participant rated pain intensity at each temperature. If the participant rated a temperature as a ‘6’ that temperature was identified as the pain-6 temperature. If they did not, the temperatures were either increased or decreased by 1°C and the determination sequence was repeated up to three times to identify the pain-6 temperature. In order to protect the participants from severe pain, two constraints were imposed: The maximum temperature used was 48°C, and if at any point a subject gave a pain rating of ‘8’ or higher, the subject was not tested at a temperature higher than that. At the end of this procedure, the pain-6 temperature was confirmed by activating the thermode once at the pain-6 temperature for 7 seconds and having the woman rate their level of pain. The pain-6 temperature was used for the test stimulus in the next steps. In addition, the pain ratings at different temperatures were used for analyses of pain sensitivity. At the end of this phase the participant relaxed for 5-minutes without the thermode.
Unconditioned Test stimulus
During this step the thermode was set at the pain-6 temperature for 30 seconds. The participant rated her pain from the test stimulus at 10, 20 and 30 seconds. After this the participant relaxed for 5-minutes without the thermode.
Conditioning stimulus
In the last step, the participant placed her non-dominant hand in a cold water bath maintained at 12°C – this was the conditioning stimulus. The instructions were to keep the hand in up to the wrist and keep the fingers apart for one minute. The participant rated her pain from the conditioning stimulus at 10, 20 and 30 s. With her non-dominant hand remaining in the cold water bath, the pain-6 temperature was now applied to the thermode on her dominant hand for 30 seconds and she rated her pain from this test stimulus at 40, 50 and 60 seconds.
CPM efficiency score
The CPM efficiency score was calculated as the average of the three pain ratings for the unconditioned test stimulus minus the average of the three pain ratings for the test stimulus when the conditioning stimulus was present. Higher positive values indicate a greater CPM efficiency.
Measures
IBS characteristics
The Rome III Diagnostic Questionnaire for the Adult Functional GI Disorders was used (Drossman, et al., 2006) was used for information on duration of IBS symptoms since onset (“When did your IBS symptoms first start?”) and by response to the question, “Usually, how severe was the pain or discomfort in your abdomen?” that was rated from 1 (very mild) to 4 (very severe).
Pain Behavior
This was measured by the Pain Behavior Short Form (PROMIS®) which asks about common pain behaviors that can be observed (thrashing), behaviors associated with pain severity (grimace, moved extremely slowly, isolate myself, irritable), verbal reports of pain rated from 1 (not at all) to 5 (very much), and one social item rated from 1 (never) to 5 (always) over the past 7 days. Content validity was tested with qualitative and quantitative approaches and good reliability was reported (Cella et al., 2010; Magasi et al., 2012). Internal consistency for this study was α = .89. The summary score is the mean of all items with higher scores reflecting more pain behavior.
Pain Impact
This was measured by the Pain Impact - Short Form (PROMIS®) which asks about the consequences of pain on five relevant aspects one’s life including: social, cognitive, emotional, physical and recreational activities. Five items assess pain over the past 7 days and rated them from 1 (not at all) to 5 (very much), and one item is rated from 1 (never) to 5 (always). Content validity was tested with qualitative and quantitative approaches and good reliability was reported (Cella, et al., 2010; Magasi, et al., 2012). Internal consistency for this study was α = .91. The summary score is the mean of all items with higher scores reflecting greater pain impact.
Psychological Distress
This was assessed by the Brief Symptom Inventory (BSI) which includes 53 items that represent symptoms of nine psychological disorders: anxiety, phobic anxiety, obsessive-compulsive, depression, somatization, interpersonal sensitivity, hostility, paranoid ideation, and psychoticism (Derogatis, 1993). The participant is asked to consider how distressed or bothered they felt during the past 7 days, and then rate the symptoms from 0 (not at all) to 4 (extremely). Validity and reliability are based on Adult Psychiatric Outpatient and Adult Non-patients (Derogatis, 1993). Internal consistency for this study was α = .911. Included were the anxiety and depression subscales and mean score for all items (Global Symptom Index [GSI]). Higher values reflect greater psychological distress.
Severity of Daily Symptoms
Participants completed a symptom diary each evening rating their symptoms based on the highest severity they experienced for each symptom over the past 24 hours. All symptoms were rated as 0 (not present), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe), and each symptom was summarized across the 14 days as percent of days with moderate to very severe symptom severity. Lower GI symptoms were abdominal pain or discomfort, pain after eating, abdominal distension, intestinal gas, bloating, constipation, diarrhea and urgency. A composite Lower GI Symptoms Scale was defined as the mean, over these 8 symptoms, of percent of days with moderate to very severe symptoms severity. A similar composite Upper GI Symptoms Scale was formed based on the symptoms heartburn, nausea, and stomach pain. The composite Somatic Pain Symptoms Scale included backache, headache, joint and muscle pain. Psychological symptoms are reported as three individual symptoms: anxiety, depression and stressed. Fatigue, a single item, was also included.
Salivary Cortisol
Saliva samples were stored at −70°C. Prior to assay, samples were thawed and centrifuged at 1500 x g for 15 min to remove particulates. Salivary cortisol concentrations were determined using a horseradish peroxidase-linked immunoassay on 96-well microtiter plates that were coated with monoclonal antibodies to cortisol (Salimetrics, State College, PA). Expected values for women age 18 to 50 ranges from 0.094 to 1.545 μg/dL.
Data Analysis
Statistics were compared using SPSS v. 15 (SPSS, Inc., Chicago, IL). A comparison of demographics, symptoms and pain sensitivity relative to temperature were compared using Chi-square for categorical data and independent t-test for continuous data. Distributions of continuous variable were not highly non normal, except for the one outlier in CPM efficiency. CPM efficiency was fairly normal with that outlier removed. Data from the familiarization phase of the CPM testing protocol were used to measure pain sensitivity. Participants rated pain intensity at several different temperatures, sometimes more than once at the same temperature. For each participant, pain rating at a given temperature was measured as the average over all the ratings at that temperature, and linear regression extrapolation was used to impute missing values if a participant did not rate pain at a given temperature. ANCOVA, controlling for baseline between the IBS and HC group, was used to test for IBS versus HC differences in pain sensitivity reported as the mean pain rating at specific temperatures 45 to 48°C and CPM efficiency.
Pearson correlation was used to measure the degree of association of baseline thermal sensitivity and CPM efficiency with these the composite symptom summary measures, as well as with the individual symptoms. These correlation analyses were done separately for IBS and HC participants.
Results
The sample included 40 women: 20 with IBS and 20 HC. Women were on average 32 years old, 75% were White, and relatively well educated. There were no differences in demographic characteristics between the HC and IBS groups (Table 1). Within the IBS group, 13 identified themselves as IBS-D, 3 as IBS-, and 4 as IBS-M. The majority of IBS participants (80%) had a diagnosis of IBS for longer than 5 years. Five women in the IBS group and three in HC group were taking a SSRI. Controlling for SSRI use did not alter any of the comparison or correlation results. Relative to the HC group women with IBS reported significantly greater GI symptoms, somatic pain symptoms (i.e., backache, muscles pain), psychological distress, and fatigue (Table 1).
Table 1.
Demographics, IBS, Pain, and Psychological Symptoms of Healthy Control (HC) and Women with Irritable Bowel Syndrome (IBS) Groups
| HC (n = 20) | IBS (n = 20) | P-valuea | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, M (SD) | 27.6 (5.5) | 27.4 (6.6) | .940 |
| White race, % | 75% | 75% | 1.00 |
| College Degree, % | 90% | 65% | .127 |
| Duration of IBS, n (%) | |||
| < 5 years | 4 (20%) | ||
| 5 – 9 years | 7 (35%) | ||
| ≥10 years | 9 (45%) | ||
| Recalled symptoms, M (SD)b | |||
| GI symptoms | |||
| Abdominal pain severity over 3 mo. | 2.8 (0.5) | ||
| Pain Impact | 2.9 (1.0) | ||
| Pain Behaviors | 2.3 (0.9) | ||
| Psychological symptoms | |||
| BSI - Global Severity Index | 0.10 (0.10) | 0.32 (0.26) | .001 |
| Anxiety | 0.12 (0.13) | 0.37 (0.39) | .007 |
| Depression | 0.10 (0.17) | 0.38 (0.54) | .032 |
| Daily symptoms, M (SD)c | |||
| GI symptoms | |||
| Abdominal pain or discomfort | 4.0 (9.8) | 28.0 (22.4) | <.001 |
| Lower GI symptoms | 1.1 (2.6) | 22.8 (16.0) | <.001 |
| Upper GI symptoms | 0.6 (2.1) | 7.2 (11.2) | .014 |
| Somatic pain symptoms | 1.1 (3.1) | 3.7 (3.6) | .017 |
| Psychological symptoms and fatigue, M(SD) | |||
| Anxiety | 1.2 (2.9) | 17.1 (20.2) | .001 |
| Depressed | 0.4 (1.9) | 7.5 (13.8) | .029 |
| Stressed | 6.5 (17.2) | 19.3 (20.2) | .037 |
| Fatigue | 5.9 (13.4) | 19.7 (17.7) | .009 |
Note. HC = Healthy Controls; IBS = Irritable Bowel Syndrome; BSI = Brief Symptom Inventory; GI = Gastrointestinal. Of the recalled symptoms abdominal pain severity was recalled over 3 months; Pain Impact and Pain Behaviors are measures from the Patient-Reported Outcomes Measurement Information System (PROMIS - short forms) and were recalled over 7 days. Psychological distress symptoms recalled over 7 days. Daily symptoms were recorded each evening, and are summarized for each person as the percent of days with moderate to very severe symptom severity.
t-test.
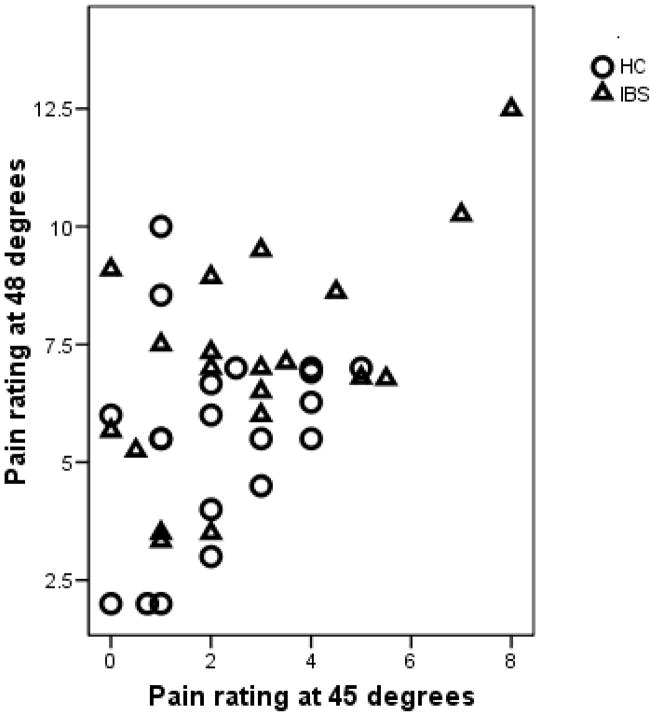
Table 2 shows the participants’ pain rating at temperatures ranging from 45 to 48°C. The average pain rating was higher in the IBS group compared to the HC group, with this difference being statistically significant at 47 and 48°C. Figure 1 shows the pain intensity ratings of each participant at 45°C and 48°C. There were two IBS participants who were very sensitive to pain, seen in the upper right. When stimulated with a relatively low temperature (45°C) these two participants rated pain as greater than ‘6’. In contrast, there were nine participants who reported low pain even at 48°C, as seen in the lower left of the plot. Since 48°C was the highest temperature allowed by the protocol, this means that the CPM testing was conducted at a temperature that was lower than ideal in that it elicited a baseline pain rating less than the target of pain-6. Based on the determination of the pain-6 temperature, for the remainder of the CPM procedure most of the subjects were tested at 48 degrees (12 HC, 8 IBS) or 47 degrees (7 HC, 7 IBS) while a few were tested at lower temperatures of 46 degrees (1 HC, 3 IBS), 45 degrees (1 IBS) or 44 degrees (1 IBS).
Table 2.
Pain Sensitivity Relative to Thermode Temperature
| HC (n = 20) | IBS (n = 20) | p-value | |
|---|---|---|---|
| Thermode temperature | |||
| 45°C | 2.16 (1.48) | 2.85 (2.22) | .255 |
| 46°C | 2.92 (1.75) | 4.10 (2.13) | .063 |
| 47°C | 4.15 (1.74) | 5.62 (1.92) | .015 |
| 48°C | 5.54 (2.14) | 6.97 (2.04) | .038 |
Note. HC = Healthy Controls; IBS = Irritable Bowel syndrome. Pain rating performed with a 0 to 10 scale.
Figure 1.
The pain ratings at 45°C and 48°C degrees. IBS participants are in triangles and HC in circles. In the upper right are two IBS participants who were highly sensitive to pain. In the lower left, there are nine participants with low sensitivity to pain even at 48°C.
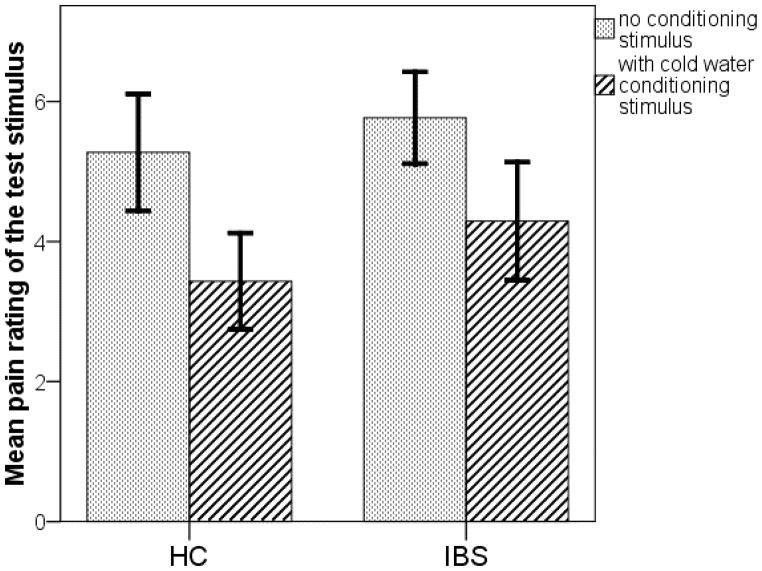
Figure 2 shows that in both the IBS and HC groups, the mean pain severity rating of the thermal stimulus was higher in the unconditioned situation than it was when the conditioning stimulus (one hand immersed in cold water) was present. Both the HC and IBS groups demonstrated a mean CPM efficiency that was significantly greater than zero (M [SD], HC, 1.84 [1.12], p<.001; IBS, 1.48 [1.86], p= .002). The mean CPM efficiency does not differ between the IBS and HC groups, p=.46. One IBS participant was an extreme outlier, with a CPM efficiency of −4.0 while the next lowest value was −0.7. This subject is also the high outlier in Figure 1. She rated the thermal pain as 6 for a temperature of 44 degrees in the familiarization phase, but gave a pain rating of 2 at that same temperature during the unconditioned test phase (under essentially the same condition as the familiarization phase) and gave a pain rating of 6 to this same 43 degrees during the conditioned stimulus (one hand in cold water). Excluding this participant does not significantly change the CPM comparison (M [SD], HC, 1.84 [1.12]; IBS, 1.76 [1.38], p = .85). Another 9 participants (HC, 6; IBS, 3) had low pain sensitivity as evidenced by reported pain levels less than 5 at a temperature of 48°C. Excluding these participants from each group did not change the conclusion of no IBS versus HC difference (M [SD], HC, 2.21 [1.05]; IBS, 1.57 [1.98], p = .28).
Figure 2.
Conditioned pain modulation. In both the HC and IBS groups, the mean pain rating for the test stimulus without conditioning (dotted) is higher than the main pain rating of the test stimulus in the presence of the conditioning stimulus (diagonal strips), demonstrate a significant CPM effect (P < .001) in both groups. Error bars represent 95% confidence intervals.
Prior to CPM testing salivary cortisol levels did not differ significantly (M [SD], HC, 0.355 [0.145] μg/dL; IBS, 0.357 [0.179] μg/dL, p = .94) or change from before to after the CPM testing (M [SD], HC, −0.052 [0.065] μg/dL; IBS, −0.030 [0.087] μg/dL, p= .38). In addition, there was no significant relationship between CPM efficiency and salivary cortisol level at baseline (r = −,19 for HC, .03 for IBS, p>.4 for both) or change from before to after CPM procedure (r = .19 for HC, .04 for IBS, p>.4 for both).
Figures 3 (A and B) show the association of CPM efficiency with lower GI symptoms and anxiety symptoms, respectively. Correlations are shown in Table 3. The outlier with a very low CPM efficiency had a large influence on the correlations; removing the outlier makes the correlation with lower GI symptom score less significant but the correlation with psychological distress symptoms, stress scores become more significant.
Figure 3.
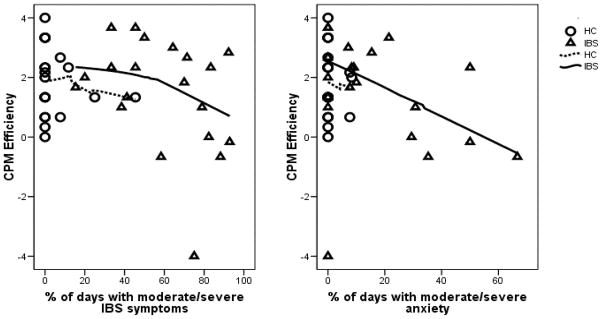
Relationship between CPM efficiency and percent of days with moderate – severe symptoms. Panel A is the percent of days with moderate to severe lower GI symptoms. Panel B is the percent of days with moderate to severe anxiety.
Table 3.
Correlation of Conditioned Pain Modulation Efficacy with Pain Impact, Pain Behavior and Daily Symptoms
| Symptoms | HC Correlationa | p | IBS Correlationa | p | IBS outlier excluded Correlationa | p |
|---|---|---|---|---|---|---|
| Recalled over 7 days | ||||||
| Pain Impact | −.27 | NS | −.21 | NS | ||
| Pain Behavior | −.31 | .18 | −.40 | .092 | ||
| Daily symptoms | ||||||
| GI symptom scores | ||||||
| Abdominal pain | −.09 | NS | −.48 | .034 | −.29 | NS |
| Lower GI symptoms | −.06 | NS | −.60 | .006 | .40 | .086 |
| Upper GI symptoms | −.01 | NS | .24 | .NS | −.09 | .NS |
| Somatic pain | −.06 | NS | .14 | NS | −.04 | NS |
| Psychological symptoms and fatigue | ||||||
| Anxiety | .08 | NS | −.31 | .18 | −.64 | .003 |
| Depressed | .31 | .18 | −.13 | NS | −.30 | NS |
| Stressed | .13 | NS | −.37 | .11 | −.63 | .004 |
| Fatigue | .02 | NS | −.30 | .20 | −.56 | .012 |
Note. HC = Healthy Controls. IBS = Irritable Bowel Syndrome.
Pearson’s correlation. Pain Behaviors and Pain Impact are from the Patient-Reported Outcomes Measurement Information System (PROMIS) - short forms.
GI = Gastrointestinal.
NS = p ≥ .20.
Two interesting things are seen in Figure 3, which prompted post-hoc analyses. First, 5 out of 20 IBS participants (25%) showed low CPM efficiency (less than or equal to zero) as compared to one participant in the HC group (5%). Second, all of the IBS participants with low CPM efficiency are in the right half of Figure 3, with moderate/severe IBS symptoms on more than half of the days. Among those IBS participants with relatively high IBS symptoms 5 out of 11 (46%) have an impaired CPM efficiency, which is significantly greater (p =.013) than the 1 out of 20 in the HC group.
DISCUSSION
In this study, we examined IBS versus HC women differences in thermal pain sensitivity and conditioned pain modulation. We found significant group differences in the temperature-determining phase (more women with IBS reporting pain at lower temperatures). Overall we failed to find a significant group difference in the CPM efficiency. However, in a post-hoc analysis of women with prospectively measured lower GI symptoms on over half the days, 5 of 11 demonstrated low CPM efficiency. The women with low CPM efficiency also reported more days with moderate to severe psychological distress and fatigue.
The observed thermal pain sensitivity results are consistent with those of King (King, et al., 2009) and Wong (Wong et al., 2010) who also found that a higher thermal temperature was required to induce the targeted pain intensity range in the HC group as compared to women with IBS. However, Heymen et al. (Heymen, et al., 2010) reported no baseline IBS versus HC differences in thermal pain sensitivity. Our findings related to group differences in the CPM testing effect are in the same direction but considerably weaker than the results of Heymen (Heymen, et al., 2010), Wong (Wong, et al., 2010) and King (King, et al., 2009), all of whom found reduced CPM efficiency among subjects with IBS. Although the study samples are similar among these studies, ours did not exclude women taking SSRIs. However, when SSRI use was controlled for in our analysis the findings did not change. In Heyman and King’s studies they did not exclude IBS women with other chronic pain conditions while the Wong study did. All the IBS participants in the Wong study had diarrhea-predominant IBS. With regard to other chronic pain conditions our study protocol specifically excluded women with moderate - severe co-morbid pain (e.g., fibromyalgia) or psychiatric conditions. This was important since Chang (Chang, et al., 2009) measuring both visceral and somatic sensitivity found that IBS patients with co-morbid fibromyalgia showed an enhanced sensitivity to unpleasant somatic stimuli. Interestingly in that study participants with only IBS showed a blunted response to somatic stimuli. In the current study we noted that although IBS women did report on the daily diary more days with backaches and muscle pain there was no significant correlation of somatic symptoms with the CPM efficiency.
Whether recruitment site accounted for the differences across studies is not known. In Heymen’s study, women were recruited from a functional GI disorder data registry as well as the community (Heymen, et al., 2010). The King study (King, et al., 2009) recruited from clinics. It is not clear how Wong’s (Wong, et al., 2010) participants were recruited. The this study we used a community recruitment approach; thus, the IBS women may represent a less symptomatic group relative to other studies.
Our results provide the first evidence that those women with IBS who do exhibit a reduced CPM efficiency are more likely to prospectively report more days with moderate to severe lower GI symptoms, anxiety, and fatigue symptoms.
The decreased ability to inhibit sensory input (reduced CPM efficiency) seen in approximately a fourth of our IBS group may explain why some IBS patients respond to ‘normal’ bowel sensations (intestinal gas) as painful. Given the potential plasticity of the CPM efficiency, constant or repeated stimulation (e.g., stress) may produce changes that result in a decreased ability to dampen peripheral input or actually enhance facilitation (Ossipov, Dussor, & Porreca, 2010). It is interesting that when one outlier was removed from the analysis a significant negative relationship of daily stress with the CPM efficiency was observed. How the perception of daily and chronic or repeated stress physiologically influence CPM efficiency is not clear. Descending pain modulatory systems are complex. The CPM efficiency is due to a spino-bulbar-spinal loop that receives input from higher centers. It has been suggested that changes in the hypothalamus contributes to the hypoactivity of the pain inhibitory system (Trimble, Johnson, Foster, & Greenwood-van Meerveld, 2007). Several studies have demonstrated that some patients with IBS show evidence of dysregulated hypothalamic-pituitary-adrenal (HPA) axis as reflected in high or low cortisol levels under basal and stress conditions (Chang, et al., 2009; Heitkemper, et al., 2012). Using salivary cortisol levels as a peripheral marker of the HPA axis we failed to find baseline group differences in response to the CPM testing. All testing procedures were conducted within the same time period in the day to control for circadian variation. The lack of the CPM testing to elicit a physiologic stress response is consistent with another study in which no difference in cardiovascular reactivity variables including heart rate and blood pressure were observed during and following the CPM testing (Campbell et al., 2008). Additional studies are warranted to elucidate the roles of the sympathetic nervous system and the HPA axis in pain modulation in IBS.
The findings of the current study need to be interpreted with caution. First, the sample size is relatively small with approximately 50% of the IBS group having lower GI symptoms that were moderate to severe on less than 50% of days. Second, almost half of the HC participants exhibited reduced pain sensitivity at 48°C temperature (thermode). Since the aim of the initial phase of the CPM testing was to identify the temperature at which pain intensity was rated “6”, the temperature for conducting the CPM testing should ideally have been greater than 48°C. The inability to increase the testing thermode temperature to greater than 48°C due to protocol may have contributed to the large number of HC participants whose pain level did not reach a rating of moderate (“6”) or higher.
An important finding of this study is that the impaired CPM efficiency observed in a subset of women with IBS is associated with prospectively measured worse lower GI symptoms, higher anxiety, and greater fatigue. Our results add to the growing evidence supporting the importance of the pain-related mechanisms in a substantial subset of women with IBS. Understanding the roles of peripheral and central mechanisms in a chronic condition such as IBS is important to the development and testing of effective pharmacologic and non-pharmacologic (e.g., cognitive behavioral therapy) therapies.
Acknowledgments
Sources of support: NINR, NIH (1RC2NR011959)
References
- Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. Pain. 2008;140(3):465–471. doi: 10.1016/j.pain.2008.09.027. S0304-3959(08)00582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, Rhudy JL. Endogenous inhibition of the nociceptive flexion reflex (NFR) and pain ratings during the menstrual cycle in healthy women. Annals of Behavioral Medicine. 2012;43(3):343–351. doi: 10.1007/s12160-012-9345-x. [DOI] [PubMed] [Google Scholar]
- Bond B, Quinlan J, Dukes GE, Mearin F, Clouse RE, Alpers DH. Irritable bowel syndrome: more than abdominal pain and bowel habit abnormalities. Clinical Gastroenterology and Hepatology. 2009;7(1):73–79. doi: 10.1016/j.cgh.2008.08.011. S1542-3565(08)00832-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Peripheral mechanisms in irritable bowel syndrome. New England Journal of Medicine. 2012;367(17):1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. Journal of Pain. 2008;9(8):759–766. doi: 10.1016/j.jpain.2008.03.010. S1526-5900(08)00502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology and Motility. 2009;21(2):149–159. doi: 10.1111/j.1365-2982.2008.01171.x. NMO1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deechakawan W, Cain KC, Jarrett ME, Burr RL, Heitkemper MM. Effect of Self-Management Intervention on Cortisol and Daily Stress Levels in Irritable Bowel Syndrome. Biological Research for Nursing. 2012 doi: 10.1177/1099800411414047. doi: 1099800411414047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. BSI: Brief Symptom Inventory; Administration, Scoring and Procedures Manual. 4. Minneapolis: National Computer Systems; 1993. [Google Scholar]
- Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG Rome III Committees. Rome III: The functional gastrointestinal disorders. 3. McLean: Degnon Associates; 2006. [Google Scholar]
- Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain, Behavior, and Immunity. 2011;25(3):386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Faresjo A, Grodzinsky E, Johansson S, Wallander MA, Timpka T, Akerlind I. A population-based case-control study of work and psychosocial problems in patients with irritable bowel syndrome--women are more seriously affected than men. American Journal of Gastroenterology. 2007;102(2):371–379. doi: 10.1111/j.1572-0241.2006.01012.x. AJG1012. [DOI] [PubMed] [Google Scholar]
- Goncalves de Medeiros MT, de Oliveira RB, dos Santos AA, de Leopoldino DM, Lima MC, Nobre RA, Nobre e Souza MA. The effects of sildenafil on rectal sensitivity and tone in patients with the irritable bowel syndrome. Alimentary Pharmacology and Therapeutics. 2012;35(5):577–586. doi: 10.1111/j.1365-2036.2011.04977.x. [DOI] [PubMed] [Google Scholar]
- Gulewitsch MD, Enck P, Hautzinger M, Schlarb AA. Irritable bowel syndrome symptoms among German students: prevalence, characteristics, and associations to somatic complaints, sleep, quality of life, and childhood abdominal pain. European Journal of Gastroenterology and Hepatology. 2011;23(4):311–316. doi: 10.1097/MEG.0b013e3283457b1e. [DOI] [PubMed] [Google Scholar]
- Heitkemper MM, Cain KC, Deechakawan W, Poppe A, Jun SE, Burr RL, Jarrett ME. Anticipation of public speaking and sleep and the hypothalamic-pituitary-adrenal axis in women with irritable bowel syndrome. Neurogastroenterology and Motility. 2012;24(7):626–e271. doi: 10.1111/j.1365-2982.2012.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clinical Journal of Pain. 2010;26(2):104–109. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. Journal of Neuroscience. 2011;31(35):12491–12500. doi: 10.1523/jneurosci.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson U, de Boussard CN, Brodda Jansen G, Bohm-Starke N. Evidence of diffuse noxious inhibitory controls (DNIC) elicited by cold noxious stimulation in patients with provoked vestibulodynia. Pain. 2007;130(1–2):31–39. doi: 10.1016/j.pain.2006.10.022. S0304-3959(06)00589-6. [DOI] [PubMed] [Google Scholar]
- Jones M, Koloski N, Boyce P, Talley NJ. Pathways connecting cognitive behavioral therapy and change in bowel symptoms of IBS. Journal of Psychosomatic Research. 2011;70(3):278–285. doi: 10.1016/j.jpsychores.2010.10.004. S0022-3999(10)00389-2. [DOI] [PubMed] [Google Scholar]
- Keszthelyi D, Troost FJ, Simren M, Ludidi S, Kruimel JW, Conchillo JM, Masclee AA. Revisiting concepts of visceral nociception in irritable bowel syndrome. European Journal of Pain (London, England) 2012;16(10):1444–1454. doi: 10.1002/j.1532-2149.2012.00147.x. [DOI] [PubMed] [Google Scholar]
- King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., 3rd Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143(3):172–178. doi: 10.1016/j.pain.2008.12.027. S0304-3959(08)00765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. American Journal of Gastroenterology. 2012;107(7):991–1000. doi: 10.1038/ajg.2012.131. ajg2012131. [DOI] [PubMed] [Google Scholar]
- Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, Cella D. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Quality of Life Research. 2012;21(5):739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131(2):352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. Journal of Clinical Psychiatry. 2011;72(2):219–224. doi: 10.4088/JCP.08m04969blu. [DOI] [PubMed] [Google Scholar]
- Nyrop KA, Palsson OS, Levy RL, Korff MV, Feld AD, Turner MJ, Whitehead WE. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Alimentary Pharmacology and Therapeutics. 2007;26(2):237–248. doi: 10.1111/j.1365-2036.2007.03370.x. APT3370. [DOI] [PubMed] [Google Scholar]
- Orr WC, Crowell MD, Lin B, Harnish MJ, Chen JD. Sleep and gastric function in irritable bowel syndrome: derailing the brain-gut axis. Gut. 1997;41(3):390–393. doi: 10.1136/gut.41.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. Journal of Clinical Investigation. 2010;120(11):3779–3787. doi: 10.1172/JCI43766. 43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche M, Bouin M, Arsenault M, Poitras P, Rainville P. Decreased pain inhibition in irritable bowel syndrome depends on altered descending modulation and higher-order brain processes. Neuroscience. 2011;195:166–175. doi: 10.1016/j.neuroscience.2011.08.040. S0306-4522(11)00982-1. [DOI] [PubMed] [Google Scholar]
- Rezaii T, Hirschberg AL, Carlstrom K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. Journal of Pain. 2012;13(7):646–655. doi: 10.1016/j.jpain.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99(2):409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103(10):2536–2543. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140(1):91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146(1–2):47–55. doi: 10.1016/j.pain.2009.06.018. S0304-3959(09)00341-8. [DOI] [PubMed] [Google Scholar]
- Trimble N, Johnson AC, Foster A, Greenwood-van Meerveld B. Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterology and Motility. 2007;19(9):754–760. doi: 10.1111/j.1365-2982.2007.00951.x. nmo951. [DOI] [PubMed] [Google Scholar]
- van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. Journal of Pain. 2010;11(5):408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. Journal of Pain. 2009;10(8):777–791. doi: 10.1016/j.jpain.2009.06.001. S1526-5900(09)00548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Rodrigues AC, King CD, Riley JL, 3rd, Schmidt S, Vierck CJ, Mauderli AP. Relationships between Irritable Bowel Syndrome Pain, Skin Temperature Indices of Autonomic Dysregulation, and Sensitivity to Thermal Cutaneous Stimulation. Pain Res Treat. 2010;2010:949027. doi: 10.1155/2010/949027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. European Journal of Pain (London, England) 2010;14(4):339. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]