Abstract
Estrogens can become endogenous carcinogens via formation of catechol estrogen quinones, which react with DNA to form specific depurinating estrogen-DNA adducts. The mutations resulting from these adducts can lead to cell transformation and the initiation of breast cancer. Estrogen metabolites, conjugates and depurinating DNA adducts in urine samples from 46 healthy control women, 12 high-risk women and 17 women with breast cancer were analyzed. The estrogen metabolites, conjugates and depurinating DNA adducts were identified and quantified by using ultra-performance liquid chromatography/tandem mass spectrometry. The levels of the ratios of depurinating DNA adducts to their respective estrogen metabolites and conjugates were significantly higher in high-risk women (p < 0.001) and women with breast cancer (p < 0.001) than in control subjects. The high-risk and breast cancer groups were not significantly different (p = 0.62). After adjusting for patient characteristics, these ratios were still significantly associated with health status. Thus, the depurinating estrogen-DNA adducts are possible biomarkers for early detection of breast cancer risk and response to preventive treatment.
Keywords: breast cancer risk, depurinating estrogen-DNA adducts, estrogen biomarkers, balance in estrogen metabolism
Development of noninvasive tests of breast cancer risk has been a major goal for more than 30 years. In this article we present bio-markers of risk that are related to the hypothesized first critical step in the initiation of breast cancer, namely, the reaction of catechol estrogen quinone metabolites with DNA.1 Prevention of cancer can be achieved by blocking this DNA damage, which generates the mutations leading to the initiation, promotion and progression of cancer.2
Exposure to estrogens is a known risk factor for breast cancer.3,4 The discovery that specific oxidative metabolites of estrogens, namely, catechol estrogen quinones, can react with DNA5–9 led to and supports the hypothesis that these metabolites can become endogenous chemical carcinogens. Some of the mutations generated by this specific DNA damage can result in the initiation of cancer.1,5 This paradigm suggests that specific, critical mutations generate abnormal cell proliferation leading to cancer.1,10–13
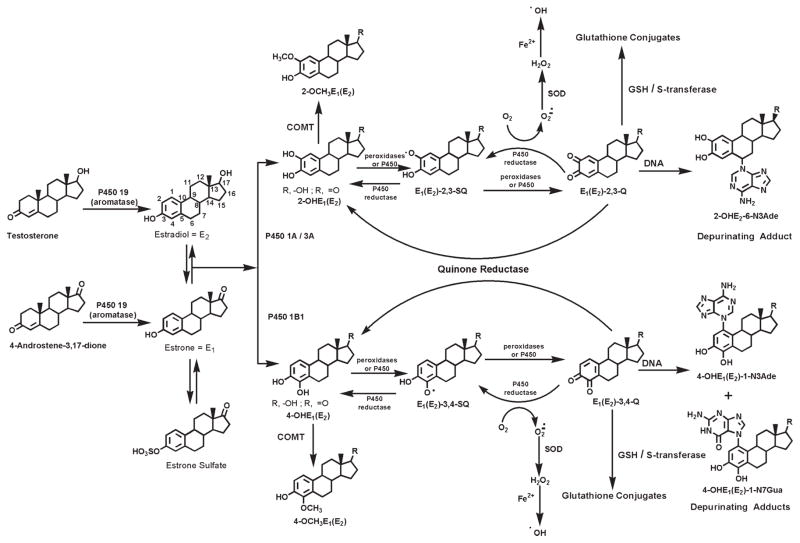
As illustrated in Figure 1, in the metabolism of catechol estrogens there are activating pathways14 that lead to the formation of the estrogen quinones, estrone (estradiol) quinones [E1(E2)-Q], which can react with DNA. There are also deactivating pathways that limit formation of the quinones and/or prevent their reaction with DNA. These are methylation of catechol estrogens,15 conjugation of the E1(E2)-Q with glutathione (GSH)16 and reduction of the quinones to catechols17 (Fig. 1).
FIGURE 1.
Biosynthesis and metabolic activation of the estrogens, E1 and E2. The metabolic activation of E1 and E2 leads to 2- and 4-catechol derivatives, which further oxidize to yield the corresponding reactive quinones. The quinones react with DNA to form depurinating DNA adducts. In the deactivation pathway, which operates in parallel, the catechol derivatives are methylated to form methoxy catechol estrogens; in addition, the quinones are reduced by quinone reductase, as well as are conjugated with GSH, and, thus, are rendered harmless. The shift in the apparent balance between these activating and deactivating pathways towards formation of depurinating DNA adducts could lead to the initiation of breast cancer.
When E1(E2)-3,4-Q react with DNA, they form predominantly the depurinating adducts 4-hydroxyestrone(estradiol)-1-N3Ade-nine [4-OHE1(E2)-1-N3Ade] and 4-hydroxyestrone(estradiol)-1-N7Guanine [4-OHE1(E2)-1-N7Gua],5–7 whereas E1(E2)-2,3-Q form much lower levels of 2-hydroxyestrone(estradiol)-6-N3Ade-nine [2-OHE1(E2)-6-N3Ade] (Figs. 1 and 2).7 Both E1(E2)-3,4-Q and E1(E2)-2,3-Q form much lower levels of stable DNA adducts than depurinating adducts.5–7 Once released from the DNA, the depurinating estrogen-DNA adducts are shed from cells into the bloodstream and, eventually, are excreted in urine.
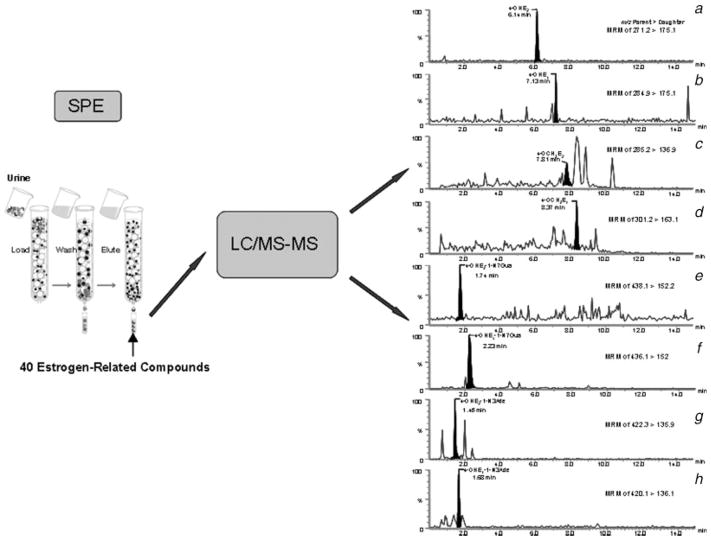
FIGURE 2.
Schematic representation of the steps carried out to purify by SPE and analyze by UPLC/MS-MS the estrogen-related compounds from urine samples. The UPLC/MS-MS chromatograms of (a) 4-OHE2, (b) 4-OHE1, (c) 4-OCH3E2, (d) 4-OCH3E1, (e) 4-OHE2-1-N7Gua, (f) 4-OHE1-1-N7Gua, (g) 4-OHE2-1-N3Ade and (h) 4-OHE1-1-N3Ade that are shown in the figure are representatives from the 40 different estrogen-related compounds seen in the urine samples.
The release of the depurinating adducts generates apurinic sites in DNA, which in turn, may induce mutations. The observation of Harvey-ras mutations within 6–12 hr after treatment of mouse skin or rat mammary glands with E2-3,4-Q suggests that these mutations arise via error-prone base excision repair.1,10,11 Similar patterns of mutations have also been observed in the big blue (BB) rat mammary gland and cultured BB rat2 embryonic cells after treatment with 4-hydroxyestradiol (4-OHE2) or E2-3,4-Q.1,12 The transforming activity of E2 and 4-OHE2 has been observed in human breast epithelial (MCF-10F) cells, which do not contain estrogen receptor-α, and it is not affected by the presence of an anti-estrogen.18–20 Furthermore, 4-OHE1(E2) are carcinogenic in the Syrian golden hamster and CD-1 mouse.21–24 All of these results support the hypothesis that estrogens initiate cancer through their genotoxicity.
Initiation of cancer by estrogens is based on estrogen metabolism in which the homeostatic balance between activating and deactivating pathways is disrupted (Fig. 1). Activating pathways are the ones that oxidize E1 and E2 to their catechol estrogen quinones, whereas the deactivating pathways are the ones that block oxidation.1 A variety of factors, such as diet, environment and lifestyle, can unbalance the equilibrium between these 2 pathways. When estrogen metabolism is balanced, the level of estrogen-DNA adducts in tissue and urine is low and/or the levels of estrogen metabolites and conjugates are high. In contrast, when estrogen metabolism is unbalanced, the level of DNA adducts in tissue and urine is high and/or the levels of estrogen metabolites and conjugates are low. It is this imbalance in estrogen metabolism, leading to relatively high levels of estrogen-DNA adducts, that may be a critical determinant of breast cancer initiation.
The above considerations led us to hypothesize that estrogen metabolites, conjugates and depurinating DNA adducts may differ between healthy women and women with breast cancer or at high risk of breast cancer. To test this hypothesis, we conducted a cross-sectional study in which 40 estrogen metabolites, conjugates and depurinating DNA adducts were analyzed in urine samples from healthy women, women at high risk for breast cancer based on Gail Model score >1.66%, and women with breast carcinoma. The Gail Model takes into account the following factors: age, age at menarche, age at first live birth, number of breast biopsies and history of atypical hyperplasia, number of first degree relatives with breast cancer (mother, sister and daughter) and race. A 5-year Gail Model score of >1.66% is considered high risk.25
Material and methods
Materials
Phenyl solid phase extraction (SPE) cartridges were purchased from Varian (Palo Alto, CA). Androstenedione (1), (Table I), testosterone (2), estrone (E1) sulfate (3), E2 (4), E1 (5), 2-OHE2 (6), 2-OHE1 (7), 16α-OHE2 (10), 16α-OHE1 (11), 2-OCH3E2 (12), 2-OCH3E1 (13), 4-OCH3E2 (14), 4-OCH3E1 (15), 2-OH-3-OCH3E2 (16) and 2-OH-3-OCH3E1 (17) were purchased from Steraloids (Newport, RI). 4-OHE2 (8) and 4-OHE1 (9) were synthesized as previously described.26 2-OHE2-1-SG (18), 2-OHE2-4-SG (19), 2-OHE1-1-SG (20), 2-OHE1-4-SG (21), 2-OHE2-(1+4)-Cys (22), 2-OHE1-1-Cys (23), 2-OHE1-4-Cys (24), 2-OHE2-1-NAcCys (25), 2-OHE2-4-NAcCys (26), 2-OHE1-1-NAcCys (27), 2-OHE1-4-NAcCys (28), 4-OHE2-2-SG (29), 4-OHE1-2-SG (30), 4-OHE2-2-Cys (31), 4-OHE1-2-Cys (32), 4-OHE2-2-NAcCys (33) and 4-OHE1-2-NAcCys (34) were synthesized by using the procedure of Cao et al.27 4-OHE2-1-N7Gua (35), 4-OHE1-1-N7Gua (36), 4-OHE2-1-N3Ade (37), 4-OHE1-1-N3Ade (38), 2-OHE2-6-N3Ade (39) and 2-OHE1-6-N3Ade (40) were synthesized by following the reported methods.6,7,28 All solvents were HPLC grade and all other chemicals used were of the highest grade available.
TABLE I.
MASS SPECTROMETRIC PARAMETERS1
| No. | Compound | Mass | ESI mode | Parent (m/z) | Daughters (m/z) | Cone (volt) | Collision | Retention time | LOD (fmol) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Androstenedione | 286.2 | Positive | 287.1 | 97.1 | 40 | 19 | 8.43 | 14 |
| 2 | Testosterone | 288.2 | Positive | 289.2 | 97.0 | 40 | 19 | 7.97 | 35 |
| 3 | E1-Sulfate | 350.1 | Negative | 249.0 | 269.0 | 50 | 28 | 6.61 | 143 |
| 4 | E2 | 272.4 | Positive | 273.2 | 135.2 | 30 | 14 | 7.74 | 184 |
| 5 | E1 | 270.1 | Positive | 271.2 | 253.2 | 25 | 14 | 8.43 | 148 |
| 6 | 2-OHE2 | 288.2 | Positive | 271.2 | 175.1 | 30 | 14 | 6.74 | 69 |
| 7 | 2-OHE1 | 286.2 | Negative | 285.0 | 160.9 | 65 | 37 | 7.3 | 18 |
| 8 | 4-OHE2 | 288.2 | Positive | 271.2 | 175.1 | 30 | 14 | 6.14 | 69 |
| 9 | 4-OHE1 | 286.2 | Negative | 284.9 | 161.0, 175.1 | 65 | 35 | 7.13 | 35 |
| 10 | 16α-OHE2 | 288.4 | Positive | 289.0 | 107.0 | 25 | 14 | 2.42 | 867 |
| 11 | 16α-OHE1 | 286.4 | Negative | 285.1 | 145.1 | 30 | 15 | 4.67 | 349 |
| 12 | 2-OCH3E2 | 302.2 | Positive | 285.2 | 136.9, 189.1 | 32 | 15 | 8.25 | 330 |
| 13 | 2-OCH3E1 | 300.2 | Positive | 301.2 | 136.9, 163.1 | 30 | 17 | 8.85 | 333 |
| 14 | 4-OCH3E2 | 302.2 | Positive | 285.2 | 136.9, 189.1 | 32 | 15 | 7.81 | 66 |
| 15 | 4-OCH3E1 | 300.2 | Positive | 301.2 | 163.1, 283.1 | 30 | 17 | 8.37 | 133 |
| 16 | 2-OH-3-OCH3E2 | 302.4 | Positive | 285.2 | 189.1 | 32 | 15 | 8.71 | 165 |
| 17 | 2-OH-3-OCH3E1 | 300.4 | Positive | 301.2 | 163.1 | 30 | 17 | 9.07 | 33 |
| 18 | 2-OHE2-1-SG | 593.7 | Positive | 594.1 | 319.1, 465.0 | 42 | 20 | 1.72 | 8.4 |
| 19 | 2-OHE2-4-SG | 593.7 | Positive | 594.0 | 319.1, 465.4 | 35 | 21 | 2.32 | 8.4 |
| 20 | 2-OHE1-1-SG | 591.0 | Positive | 592.1 | 316.8 | 45 | 22 | 2.65 | 1.7 |
| 21 | 2-OHE1-4-SG | 591.0 | Positive | 592.2 | 317.1, 463.2 | 40 | 22 | 2.65 | 1.7 |
| 22 | 2-OHE2-1+4-Cys | 407.2 | Positive | 408.2 | 319.0 | 30 | 17 | 1.73 | 12 |
| 23 | 2-OHE1-1-Cys | 405.2 | Positive | 406.0 | 316.9 | 35 | 15 | 3.25 | 6.2 |
| 24 | 2-OHE1-4-Cys | 405.2 | Positive | 406.2 | 317.1 | 30 | 17 | 3.25 | 6.2 |
| 25 | 2-OHE2-1-NAcCys | 449.2 | Positive | 450.1 | 162.0, 287.4 | 25 | 14 | 4.07 | 5.6 |
| 26 | 2-OHE2-4-NAcCys | 449.2 | Positive | 450.2 | 162.0, 287.2 | 30 | 14 | 4.07 | 5.6 |
| 27 | 2-OHE1-1-NAcCys | 447.2 | Positive | 448.1 | 162.0, 285.4 | 30 | 13 | 6.05 | 5.6 |
| 28 | 2-OHE1-4-NAcCys | 447.2 | Positive | 448.0 | 162.0, 284.9 | 35 | 14 | 6.05 | 5.6 |
| 29 | 4-OHE2-2-SG | 593.2 | Positive | 594.4 | 318.9, 464.8 | 42 | 20 | 2.33 | 8.4 |
| 30 | 4-OHE1-2-SG | 591.2 | Positive | 592.3 | 317.1, 462.9 | 45 | 22 | 2.65 | 8.5 |
| 31 | 4-OHE2-2-Cys | 407.2 | Positive | 408.0 | 318.9, 286.1 | 40 | 16 | 2.24 | 2.4 |
| 32 | 4-OHE1-2-Cys | 405.2 | Positive | 406.0 | 316.9, 389.0 | 35 | 15 | 2.84 | 6.2 |
| 33 | 4-OHE2-2-NAcCys | 449.2 | Positive | 450.1 | 162.1 | 35 | 15 | 5.91 | 5.6 |
| 34 | 4-OHE1-2-NAcCys | 447.2 | Positive | 448.3 | 161.8 | 35 | 14 | 6.64 | 2.2 |
| 35 | 4-OHE2-1-N7Gua | 437.2 | Positive | 438.1 | 152.2, 272.0 | 62 | 38 | 1.74 | 2.3 |
| 36 | 4-OHE1-1-N7Gua | 435.2 | Positive | 436.1 | 152.0, 271.9 | 62 | 38 | 2.23 | 2.2 |
| 37 | 4-OHE2-1-N3Ade | 421.2 | Positive | 422.3 | 135.9, 257.1 | 62 | 45 | 1.45 | 5.9 |
| 38 | 4-OHE1-1-N3Ade | 419.2 | Positive | 420.1 | 296.0, 136.1 | 60 | 44 | 1.68 | 2.4 |
| 39 | 2-OHE2-6-N3Ade | 421.1 | Positive | 422.2 | 136.0, 287.0 | 26 | 10 | 1.05 | 1.2 |
| 40 | 2-OHE1-6-N3Ade | 419.1 | Positive | 420.0 | 135.9 | 26 | 10 | 1.41 | 2.4 |
List of the 40 estrogen-related compounds with the masses of parent and daughter ions and the ionization mode that were used for MRM method optimization. The last column indicates the limit of detection for each compound.
Study population
We collected urine from 75 women at 3 different sites: (i) at the Center for Mammographic Screening at the University of Naples, Italy (42 women), (ii) at the Breast Diagnostic Clinic and Oncology Breast Clinic of the Mayo Clinic, Rochester, MN (18 women) and (iii) at the Olson Center for Women’s Health, University of Nebraska Medical Center (UNMC), Omaha, NE (15 women). Women were recruited between March 2005 and September 2006 and their ages ranged between 34 and 73 years—healthy women: range, 34–67; mean, 50 ± 8; high-risk women: range, 44–64; mean, 52± 6; women with breast cancer: range, 34–73; mean, 54 ± 10.
All women recruited at the University of Naples were healthy (they did not receive a diagnosis of breast cancer at the time of their mammographic test). Among the women recruited at the Mayo Clinic, 12 were classified as high-risk women (Gail Model score = 1.67%–11.7%) and 6 were breast cancer cases. At UNMC, 4 women were healthy, that is, had no known cancer, and 11 were diagnosed with breast cancer. None of the subjects received estrogen-containing treatment for at least 3 months prior to providing a urine sample. The 3 groups were frequency matched on age, race and menopausal status.
All procedures were approved by the University of Naples, Mayo Clinic and UNMC Institutional Review Boards. Signed consents included authorization to collect and bank urine samples and collect demographic and clinical information.
Sample collection
A standardized method was followed to collect all of the urine samples. A spot urine sample of about 50 ml was collected from each participant and 1 mg/ml ascorbic acid was added to prevent oxidation of the catechol moieties in the various estrogen compounds. The urine samples were aliquoted, frozen and four 10-ml aliquots were transferred to the Eppley Institute, UNMC, on dry ice and were stored at −80°C until analysis. Thus, each analytical sample was thawed only once prior to analysis.
Solid-phase extraction of urine
Two milliliter aliquots of urine were partially purified by SPE. The SPE was performed using a 20-port SPE vacuum manifold with phenyl cartridges (Fig. 2). Urine samples were adjusted to pH 7 with 1 M NaOH or 1 M HCl. For method development and validation, 2-ml aliquots of charcoal-treated human urine samples were spiked with a total of 250, 500 or 1,000 pg of the 40 estrogen-related compounds (final concentration 0.125, 0.25 and 0.50 pg/μl) and loaded onto the phenyl 100-mg cartridges preconditioned with CH3OH and the loading buffer, 10 mM ammonium formate, pH 7. The cartridges were washed with the loading buffer, and then the compounds of interest were eluted from the cartridge by using an elution buffer, methanol/10 mM ammonium formate, pH 7 (90:10) with 1% acetic acid. This procedure led to enrichment of the 40 estrogen-related compounds after elution. Charcoal-treated urine (2 ml) was used in controls, and the eluates from the SPE cartridges were spiked with 250, 500 or 1,000 pg of the 40 estrogen-related compounds. The eluates from both the experimental and control samples were concentrated using a Speed-Vac and lypholizer, and subjected to ultraperformance liquid chromatography/tandem mass spectrometry (UPLC/MS-MS) analysis. To determine the recovery of the standards by the SPE method, comparison was made between the corresponding concentrations of experimental and control samples (Fig. 3). Study samples were cleaned in duplicate by using the above optimized SPE conditions and analyzed by UPLC/MS-MS.
FIGURE 3.
SPE recovery of standard 40 estrogen-related compounds. The 2-ml aliquots of activated charcoal-treated human urine samples were spiked with the total (a) 250, (b) 500 and (c) 1,000 pg of 40 estrogen-related compounds before and after (control) passing over phenyl SPE cartridges. The recovery of each compound was determined by comparing the experimental values to the controls.
UPLC/MS-MS analysis of urine samples
The 40 analytes (Table I) included the androgens androstenedione and testosterone; the estrogens E1 sulfate, E1 and E2; the catechol estrogens 2-OHE1(E2) and 4-OHE1(E2); the 16α-OHE1(E2); the methylated 2- and 4-catechol estrogens; the 2- and 4-catechol estrogens conjugated with GSH, cysteine (Cys) or N-acetylcys-teine (NAcCys); and the depurinating DNA adducts of 4-OHE1(E2) and 2-OHE1(E2). All of the estrogen compounds were analyzed as both E1 and E2 derivatives because the interconversion of these 2 estrogens is carried out continuously by 17β-estradiol dehydrogenase.
All experiments were performed on a Waters (Milford, MA) Quattro Micro triple quadrupole mass spectrometer by using electrospray ionization (ESI) in positive ion (PI) and negative ion (NI) mode, with an ESI-MS capillary voltage of 3.0 kV, an extractor cone voltage of 2 V, and a detector voltage of 650 V. Desolvation gas flow was maintained at 600 l/h. Cone gas flow was set at 60 l/h. Desolvation temperature and source temperature were set to 200 and 100°C, respectively. For all the studies, a methanol:-water (1:1) mixture with 0.1% formic acid was used as the carrier solution. ESI interface tuning and mass calibration were accomplished in the PI mode by using a standard sodium iodide-rubidium iodide solution. The test sample (compounds 1 through 40) was introduced to the source at a flow rate of 10 μl/min by using an inbuilt pump. PI or NI detection was used in cases where the sample was readily ionized to cation or anion, respectively. The masses of parent ion and daughter ions were obtained in the MS and MS-MS operations. The parent and daughter ion data obtained for each compound were used to generate the multiple reaction monitoring (MRM) method for UPLC/MS-MS operation (Table I).
Measurements of estrogen-related compounds in urine extracts were conducted by using UPLC/MS-MS. UPLC/MS-MS analyses were carried out with a Waters Acquity UPLC system connected with the high-performance Quattro Micro triple quadrupole mass spectrometer. Analytical separations on the UPLC system were conducted using an Acquity UPLC BEH C18 1.7 μm column (1 × 100 mm) at a flow rate of 0.15 ml/min. The gradient started with 80% A (0.1% formic acid in H2O) and 20% B (0.1% formic acid in CH3CN), changed to 79% A over 4 min, followed by a 6-min linear gradient to 45% A, resulting in a total separation time of 10 min. The elutions from the UPLC column were introduced to the Quattro Micro mass spectrometer.
The ionization method used for MS analysis was ESI in both the PI and NI mode. MS-MS was performed in the MRM mode (see above), and resulting data were processed by using QuanLynx software (Waters) to quantify the estrogen metabolites. To calculate limits of detection, various concentrations, 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, 10, 25, 50 and 100 pg/μl, of the analyte were injected to UPLC/MS-MS. The injected amount that resulted in a peak with a height at least 2 or 3 times as high as the baseline noise level was used as the limit of detection (Table I). Pure standards were used to optimize the UPLC/MS conditions prior to analysis. After UPLC analysis, the mean value was calculated for all the compounds obtained from each sample.
Statistical methods
Estrogen-related compounds were compared for control versus high risk and for control versus breast cancer using a Mann–Whitney test, with p-values adjusted for the 2 multiple comparisons using the Bonferroni method. To account for the multiple hypothesis tests conducted for these variables, a p-value <0.01 was interpreted as statistically significant. The log-transformed sum of the ratios of depurinating adducts to the corresponding metabolites and conjugates was compared using a one way ANOVA, and post hoc comparisons were made using the method of Bonferroni. Linear regression was used to assess the association between disease status and ratio adjusted for age at recruitment, age at menarche, menopausal status (categorical) and parity for the 56 subjects with patient characteristics available. All the statistics and p-values were calculated using SPSS software (SPSS, Chicago, IL).
Results and discussion
Analysis of urine samples
After partial purification of the urine samples by SPE (Fig. 2), we analyzed the 40 estrogen-related compounds using UPLC/MS-MS. The advantage of having MS detector in MRM mode over conventional high pressure liquid chromatography analysis is that number of channels in the detector could be set to specifically and separately identify all the estrogen related compounds (Fig. 2). Each metabolite was detected and identified based on the parameters that are unique to them, such as mass (parent and daughter), retention time and ionization mode (positive and negative) (Table I). The typical spectra of representative estrogen derivatives, which were obtained in a single injection, are shown in Figure 2. The levels of estrogen-related compounds for a high risk woman, measured from single injection, are presented in Table II.
TABLE II.
REPRESENTATIVE METABOLIC PROFILE OF A URINE SAMPLE OBTAINED FROM A HIGH RISK WOMAN.1
| No. | Compound | pmole/mg creatinine mean, n = 2 | Total pmole/mg creatinine |
|---|---|---|---|
| 1 | Androstenedione | 1.56 | 1.56 |
| 2 | Testosterone | 0.24 | 0.24 |
| 3 | E14 Sulfate | 1.81 | 1.81 |
| 4 | E24 | 5.29 | 15.93 |
| 5 | E1 | 10.64 | |
| 6 | 2-OHE2 | 3.09 | 3.15 |
| 7 | 2-OHE1 | 0.05 | |
| 8 | 4-OHE2 | 2.64 | 2.91 |
| 9 | 4-OHE1 | 0.27 | |
| 10 | 16α-OHE2 | 12.12 | 38.64 |
| 11 | 16α-OHE1 | 26.52 | |
| 12 | 2-OCH3E2 | 1.95 | 49.81 |
| 13 | 2-OCH3E1 | 47.87 | |
| 14 | 4-OCH3E2 | 0.41 | 5.08 |
| 15 | 4-OCH3E1 | 4.67 | |
| 16 | 2-OH-3-OCH3E2 | 1.91 | 10.27 |
| 17 | 2-OH-3-OCH3E1 | 8.36 | |
| 18 | 2-OHE2-1-SG | 0.17 | 3.105 |
| 19 | 2-OHE2-4-SG | 0.17 | |
| 20 | 2-OHE1-1-SG | 0.49 | |
| 21 | 2-OHE1-4-SG | 0.47 | |
| 22 | 2-OHE2-1+4-Cys | 0.27 | |
| 23 | 2-OHE1-1-Cys | 0.10 | |
| 24 | 2-OHE1-4-Cys | 0.44 | |
| 25 | 2-OHE2-1-NAcCys | 0.07 | |
| 26 | 2-OHE2-4-NAcCys | 0.07 | |
| 27 | 2-OHE1-1-NAcCys | 0.43 | |
| 28 | 2-OHE1-4-NAcCys | 0.43 | |
| 29 | 4-OHE2-2-SG | 0.51 | 1.776 |
| 30 | 4-OHE1-2-SG | 0.50 | |
| 31 | 4-OHE2-2-Cys | 0.13 | |
| 32 | 4-OHE1-2-Cys | 0.06 | |
| 33 | 4-OHE2-2-NAcCys | 0.29 | |
| 34 | 4-OHE1-2-NAcCys | 0.28 | |
| 35 | 4-OHE2-1-N7Gua | 0.48 | 2.81 |
| 36 | 4-OHE1-1-N7Gua | 2.33 | |
| 37 | 4-OHE2-1-N3Ade | 137.78 | 137.90 |
| 38 | 4-OHE1-1-N3Ade | 0.13 | |
| 39 | 2-OHE2-6-N3Ade | 0.06 | 0.07 |
| 40 | 2-OHE1-6-N3Ade | 0.02 | |
| (Ratio-4)2 × 1,000 | 935 | ||
| (Ratio-2)3 × 1,000 | 1 | ||
| (Ratio-4) + (Ratio-2) × 1,000 | 936 | ||
Typically, each 2-ml urine sample was analyzed at least 2 times. The data obtained from LC/MS-MS were processed and normalized to creatinine levels. Since the E1 and E2 derivatives are interconvertible, the total amount for each E1 plus E2 derivative in the various categories are presented in the last column and used for calculating the final ratios of depurinating adducts to the respective metabolites and conjugates.
Free E2 and E1 in the urine sample.
All 2-OHE1(E2) conjugates.
All 4-OHE1(E2) conjugates.
Treatment of urine with glucuronidase/sulfatase led to significant increases (10 to 20-fold) in the levels of E1 and E2, while the levels of estrogen metabolites, conjugates and adducts changed marginally and in many cases decreased because of the incubation for 8 hr at 37°C. To avoid artifacts and errors that are introduced by maintaining the urine samples at 37°C for 8 hr, we carried out all the analyses without glucuronidase/sulfatase treatment. Therefore, the observed levels of E1 and E2, as reported in Table II, for example, were 10 to 20-fold lower than the total values. Since estrone and estradiol are constantly inter-converting, we have combined estrone and estradiol values of all the derivatives (Tables II and III). The GSH conjugates of estrogen quinones are further converted to Cys and NAcCys conjugates via the mercapturic acid biosynthesis pathway.29 Hence we have combined all the values of 2 conjugates and 4 conjugates (Tables II and III), which reflect the total protection by GSH from 2 or 4 quinones, respectively. The results presented here clearly demonstrate the ability of SPE combined with UPLC/MS-MS analysis to resolve, identify and quantify 40 estrogen-related compounds with accuracy and speed.
TABLE III.
URINARY LEVELS OF ESTROGEN COMPOUNDS IN HEALTHY WOMEN, HIGH-RISK WOMEN AND WOMEN WITH BREAST CANCER
| No. | Compound | Control (n = 46)
|
High Risk (n = 12)
|
Breast Cancer (n = 18)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Min–Max | Median | Min–Max | p-value3 | Median | Min–Max | p-value4 | ||
| 1 | Androstenedione | 9.9 | 2.1–108 | 4.2 | 1.3–11.5 | 0.0033 | 5.5 | 0.4–95.1 | 0.047 |
| 2 | Testosterone | 2.2 | 0.2–16.5 | 0.8 | 0.2–2.8 | 0.0085 | 1.1 | 0.5–3.7 | 0.050 |
| 3 | E1-Sulfate | 5.0 | 0.1–382 | 2.4 | 0.1–10.6 | 0.087 | 1.1 | 0.1–121 | 0.032 |
| 4 | E2 | 31.7 | 9.1–3865 | 11.4 | 4.7–80.0 | 0.007 | 28.1 | 3.6–151 | 0.943 |
| 5 | E1 | ||||||||
| 6 | 2-OHE2 | 10.4 | 1.7–564 | 7.3 | 1.6–26.5 | 0.035 | 5.6 | 0.0–38.8 | 0.006 |
| 7 | 2-OHE1 | ||||||||
| 8 | 4-OHE2 | 12.4 | 2.4–157 | 8.1 | 2.9–43.3 | 0.138 | 5.2 | 0.0–28.0 | 0.008 |
| 9 | 4-OHE1 | ||||||||
| 10 | 16α-OHE2 | 168 | 10.3–638 | 33.7 | 0.0–279 | 0.001 | 10.9 | 0.0–86.3 | <0.001 |
| 11 | 16α-OHE1 | ||||||||
| 12 | 2-OCH3E2 | 49.7 | 4.7–568 | 31.1 | 6.5–452 | 0.275 | 26.8 | 2.2–171 | 0.044 |
| 13 | 2-OCH3E1 | ||||||||
| 14 | 4-OCH3E2 | 73.1 | 12.6–3979 | 5.9 | 1.6–37.1 | <0.001 | 21.5 | 4.5–53.2 | <0.001 |
| 15 | 4-OCH3E1 | ||||||||
| 16 | 2-OH-3-OCH3E2 | ||||||||
| 17 | 2-OH-3-OCH3E1 | ||||||||
| 18 | 2-OHE2-1-SG | 11.21 | 0.8–79.81 | 4.61 | 1.2–18.71 | 0.0051 | 3.11 | 1.1–16.91 | 0.0011 |
| 19 | 2-OHE2-4-SG | ||||||||
| 20 | 2-OHE1-1-SG | ||||||||
| 21 | 2-OHE1-4-SG | ||||||||
| 22 | 2-OHE2-1+4-Cys | ||||||||
| 23 | 2-OHE1-1-Cys | ||||||||
| 24 | 2-OHE1-4-Cys | ||||||||
| 25 | 2-OHE2-1-NAcCys | ||||||||
| 26 | 2-OHE2-4-NAcCys | ||||||||
| 27 | 2-OHE1-1-NAcCys | ||||||||
| 28 | 2-OHE1-4-NAcCys | ||||||||
| 29 | 4-OHE2-2-SG | 2.72 | 0.7–24.62 | 1.42 | 0.6–6.82 | 0.0322 | 1.32 | 0.4–8.92 | 0.0272 |
| 30 | 4-OHE1-2-SG | ||||||||
| 31 | 4-OHE2-2-Cys | ||||||||
| 32 | 4-OHE1-2-Cys | ||||||||
| 33 | 4-OHE2-2-NAcCys | ||||||||
| 34 | 4-OHE1-2-NAcCys | ||||||||
| 35 | 4-OHE2-1-N7Gua | 0.7 | 0.0–4.8 | 1.2 | 0.2–2106 | 0.238 | 1.6 | 0.4–11.8 | 0.007 |
| 36 | 4-OHE1-1-N7Gua | ||||||||
| 37 | 4-OHE2-1-N3Ade | 0.7 | 0.0–18.8 | 1.8 | 0.5–138 | 0.007 | 1.2 | 0.1–288 | 0.085 |
| 38 | 4-OHE1-1-N3Ade | ||||||||
| 39 | 2-OHE2-6-N3Ade | 0.1 | 0.0–6.5 | 0.1 | 0.0–0.7 | 0.999 | 0.1 | 0.0–5.4 | 0.960 |
| 40 | 2-OHE1-6-N3Ade | ||||||||
All 2-OHE1(E2) conjugates.
All 4-OHE1(E2) conjugates.
Bonferroni-adjusted p-value for comparing control versus high risk using Mann–Whitney test.
Bonferroni-adjusted p-value for comparing control versus breast cancer by using Mann–Whitney test.
Significant p-values are shown in bold.
The values obtained for the various estrogen-related compounds in 3 groups of women were processed in 2 different ways. First, median values were calculated for all the compounds and their levels were examined in the 3 groups of women (Table III). Then, we used the ratio of depurinating N3Ade and N7Gua adducts to the sum of their respective estrogen metabolites and conjugates in urine samples because the ratio reflects the degree of imbalance in estrogen metabolism that can lead to cancer initiation (Fig. 4). A high ratio of adducts to their respective metabolites and conjugates represents relatively more DNA damage. In contrast, a low ratio of adducts to their respective metabolites and conjugates means that relatively little of the estrogen metabolites reacted with DNA.
FIGURE 4.

Median values of the urinary estrogen-related compounds in the 3 groups of women
Using the newly developed SPE/UPLC/MS-MS methodology, we have analyzed urine samples of various women’s groups for estrogen-related compounds. The data obtained were used to calculate median values for each of the 40 compounds (Table III).
The median andostenedione, testosterone, E2/E1, 16α-OHE2/ 16α-OHE1, 4-OCH3E2/4-OCH3E1, 2-OHE1(E2) GSH conjugate and derivative values were higher for controls compared to high risk participants, and the median 4-OHE2-1-N3Ade/4-OHE1-1-N3Ade values were lower for controls compared to high risk participants. Compared to breast cancer participants, the median 2-OHE2/2-OHE1, 4-OHE2/4-OHE1, 16α-OHE2/16α-OHE1, 4-OCH3E2/4-OCH3E1, 2-OHE1(E2) GSH conjugate and derivative values were higher for controls, while the median 4-OHE2-1-N7Gua/4-OHE1-1-N7Gua values were lower for controls. Of particular interest are the significantly lower levels of the methoxycatechol estrogens in the women with breast cancer or at high risk compared to the control women, because this represents a major protective pathway in estrogen metabolism. In addition, the levels of the 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua adducts are higher in the women with breast cancer or at high risk than in the control women, although only 2 of the differences are statistically significant.
Depurinating estrogen-DNA adducts in the 3 groups of women
In the second analysis, the ratios of depurinating N3Ade and N7Gua adducts to the sum of estrogen metabolites and conjugates in urine samples from healthy control women are generally low (Fig. 4). In contrast, high ratios of these adducts to estrogen metabolites and conjugates were observed in urine from high-risk women (Gail Model score >1.66%) and women with breast carcinoma. In general, the value obtained from the high-risk women and women with breast carcinoma derives from the ratio between a high level of adducts and low levels of metabolites and conjugates. In some women, however, the level of adducts was not particularly high, but the levels of metabolites and conjugates were very low, suggesting that a substantial proportion of the metabolites was converted to adducts.
In the sum of the ratios of depurinating adducts to estrogen metabolites and conjugates, the preponderant role is played by the N3Ade and N7Gua adducts of 4-OHE1(E2), whereas the adducts of 2-OHE1(E2) play a very minor role. For example, for the high-risk subject presented in Table II, the overall adduct ratio is 936, but the contribution of 2-OHE1(E2)-6-N3Ade is 1, whereas the contribution of 4-OHE1(E2)-1-N3Ade plus 4-OHE1(E2)-1-N7Gua is 935. In general, the average contribution of the 2-OHE1(E2)-6-N3Ade adducts is ~2.5% of the total, whereas the predominant contribution of ~97.5% derives from the 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua adducts. The observation of high levels of depurinating estrogen-DNA adducts in urine from high-risk women, as well as subjects with breast carcinoma (Fig. 4), is consistent with the hypothesis that these adducts are a causative factor in the etiology of breast cancer.
Analysis by subject characteristics
We first analyzed the data using the ratio of depurinating N3Ade and N7Gua adducts to the sum of their respective estrogen metabolites and conjugates in urine samples as a continuous variable. Analysis using one-way ANOVA revealed a significant difference among the groups (p < 0.001). Additional post hoc analysis using a Bonferroni correction for multiple comparisons revealed significantly higher means for high risk subjects [mean 336.45, standard deviation (SD) 331.92] compared to controls (mean 20.51, SD 37.01, p < 0.001) and for breast cancer patients (mean 176.28, SD 205.68, p < 0.001). The mean for patients known to be at high risk was not significantly different from that of the breast cancer group (p = 0.62).
A limitation of the study is that most of the group of healthy women (42 of 46) were Italian, whereas the remaining healthy women, high-risk women and women with breast cancer were American. All of the subjects in our study, however, were Caucasian. The 3 groups (healthy, high-risk and breast cancer) had similar mean age at recruitment, mean age at menarche and menopausal status (Table IV). These similarities in subject characteristics support the validity of comparing the ratios of adducts to their respective metabolites and conjugates in these 3 groups of women.
TABLE IV.
SUBJECT CHARACTERISTICS
| Characteristic | Health status
|
||
|---|---|---|---|
| Healthy (n = 37) | High risk (n = 12) | Breast cancer (n = 7) | |
| Age at recruitment in years, mean (SD) | 49 (7.85) | 52 (6.09) | 57 (12.16) |
| Age at menarche in years, mean (SD) | 12 (1.45) | 12 (1.44) | 13 (1.25) |
| Menopausal Status, n (%) | |||
| Premenopausal | 17 (46%) | 6 (50%) | 3 (43%) |
| Postmenopausal | 20 (54%) | 6 (50%) | 4 (57%) |
| Parity | |||
| 0 | 6 (16%) | 0 (0%) | 0 (0%) |
| 1 | 3 (8%) | 0 (0%) | 0 (0%) |
| 2 | 13 (35%) | 7 (58%) | 4 (57%) |
| 3 | 11 (30%) | 3 (25%) | 0 (0%) |
| ≥4 | 4 (11%) | 2 (17%) | 3 (43%) |
Subject characteristics of age at recruitment, age at menarche, menopausal status, and parity were available for 56 of the 75 subjects (Table IV). The mean age of our entirely Caucasian sample was 50 years (SD 8.5). The average age at menarche was 12.0 years (SD 1.4). Only 11% of the women were nulliparous and 43% had at least 2 children. Twenty-six (46%) women were pre-menopausal at recruitment, 30 (54%) were postmenopausal (they did not have menstrual cycles in the last 12 months before recruitment). Analysis using one way ANOVA revealed that health status, that is breast cancer cases versus high risk and healthy individuals, was significantly associated with age at recruitment (p = 0.048). Specifically, the mean age (years) at recruitment for healthy women was 49 (SD 7.8), 52 (SD 6.1) for women at high risk and 57 (SD 12.2) for breast cancer cases. Age at menarche was not statistically different across the disease status groups (p = 0.534). Analysis using a χ2 test did not reveal an association between health status and menopausal status (p = 0.95) or parity (parous vs. nulliparous) (p = 0.15).
The correlation coefficient was used to examine the association between the ratio and subject characteristics. We observed evidence of significant correlation between parity and ratio (r = 0.36, p = 0.007) and marginally significant correlation between the ratio and menopausal status (r = 0.26, p = 0.06). Age at recruitment and age at menarche were not significantly associated with the ratio.
Linear regression was used to assess the association between disease status and ratio adjusted for age at recruitment, age at menarche, menopausal status (categorical) and parity for the 56 subjects with patient characteristics available (Table V). After accounting for these characteristics, the ratio was significantly associated with health status. Specifically, the multivariate coefficient for disease status (108.6) was statistically significant (p = 0.007) in a model that explained 10% (p = 0.040) of variance in the ratio after accounting for covariates. All other covariates did not reach the usual level of significance of 0.05 (Table V).
TABLE V.
RESULTS OF UNIVARIATE MULTIVARIATE LINEAR REGRESSION OF RATIO
| Covariate | Univariate regression
|
Multivariate regression
|
||
|---|---|---|---|---|
| Regression coefficient | p-value | Regression coefficient | p-value | |
| Health status | 103.60 | 0.005 | 108.56 | 0.007 |
| Postmenopausal | 35.66 | 0.51 | 41.18 | 0.44 |
| Parity | 15.01 | 0.42 | −7.29 | 0.71 |
Interpretation of results
The observation of high ratios of depurinating estrogen-DNA adducts to their corresponding metabolites and conjugates in urine samples from both high-risk women and women with breast cancer supports the hypothesis that formation of estrogen-DNA adducts is the first critical step in the initiation of breast cancer.1 In addition, these results suggest that this assay may provide a diagnostic tool for early detection of breast cancer risk. At this point, we do not know how far in advance this assay would predict the development of a detectable tumor. Further studies are required to address this question.
In addition, we can hypothesize that the ratio of depurinating estrogen-DNA adducts to their metabolites and conjugates can be used to monitor the efficacy of putative preventive compounds in balancing estrogen activation and deactivation. Minimizing formation of catechol estrogen quinones and/or their reaction with DNA should reduce the risk of developing breast cancer.
Conclusions
UPLC/MS-MS can be used to analyze depurinating estrogen-DNA adducts, estrogen metabolites and estrogen conjugates in 2-ml urine specimens. The ratio of adducts to their corresponding metabolites and conjugates provides a biomarker that can be used to distinguish women known to be at high risk of developing breast cancer (Gail Model score >1.66%) and those with breast cancer from healthy control women. The development of such bio-markers could be invaluable in assessing breast cancer risk and response to preventive treatment.
Acknowledgments
Grant sponsor: National Cancer Institute (U.S. Public Health Service); Grant numbers: P01 CA49210, P30 CA36727, P30 CA15083; Grant sponsor: U.S. Army (Breast Cancer Research Program); Grant number: DAMD 17-03-1-0229; Grant sponsor: National Institutes of Health; Grant number: P30 CA036727-22; Grant sponsor: AIRC (Associazione Italiana Ricerca sul Cancro), Milan, Italy.
The authors thank Dr. M. Saeed and Dr. M. Zahid for the synthesis of estrogen metabolites, conjugates and DNA adducts.
Abbreviations
- Cys
cysteine
- ESI
electrospray ionization
- E1(E2)-Q
estrone(estradiol)-quinones
- GSH
glutathione
- 4-OHE2
4-hydroxyestra-diol
- 4-OHE1(E2)-1-N3Ade
4-hydroxyestrone(estradiol)-1-N3Adenine
- 4-OHE1(E2)-1-N7Gua
4-hydroxyestrone(estradiol)-1-N7Guanine
- 2-OHE1(E2)-6-N3Ade
2-hydroxyestrone(estradiol)-6-N3Adenine
- MRM
multiple reaction monitoring
- NAcCys
N-acetylcysteine
- NI
negative ion
- PI
positive ion
- SPE
solid-phase extraction
- UPLC/MS-MS
ultraperformance liquid chromatography/tandem mass spectrometry
References
- 1.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Bio-phys Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–7. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 3.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 4.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–65. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–42. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li KM, Todorovic R, Devanesan P, Higginbotham S, Kofeler H, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25:289–97. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 7.Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E. The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem Res Toxicol. 2006;19:164–72. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- 8.Saeed M, Rogan E, Fernandez SV, Sheriff F, Russo J, Cavalieri E. Formation of depurinating N3Adenine and N7Guanine adducts by MCF-10F cells cultured in the presence of 4-hydroxyestradiol. Int J Cancer. 2007;120:1821–4. doi: 10.1002/ijc.22399. [DOI] [PubMed] [Google Scholar]
- 9.Markushin Y, Zhong W, Cavalieri EL, Rogan EG, Small GJ, Yeung ES, Jankowiak R. Spectral characterization of catechol estrogen quinone (CEQ)-derived DNA adducts and their identification in human breast tissue extract. Chem Res Toxicol. 2003;16:1107–17. doi: 10.1021/tx0340854. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarti D, Mailander PC, Li KM, Higginbotham S, Zhang HL, Gross ML, Meza JL, Cavalieri EL, Rogan EG. Evidence that a burst of DNA depurination in SENCAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–53. doi: 10.1038/sj.onc.1204969. [DOI] [PubMed] [Google Scholar]
- 11.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G. C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J Steroid Biochem Mol Biol. 2006;101:204–15. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Kosinska W, Khmelnitsky M, Cavalieri EL, Rogan EG, Chakravarti D, Sacks PG, Guttenplan JB. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem Res Toxicol. 2006;19:475–9. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int J Cancer. 2006;118:1862–8. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 14.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 β-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA. 1996;93:9776–81. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 16.Cavalieri EL, Kumar S, Todorovic R, Higginbotham S, Badawi AF, Rogan EG. Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: implications for estrogen-induced initiation of renal tumors. Chem Res Toxicol. 2001;14:1041–50. doi: 10.1021/tx010042g. [DOI] [PubMed] [Google Scholar]
- 17.Gaikwad NW, Rogan EG, Cavalieri EL. Evidence by ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic Biol Med. 2007;43:1289–98. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 19.Lareef MH, Garber J, Russo PA, Russo IH, Heulings R, Russo J. The estrogen antagonist ICI-182–780 does not inhibit the transformation phenotypes induced by 17-β-estradiol and 4-OH estradiol in human breast epithelial cells. Int J Oncol. 2005;26:423–9. [PubMed] [Google Scholar]
- 20.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-β-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–34. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 21.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24:353–6. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 22.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed Proc. 1987;46:1858–63. [PubMed] [Google Scholar]
- 23.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–7. [PubMed] [Google Scholar]
- 24.Yue W, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, Korach KS, Devanesan P, Todorovic R, Rogan EG, Cavalieri EL. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol. 2003;86:477–86. doi: 10.1016/s0960-0760(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 25.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 26.Saeed M, Zahid M, Rogan E, Cavalieri E. Synthesis of the catechols of natural and synthetic estrogens by using 2-iodoxybenzoic acid (IBX) as the oxidizing agent. Steroids. 2005;70:173–8. doi: 10.1016/j.steroids.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine, and glutathione. Chem Res Toxicol. 1998;11:909–16. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 28.Stack DE, Byun J, Gross ML, Rogan EG, Cavalieri EL. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem Res Toxicol. 1996;9:851–9. doi: 10.1021/tx960002q. [DOI] [PubMed] [Google Scholar]
- 29.Boyland E, Chasseaud LF. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]