Summary
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a complex inflammatory condition that affects a large proportion of the population world-wide and is associated with high cost of management and significant morbidity. Yet, there is a lack of population-based epidemiologic studies using current definitions of CRSwNP, and the mechanisms that drive pathogenesis in this disease remain unclear. In this review, we summarize the current evidence for the plethora of factors that likely contribute to CRSwNP pathogenesis. Defects in the innate function of the airway epithelial barrier, including diminished expression of antimicrobial products and loss of barrier integrity, combined with colonization by fungi and bacteria likely play a critical role in the development of chronic inflammation in CRSwNP. This chronic inflammation is characterized by elevated expression of many key inflammatory cytokines and chemokines, including IL-5, thymic stromal lymphopoietin and CCL11, that help to initiate and perpetuate this chronic inflammatory response. Together, these factors likely combine to drive the influx of a variety of immune cells, including eosinophils, mast cells, group 2 innate lymphoid cells and lymphocytes, which participate in the chronic inflammatory response within the nasal polyps. Importantly, however, future studies are needed to demonstrate the necessity and sufficiency of these potential drivers of disease in CRSwNP. In addition to the development of new tools and models to aid mechanistic studies, the field of CRSwNP research also needs the type of robust epidemiologic data that has served the asthma community so well. Given the high prevalence, costs and morbidity, there is a great need for continued research into CRS that could facilitate the development of novel therapeutic strategies to improve treatment for patients who suffer from this disease.
Introduction
The purpose of this review is to summarize the current state of knowledge regarding the pathogenesis of nasal polyposis. In this review, we focus on chronic rhinosinusitis with nasal polyps (CRSwNP), rather than other conditions associated with nasal polyp formation such as antrochoanal polyps, cystic fibrosis or allergic fungal sinusitis, due to the significantly higher prevalence and associated costs of management of CRSwNP.
Epidemiology of CRSwNP
Chronic rhinosinusitis (CRS) is characterized by the presence of four cardinal symptoms: nasal obstruction, drainage, smell loss and facial pain or pressure, which last for at least 3 months. In some patients with CRS, exuberant hyperplastic inflammatory growth of nasal polyps into the nasal airway is observed [1–3]. There is a paucity of large population-based epidemiologic studies conducted using current definitions of CRS, and fewer studies yet that specifically delineate the proportion of patients who have CRSwNP. Two recent population-based studies conducted in Europe and South Korea that used both symptom histories and endoscopic exams estimated the prevalence of CRS in the general population at 10.9% and 7.0% of adults, respectively, but they did not define the proportion of CRS patients with nasal polyps [4, 5]. Studies that have specifically examined CRSwNP suggest its population prevalence is around 2.7% of adults in a single municipality of Sweden [6], and its incidence has been estimated at 0.63 or 0.83 patients per thousand per year based on studies in a Danish county or in central Pennsylvania, respectively [7, 8]. Based on these studies, there is a predominance of men with CRSwNP, with a male to female ratio varying from 1.3 to 2.2, and a peak incidence between the ages of 45 and 65. While CRSwNP is the less common form of CRS, we have noted that the proportion of CRS patients with nasal polyps is substantially higher in surgical cohorts of tertiary care patients who had failed medical management (~ 50%) when compared with incident cases where the proportion of CRS patients with nasal polyps may be as low as 7% [9, 10]. Together, these studies suggest that compared to CRSsNP, CRSwNP may persist for a longer duration, be relatively recalcitrant to medical management, more frequently lead to surgical management and/or require more surgeries to address. While total CRS management costs are estimated to be over $8 billion annually in the United States [11], there are no studies, to our knowledge, that have provided estimates of direct costs associated with CRSwNP management. In addition to direct costs of management, studies have shown that patients with CRS seen in a tertiary care setting suffer a significantly impaired quality of life comparable to congestive heart failure and chronic obstructive pulmonary disease [12]. Although our unpublished data suggest that patients with CRSwNP may have marginally less impairment of quality of life than those without polyps, other studies have shown that patients with CRSwNP are more bothered by rhinorrhoea and smell/taste disturbances and less bothered by facial pain [13]. Certainly, it is well established that patients with CRSwNP do have more frequent impairment from co-morbid conditions such as asthma and atopic multi-sensitization than the general population [8, 10]. While epidemiologic study of CRSwNP is in its infancy, sorely needed studies in primary care populations will hopefully yield important information regarding the environmental or occupational factors that drive disease formation and persistence.
Aspirin-exacerbated respiratory disease (AERD)
It is important to emphasize that there is a specific subset of patients with CRSwNP with severe disease that suffers from aspirin-exacerbated respiratory disease (AERD), otherwise known as Samter's triad. These patients have asthma and CRSwNP and develop exacerbated airway responses following ingestion of medications that inhibit the cyclooxygenase (COX) 1 enzyme. Although patients with AERD often present with the most severe manifestations of both asthma and nasal polyposis, this disease is less common than either CRSwNP or asthma and is currently understudied. The prevalence of AERD has been estimated to be as much as 0.6–2.5% in the general population, with higher prevalence among patients with asthma and CRSwNP [14–16]. However, the published work on AERD has been hampered by differing definitions of AERD used by different groups of investigators. For example, while some require documented airway reactions to COX-1 inhibitors along with asthma and CRSwNP for AERD diagnosis, others do not specify the type of reaction to COX-1 inhibitors in their patients, and some studies do not require patients to have CRSwNP for their definition of AERD. Thus, the population prevalence estimates above may not truly reflect the population of patients who have the triad of asthma, CRSwNP and airway responses to COX-1 inhibitors. As such it is unclear whether AERD represents the severe end of the CRSwNP disease spectrum, or whether it is a distinct clinical entity. However, it is clear that patients with AERD uniquely respond to aspirin desensitization therapy [17] and have higher baseline levels of urinary leukotriene E4 [18], suggesting that distinct molecular mechanisms, such as dysregulation of arachidonic acid metabolism, drive this unique disease [19–21]. The pathogenesis of AERD is not discussed further in this review.
Allergic fungal rhinosinusitis (AFRS)
Patients with allergic fungal rhinosinusitis (AFRS) represent another distinct subset of CRSwNP. These patients are often atopic and have highly elevated serum IgE levels [22], and they can be distinguished from other patients with CRSwNP by the presence of eosinophilic mucin without fungal invasion of the mucosa, mucus with positive fungal staining and often bony erosion [23]. Interestingly, the estimated prevalence of AFRS varies widely across different geographic regions of the United States such that AFRS has been estimated to account for > 20% of all patients undergoing endoscopic sinus procedures in southern areas of the country, such as Memphis, but only 0–4% of these same patients in northern regions of the country [24]. Like AERD, it is not clear whether AFRS is driven by distinct pathogenic mechanisms from CRSwNP or is simply part of the CRSwNP disease spectrum [23], and we will not discuss the pathogenesis of AFRS further in this review.
Eastern vs. western presentations of CRSwNP
Chronic rhinosinusitis with nasal polyps in Western populations is often characterized by type 2 inflammation, with elevated levels of type 2 cytokines, such as IL-5 and IL-13, along with eosinophilia. However, there is accumulating evidence from Asian countries, especially in China, Korea and Japan, that CRSwNP in their populations may be characterized by a mixed type 1 or type 3 inflammation, with a more neutrophilic inflammation than observed in NP in patients from the United States or Europe [25]. The mechanisms that drive these different phenotypes are unclear at this time. Interestingly, recent work from our group found that tissue from NP from second-generation Asian patients born in and living in the United States was considerably less eosinophilic than NP tissue from Caucasian, African American and Hispanic patients living in the greater Chicago area, suggesting that genetic differences may play a critical role in the development of eosinophilia in NP [26]. Due to space constraints, we will focus the discussion in this review on the pathogenesis of NP associated with type 2 inflammation.
Pathogenesis of CRSwNP
Potential role for microbial colonization
It has been well documented that the upper airways or sinuses of patients with CRSwNP are often chronically colonized with fungi and bacteria and that these microbes may play an important role in CRSwNP pathogenesis [27]. Although there are numerous hypothetical mechanisms that could account for this colonization, it is not yet clear whether this accumulation of microbes is an initial cause of CRSwNP or a downstream effect of the underlying inflammatory disease mechanism. A fungal hypothesis was suggested by Ponikau et al. to be the main cause of all CRS pathologies [28–30]. In brief, it was proposed that exposure to, and colonization by, alternaria and other fungal species could drive many of the cardinal features of CRS, including epithelial damage and activation of adaptive immune responses. However, it has become clear that while fungi may play a role in a subset of patients, such as those with AFRS, they do not play a central role in the majority of patients with CRS [27]. Staphylococcus aureus (SA) is another microbe that has been the focus of much research in CRSwNP. Many investigators believe that SA and its superantigens play a critical role in the pathogenesis of CRSwNP, as has been established in atopic dermatitis [31, 32]. Superantigens have the potential to activate large numbers of T cells, due to their ability to bind a large fraction of T cell receptors via the Vβ region, independent of peptide processing and MHCII recognition mechanisms required by traditional antigens. Elevated levels of superantigen-binding T cells in NP have been identified by flow cytometry [33–35], and it has been demonstrated that some patients with CRSwNP have superantigen-specific IgE in their NP [36, 37]. These data suggest that SA plays at least some role in the chronic inflammatory response of patients with CRSwNP.
Defects in airway epithelial cell innate immune functions associated with CRSwNP
The epithelial layer of the airways provides a first line of defence against foreign antigens and is critical for maintaining homeostasis of the underlying tissue mucosa. Perhaps the most basic function of this epithelial layer is its ability to form tight junctions between cells that create a physical barrier between the airway lumen, which is constantly exposed to foreign antigens, and the underlying submucosal tissue. It is well documented that defects in airway epithelial barrier function are associated with chronic airway diseases such as asthma [38]. Importantly, defects in the sinonasal epithelial barrier of patients with CRSwNP have also been documented [39–42]. These include diminished expression, or mislocalization, of tight junction proteins and increased epithelial permeability. To date, it has been demonstrated that type 2 cytokines, such as IL-4 and IL-13, may play an important role in decreasing tight junction function [39]. Pothoven et al. [43] have demonstrated that pro-inflammatory cytokines, such as oncostatin M (OSM), may also play a role in disrupting tight junctions and increasing epithelial permeability. As has been shown in asthma, these defects in barrier function could play a critical role in the pathogenesis of CRSwNP by allowing an influx of foreign antigens into the submucosa where they may trigger or exacerbate an inflammatory response.
Another mechanism used by the airway epithelial layer to prevent the interaction of lumenal antigens and pathogenic organisms with underlying tissue is the production of mucus and clearance of antigens by cilia. This mechanism also appears to be defective in CRSwNP [44]. Overproduction of mucus can lead to an imbalance in the air-surface liquid levels that make it difficult for cilia to efficiently clear away antigens and debris. This can result in the accumulation of foreign antigens in the lumen that can contribute to inflammation [45–47]. Some of the molecules that are altered in CRSwNP, and that may cause dysfunction in the formation of nasal lining fluid, include pendrin, periostin and PLUNC family members, that can affect uptake or extrusion of various electrolytes and maintenance of the proper sol and gel phase of the mucus blanket [45]. Interestingly, individuals that are carriers of one mutated CFTR gene, the well-known ABC transporter of chloride and isocyanate associated with cystic fibrosis (CF), but do not have CF, are at considerably increased risk to develop CRSwNP, further supporting the concept that the components of the nasal lining fluid may be disturbed in CRS [46].
In addition to their constitutive barrier and clearance functions, airway epithelial cells have inducible innate immune functions that are important for recognition of potential pathogens, killing of these microbes and alerting immune cells in the underlying mucosa of potential danger. Airway epithelial cells express pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), that function to sense potential pathogens and initiate downstream immune responses [47]. Several groups have assessed expression of TLRs in either whole NP or epithelial cells from patients with CRSwNP in order to attempt to determine whether there are defects in the expression of these important receptors that are associated with CRSwNP. Unfortunately, the results from these studies are often conflicting, and a clear picture of the role of TLR signalling in CRSwNP pathogenesis has not emerged. For example, some studies have reported elevated expression of TLR2, TLR4, TLR7 and/or TLR9 [48–51], while others found no changes in expression of TLRs compared to controls, or decreased expression of these same molecules [52, 53]. As a result, some suggest that increased TLR expression may contribute to CRSwNP pathogenesis by triggering unnecessary inflammatory responses to commensal microbes, while others suggest that decreased expression of TLRs may contribute to disease by failing to properly control the pathogenic organisms. Some of these discrepancies may be due to the use of whole tissues vs. epithelial scrapings, or due to differences in the anatomical source of the control tissue that was used for comparison. It is also important to note that all of these studies based their conclusions on mRNA expression or protein staining by immunohistochemistry or flow cytometry, and it is not clear whether the activation and downstream signalling mechanisms of TLRs are altered in disease. In one study that found decreased expression of TLR9 in epithelial cells from CRSwNP, there was no apparent defect in the ability of those cells to up-regulate inflammatory genes upon TLR9 activation, suggesting that the changes in protein expression found did not have functional consequences [53]. Interestingly, one study from Korea found that two different polymorphisms in the TLR2 gene were associated with increased risk of CRS, although it is not known whether these polymorphisms result in any biologically relevant functional consequences, and these investigators did not separate patients with CRS into those with or without polyps for this study [54]. Given the potential important role that TLRs could play in CRSwNP pathogenesis, further studies aimed at elucidating their function in disease are warranted.
Another important inducible immune function of airway epithelial cells is their ability to produce potent antimicrobial peptides. These peptides can function to directly kill specific pathogens and/or induce an inflammatory response. Several families of antimicrobial peptides have been shown to be expressed by airway epithelial cells, and each has its own unique target and function. We, and others, have found decreased expression of antimicrobial products, including S100A7 and PLUNC family proteins, in nasal lavage and tissue from patients with CRSwNP [45, 55, 56]. Currently, it appears that decreased production of some antimicrobial products may be due to an overall reduction in the numbers of submucosal glands in NP, the increased presence of type 2 cytokines, and/or defects in the activation of STAT3, a key transcription factor for some of these peptides [45, 57, 58]. Regardless of the mechanisms responsible, loss of expression of these key molecules may result in a situation in which the sinonasal epithelium of CRSwNP is unable to provide adequate defence against pathogens, which could contribute to the accumulation of microbes within the lumen.
Altered expression of cytokines and chemokines in CRSwNP
There are numerous cytokines and chemokines that have been reported to be expressed at altered levels in CRSwNP tissues. Unfortunately, the sheer number of different mediators is too numerous for us to discuss the importance of each in detail in this brief review. We have summarized the major cytokines and chemokines whose expression is thought to be altered in CRSwNP in Table 1, and we will briefly discuss the potential role in pathogenesis of a few of these important mediators here.
Table 1.
Chemokines in CRS
| Chemokines | Potential role in CRSwNP | Finding | Source | Technique | Patient population | References |
|---|---|---|---|---|---|---|
| CCL2 (MCP-1) | Monocyte, T cell and DC recruitment | Not different between NP and control or CRSsNP | Tissue | Multiplex bead array | USA | C. J. Ocampo, unpublished observations |
| CCL5 (RANTES) | Eosinophil recruitment | Not different between NP and control | Tissue | Semi-quantitative PCR | Germany | Barrels et al. [59] |
| Not different between NP and control | Tissue | ELISA | Germany | Bachert et al. [106] | ||
| Decreased in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| CCL7 (MCP-3) | Monocyte recruitment | Not detectible in NP or control | Tissue | Semi-quantitative PCR | Germany | Barrels et al. [59] |
| CCL11 (Eotaxin-1) | Eosinophil recruitment | Not different between NP and control | Tissue | Semi-quantitative PCR | Germany | Barrels et al. [59] |
| Elevated in NP compared to control | Tissue | ELISA | Belgium | Bachert et al. [60] | ||
| Elevated in NP compared to control | Tissue | ELISA | Germany | Olze et al. [107] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | ELISA | Belgium | Van Zele et al. [87] | ||
| Elevated in NP compared to control | Tissue | ELISA | Belgium* | Zhang et al. [90] | ||
| Not different between NP and control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Elevated in CRSwNP compared to control and CRSsNP | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| CCL13 (MCP-4) | Eosinophil and basophil recruitment | Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations |
| CCL17 (TARC) | T cell recruitment | Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations |
| CCL18 (PARC) | Lymphocyte and DC recruitment | Elevated in NP compared to control and CRSsNP | Tissue | PCR and ELISA | USA | Peterson et al. [64] |
| CCL23 (MIP-3) | Monocyte and macrophage recruitment | Elevated in NP compared to control and CRSsNP | Tissue | PCR | USA | Poposki, 2011 [109] |
| CCL24 (Eotaxin-2) | Eosinophil recruitment | Elevated in NP compared to control | Tissue | ELISA | Germany | Olze et al. [107] |
| Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| CCL26 (Eotaxin-3) | Eosinophil recruitment | Elevated in NP compared to control | Tissue | ELISA | Germany | Olze et al. [107] |
| Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| CXCL9 (MIG) | T cell recruitment | Elevated in CRSwNP compared to control and CRSsNP | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] |
| CXCL1 (GRO-a) | T cell recruitment | Not different between NP and control | Tissue | ELISA | Germany | Bachert et al. [106] |
| CXCL12 (SDF-1a) | B cell recruitment | Elevated in NP compared to control and CRSsNP | Tissue | PCR and ELISA | USA | Patadia et al. [65] |
| CXCL13 (BLC) | B cell recruitment | Elevated in NP compared to control and CRSsNP | Tissue | PCR and ELISA | USA | Patadia et al. [65] |
| Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations |
This work investigated patients from Belgium and China; for clarity, we have separated the results from these two distinct populations.
Chemokines important for the attraction of eosinophils, neutrophils, macrophages, dendritic cells, T cells and B cells, have all been reported to be elevated in CRSwNP tissues. Given the large increase in eosinophils, as well as their ability to promote type 2 inflammation, many groups have focused on studying the mechanisms that account for the accumulation of these cells in NP. Eotaxin 1 (CCL11), along with eotaxins 2 and 3 (CCL24 and CCL26, respectively), is among the main CCR3-activating chemokines responsible for recruitment of eosinophils, which have been shown to be highly elevated in NP [59–61]. Importantly, the expression of CCL11 by NP-derived fibroblasts, or airway epithelial cells, has been shown to be increased by the combination of IL-4, or IL-13, and TNF in vitro, suggesting that there may be a positive feedback loop for eosinophil recruitment that is further enhanced in a pro-inflammatory type 2 inflammatory environment [62, 63]. Pulmonary and activation-related chemokine (PARC; CCL18) is another chemokine associated with type 2 inflammation and has been shown to recruit naïve T cells, B cells and immature dendritic cells. Recently, this important chemokine was also shown to be elevated in NP and positively correlated with levels of M2 macrophages, suggesting that it may play an important role in facilitating type 2 responses in NP [64]. Two other chemokines that are important for the recruitment of lymphocytes, including B cells, have also been reported to be elevated in NP. Both stromal cell-derived factor 1 (SDF-1; CXCL12) and B lymphocyte chemoattractant (BLC; CXCL13) were found to be elevated in NP, and CXCL13 significantly positively correlated with expression of B cell markers in NP [65]. Altogether, the evidence suggests that a variety of chemokines are highly elevated and may play critical roles in facilitating the recruitment of key immune effector cells to NP that can then promote chronic type 2 inflammatory responses. Studies aimed at elucidating the relative importance of these chemokines during ongoing inflammation could provide insights for the development of treatments to target these molecules and reduce infiltration of inflammatory cells.
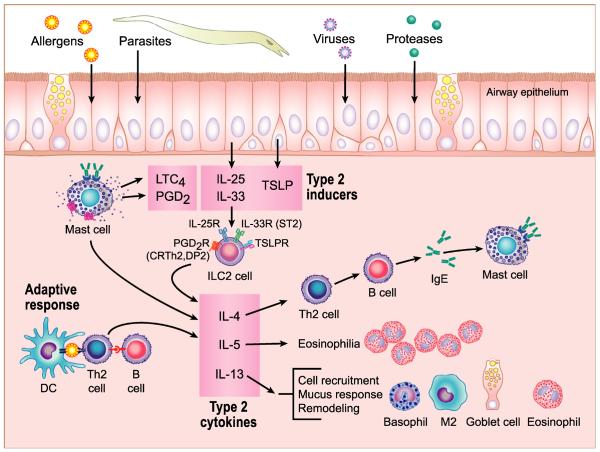
Cytokines that play an important role in the skewing of adaptive immune responses and the survival of a variety of immune effector cells are also highly elevated in NP. Due to space limitations, we will not discuss the role of traditional type 2 cytokines (IL-4, IL-5 and IL-13; see Figure 3 and Table 2), but will instead focus on more recently identified cytokines that could play an important role in CRSwNP. It is important to point out, however, that recent unpublished studies by Sanofi/Regeneron (multiple press releases are available) indicate that dupilumab, an antibody against the alpha chain of the IL-4 and IL-13 receptors, has impressive beneficial effects in polypoid CRS, supporting an important role for cells that respond to these type 2 cytokines. It has been clear for some time that epithelial-derived chemokines play an important role in driving type 2 inflammatory responses. More recently, three epithelial cytokines in particular have been implicated in skewing towards type 2 inflammatory conditions. These are IL-25 (IL-17E), IL-33 and thymic stromal lymphopoietin (TSLP). Currently, there is little evidence to support a role for IL-25 in CRSwNP [66], and a potential role for IL-33 is still somewhat controversial, as it is not clear whether this cytokine is elevated in NP or not [67–69]. In addition, our own data indicate that IL-33 is not elevated in nasal polyps (A. Kato et al., unpublished observations). However, it is clear that the expression of TSLP mRNA is elevated in NP, and activity of TSLP is also enhanced in NP [67, 70]. TSLP potentially plays an important role in establishing a type 2 inflammatory milieu within NP and may enhance the activation of group 2 innate lymphoid cells (ILC2) and the Th2-skewing potential of NP-resident DCs. Finally, elevations of the B cell activating factor of the TNF family (BAFF) have also been documented in NP [71]. This cytokine is known to play an important role in the activation and survival of B cells in secondary lymphoid organs and could play an important role in the accumulation of activated B cell subsets and antibodies in NP. Future studies aimed at clarifying the role of TSLP and BAFF, along with the potential role of IL-33, would be of great value.
Fig. 3.
Predominant role of type 2 inflammation in the mediation of CRSwNP. Type 2 cytokines are traditionally viewed as being derived from Th2 cells within an adaptive immune response. Th2 cells are activated by antigen-presenting cells (APC), including DC, B cells and others, along with a Th2-skewing cytokine milieu (lower left – APC shown are DC and B cell). The namesake cytokines of Th2 cells include IL-4, IL-5 and IL-13; these cytokines drive the recruitment and/or activation of mast cells, eosinophils, basophils, goblet cells, M2 macrophages, B cells, etc., as well as many of the tissue responses resulting from the factors that these inflammatory cells produce. Mast cells are further activated by specific antigen after local or systemic synthesis of antigen-specific IgE. Recent findings have shown that type 2 cytokines can also be produced by group 2 innate lymphoid cells, known as ILC2, that do not require direct activation by APC and antigen. ILC2 nonetheless produces a similar spectrum of cytokines and elicits similar effector responses as Th2 cells. ILC2 can be activated by proteases, pathogens and other stimuli. Multiple pathways by which ILC2 are activated by such stimuli have been described, especially in murine systems, and the precise mechanisms by which ILC2 are activated in CRS are under intensive scrutiny (see top of the figure).
Table 2.
Cytokines in CRS
| Cytokines | Potential role in CRSwNP | Finding | Source | Technique | Patient population | References |
|---|---|---|---|---|---|---|
| IL-1RA | Decreased in NP compared to control | Tissue | ELISA | Germany | Bachert et al. [106] | |
| IL-1β | Promote inflammation | Decreased in NP compared to control | Tissue | ELISA | Germany | Bachert et al. [106] |
| Elevated in NP compared to control | Tissue | IHC | USA | Lennardet al. [110] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | Belgium | Van Zele et al. [87] | ||
| Not different between NP and control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Not different between NP and control | Tissue | ELISA | Belgium* | Zhang et al. [90] | ||
| Elevated in NP compared to control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | Belgium | Van Crombruggen et al. [112] | ||
| Elevated in CRSwNP compared to control and CRSsNP | Tissue | ELISA | Australia | Foreman et al. [113] | ||
| Decreased in CRSwNP compared to CRSsNP; not different between CRSwNP and control | Tissue | ELISA | Japan | Sejima et al. [114] | ||
| Increased in NP compared to control; not different between NP and CRSsNP | Tissue | ELISA | China | Luo et al. [115] | ||
| IL-3 | Eosinophil survival | Elevated IL-3+ cells in CHS compared to controls | Tissue | In situ hybridization | Denver | Hamilos et al. [116] |
| Not detectible | Tissue | ELISA | Germany | Bachert et al. [106] | ||
| IL-4 | Promote type 2 inflammation | Not detectible | Tissue | ELISA | Germany | Bachert et al. [106] |
| Not different between NP and control | Tissue | ELISA | Belgium | Bachert et al. [60] | ||
| Not different between NP and control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Elevated in CRSwNP compared to control; not different between | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] | ||
| CRSwNP and CRSsNP | ||||||
| Elevated in CRSwNP compared to control; not different between | Tissue and Mucus | Multiplex bead array | USA | Oyer et al. [117] | ||
| CRSwNP and CRSsNP | ||||||
| Elevated in NP compared to control and CRSsNP | Tissue | PCR | China | Zhang et al. [51] | ||
| Not different between NP and control; Elevated in NP compared to CRSsNP | Tissue | Muliplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| IL-5 | Eosinophil survival and/or recruitment | Elevated in NP compared to control | Tissue | ELISA | Germany | Bachert et al. [106] |
| Not different between NP and control | Tissue | IHC | USA | Lennard et al. [110] | ||
| Elevated in NP compared to control | Tissue | ELISA | Belgium | Bachert et al. [60] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | ELISA | Belgium | Van Zele et al. [87] | ||
| Elevated in NP compared to control | Tissue | PCR | Germany | Chenet al. [118] | ||
| Elevated in NP compared to control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Elevated in NP compared to control | Tissue | ELISA | Belgium* | Zhang et al. [90] | ||
| Not different between NP and control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | ELISA | Belgium | Van Crombruggen et al. [112] | ||
| Elevated in CRSwNP compared to control and CRSsNP | Tissue | ELISA | Australia | Foreman et al. [113] | ||
| Elevated in CRSwNP compared to control and CRSsNP | Tissue | ELISA | Japan | Sejima et al. [114] | ||
| Increased in NP compared to control; not different between NP and CRSsNP | Tissue | ELISA | China | Luo et al. [115] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | PCR | Australia | Miljkovic et al. [66] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| IL-6 | Promote inflammation | Not different between NP and control | Epi cell secretion | PCR, ELISA | Spain | Mullol et al. [119] |
| Not different between NP and control | Tissue | ELISA | Germany | Bachert et al. [106] | ||
| Elevated in NP compared to control | Tissue | IHC | USA | Lennard et al. [110] | ||
| Not different between NP and control | Tissue | ELISA | Belgium* | Zhang et al. [90] | ||
| Elevated in NP compared to control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | ELISA | USA | Peters et al. [57] | ||
| Elevated in CRSwNP compared to control and CRSsNP | Tissue | ELISA | Australia | Foreman et al. [113] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | Japan | Sejima et al. [114] | ||
| Elevated in CRSwNP compared to control; not different between CRSwNP and CRSsNP | Tissue and Mucus | Multiplex bead array | USA | Oyer et al. [117] | ||
| Increased in NP compared to control and CRSsNP | Tissue | ELISA | China | Luo et al. [115] | ||
| Not different between NP and control or CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| IL-8 | Promote inflammation | Elevated in NP compared to control | Epi cell secretion | PCR, ELISA | Spain | Mullol et al. [119] |
| Not different between NP and control | Tissue | ELISA | Germany | Bachert et al. [106] | ||
| Elevated in wNP compared to control | Tissue | IHC | USA | Lennard et al. [110] | ||
| Elevated in NP compared to control; not different between NP and CRSsNP | Tissue | ELISA | Belgium | Van Zele et al. [87] | ||
| Not different between NP and control | Tissue | PCR | Germany | Chen et al. [118] | ||
| Not different between NP and control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Elevated in CRSwNP compared to control; not different between CRSwNP and CRSsNP | Tissue | ELISA | Japan | Sejima et al. [114] | ||
| Elevated in CRSwNP compared to control; not different between CRSwNP and CRSsNP | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] | ||
| Decreased in NP compared to sNP | Tissue | PCR | China | Shi et al. [120] | ||
| Increased in NP compared to control; not different between NP and CRSsNP | Tissue | ELISA | China | Luo et al. [115] | ||
| IL-10 | Dampen inflammation | Not different between NP and control | Tissue | ELISA | Germany | Bachert et al. [106] |
| Elevated in NP compared to control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Elevated in CRSwNP compared to control and CRSsNP in mucus; Elevated in CRSwNP compared to control in tissue; not different between CRSwNP and CRSsNP in tissue | Tissue and Mucus | Multiplex bead array | USA | Oyer et al. [117] | ||
| Not different between NP and control or CRSsNP | Tissue | PCR | China | Zhang et al. [51] | ||
| Elevated in NP compared to control; not different between NP and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| IL-13 | Promote type 2 inflammation and/or mucus production | Increased in NP compared to control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] |
| Not different between CRSwNP and control or CRSsNP | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] | ||
| Elevated in NP compared to control; not different between NP and CRSsNP | Tissue | PCR | Australia | Miljkovic et al. [66] | ||
| Elevated in NP compared to control and CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| IL-17A | Promote type 3 inflammation | Not different between NP and control | Tissue | ELISA | Belgium* | Zhang et al. [90] |
| Elevated in NP compared to control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Not different between CRSwNP and control or CRSsNP | Tissue | ELISA | Australia | Foreman et al. [113] | ||
| Elevated in CRSwNP compared to control; not different between CRSwNP and CRSsNP | Tissue and Mucus | Multiplex bead array | USA | Oyer et al. [117] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | China | Luo et al. [115] | ||
| Elevated in NP compared to control | Tissue | PCR | China | Wang et al. [121] | ||
| IL-25 | Promote type 2 inflammation | Decreased in NP compared to control and CRSsNP | Tissue | PCR | Australia | Miljkovic et al. [66] |
| IL-33 | Promote type 2 inflammation | Detected in NP epithelial cells | Epithelial cells | PCR | USA | Reh et al. [68] |
| No difference at baseline | Epithelial cells | IHC | USA | Paris et al. [122] | ||
| Not different between NP and control or CRSsNP | Tissue | PCR | Australia | Miljkovic et al. [66] | ||
| Not different between NP and control or CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| BAFF | B cell survival and/or activation | Elevated in NP compared to control and CRSsNP | Tissue | PCR, IHC and ELISA | USA | Kato et al. [71] |
| GM-CSF | Eosinophil survival | Elevated GM-CSF+ cells in CHS compared to controls | Tissue | In situ hybridization | Denver | Hamilos et al. [116] |
| Not different between NP and control | Epi cell secretion | In vitro Eos survival assay and ELISA | Spain | Xaubet et al. [123] | ||
| Elevated in NP compared to control | Epi cell secretion | PCR, ELISA | Spain | Mullol et al. [119] | ||
| Not detectible | Tissue | ELISA | Germany | Bachert et al. [106] | ||
| Not different between NP and control or CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations. | ||
| IFN-γ | Promote type 1 inflammation | Decreased in NP compared to CRSsNP; not different between NP and control | Tissue | ELISA | Belgium | Van Zele et al. [87] |
| Not different between NP and control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Not different between NP and control | Tissue | ELISA | Belgium* | Zhang et al. [90] | ||
| Elevated in NP compared to control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | Belgium | Van Crombruggen et al. [112] | ||
| Not different between CRSwNP and control or CRSsNP | Tissue | ELISA | Australia | Foreman et al. [113] | ||
| Not different between CRSwNP and control or CRSsNP | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | China | Luo et al. [115] | ||
| Not different between NP and control or CRSsNP | Tissue | PCR | China | Zhang et al. [51] | ||
| Not different between NP and control or CRSsNP | Tissue | Multiplex bead array | USA | C. J.Ocampo, unpublished observations | ||
| TGF-β1 | Tissue remodelling | Decreased in NP compared to CRSsNP | Tissue | PCR, IHC and ELISA | Belgium | Watelet et al. [124] |
| Not different between NP and control or CRSsNP | Tissue | ELISA | Belgium | Van Zele et al. [87] | ||
| Not different between NP and control | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Lower in NP compared to control | Tissue | ELISA | Belgium* | Zhang et al. [90] | ||
| Lower in NP compared to control | Tissue | ELISA | China* | Zhang et al. [90] | ||
| Decreased in CRSwNP compared to control and CRSsNP | Tissue | ELISA | Belgium | Van Bruaene et al. [125] | ||
| Decreased in NP compared to CRSsNP; not different between NP and control | Tissue | ELISA | Belgium | Van Crombruggen et al. [112] | ||
| Not different between CRSwNP and control or CRSsNP | Tissue | ELISA | Australia | Foreman et al. [113] | ||
| Decreased in CRSwNP compared to control and CRSsNP | Tissue | ELISA | Japan | Sejima et al. [114] | ||
| Decreased in NP compared to control | Tissue | PCR | China | Shi et al. [120] | ||
| Decreased in NP compared to CRSsNP; not different between NP and control | Tissue | ELISA | China | Luo et al. [115] | ||
| TNF | Promote inflammation | Not different between NP and control | Epi cell secretion | PCR, ELISA | Spain | Mullol et al. [119] |
| Not different between NP and control | Tissue | ELISA | Germany | Bachert et al. [106] | ||
| Elevated in NP compared to control | Tissue | IHC | USA | Lennard et al. [110] | ||
| Not different between NP and control or CRSsNP | Tissue | ELISA | Belgium | Van Zele et al. [87] | ||
| Elevated in CRSwNP compared to control; not different between CRSwNP and CRSsNP | Tissue culture supernatant | Multispot assay | Belgium | Patou et al. [111] | ||
| Elevated in wNP | Nasal secretion | Multiplex bead array | USA | Cho et al. [108] | ||
| Not different between CRSwNP and control or CRSsNP in tissue; Elevated in CRSwNP compared to control in mucus; not different between CRSwNP and CRSsNP in mucus | Tissue and Mucus | Multiplex bead array | USA | Oyer et al. [17] | ||
| Not different between NP and control or CRSsNP | Tissue | PCR | China | Zhang et al. [51] | ||
| TSLP | Promote type 2 inflammation | Enhanced TSLP activity in NP compared to control | Tissue culture supernatant | In vitro activity assay | Canada | Allakhverdi et al. [67] |
| Enhanced TSLP activity in NP compared to control and CRSsNP | Tissue extracts | In vitro activity assay | USA | Nagarkar et al. [126] | ||
| Not different between NP and control and CRSsNP | Tissue | PCR | Australia | Miljkovic et al. [66] |
This work investigated patients from Belgium and China; for clarity, we have separated the results from these two distinct populations.
Innate immune effector cells in CRSwNP pathogenesis
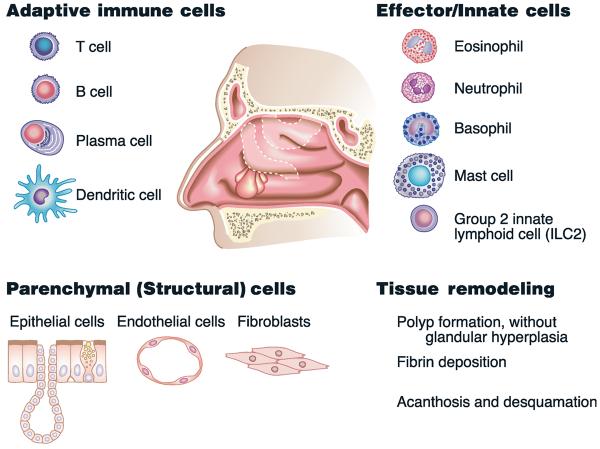
As mentioned above, cytokines and chemokines are important for initiating and perpetuating tissue inflammatory responses. Given the increased expression of many of these pro-inflammatory mediators in NP of patients with CRSwNP (Tables 1 and 2), it is not surprising that a variety of innate immune effector cells are elevated in the NP of these patients compared to control tissues (Fig. 1). This includes monocytes, macrophages, dendritic cells, neutrophils, mast cells, eosinophils, basophils and ILC2s. Due to space limitations, we cannot expand in depth on all of these important cell types, but will instead focus on a few interesting findings from the recent literature.
Fig. 1.
Summary of the events and cell types involved in the pathogenesis of chronic rhinosinusitis with nasal polyps. Immune cells shown on the top of the figure are elevated in polyp tissue from most European and American patients and a subset of Asian patients. Recent studies discussed in the text provide the basis for a model in which local expansion of T cells, B cells and plasma cells occurs with the help of local dendritic cells, resulting in the production of cytokines and immunoglobulins at high levels in the tissue (top left). Recruitment and/or activation of effector cells, shown on the top right, is likely a consequence of the activation of adaptive immune cells, tissue cells (e.g. epithelium and endothelium) and innate lymphoid cells. Activation of structural cells can contribute to cellular recruitment, leak and activation of plasma proteins, deposition of fibrin and modification of the extracellular matrix. The actions of both the immune and the structural cells lead to tissue remodelling, as summarized in part on the lower right.
Mast cells are important sentinels of the immune system, and they are ideally located to detect and rapidly respond to pathogens at mucosal surfaces. Recent work by Takabayashi et al. has confirmed earlier reports of elevated mast cells in NP and has also identified elevated levels of unique subsets of mast cells in NP [72, 73]. This work revealed that mast cells associated with the epithelium expressed both tryptase and carboxypeptidase A3, while those associated with the glandular epithelium in NP expressed these proteases in conjunction with chymase. These results are particularly interesting because chymase is known to induce secretion of mucus, and the close localization of the chymase+ mast cells to glandular epithelium may play a critical role in the overproduction of mucus in CRSwNP [72]. Like mast cells, basophils also play an important role in type 2 immune responses. Until recently, however, there were no published studies addressing whether basophils play a role in CRSwNP. Work by Mahdavinia et al. [74] has now reported that basophils are indeed elevated in NP compared to control sinus tissues. Interestingly, basophil levels were significantly positively correlated with eosinophil levels in NP, suggesting a coordinated recruitment or survival of these two important effector cells in type 2 immune responses in NP.
It is well documented that eosinophils are highly elevated in NP tissues as well. These cells are known to produce toxic cationic proteins that can injure epithelial cells, and have been associated with CRSwNP for over a century [75, 76]. More recently, it has been established that not only do eosinophils produce a variety of proinflammatory molecules that can contribute to type 2 inflammation, they also play an important role in the long-term maintenance of plasma cells and antibody production in the bone marrow and have been shown to activate T cells during inflammatory responses [77, 78]. Given the elevations of activated T and B cell subsets and antibody production found in NP, eosinophils may do more than simply promote type 2 immune responses in NP. Tan et al. have recently found that local eosinophils may further contribute to the anosmia from which many patients with CRSwNP suffer (unpublished observations). They were able to show that increased levels of eosinophils within the olfactory tissue of the sinuses significantly positively correlated with loss of smell in those patients. Interestingly, an antibody against IL-5 has been shown to have some efficacy in CRSwNP, including improvement of smell loss, presumably by reducing eosinophil numbers [79]. Altogether, these data suggest that eosinophils play many important roles in driving various aspects of CRSwNP disease and that research focused on targeting these cells specifically could be very beneficial.
Innate lymphoid cells (ILCs) are a newly identified class of innate immune cells that have recently been characterized. These cells share many features of CD4+ T cells, but they lack expression of all lineage markers and do not express adaptive immune cell receptors, such as the T cell receptor. Recently, Spits et al. demonstrated that group 2 ILCs (ILC2s) were significantly elevated in NP compared to control tissue [80]. As their name suggests, ILC2s are closely associated with type 2 inflammatory responses and have been shown to play critical roles in parasite expulsion and allergic airway responses in murine models [81–83]. These cells are capable of producing large amounts of IL-5 and IL-13 and can be activated by the epithelial-derived cytokines IL-25, IL-33 and TSLP (see Fig. 1). Some reports suggest that ILC2s may be very important at early stages of the immune response and may facilitate the development of the type 2 milieu through the production of cytokines and/or direct interactions with other cells, such as T cells [84, 85]. This initial ILC2 activation then affects multiple other cell types and results in skewing of the entire response to a type 2 response. Further studies are needed to more fully elucidate whether ILC2s play an important ongoing role in CRSwNP pathogenesis and whether they are critical in the early stages of disease.
Adaptive immunity
Given the pro-inflammatory environment created within NP by the epithelium and innate immune effector cells, it is no surprise that there are also several reports of elevations in adaptive immune cells (Fig. 1). Many studies have focused on the potential role of Th2 cells in CRSwNP, and elevations in T cells have been well documented in NP [27, 86, 87]. The presence of activated T cells within NP could play many important roles in CRSwNP pathogenesis. These cells are important for the activation of B cell responses and can produce pro-inflammatory cytokines within the tissue. Given the abundance of DC subsets in NP, it is also likely that some T cells can be reactivated and expanded locally [88, 89]. The elevated presence of T cell-attracting chemokines in NP also suggests that new T cells could be continually trafficking to this site to participate in the inflammatory response. Aside from the elevated frequencies of T cells with receptors capable of binding SA superantigens discussed above [33, 34], few studies have investigated the specificity of the NP-resident T cells. Many early studies aimed at quantifying T cells in NP used semi-quantitative methods, such as immunohistochemistry (IHC) to identify CD3+ T cells in NP tissues. More recently, investigators have turned to using flow cytometry to more accurately count and identify different T cell subsets. Studies using flow cytometry have revealed elevated levels of Th2 cells in NP, both by expression of CCR4 and by intra-cellular staining of IL-4 and IL-5. Interestingly, a recent study by Derycke et al. [86] found that Th cell subsets from sinus tissues of CRSsNP and CRSwNP were actually quite similar. This study did find that CRSwNP tissue, especially from patients who also had asthma, did have higher levels of Th2 cells, but it also showed that there were many different subsets of cytokine-positive Th cells in both CRSsNP and CRSwNP tissues, including IFN-γ+, IL-17+ and IL-10+ subsets. These data suggest that the inflammatory environment within NP tissue may be more complex than a simple Th2 response. In addition, we now know that ILC2s are elevated in NP and express many of the same markers that were previously thought to be exclusive to Th2 cells, including GATA-3, CRTH2, IL-5 and IL-13. Thus, previous studies reporting elevated Th2 cells, or a Th2 inflammatory environment, may have actually been quantifying both ILC2 and Th2 cells, and the environment within NP may be more accurately classified as `type 2', as both of these cell types make contributions.
Other studies have focused on determining whether there is a deficiency in regulatory T cells in NP or the peripheral blood of patients with CRSwNP. Again, early studies relied heavily on mRNA expression or IHC staining of FoxP3 to identify potential regulatory T cells, and more recent studies have used flow cytometry to more accurately identify these cells. Interestingly, early data suggested there was a decrease in FoxP3 expression in NP tissue compared to controls, along with decreased levels of regulatory cytokines IL-10 and TGF-b in NP tissue [90–92]. However, a recent study found elevated levels of FoxP3+ regulatory T cells in NP, and no differences in regulatory cytokine expression in NP [93]. It is important to remember that in humans, FoxP3 can be expressed by activated effector T cells; thus, studies that examined only mRNA or single protein expression may not have identified true FoxP3+ regulatory T cells. In addition, elevations of FoxP3+ regulatory T cells in an inflammatory environment may be an unsuccessful attempt by the immune system to control the inflammation. Importantly, some subsets of inducible regulatory T cells do not express FoxP3; thus, the current literature does not adequately address whether there are any differences in these potentially important subsets of regulatory T cells in patients with CRSwNP.
In addition to T cells, significant elevations in B cells in NP have also been demonstrated by several groups [94, 95]. This finding fits well with the reported elevations of the B cell-attracting chemokines CXCL12 and CXCL13 in NP. Again, early studies focused on basic identification of B cells by IHC and CD19 or CD20 expression. More recently, it has become apparent that the elevated levels of B cells in NP are not just re-circulating memory B cells, but instead, they represent several different B cell subsets, including naïve B cells, plasmablasts and plasma cells [94, 95]. Gevaert et al. [96] reported evidence for the formation of lymphoid follicle-like structures in NP, which could promote the local activation of B cells, but we have searched extensively for these structures, and although they can be found in polyp tissue, we cannot find any convincing evidence that they occur more frequently in NP than they do in control tissue from non-CRS patients (Hulse, Carter et al., unpublished observations). Instead, we have found evidence that the local B cell response in NP may be similar to the extrafollicular response that is known to occur in lymph nodes and spleen. In support of this hypothesis, we have found significantly elevated expression of Epstein-Barr virus-induced protein 2 (EBI2), which is critical for the development of extrafollicular B cell responses, in NP compared to control sinus tissue [95].
Total antibody levels are highly elevated in NP, but not within the systemic circulation, of patients with CRSwNP [60, 94, 95]. Our preliminary data suggest that the antibody repertoire in NP is polyclonal, and the antibody variable regions have not accumulated a large number of mutations (Hulse et al., unpublished observations). Together with the lack of evidence for increased formation of follicles in NP, these data further support the notion that the B cell response in NP resembles an extrafollicular response. While the antigen specificity of these antibodies is largely unknown, it has been demonstrated that some of the IgE in NP is specific for SEB [36], and some of the IgG and IgA are autoantigen specific [97, 98]. Given that a high proportion of patients with CRS are systemically atopic (likely over 50%), it is not surprising that some of the IgE found in CRSwNP nasal tissue is specific to aeroallergens [99, 100]. Interestingly, these studies indicate that even patients with CRSwNP who are not systemically sensitized have local elevations of IgE in their nasal tissues, which is similar to what has been found in patients with allergic rhinitis [101]. There is also evidence that at least some of the antibody-secreting B cells are directly activated within the NP. Gevaert et al. [102] have reported elevated expression of markers of local class switch recombination in NP tissues, including expression of ε germline transcripts and RAG genes that are important for this process. We have recently confirmed this finding and discovered evidence for local switching to other isotypes and elevated expression of AID (Hulse et al., unpublished observations).
Whether locally produced antibodies contribute directly to CRSwNP pathogenesis is unclear. Recently, Gevaert et al. investigated the effects of the anit-IgE antibody omalizumab, compared to placebo, in a small cohort of patients with CRSwNP. They found that treatment with omalizumab significantly reduced the nasal polyp score, as well as several quality of life measures, compared to placebo, suggesting that IgE may play an important role in CRSwNP pathogenesis in some patients [103]. Given the large number of cells that express Fc receptors in NP, it seems likely that antibodies of several isotypes could play an important role in CRSwNP pathogenesis through the activation of these cells locally. Moreover, we have evidence of elevated levels of markers of complement activation via the classical (antibody mediated) pathway (Tan et al., unpublished observation). Together with elevated antibody levels, activated complement could also contribute to the chronic inflammation in NP. Finally, B cells themselves may also play an important role in CRSwNP pathogenesis aside from their immunoglobulin products. They can act as antigen-presenting cells for T cells and can secrete pro-inflammatory cytokines.
Tissue remodelling
Edematous stromal tissue and the formation of pseudocysts are common features of NP, and it has been suggested that the retention of plasma proteins, such as albumin, may contribute to NP growth [27]. Recent work by Takabayashi et al. [104, 105] has provided some insight into the mechanisms that may drive the formation of NP (Fig. 2). This work found that levels of fibrin and an initiator of the coagulation cascade, Factor XIII-A, were both significantly elevated in NP. Further, they found that levels of tissue plasminogen activator (tPA), an important enzyme involved in the breakdown of fibrin, along with a marker of fibrin breakdown (D-dimers) were decreased in NP. Importantly, Factor XIII-A was shown to be produced by M2 macrophages, which develop under type 2 inflammatory conditions and are elevated in NP, and levels of tPA were diminished in epithelial cells in vitro in the presence of type 2 cytokines [104]. Together, these data suggest that the type 2 inflammatory milieu within NP may contribute to the remodelling and growth of NP, as well as to the chronic inflammatory state of the tissue.
Fig. 2.
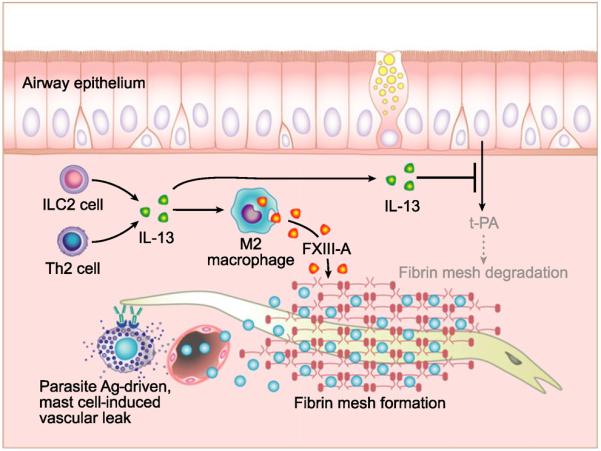
Theoretical role of local activation of fibrin deposition by type 2 inflammatory responses in type 2 diseases. Recent studies by Takabayashi et al. have shown that type 2 cytokines can promote deposition of fibrin by two important mechanisms. The first is the enhancement of fibrin cross-linking by the induction of factor XIII-A (FXIII-A in the figure) in M2 macrophages. This factor cross-links fibrin that has been liberated from fibrinogen by the action of thrombin or other fibrinogen activators. The second mechanism is by diminishing levels of tissue plasminogen activator (tPA), which is a major enzyme in the dissolution of fibrin clots or cross-linked fibrin. Within airways, tPA is expressed by epithelial cells, and IL-13 and IL-4 diminish expression of this important fibrinolytic enzyme. We hypothesize that these mechanisms may have originally evolved as part of the normal antiparasite response, but are inappropriately activated in CRSwNP and may contribute to polyp formation.
Summary and conclusions
In summary, CRSwNP is a complex inflammatory condition, and many factors likely contribute to its pathogenesis. Defects in the basic functions of the airway epithelium coupled with elevated pro-inflammatory cytokines and chemokines likely combine to drive the chronic adaptive immune response found in NP (Fig. 3). Although CRSwNP provides investigators with a unique opportunity to study an ongoing inflammatory response in a human system, studies of the mechanisms that are responsible for disease pathogenesis have been limited by a lack of animal models and a reliance on descriptive and correlative data. As such, there is a paucity of data that demonstrates the necessity or sufficiency of any of these potential drivers of CRSwNP, and clinical studies in patients with new drugs that target defined cytokines, cytokine receptors or signalling molecules are sorely needed. In addition to the development of new tools and models to aid mechanistic studies, the field of CRSwNP research also needs the robust epidemiologic data that has served the asthma community so well. Given the high prevalence, costs and morbidity associated with this disease, there is a great need for continued research into CRS that could facilitate the development of novel therapeutic strategies to improve treatment for patients who suffer from this disease.
Acknowledgement
The authors wish to thank Ms. Jacqueline Schaffer for the illustrations included in this review.
Funding The research was funded by NIH R37 HL068546, R01 HL078860, R01 AI072570, P01 106683-01, K12 HD055884, T32 AI083216-04, K23 DC012067 and the Ernest S. Bazley Trust.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.WJ Fokkens, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86:427–43. doi: 10.4065/mcp.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan BK, Kern RC, Schleimer RP, Schwartz BS. Chronic rhinosinusitis: the unrecognized epidemic. Am J Respir Crit Care Med. 2013;188:1275–7. doi: 10.1164/rccm.201308-1500ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–8. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–21. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 6.Johansson L, Akerlund A, Holmberg K, Melen I, Bende M. Prevalence of nasal polyps in adults: the Skovde population-based study. Ann Otol Rhinol Laryngol. 2003;112:625–9. doi: 10.1177/000348940311200709. [DOI] [PubMed] [Google Scholar]
- 7.Larsen K, Tos M. The estimated incidence of symptomatic nasal polyps. Acta Otolaryngol. 2002;122:179–82. doi: 10.1080/00016480252814199. [DOI] [PubMed] [Google Scholar]
- 8.Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–60. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braakman I, Verest O, Pijning T, Meijer DK, Groothuis GM. Zonal distribution of the cation lucigenin in rat liver: influence of taurocholate. Mol Pharmacol. 1989;36:532–6. [PubMed] [Google Scholar]
- 10.Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:88–94. doi: 10.1002/alr.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144:440–5. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 12.Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121:2672–8. doi: 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123:57–63. doi: 10.1002/lary.23671. [DOI] [PubMed] [Google Scholar]
- 14.Chang JE, White A, Simon RA, Stevenson DD. Aspirin-exacerbated respiratory disease: burden of disease. Allergy Asthma Proc. 2012;33:117–21. doi: 10.2500/aap.2012.33.3541. [DOI] [PubMed] [Google Scholar]
- 15.Bavbek S, Dursun B, Dursun E, Korkmaz H, Sertkaya Karasoy D. The prevalence of aspirin hypersensitivity in patients with nasal polyposis and contributing factors. Am J Rhinol Allergy. 2011;25:411–5. doi: 10.2500/ajra.2011.25.3660. [DOI] [PubMed] [Google Scholar]
- 16.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease: update on pathogenesis and desensitization. Semin Respir Crit Care Med. 2012;33:588–94. doi: 10.1055/s-0032-1325618. [DOI] [PubMed] [Google Scholar]
- 17.White AA, Stevenson DD. Aspirin desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:211–22. doi: 10.1016/j.iac.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–9. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 19.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33:195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullol J, Picado C. Rhinosinusitis and nasal polyps in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:163–76. doi: 10.1016/j.iac.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski ML, Makowska JS, Blanca M, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) – classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011;66:818–29. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 22.Bakhshaee M, Fereidouni M, Mohajer MN, Majidi MR, Azad FJ, Moghiman T. The prevalence of allergic fungal rhinosinusitis in sinonasal polyposis. Eur Arch Otorhinolaryngol. 2013;270:3095–8. doi: 10.1007/s00405-013-2449-5. [DOI] [PubMed] [Google Scholar]
- 23.Laury AM, Wise SK. Chapter 7: allergic fungal rhinosinusitis. Am J Rhinol Allergy. 2013;27(Suppl 1):S26–7. doi: 10.2500/ajra.2013.27.3891. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson BJ, Barnes L, Bernstein JM, et al. Geographic variation in allergic fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:441–9. doi: 10.1016/s0030-6665(00)80018-3. [DOI] [PubMed] [Google Scholar]
- 25.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdavinia M, Suh LA, Carter RG, et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol. 2014;133:1759–63. doi: 10.1016/j.jaci.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- 28.Sasama J, Sherris DA, Shin SH, Kephart GM, Kern EB, Ponikau JU. New paradigm for the roles of fungi and eosinophils in chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2005;13:2–8. doi: 10.1097/00020840-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Davis LJ, Kita H. Pathogenesis of chronic rhinosinusitis: role of airborne fungi and bacteria. Immunol Allergy Clin North Am. 2004;24:59–73. doi: 10.1016/S0889-8561(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 30.Ponikau JU, Sherris DA, Kephart GM, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–9. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:34–8. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein JM, Ballow M, Schlievert PM, Rich G, Allen C, Dryja D. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol. 2003;17:321–6. [PubMed] [Google Scholar]
- 33.Conley DB, Tripathi A, Seiberling KA, et al. Superantigens and chronic rhinosinusitis: skewing of T-cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am J Rhinol. 2006;20:534–9. doi: 10.2500/ajr.2006.20.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conley DB, Tripathi A, Seiberling KA, et al. Superantigens and chronic rhinosinusitis II: analysis of T-cell receptor V beta domains in nasal polyps. Am J Rhinol. 2006;20:451–5. doi: 10.2500/ajr.2006.20.2880. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Shi P, Yue Z, et al. Superantigens and the expression of T-cell receptor repertoire in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2008;128:901–8. doi: 10.1080/00016480701760122. [DOI] [PubMed] [Google Scholar]
- 36.Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–8. 68, e1–6. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–3. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–56. e1–12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 39.Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012;130:1087–96. e10. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 40.Bernstein JM, Yankaskas JR. Increased ion transport in cultured nasal polyp epithelial cells. Arch Otolaryngol Head Neck Surg. 1994;120:993–6. doi: 10.1001/archotol.1994.01880330071013. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein JM, Gorfien J, Noble B, Yankaskas JR. Nasal polyposis: immunohistochemistry and bioelectrical findings (a hypothesis for the development of nasal polyps) J Allergy Clin Immunol. 1997;99:165–75. doi: 10.1016/s0091-6749(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda M, Niisato N, Miyazaki H, et al. Epithelial ion transport of human nasal polyp and paranasal sinus mucosa. Am J Respir Cell Mol Biol. 2007;36:466–72. doi: 10.1165/rcmb.2006-0064OC. [DOI] [PubMed] [Google Scholar]
- 43.Pothoven K, O'Campo C, Suh L, et al. Oncostatin M is elevated in chronic rhinosinusitis and decreases barrier function in human airway epithelium. J Allergy Clin Immunol. 2014;133:AB237. [Google Scholar]
- 44.Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:1–6. doi: 10.2500/ajra.2012.26.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seshadri S, Lin DC, Rosati M, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67:920–8. doi: 10.1111/j.1398-9995.2012.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Kim J, McWilliams R, Cutting GR. Increased prevalence of chronic rhinosinusitis in carriers of a cystic fibrosis mutation. Arch Otolaryngol Head Neck Surg. 2005;131:237–40. doi: 10.1001/archotol.131.3.237. [DOI] [PubMed] [Google Scholar]
- 47.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 48.Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–44. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao CY, Wang X, Liu M, Jin DJ. Microarray gene analysis of Toll-like receptor signaling elements in chronic rhinosinusitis with nasal polyps. Int Arch Allergy Immunol. 2011;156:297–304. doi: 10.1159/000323767. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Zhou B, Wang C, et al. Biofilm formation and Toll-like receptor 2, Toll-like receptor 4, and NF-kappaB expression in sinus tissues of patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:104–9. doi: 10.2500/ajra.2012.26.3718. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Wang CS, Han DM, et al. Differential expression of Toll-like receptor pathway genes in chronic rhinosinusitis with or without nasal polyps. Acta Otolaryngol. 2013;133:165–73. doi: 10.3109/00016489.2012.717713. [DOI] [PubMed] [Google Scholar]
- 52.Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol. 2006;20:117–23. [PMC free article] [PubMed] [Google Scholar]
- 53.Ramanathan M, Jr, Lee WK, Dubin MG, Lin S, Spannhake EW, Lane AP. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol. 2007;21:110–6. doi: 10.2500/ajr.2007.21.2997. [DOI] [PubMed] [Google Scholar]
- 54.Park CS, Cho JH, Park YJ. Toll-like receptor 2 gene polymorphisms in a Korean population: association with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2011;144:96–100. doi: 10.1177/0194599810390881. [DOI] [PubMed] [Google Scholar]
- 55.Tieu DD, Peters AT, Carter RG, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–75. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Y, Xia W, Ye X, et al. The antimicrobial protein short palate, lung, and nasal epithelium clone 1 (SPLUNC1) is differentially modulated in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2014;133:420–8. doi: 10.1016/j.jaci.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 57.Peters AT, Kato A, Zhang N, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. e10. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hulse KE, Chaung K, Seshadri S, et al. Suppressor of cytokine signaling 3 expression is diminished in sinonasal tissues from patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2014;133:275–7. e1. doi: 10.1016/j.jaci.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartels J, Maune S, Meyer JE, et al. Increased eotaxin-mRNA expression in non-atopic and atopic nasal polyps: comparison to RANTES and MCP-3 expression. Rhinology. 1997;35:171–4. [PubMed] [Google Scholar]
- 60.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 61.Jahnsen FL, Haye R, Gran E, Brandtzaeg P, Johansen FE. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J Immunol. 1999;163:1545–51. [PubMed] [Google Scholar]
- 62.Yoshifuku K, Matsune S, Ohori J, Sagara Y, Fukuiwa T, Kurono Y. IL-4 and TNF-alpha increased the secretion of eotaxin from cultured fibroblasts of nasal polyps with eosinophil infiltration. Rhinology. 2007;45:235–41. [PubMed] [Google Scholar]
- 63.Matsukura S, Stellato C, Georas SN, et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 2001;24:755–61. doi: 10.1165/ajrcmb.24.6.4351. [DOI] [PubMed] [Google Scholar]
- 64.Peterson S, Poposki JA, Nagarkar DR, et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129:119–27. e1–9. doi: 10.1016/j.jaci.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patadia M, Dixon J, Conley D, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:11–6. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miljkovic D, Bassiouni A, Cooksley C, et al. Association between Group 2 Innate Lymphoid Cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–61. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 67.Allakhverdi Z, Comeau MR, Smith DE, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy. 2010;24:105–9. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baba S, Kondo K, Kanaya K, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014;124:E115–22. doi: 10.1002/lary.24462. [DOI] [PubMed] [Google Scholar]
- 70.Nagarkar DR, Poposki JA, Comeau MR, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–32. e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 92, e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takabayashi T, Kato A, Peters AT, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–20. e5. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy. 2005;87:111–29. doi: 10.1159/000087639. [DOI] [PubMed] [Google Scholar]
- 74.Mahdavinia M, Carter RG, Ocampo CJ, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014;133:1759–63. doi: 10.1016/j.jaci.2013.12.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin LB, Kita H, Leiferman KM, Gleich GJ. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int Arch Allergy Immunol. 1996;109:207–15. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]
- 76.Schleimer RP, Kato A, Kern R. Eosinophils and chronic rhinosinusitis. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Elsevier; San Diego, CA: 2012. pp. 508–18. [Google Scholar]
- 77.Chu VT, Frohlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 78.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–90. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 80.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 81.Koyasu S, Moro K, Tanabe M, Takeuchi T. Natural helper cells: a new player in the innate immune response against helminth infection. Adv Immunol. 2010;108:21–44. doi: 10.1016/B978-0-12-380995-7.00002-1. [DOI] [PubMed] [Google Scholar]
- 82.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barlow JL, Bellosi A, Hardman CS, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–8. e1–4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 84.Mjosberg J, Eidsmo L. Update on innate lymphoid cells in atopic and non-atopic inflammation in the airways and skin. Clin Exp Allergy. 2014;44:1033–43. doi: 10.1111/cea.12353. [DOI] [PubMed] [Google Scholar]
- 85.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–74. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Derycke L, Eyerich S, Van Crombruggen K, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS ONE. 2014;9:e97581. doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflamma-tory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 88.Peterson S, Welch K, Poposki J, et al. Elevated presence of dendritic cell subsets in chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:AB60. [Google Scholar]
- 89.Pezato R, Perez-Novo CA, Holtappels G, et al. The expression of dendritic cell subsets in severe chronic rhinosinusitis with nasal polyps is altered. Immunobiology. 2014;219:729–36. doi: 10.1016/j.imbio.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Van Bruaene N, Perez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. 41, e1–3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Li HB, Cai KM, Liu Z, et al. Foxp3+ T regulatory cells (Tregs) are increased in nasal polyps (NP) after treatment with intranasal steroid. Clin Immunol. 2008;129:394–400. doi: 10.1016/j.clim.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 93.Pant H, Hughes A, Schembri M, Miljkovic D, Krumbiegel D. CD4(+) and CD8(+) regulatory T cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy. 2014;28:e83–9. doi: 10.2500/ajra.2013.28.4014. [DOI] [PubMed] [Google Scholar]
- 94.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–7. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 95.Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 97.Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoanti-bodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206. e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeffe JS, Seshadri S, Hamill KJ, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngo-scope. 2013;123:2104–11. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabirov A, Hamilton RG, Jacobs JB, Hillman DE, Lebowitz RA, Watts JD. Role of local immunoglobulin E specific for Alternaria alternata in the pathogenesis of nasal polyposis. Laryngoscope. 2008;118:4–9. doi: 10.1097/MLG.0b013e3181567a7a. [DOI] [PubMed] [Google Scholar]
- 100.Suh KS, Park HS, Nahm DH, Kim YK, Lee YM, Park K. Role of IgG, IgA, and IgE antibodies in nasal polyp tissue: their relationships with eosinophilic infiltration and degranulation. J Korean Med Sci. 2002;17:375–80. doi: 10.3346/jkms.2002.17.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Powe DG, Jagger C, Kleinjan A, Carney AS, Jenkins D, Jones NS. `Entopy': localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003;33:1374–9. doi: 10.1046/j.1365-2222.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- 102.Gevaert P, Nouri-Aria KT, Wu H, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 103.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–6. e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 104.Takabayashi T, Kato A, Peters AT, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takabayashi T, Kato A, Peters AT, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–92. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–42. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 107.Olze H, Forster U, Zuberbier T, Morawietz L, Luger EO. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006;44:145–50. [PubMed] [Google Scholar]
- 108.Cho DY, Nayak JV, Bravo DT, et al. Expression of dual oxidases and secreted cytokines in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:376–83. doi: 10.1002/alr.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poposki JA, Uzzaman A, Nagarkar DR, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–81. e4. doi: 10.1016/j.jaci.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000;14:367–73. doi: 10.2500/105065800779954329. [DOI] [PubMed] [Google Scholar]
- 111.Patou J, Gevaert P, Van Zele T, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008;121:110–5. doi: 10.1016/j.jaci.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 112.Van Crombruggen K, Van Bruaene N, Holtappels G, Bachert C. Chronic sinusitis and rhinitis: clinical terminology “Chronic Rhinosinusitis” further supported. Rhinology. 2010;48:54–8. doi: 10.4193/Rhin09.078. [DOI] [PubMed] [Google Scholar]
- 113.Foreman A, Holtappels G, Psaltis AJ, et al. Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy. 2011;66:1449–56. doi: 10.1111/j.1398-9995.2011.02678.x. [DOI] [PubMed] [Google Scholar]
- 114.Sejima T, Holtappels G, Kikuchi H, Imayoshi S, Ichimura K, Bachert C. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int. 2012;61:115–22. doi: 10.2332/allergolint.10-OA-0290. [DOI] [PubMed] [Google Scholar]
- 115.Luo B, Feng L, Jintao D, et al. Immunopathology features of chronic rhinosinusitis in high-altitude dwelling Tibetans. Allergy Rhinol. 2013;4:e69–76. doi: 10.2500/ar.2013.4.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamilos DL, Leung DY, Wood R, et al. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte-macrophage colony-stimulating factor and interleukin-3. J Allergy Clin Immunol. 1993;92:39–48. doi: 10.1016/0091-6749(93)90035-e. [DOI] [PubMed] [Google Scholar]
- 117.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013;123:E72–8. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 118.Chen YS, Langhammer T, Westhofen M, Lorenzen J. Relationship between matrix metalloproteinases MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinases-1 and IL-5, IL-8 in nasal polyps. Allergy. 2007;62:66–72. doi: 10.1111/j.1398-9995.2006.01255.x. [DOI] [PubMed] [Google Scholar]
- 119.Mullol J, Xaubet A, Gaya A, et al. Cytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polyps. Clin Exp Allergy. 1995;25:607–15. doi: 10.1111/j.1365-2222.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 120.Shi LL, Xiong P, Zhang L, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013;68:101–9. doi: 10.1111/all.12064. [DOI] [PubMed] [Google Scholar]
- 121.Wang H, Bai J, Ding M, et al. Interleukin-17A contributes to the expression of serum amyloid A in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol. 2013;270:1867–72. doi: 10.1007/s00405-012-2295-x. [DOI] [PubMed] [Google Scholar]
- 122.Paris G, Pozharskaya T, Asempa T, Lane AP. Damage-associated molecular patterns stimulate interleukin-33 expression in nasal polyp epithelial cells. Int Forum Allergy Rhinol. 2014;4:15–21. doi: 10.1002/alr.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xaubet A, Mullol J, Lopez E, et al. Comparison of the role of nasal polyp and normal nasal mucosal epithelial cells on in vitro eosinophil survival. Mediation by GM-CSF and inhibition by dexamethasone. Clin Exp Allergy. 1994;24:307–17. doi: 10.1111/j.1365-2222.1994.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 124.Watelet JB, Claeys C, Perez-Novo C, Gevaert P, Van Cauwenberge P, Bachert C. Transforming growth factor beta1 in nasal remodeling: differences between chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2004;18:267–72. [PubMed] [Google Scholar]
- 125.Van Bruaene N, Derycke L, Perez-Novo CA, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–9. e1–2. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 126.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600. e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]