Abstract
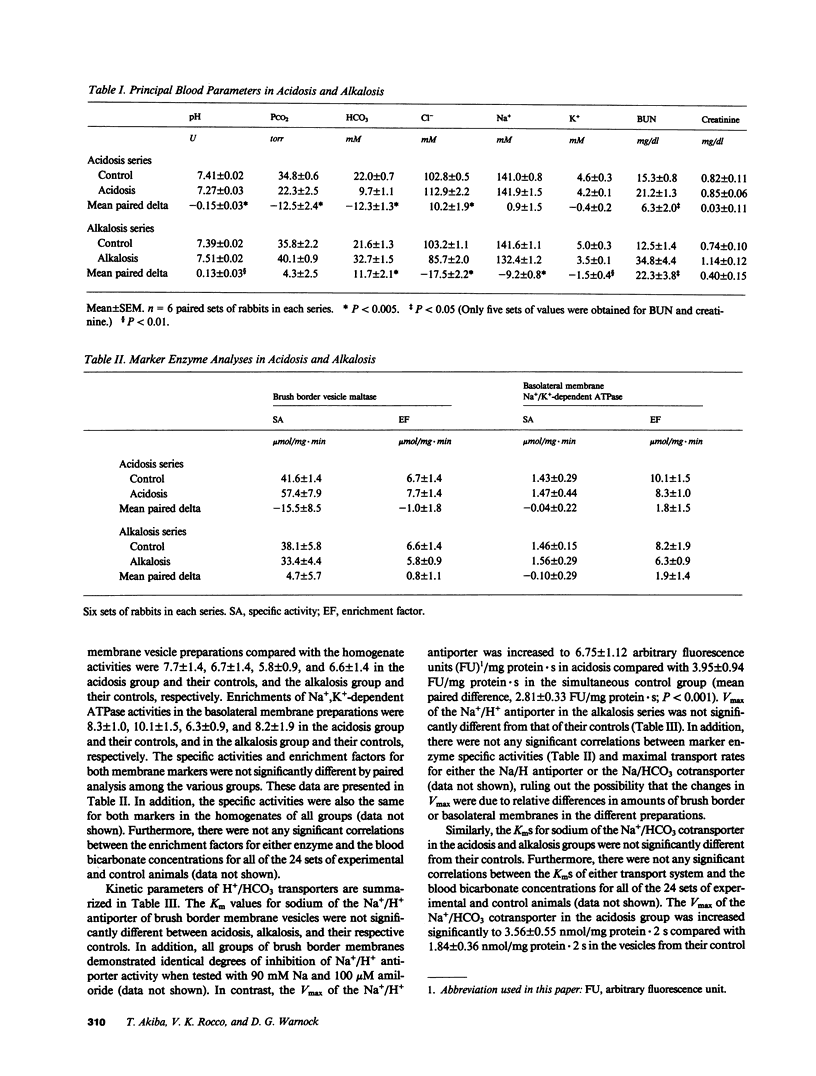
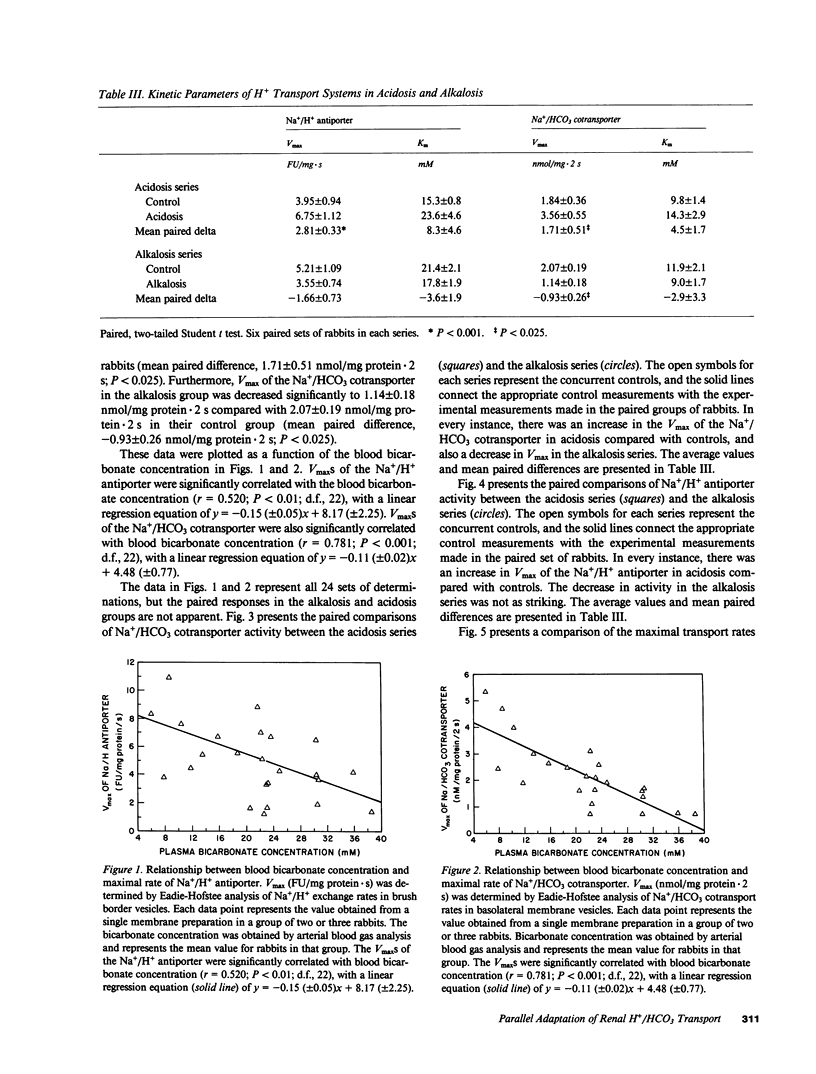
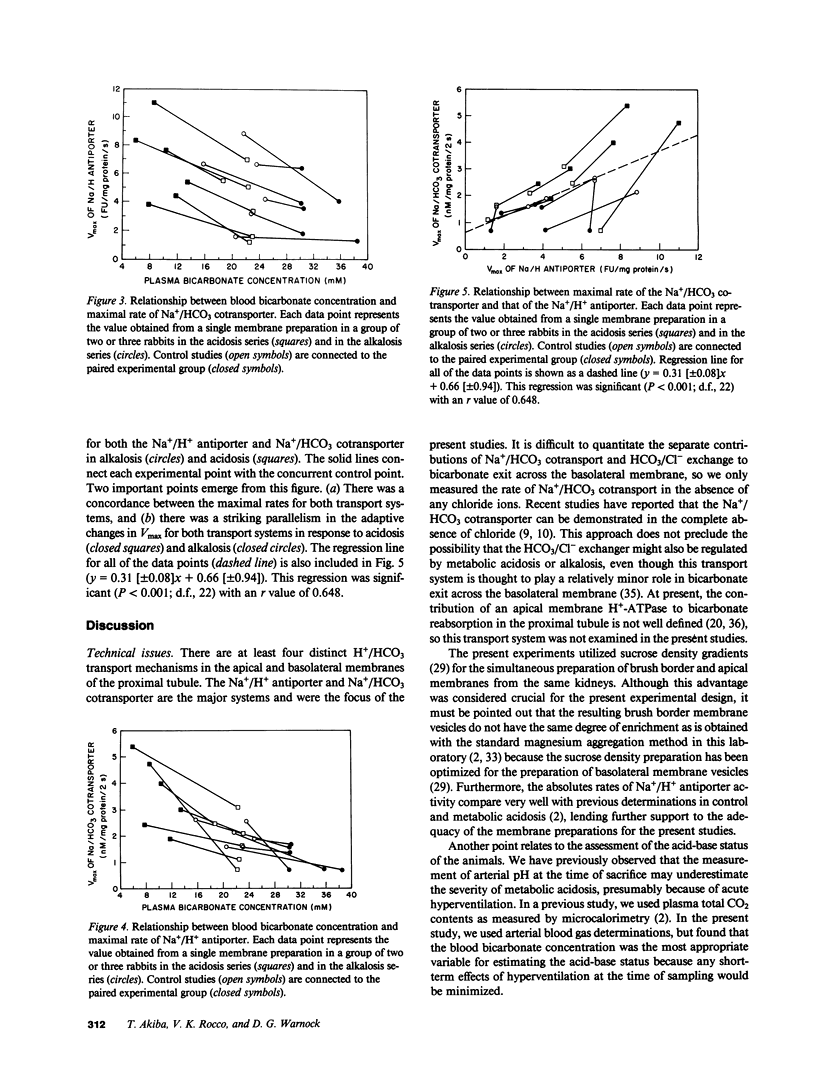
Recent studies have shown that the bicarbonate reabsorptive capacity of the proximal tubule is increased in metabolic acidosis. For net bicarbonate reabsorption to be regulated, there may be changes in the rate of apical H+ secretion as well as in the basolateral base exit step. The present studies examined the rate of Na+/H+ exchange (acridine orange method) and Na+/HCO3 cotransport (22Na uptake) in apical and basolateral membranes prepared from the rabbit renal cortex by sucrose density gradient centrifugation. NH4Cl loading was used to produce acidosis (arterial pH, 7.27 +/- 0.03), and Cl-deficient diet with furosemide was used to produce alkalosis (arterial pH, 7.51 +/- 0.02). Maximal transport rate (Vmax) of Na+/H+ antiporter and Na+/HCO3 cotransporter were inversely related with plasma bicarbonate concentration from 6 to 39 mM. Furthermore, the maximal transport rates of both systems varied in parallel; when Vmax for the Na+/HCO3 cotransporter was plotted against Vmax for the Na+/H+ antiporter for each of the 24 groups of rabbits, the regression coefficient (r) was 0.648 (P less than 0.001). There was no effect of acidosis or alkalosis on affinity for Na+ of either transporter. We conclude that both apical and basolateral H+/HCO3 transporters adapt during acid-base disturbances, and that the maximal transport rates of both systems vary in parallel during such acid-base perturbations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Alpern R. J., Eveloff J., Calamina J., Warnock D. G. Electrogenic sodium/bicarbonate cotransport in rabbit renal cortical basolateral membrane vesicles. J Clin Invest. 1986 Dec;78(6):1472–1478. doi: 10.1172/JCI112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest. 1986 Aug;78(2):502–510. doi: 10.1172/JCI112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Biagi B. A. Effects of the anion transport inhibitor, SITS, on the proximal straight tubule of the rabbit perfused in vitro. J Membr Biol. 1985;88(1):25–31. doi: 10.1007/BF01871210. [DOI] [PubMed] [Google Scholar]
- Blumenthal S. S., Ware R. A., Kleinman J. G. Proximal tubule hydrogen ion transport processes in diuretic-induced metabolic alkalosis. J Lab Clin Med. 1985 Jul;106(1):17–22. [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt B. C., Sato K., Frömter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. I. Basic observations. Pflugers Arch. 1984 May;401(1):34–42. doi: 10.1007/BF00581530. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Alpern R. J. Regulation of proximal bicarbonate reabsorption. Am J Physiol. 1984 Sep;247(3 Pt 2):F387–F395. doi: 10.1152/ajprenal.1984.247.3.F387. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Liu F. Y. Metabolic alkalosis in the rat. Evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest. 1983 May;71(5):1141–1160. doi: 10.1172/JCI110864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan M. G., Rector F. C., Jr Proximal reabsorption during metabolic acidosis in the rat. Am J Physiol. 1982 May;242(5):F499–F507. doi: 10.1152/ajprenal.1982.242.5.F499. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Klahr S., Hammerman M. R. Metabolic acidosis and parathyroidectomy increase Na+-H+ exchange in brush border vesicles. Am J Physiol. 1983 Aug;245(2):F217–F222. doi: 10.1152/ajprenal.1983.245.2.F217. [DOI] [PubMed] [Google Scholar]
- Grassl S. M., Aronson P. S. Na+/HCO3-co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem. 1986 Jul 5;261(19):8778–8783. [PubMed] [Google Scholar]
- Gurich R. W., Warnock D. G. Electrically neutral Na+-H+ exchange in endosomes obtained from rabbit renal cortex. Am J Physiol. 1986 Oct;251(4 Pt 2):F702–F709. doi: 10.1152/ajprenal.1986.251.4.F702. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Seifter J. L., Brenner B. M. Adaptation of Na+-H+ exchange in renal microvillus membrane vesicles. Role of dietary protein and uninephrectomy. J Clin Invest. 1984 Dec;74(6):1979–1987. doi: 10.1172/JCI111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E. Proton/hydroxyl permeability of proximal tubule brush border vesicles. Am J Physiol. 1985 Jan;248(1 Pt 2):F78–F86. doi: 10.1152/ajprenal.1985.248.1.F78. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Yee V. J., Warnock D. G. Asymmetric distribution of the Na+/H+ antiporter in the renal proximal tubule epithelial cell. J Biol Chem. 1983 Nov 25;258(22):13513–13516. [PubMed] [Google Scholar]
- Jentsch T. J., Schill B. S., Schwartz P., Matthes H., Keller S. K., Wiederholt M. Kidney epithelial cells of monkey origin (BSC-1) express a sodium bicarbonate cotransport. Characterization by 22Na+ flux measurements. J Biol Chem. 1985 Dec 15;260(29):15554–15560. [PubMed] [Google Scholar]
- Kinsella J. L., Freiberg J. M., Sacktor B. Glucocorticoid activation of Na+/H+ exchange in renal brush border vesicles: kinetic effects. Am J Physiol. 1985 Feb;248(2 Pt 2):F233–F239. doi: 10.1152/ajprenal.1985.248.2.F233. [DOI] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange in isolated renal brush-border membrane vesicles in response to metabolic acidosis. Kinetic effects. J Biol Chem. 1984 Nov 10;259(21):13224–13227. [PubMed] [Google Scholar]
- Kunau R. T., Jr, Hart J. I., Walker K. A. Effect of metabolic acidosis on proximal tubular total CO2 absorption. Am J Physiol. 1985 Jul;249(1 Pt 2):F62–F68. doi: 10.1152/ajprenal.1985.249.1.F62. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Axial heterogeneity of bicarbonate, chloride, and water transport in the rat proximal convoluted tubule. Effects of change in luminal flow rate and of alkalemia. J Clin Invest. 1986 Dec;78(6):1547–1557. doi: 10.1172/JCI112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox D. A., Gennari F. J. Load dependence of proximal tubular bicarbonate reabsorption in chronic metabolic alkalosis in the rat. J Clin Invest. 1986 Mar;77(3):709–716. doi: 10.1172/JCI112365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madias N. E., Zelman S. J. The renal response to chronic mineral acid feeding: a re-examination of the role of systemic pH. Kidney Int. 1986 Mar;29(3):667–674. doi: 10.1038/ki.1986.50. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Cohen B., Guggino W. B., Giebisch G. Electrical effects of potassium and bicarbonate on proximal tubule cells of Necturus. J Membr Biol. 1984;79(2):145–152. doi: 10.1007/BF01872118. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Schoner W., von Ilberg C., Kramer R., Seubert W. On the mechanism of Na+- and K+-stimulated hydrolysis of adenosine triphosphate. 1. Purification and properties of a Na+-and K+-activated ATPase from ox brain. Eur J Biochem. 1967 May;1(3):334–343. doi: 10.1007/978-3-662-25813-2_45. [DOI] [PubMed] [Google Scholar]
- Schwartz W. B., Cohen J. J. The nature of the renal response to chronic disorders of acid-base equilibrium. Am J Med. 1978 Mar;64(3):417–428. doi: 10.1016/0002-9343(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- Trivedi B., Tannen R. L. Effect of respiratory acidosis on intracellular pH of the proximal tubule. Am J Physiol. 1986 Jun;250(6 Pt 2):F1039–F1045. doi: 10.1152/ajprenal.1986.250.6.F1039. [DOI] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol. 1982 Apr;242(4):F406–F414. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- Vigne P., Jean T., Barbry P., Frelin C., Fine L. G., Lazdunski M. [3H]Ethylpropylamiloride, a ligand to analyze the properties of the Na+/H+ exchange system in the membranes of normal and hypertrophied kidneys. J Biol Chem. 1985 Nov 15;260(26):14120–14125. [PubMed] [Google Scholar]
- Warnock D. G., Reenstra W. W., Yee V. J. Na+/H+ antiporter of brush border vesicles: studies with acridine orange uptake. Am J Physiol. 1982 Jun;242(6):F733–F739. doi: 10.1152/ajprenal.1982.242.6.F733. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]



