Abstract
miR-122 is an abundant, liver-specific microRNA that is required for efficient amplification of hepatitis C virus (HCV) RNA. Recent studies with a miR-122-specific locked nucleic acid antagomir have shown it to be an important host target for therapeutic intervention. However, considerable controversy exists concerning the mechanisms underlying the dependence of HCV replication on miR-122. We studied the impact of miR-122 on the rate of [32P]-incorporation into positive-strand viral RNA by membrane-bound replicase complexes isolated from cells containing HCV RNA replicons. [32P]-incorporation in this cell-free system represents primarily the elongation phase of RNA synthesis, with little or no de novo initiation, and was not affected by the addition of either excess miR-122 or a miR-122-specific antisense oligonucleotide that suppresses replication in vivo. We also found no evidence that detectable quantities of miR-122 are specifically associated with replicase complexes in vivo. These results are consistent with miR-122 acting at an alternative step in the viral life cycle, promoting cap-independent viral translation, enhancing viral RNA stability, or facilitating de novo initiation of viral RNA synthesis.
Hepatitis C virus (HCV) is a positive-strand virus classified within the family Flaviviridae. It has an RNA genome ~9.6 kb in length containing a single long open reading frame flanked by 5′- and 3′-nontranslated regions (NTRs) (reviewed in Moradpour et al., 2007). As with other positive-strand RNA viruses, the synthesis of HCV RNA is believed to take place in association with intracellular membranes which form a “membranous web” that is thought to be the site of new viral RNA synthesis (Gosert et al., 2003). RNA synthesis is catalyzed by NS5B which possesses RNA-dependent RNA polymerase activity in vitro (Behrens et al., 1996). Recombinant NS5B is capable of de novo initiation of RNA synthesis, but lacks template specificity due to the absence of other viral and host-derived proteins that in vivo constitute a multi-subunit, membrane-associated HCV replication complex (the HCV replicase). A unique feature of HCV genome amplification is its dependence on a liver-specific micro-RNA (miRNA), miR-122 (Jopling et al., 2005). miR-122 positively regulates the abundance of HCV RNA in Huh-7 cells, and its functional sequestration by transfection of an antisense oligonucleotide reduces HCV RNA abundance and inhibits replication of infectious virus (Jopling et al., 2005; Randall et al., 2007). Importantly, therapeutic silencing of miR-122 with a locked nucleic acid (LNA) antagomir was shown recently to have a profound antiviral effect in HCV-infected chimpanzees (Lanford et al., 2010).
The positive-sense HCV RNA genome contains at least three potential miR-122 seed sequence-binding sites. Two sites within the 5′-NTR, just upstream of an internal ribosome entry site (IRES), have been shown to bind miR-122 (Jopling et al., 2005, 2008), while a third potential site exists within the 3′-NTR. Point mutations within the 5′-NTR sites abolish HCV RNA replication, but replication can be rescued by transfection of a complementary mutant miR-122 (Jopling et al., 2005, 2008). These data indicate that miR-122 interacts directly with the viral RNA, but how it regulates the abundance of HCV RNA is poorly understood. Norman and Sarnow (2010) suggested recently that miR-122 does not directly modulate the synthesis of viral RNA in cells. However, the potential regulation of in vitro synthesis of viral RNA by isolated replication complexes has not been examined. We set out to answer this question, and to determine if miR-122 specifically associates with the replicase complex in vivo.
Membrane fractions containing synthetically active replicase complexes can be isolated by differential centrifugation of extracts from Huh-7 cells containing HCV replicons (Ali et al., 2002; Hardy et al., 2003). We prepared such a heavy membrane fraction (16 000xG pellet) from cells containing subgenomic and genome-length genotype 1a replicons (Yi and Lemon, 2004). This fraction contained viral nonstructural proteins, including NS5B, and was also enriched in ER proteins, calnexin and Rab1b (data not shown). When incubated at 37°C with a standard transcription mixture containing [α-32P]-CTP, RNA products corresponding to the length of the respective replicon could be resolved by denaturing agarose gel electrophoresis (Fig. 1A, lane 14). Similar products were not obtained with incubation of a related supernatant fraction, or P16 fractions prepared from normal Huh-7 cells (data not shown). Previous studies suggest that preassembled, membrane-bound replicase complexes isolated in this fashion predominantly produce copies of positive-strand RNA from the endogenous RNA template (Hardy et al., 2003). The RNA product thus represents the elongation of nascent HCV transcripts initiated in vivo but arrested on cell lysis. We confirmed this by RNA protection assays, done as described by Egger et al. (1996) with unlabeled RNA probes complementary to the 5′ and 3′ ends of positive-strand RNA (data not shown).
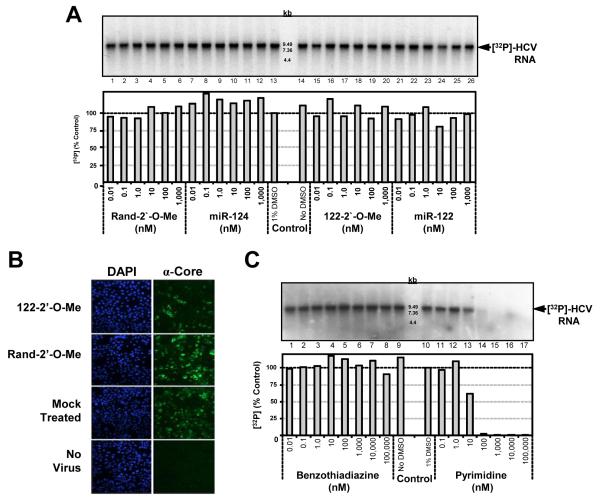
Figure 1. Effect of miR-122 on the elongation phase of HCV RNA synthesis by cell-free replicases.
(A) Various concentrations of miR-122 or other oligonucleotides (Table 1) were added to the P16 fraction from subgenomic replicon cells in the cell-free replicase reaction. Control reactions were carried out either in the absence (lane 14) or presence (lane 13) of 1% DMSO. Radiolabeled RNA was extracted, and resolved on a 1% agarose gel containing glyoxal, followed by autoradiography (top panel) or PhosphoImager quantitation (lower panel). (B) Huh-7.5 cells were transfected with 50 nM oligonucleotide, and 24 hrs later infected with HJ3-5 virus, a chimeric HCV, at an m.o.i. of 1. Cells were immunostained for core antigen 72 hrs after infection. Nuclei were labeled with DAPI. (C) Increasing concentrations of an allosteric benzothiadiazine or active-site pyrimidine inhibitor were incubated with the P16 fraction.
Neither supplementing reaction mixes with excess miR-122, or functionally inactivating miR-122 by adding a complementary 2′-O-methylated oligonucleotide (122-2′-O-Me)(Table 1), had any effect on [32P] incorporation (Fig. 1A). Micromolar concentrations of the antisense 122-2′-O-Me did not inhibit the reaction, even though transfection of cells with as little as 50 nM was sufficient to inhibit replication of infectious HCV in Huh-7 cells (Fig. 1B). To confirm the specificity of the RNA synthesized in this reaction, we determined whether [32P] incorporation was blocked by small molecule NS5B inhibitors: an allosteric benzothiadiazine inhibitor, and a pyrimidine pyrophosphate mimic that acts as an active-site inhibitor (Summa et al., 2004; Tomei et al., 2004). The IC50 was 15.5 μM for the pyrimidine and 3.4 μM for the benzothiadiazine in genotype 1a replicon cells (Bourne et al., 2005) (Table 2). Consistent with this, the pyrimidine inhibitor caused a 40% reduction in [32P] incorporation at 10 nM, and completely blocked RNA synthesis at higher concentrations (Fig. 1C). These data confirm that RNA synthesis was catalyzed by the authentic NS5B polymerase. In contrast, even high concentrations of the allosteric benzothiadiazine inhibitor (up to 100 μM) failed to inhibit [32P] incorporation (Fig. 1C). These results are consistent with previous studies showing that allosteric non-nucleoside inhibitors act at an early step in the initiation of viral RNA synthesis and may fail to block elongation (Liu et al., 2006; Ma et al., 2005; Tomei et al., 2004).
Table 1.
Oligonucleotides
| RNA oligonucleotide1 |
Sequence |
|---|---|
| miR-122 | 5′-UGG AGU GUG ACA AUG GUG UUU GU-3′ |
| miR-122* | 5′-AAA CGC CAU UAU CAC ACU AAA UA-3′ |
| miR-124 | 5′-UUA AGG CAC GCG GUG AAU GCC A-3′ |
| miR-124* | 5′-CCG UGU UCA CAG CGG ACC UUG A-3′ |
| 122-2′-O-Me | 5′-AGA CAC AAA CAC CAU UGU CAC ACU CCA CAG C-3′ |
| Rand-2′-O-Me | 5′-CAC GUU AAA ACC AUA CGC ACU ACG AAA CCC C-3′ |
miR-122 and miR-124 were transfected as duplex RNAs generated by annealing equimolar amounts of miR-122 and miR-122*, and miR-124 and miR-124*, respectively.
Table 2.
Antiviral activities of NS5B inhibitors against the subgenomic HCV replicon
| Compound | IC501 | CC501 |
|---|---|---|
| Pyrimidine (L-000874792) (βM) | 15.5 | >20.0 |
| Benzothiadiazine (L-001117921) (βM) | 3.4 | >20.0 |
| Α2b-Interferon (I.U./mL) | 2.1 | >500 |
IC50, 50% reduction in HCV RNA abundance; CC50, 50% cytotoxicity by MTT assay.
These results indicate that miR-122 does not regulate the elongation phase of HCV RNA synthesis. There are two possible explanations for this. First, the fully assembled HCV replicase simply may not require miR-122 to mediate elongation. An alternative interpretation is that miR-122 may indeed be required for elongation, but that the membranous environment of the replicase renders it inaccessible to either supplementation or sequestration by miR-122-specific oligonucleotides added to the reaction mix. Such an interpretation is consistent with prior observations that isolated replicase complexes cannot use an exogenous viral RNA as a template when supplied in trans, and that the endogenous viral RNA template within the replication complex is inaccessible to enzymatic degradation (Ali et al., 2002; Lai et al., 2003; Miyanari et al., 2003). If so, however, this is not an artifact of the in vitro system in which we studied isolated replicase complexes: the same constraints on accessing the fully-formed replicase would exist within the infected cell in which these membranous complexes normally reside. Our results thus indicate that if the available abundance of free miR-122 directly influences the rate of viral RNA synthesis, it must do so during an early step in replicase assembly or initiation of RNA synthesis, steps that are specifically antagonized by non-nucleoside inhibitors of the NS5B polymerase, as discussed above.
As an alternative approach to defining a role for miR-122 in viral RNA synthesis, we transfected replicon cells with the antisense 122-2′-O-Me oligonucleotide 24 hrs prior to lysis and isolation of replicase complexes. If miR-122 were directly required for RNA synthesis and incorporated into the replicase complex at assembly, complexes formed after transfection of the antisense 122-2′-O-Me should demonstrate substantially reduced specific RNA synthetic activity and less [32P] incorporation. While we observed a reduction in the abundance of replicase complexes after transfecting the antisense oligonucleotide, as would be expected, the complexes were not grossly reduced in their ability to incorporate [32P] (data not shown). While such an experiment is admittedly difficult to rigorously control, these results suggest that miR-122 is required either at or before the formation of replicase complexes.
To directly investigate the possible association of miR-122 with the replicase, we prepared P16 fractions from both naïve Huh-7 and HCV replicon cells, and layered them onto 20-70% sucrose gradients that were then centrifuged to equilibrium. The cellular protein content peaked in fractions 9-11 of these gradients, while total RNA content peaked in fraction 11 (Fig. 2A). Functional HCV replication complexes were most abundant in fraction 7 (density ~1.17 gm/cm3) with lesser amounts in adjacent fractions (Fig. 2B). Viral RNA was detected by RT-PCR in fractions 7-8. Using an RNase protection assay, we found miR-122 to be concentrated in fractions 11-12 which had a considerably higher density (~1.22 gm/cm3) (Fig. 2D, right panel). Overall, the distribution of miR-122 within these gradients was similar to that of argonaute 2 (Ago2, Fig. 2C), a component of the miRNA-induced silencing complex, and distinctly different from the HCV replicase. Similar results were obtained with rate-zonal gradients (data not shown). Importantly, there was no difference in the distribution of miR-122 between normal cells and cells containing HCV replicons. To determine whether any amount of miR-122 could be shown to band at the density of the viral replicase, we concentrated material in fractions with peak replicase activity, and subjected it to a second round of centrifugation in identical equilibrium gradients. While these results confirmed that a minor fraction of miR-122 possesses a buoyant density similar to the replicase, this probable membrane-bound miR-122 fraction was also present in normal Huh-7 cells.
Figure 2. Distribution of miR-122 in equilibrium density gradients.
P16 fractions were layered on top of a linear 20-70% sucrose gradient, and centrifuged at 35 000 rpm for 18 hrs at 4°C in an SW60 rotor. (A) Fractions were analyzed for density and total protein (left panel) and total RNA (right panel). (B) Fractions were concentrated by ultracentrifugation (TLS-55 rotor, 52 000 rpm for 2 hrs at 4°C) and increasing quantities tested for replicase activity (top panel). HCV RNA was detected in a one-step RT-PCR assay (Qiagen), using the primers: 5′-ATC CGC TTG TGG CAG AGG AG, and 5′-CCT GGA GAG TAA CTG TGG AGT (bottom panel). (C) Concentrated fractions were analyzed by immunoblot for Ago2, NS3, and NS5B proteins. (D) miR122 was detected by an RNase protection assay (mirVana miRNA Detection Kit, Applied Biosystems); protected fragments were resolved on 15% polyacrylamide/8M urea gels, and imaged by autoradiography. Undigested probe (UDP) and protected probe (PP) bands are indicated.
In summary, the elongation phase of HCV RNA synthesis is not affected by changes in the abundance of miR-122. While we demonstrated this in a cell-free replicase assay, it is also likely to be true within infected cells as discussed above. However, our results do not rule out a role for miR-122 in the initiation of viral RNA synthesis. Just as the allosteric benzothiadiazine inhibitor was unable to inhibit [32P] incorporation in the cell-free replicase assay (Fig. 1C), this assay may have failed to detect a role for miR-122 in initiation of RNA synthesis. Norman and Sarnow (2010) recently reported that a miR-122-specific antisense LNA oligonucleotide failed to slow the incorporation of 4-thiouridine (4SU) into nascent HCV RNA in replicon cells. However, these results may have been subject to the same limitations as the cell-free assay, as the extent to which 4SU incorporation reflects de novo initiation vs. elongation under the conditions used by Norman and Sarnow (2010) is not clear. Our efforts to show a specific association of miR-122 with isolated replicase complexes used a ribonuclease protection assay with sub-femtomolar sensitivity, but were confounded by the presence in normal cells of a small miR-122 fraction with buoyant density similar to that of the replicase. Since there are two miR-122 binding sites on the viral RNA, as few as 2 copies of miR-122 per positive-strand molecule might be sufficient to promote RNA synthesis. Each membrane vesicle with an active replicase complex has been estimated to contain at least one negative-strand RNA molecule and as few as 2-10 positive-strand molecules (Quinkert et al., 2005). Given that Huh-7 cells contain up to 70,000 copies of miR-122 (Chang et al., 2004), a change in subcellular distribution may be too subtle to detect on these gradients.
Three different mechanisms have been proposed to explain the positive impact of miR-122 on viral RNA abundance: first, an increase in viral RNA translation; second, a direct enhancement of viral RNA synthesis, and third, an increase in HCV RNA stability (Jopling et al., 2005). The effect of miR-122 on HCV translation is controversial, with several conflicting reports in the literature (Henke et al., 2008; Jopling et al., 2005, 2008). Henke et al. (2008) demonstrated small miR-122-mediated increases in translation of genomic RNA, and proposed a mechanism involving accelerated association of viral RNA with ribosomes. We recently confirmed that the interaction of miR-122 with HCV RNA exerts a specific, positive modulatory effect on translation of the viral genome (Jangra et al., 2010). However, our results also demonstrated that this effect is insufficient in magnitude to explain the profound requirement for miR-122 in genome amplification. We have shown here that RNA synthesis is not altered in the elongation phase, consistent with results reported recently by Norman and Sarnow (2010). We believe, however, that a miR-122-specific effect on the initiation of RNA synthesis is still to be excluded. Stabilization of viral RNA by miR-122 also remains a possibility, and will be extraordinarily difficult to demonstrate given the limited experimental systems available for study.
Acknowledgements
We are grateful to Raffaele de Francesco for providing small molecule inhibitors of NS5B and MinKyung Yi for HCV replicons. This work was supported in part by the National Institutes of Health: U19-AI40035, P20-CA150343, and N01-AI25488.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ali N, Tardif KD, Siddiqui A. Cell-free replication of the hepatitis C virus subgenomic replicon. J. Virol. 2002;76:12001–12007. doi: 10.1128/JVI.76.23.12001-12007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens SE, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15:12–22. [PMC free article] [PubMed] [Google Scholar]
- Bourne N, Pyles RB, Yi M, Veselenak RL, Davis MM, Lemon SM. Screening for hepatitis C virus antiviral activity with a cell-based secreted alkaline phosphatase reporter replicon system. Antiviral Res. 2005;67:76–82. doi: 10.1016/j.antiviral.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, Zaret KS, Taylor JM. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- Egger D, Pasamontes L, Bolten R, Boyko V, Bienz K. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J Virol. 1996;70:8675–8683. doi: 10.1128/jvi.70.12.8675-8683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R, Egger D, Lohmann V, Bartenschlager R, Blum HE, Bienz K, Moradpour D. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J Virol. 2003;77:5487–5492. doi: 10.1128/JVI.77.9.5487-5492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R, Marcotrigiano J, Blight KJ, Majors JE, Rice CM. Hepatitis C virus RNA synthesis in a cell-free system isolated from replicon-containing hepatoma cells. J Virol. 2003;77:2029–2037. doi: 10.1128/JVI.77.3.2029-2037.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke J, Goergen D, Zheng J, Song Y, Schüttler CG, Fehr C, Jünemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra RK, Yi M, Lemon SM. Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J. Virol. 2010;84:6615–6625. doi: 10.1128/JVI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Schütz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai VC, Dempsey S, JY, Hong Z, Zhong W. In vitro RNA replication directed by replicase complexes isolated from the subgenomic replicon cells of hepatitis C virus. J Virol. 2003;77:2295–2300. doi: 10.1128/JVI.77.3.2295-2300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang WW, Pratt J, Rockway T, Harris K, Vasavanonda S, Tripathi R, Pithawalla R, Kati WM. Mechanistic study of HCV polymerase inhibitors at individual steps of the polymerization reaction. Biochemistry. 2006;45:11312–11323. doi: 10.1021/bi060511j. [DOI] [PubMed] [Google Scholar]
- Ma H, Leveque V, De Witte A, Li W, Hendricks T, Clausen S, Cammack N, Klumpp K. Inhibition of native hepatitis C virus replicase by nucleotide and nonnucleoside inhibitors. Virology. 2005;332:8–15. doi: 10.1016/j.virol.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Miyanari Y, Hijikata M, Yamaji M, Hosaka M, Takahashi H, Shimotohno K. Hepatitis C virus non-structural proteins in the probable membranous compartment function in viral genome replication. J. Biol. Chem. 2003;278:50301–50308. doi: 10.1074/jbc.M305684200. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat. Rev. Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Norman KL, Sarnow P. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms. J. Virol. 2010;84:666–670. doi: 10.1128/JVI.01156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinkert D, Bartenschlager R, Lohmann V. Quantitative analysis of the hepatitis C virus replication complex. J. Virol. 2005;79:13594–13605. doi: 10.1128/JVI.79.21.13594-13605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa V, Petrocchi A, Matassa V, Taliani M, Laufer R, De Francesco R, Altamura S, Pace P. HCV NS5b RNA-dependent RNA polymerase inhibitors: from alpha,gamma-diketoacids to 4,5-dihydroxypyrimidine- or 3-methyl-5-hydroxypyrimidinonecarboxylic acids. Design and synthesis. J. Med. Chem. 2004;47:5336–5339. doi: 10.1021/jm0494669. [DOI] [PubMed] [Google Scholar]
- Tomei L, Altamura S, Bartholomew L, Bisbocci M, Bailey C, Bosserman M, Cellucci A, Forte E, Incitti I, Orsatti L, Koch U, De Francesco R, Olsen DB, Carroll SS, Migliaccio G. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 2004;78:938–946. doi: 10.1128/JVI.78.2.938-946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Lemon SM. Adaptive mutations producing efficient replication of genotype 1a hepatitis C virus RNA in normal Huh7 cells. J. Virol. 2004;78:7904–7915. doi: 10.1128/JVI.78.15.7904-7915.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]