Summary
The epithelial to mesenchymal transition (EMT) is the breakdown of epithelial cell morphology that gives way to a more mobile, mesenchymal phenotype. Although this process is fundamental to the development of multicellular organisms, it is also a key occurrence in many diseases, including cancers of epithelial origin (1). E-cadherin is a central component of adherens junctions (AJs), which act as structural and signaling hubs in epithelial cells that oppose EMT. The loss of E-cadherin from the plasma membrane is an early indication of EMT and a marker of poor prognosis in many cancers (2–4), making the trafficking of E-cadherin an area of great interest. Recent work from the authors’ laboratory has established the role of type Iγ phosphatidylinositol 4-phosphate 5-kinase (PIPKIγ) in the trafficking of E-cadherin by studying the surface accessibility of E-cadherin in endocytosis and recycling assays. Additionally, immunofluorescence data demonstrated that cells lacking PIPKIγ lost E-cadherin at the plasma membrane. The biochemical and microscopic techniques used to investigate the trafficking of E-cadherin are presented herein.
Keywords: E-cadherin, endocytosis, PIPKIγ, plasma membrane targeting, recycling, transport, type Iγ phosphatidylinositol 4-phosphate 5-kinase trafficking
1. Introduction
Adherens junctions (AJs) are integral components of epithelia that serve as both structural and signaling centers for the cell (5,6). Cell–cell contacts formed by AJs maintain the polarity and function of epithelial cells, and the loss of AJs is recognized as an early event in the epithelial to mesenchymal transition (EMT; 7), which is a hallmark of many epithelial cancers (8). Cadherins are Ca2+-dependent homodimeric cell–cell adhesion receptors that are central to the structural stability and signaling capability of AJs. Cadherins interact with the p120-, β- and α- catenins. These catenins serve not only as scaffolds between these intercellular receptors and the cytoskeleton but also as immediate effectors governing actin dynamics, AJ stability and the Wnt, MAPK, NFκb, and sonic hedgehog pathways (5,9,10). The loss of E-cadherin from the plasma membrane is an indicator of poor prognosis in cancers of epithelial origin, which makes its mechanisms of assembly and disassembly into AJs an area of considerable interest (1).
Recent work in our lab has determined that type Iγ phosphatidylinositol 4-phosphate 5-kinase (PIPKIγ) regulates the transport of E-cadherin to and from the cell surface and thus the formation and disassembly of AJs (11). To study the role of PIPKIγ on the transport of E-cadherin to and from the plasma membrane, the authors’ laboratory used biochemical and microscopic means to label and visualize both cell surface and internalized receptors (11,12). The methods used to track E-cadherin assembly and endocytosis are described herein.
2. Materials
2.1. Cell Culture and Lysis
Madin-Darby Canine Kidney (MDCK) growth media: Dulbecco’s Modified Eagle’s Medium (DMEM; Cellgro) supplemented with 10% fetal bovine serum (FBS; Gibco), stored at 4°C.
The trypsin (0.25%) ethylenediamine-tetraacetic acid (EDTA; 2.2 mM) solution was in Hank’s Buffered Salt Solution (HBSS) without sodium bicarbonate, calcium, or magnesium (Cellgro) stored in aliquots at –20°C for long-term storage, and 4°C for short-term (a few weeks) storage.
MDCK cells were cultured in a 24-mm trans well with 3 µm pore and a polycarbonate membrane insert from Corning.
Phosphate-buffered saline (PBS) solution is 137 mM NaCl, 2.7 mM KCl 10 mM Na2HPO4 and 1.8 KH2PO4, adjusted to pH 7.4 with HCl. It may be stored at room temperature as an autoclaved 10× stock and diluted as needed.
Lysis buffer: 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 1 mM phenylmethanesulphonyl fluoride (PMSF), and 10% glycerol with an EDTA-free protease inhibitor (PI) tablet (Roche). The PI tablets are stored at 4°C.
Sample buffer (5×): 60 mM Tris-HCl, pH 6.8, 25% glycerol, 2% sodium dodecyl sulfate (SDS), 70 mM β-mercaptoethanol and 0.03% bromophenol blue stored in aliquots at –20°C.
Disposable cell lifter (Fisher).
2.2. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The gel system we use is a Mini PROTEAN 3 Cell electrophoresis system from Bio-Rad.
Buffer A: 3 M Tris-HCl, pH 8.9 stored at room temperature.
Buffer B: 1 M Tris-HCl, pH 6.7 stored at room temperature.
Molecular-weight marker: Pageruler Prestained Protein Marker (Fermentas) stored at –20°C.
Running buffer (10×): 200 mM Tris, 1.92 M glycine, and 1% SDS in water and is stored at room temperature. Dilute to 1× in water.
Ammonium persulfate (APS) solution (10%) is made in water and may be stored at 4°C for weeks at a time.
Acrylamide/bis solution, (30%) 29:1 (3.3%C) is purchased from Bio-Rad and stored at 4°C.
N,N,N′,N′-Tetramethylethylenediamine (TEMED) is purchased from FisherBiotech and stored at 4°C.
Needle (27.5 gage) and 1-mL syringe (Becton Dickinson) may both be stored at room temperature.
2.3. Western Blotting
PBS-T is phosphate-buffered saline (PBS) with 0.1% Tween-20 (Sigma-Aldrich) which may be stored at room temperature. Check the solution regularly for bacterial growth, which will appear as insoluble floc.
Blocking buffer (BB) is 5% (w/v) nonfat dry milk in PBS-T (LabScientific). The dry milk is stored at room temperature, but the BB is stored at 4°C.
Transfer buffer (TB): 20 mM Tris-HCl, 200 mM glycine, 10% methanol in water and is stored at 4°C.
Purified mouse anti E-cadherin antibody is available from BD Transduction laboratories and is stored at −20°C.
Peroxidase-conjugated AffiniPure Donkey Anti-mouse immunoglobulin (Ig)G is available from Jackson ImmunoResearch and is stored at −20°C.
SuperSignal west pico chemiluminescent substrate is available from Pierce and is stored at room temperature.
Polyvinylidene fluoride (PVDF) is available from Millipore and is stored at room temperature.
Chromatography paper, 3MM, or Whatmann paper, is available from Whatmann (Maidstone).
Film: x-Ray Film from Research Products International.
NIH ImageJ version 1.36b is available from http://rsb.info.nih.gov/ij/.
2.4. Immunostaining
Corning Micro Slides (Plain) were purchased from Corning Glass Works.
Cover slips (22 × 22 × 0.15 mm) were purchased from Fisher.
Blocking solution: 3% bovine serum albumin (BSA) in PBS.
Paraformaldehyde (PFM)/PBS solution is made as an 8% solution in PBS and stored frozen at −20°C. Thaw as needed and dilute to 4% in PBS. Insoluble precipitate may be resuspended in a 60°C water bath, but be careful to allow the solution to return to room temperature before use.
The buffer used to permeabilize the cells is PBS with 0.2% Triton X-100.
Alexa Fluor 488 goat anti-mouse IgG (2 mg/mL stock; Molecular Probes) may be diluted 1:400 in blocking solution to 50 µg/mL.
4,5-Diamidino-2-phenylindole (DAPI) is stored as a 10-mg/mL solution in water at 4°C, and may be diluted as needed.
The mounting medium used is Vectashield from Vector labs.
2.5. Reversible Biotinylation and Endocytosis
Sulfo-N-hydroxysulfosuccinimide (NHS) SS-biotin (Pierce) is diluted to 1 mg/mL in 4°C PBS shortly before use. Solid Sulfo-NHS SS-biotin is sensitive to moisture and should be stored at −20°C in a desiccator. When removing the compound from the freezer, remove the entire desiccator and allow it to come to room temperature before removing and opening the biotin. This will prevent condensation from forming on the cold Sulfo-NHS SS-biotin powder when the container is opened.
Sulfo-NHS-SS-biotin blocking reagent is 50 mM NH4Cl in PBS with 1 mM MgCl2 and 0.1 mM CaCl2.
HBBS solution (Cellgro) with 0.5 mM ethyleneglycol-tetraacetic acid (EGTA) is stored at room temperature. The EGTA should be sterile filtered (0.2 µm from Nalgene) before adding to the HBBS.
Glutathione solution should be made with fresh glutathione (60 mM) and 0.83 M NaCl, 0.83 M NaOH and 1% BSA which may all be diluted from stock solutions.
3. Methods
E-cadherin trafficking to and from the plasma membrane may be tracked using biochemical and microscopic means, three methods of which are described in detail here. To follow the internalization of E-cadherin, first the surface accessible protein must be biotinylated, allowing those proteins to be selectively precipitated using streptavidin-coated beads. Endocytosis of E-cadherin is induced by using EGTA to strip the cellular environment of its Ca2+, which cadherins require for their homotypic interaction. As the biotinylated E-cadherin is internalized, it is protected from the glutathione washes, which strip the disulfide linked biotin from the sulfo- NHS group that links it to any surface accessible E-cadherin. When the cell lysate is probed with streptavidin beads, only the E-cadherin that was internalized at the time of the glutathione wash will be precipitated.
Quantifying the amount of internalized E-cadherin at various time points against the level of total E-cadherin biotinylated will allow you to determine the effect of your protocol on the rate of E-cadherin endocytosis. The rate of E-cadherin recycling is determined by inducing endocytosis of E-cadherin, and biotinylating it as it returns to the plasma membrane following the calcium rescue.
Visualizing the intracellular localization of E-cadherin is equally important for understanding its endocytosis and recycling. This may be accomplished using indirect immunofluorescence, which can unveil a wealth of information beyond what the biotinylation data can provide. Co-staining of E-cadherin with your protein of interest can help determine where in the cell two proteins may be co-localizing and at what stage in the endocytic process.
3.1. Biotin Conjugated E-Cadherin Endocytosis Assay
MDCK cells are grown to confluence in 24-mm diameter transwells (Sarstedt) while the protein of interest is expressed at appropriate levels according to your protocol.
Remove the media from the cells and wash them once with cold PBS. Treat the apical and basal surfaces of the cells with 1 mg/mL sulfo-NHS SS-biotin in cold PBS at 4°C for 30 to 60 min to biotinylate the exposed cell-surface proteins. Quench the free sulfo-NHS-SS-biotin by washing the cells in sulfo-NHS-SS-biotin blocking reagent twice for 5 min. This is followed by several washes with 4°C PBS.
Induce endocytosis of E-cadherin by replacing the media in the transwell with HBBS solution (Cellgro) with 0.5 mM EGTA for 0, 15, 30, 60, 90, and 120 min at 18°C (see Note 2).
Incubate the cells from each time point in two 20-min washes of glutathione solution at 4°C to remove the biotin conjugated to all proteins still at the cell surface (see Notes 3 and 4). One well from each condition used in your protocol should be excluded from this wash to be used as a positive control for total cell-surface biotinylated E-cadherin.
Lyse the cells in 500 µL lysis buffer containing PIs (and no reducing agents) and scrape them from the transwell using a disposable cell lifter (Fisher). Transfer the lysate to a 1.5 mL low-retention microcentrifuge tube and rotate the tube at 4°C for 1 h. Next, clear the lysate of insoluble debris by centrifuging at 16,000g (in an 18-place rotor Eppendorf 5415C) for 15 min and aspirating the supernatant into a fresh 1.5-mL centrifuge tube. Save 20 µL of the cleared lysate for each condition in a separate tube as an input sample.
While the lysates are clearing, equilibrate the streptavidin affinity gel (EZview Red streptavidin affinity gel from Sigma) by briefly vortexing the gel in 750 µL of lysis buffer and spinning it down for 1 min at 4,500g (in an Eppendorf 5415C). Repeat the above wash. For each affinity pull-down, use approx 10 µL settled bead volume of gel, the transfer of which may be made easier by using a cut pipet tip (see Notes 5 and 6). Aspirate the wash lysates and resuspend the gel with an equal volume of lysis buffer, creating agel slurry.
Add 20 µL of the slurry to the cleared lysates and allow them to rotate together for 4 h to precipitate the biotin-labeled protein.
Spin-down the tubes for 1 min at 4,500g and aspirate the supernatant. Resuspend the gel in 1 mL of lysis buffer before spinning again. Repeat this wash three to fourtimes.
Aspirate the final wash solution and dry the beads with a 27.5-gage needle and a 1-mL syringe (Becton Dickinson). Resuspend the gel in 60 µL of sample buffer and heat the tubes to 100°C for 10 min.
3.2. Biotin-Conjugated E-Cadherin Recycling Assay
MDCK stable cell lines are grown to confluence in 24-mm diameter transwells (Corning) while the protein of interest is expressed at appropriate levels according to the institution’s protocol.
The endocytosis of E-cadherin is induced by changing the MDCK media to HBBS with 2 mM EGTA for 40 min at 37°C.
The E-cadherin is recycled back to the plasma membrane upon calcium rescue, which is achieved by changing the media back to DMEM supplemented with 10% FBS for 0, 5, 15, 30, 45, or 60 min.
At the times indicated, wash the cells twice with PBS at 4°C then add 1 mg/mL sulfo-NHS SS-biotin PBS for 60 min at 4°C to biotinylate the exposed cell-surface proteins. Quench the free Sulfo-NHS-SS-biotin by washing the cells in Sulfo-NHS-SS-biotin blocking reagent twice for 5 min. This is followed by several washes with PBS at 4°C.
The cells are lysed and biotin-labeled proteins precipitated as described in steps 5 to 9 of the endocytosis assay, and analyzed as described here.
3.3. Western Blotting for E-Cadherin
3.3.1. Pouring the Gels
These instructions are based on the Mini PROTEAN 3 Cell system from Bio-Rad, and are easily adaptable to other gel systems.
The glass plates should be thoroughly washed with a water soluble detergent such as Alconox (Alconox Inc.) and rinsed clean with distilled water to avoid the buildup of impurities. Before pouring the gels the plates should be cleaned again with 95% ethanol and a Kimwipe (Kimberly-Clark).
A 7.5% gel of 1.5-mm thickness may be prepared with 5 mL of water, 1 mL of Buffer A, 2 mL 30% acrylamide/bis solution, 29:1, 80 µL of a 10% SDS solution, 80 µL of a 10% ammonium sulfate solution, and 5 µL TEMED. Once poured, the gel should be covered with a small amount of 95% ethanol (see Notes 7– 9) (13).
After the resolving gel has solidified (~15 min) the ethanol should be poured off, and any remaining ethanol should be removed with a clean piece of 3 MM chromatography paper (Whatmann paper). Next, a 5% stacking gel may be prepared with 1.4 mL water, 250 µL Buffer B, 330 µL acrylamide (30%), 20 µL ammonium sulfate (10%), 20 µL SDS (20%), 2 µL TEMED. The gel comb should be inserted immediately after the stacking gel is poured on top of the resolving gel. Once the gel has solidified the comb may be removed and the wells should be rinsed with distilled water.
3.3.2. Running the Gels
Load the gels into the electrophoresis module and fill the chamber and the tank with 500 mL running buffer.
Load a prestained molecular-weight marker onto the far left lane of the gel, followed by 20 µL of each sample into the SDS-PAGE gel along with a prestained molecular-weight marker. Run the gel at 100 V for 20 min, or until the dye front has passed through the stacking gel. The gel may now be run at 200 V until the dye front enters the running buffer.
3.3.3. Transferring the Protein to a Membrane
After the dye front has left the gel, remove the lid from the gel box and rinse all of the components in deionized water. In a tub of transfer buffer, submerge two sponges and two pieces of Whatmann paper per gel to be transferred. Force out any pockets of air in the sponges or the paper. While assembling the transfer sandwich it is important to make sure no bubbles are resting between layers. Place an open transfer cassette (black side down) in the tub with the buffer and layer a sponge and a piece of 3 MM paper in the cassette. Remove a gel from its glass plates and place it face down on the submerged Whatmann paper.
Designate a corner of the nitrocellulose membrane as the upper left-hand corner by cutting it off with clean scissors. Only handle the membrane with tweezers or forceps to prevent foreign proteins from being deposited on the membrane. Pretreat the PVDF by rinsing it in methanol for 30 s. Remove the membrane from the methanol and wash off the excess methanol in the TB. Place the membrane on the submerged gel face down so that the cut upper left-hand corner of the membrane is on your right. Layer the second wet piece of Whatmann paper on the membrane, followed by the second sponge. Squeeze out any air bubbles that may have developed between layers while you were assembling the sandwich.
Place the transfer cassettes in the transfer assembly in the proper orientation (black to black) along with an ice block and a stir bar to help maintain the temperature of the TB. Place the assembled transfer apparatus in a cold room (4°C) on a magnetic stir plate and set the plate to spin at a low speed. Set the power supply to transfer at 100 V for 90 min (see Note 10).
3.3.4. Blotting for E-Cadherin
Once the transfer is complete, the transfer sandwich may be opened, and excess portions of the membrane may be trimmed off. Move this membrane into a blotting container containing enough PBS-T (PBS with 0.1% Tween-20) to submerge the blots. Set the box on a shaker or rocker, and allow it to wash for 5 min.
Replace the PBS-T with 5% milk PBS-T and block the membrane for 45 min. Replace the blocking buffer with 5% milk PBS-T with mouse anti-E-cadherin antibody (BD Transduction Laboratories) diluted 1:2,500 to a final concentration of 0.1 µg/mL and blot for 1 h on the rocker at room temperature.
Wash the blot in PBS-T three times for 5 min each on the rocker.
Decant the wash solution and add more milk-PBS-T with peroxidase-conjugated AffiniPure Donkey Anti-mouse IgG at 80 ng/mL at room temperature for 30 min.
Wash the blot in PBS-T three times for 5 min each on the rocker.
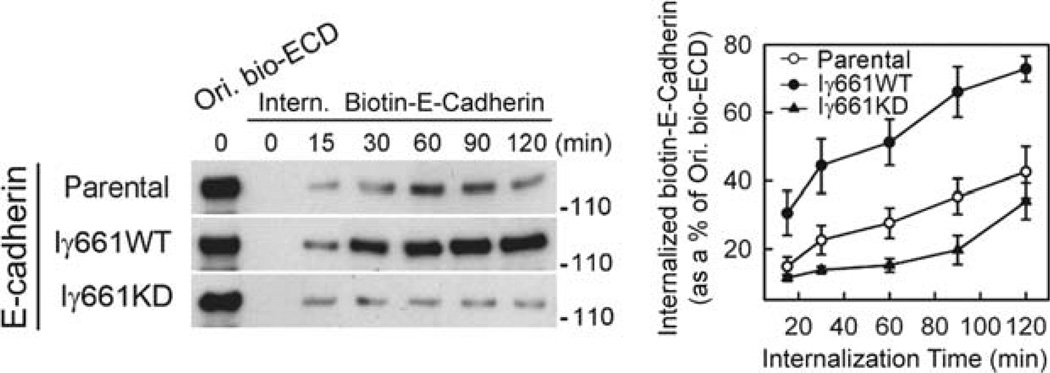
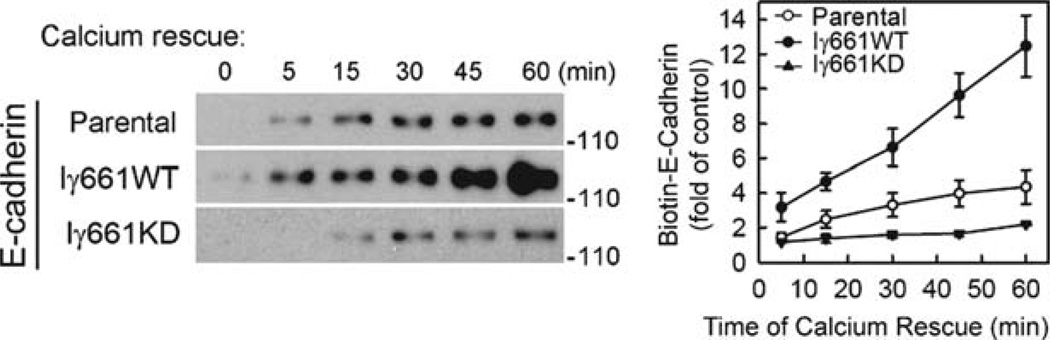
Lay the blots on Parafilm “M” (Pechiney Plastic Packaging) face up (cut corner on the upper left-hand side) and expose the blot to SuperSignal west pico chemiluminescent substrate (Pierce) for 5 min or another enhanced chemiluminescent reagent for an appropriate length of time. Next, place the membrane between two pieces of transparency film in a cassette and expose the blot to x-ray film in a dark room. Commonly the exposure times for this experiment will range between 10 and 60 s. Examples of blots assaying the endocytosis and recycling of E-cadherin are shown in Figs. 1 and 2.
These results may be quantified to determine the percentage of E-cadherin internalization by scanning densitometry of the films. The densitometry may be determined using programs such as ImageJ (NIH), allowing for the quantitative comparison of the E-cadherin in the glutathione-washed time points to the lane containing the E-cadherin that never endured the glutathione wash.
Fig. 1.
Overexpression of wild-type PIPKIγ661 accelerates E-cadherin internalization, while expression of kinase dead (KD) PIPKIγ661 impedes it (11). MDCK cells expressing PIPKIγ661 wild-type and kinase dead were biotinylated (Ori. Bio-ECD) with sulfo-NHS-SS-biotin followed by EGTA treatment to induce endocytosis. The cells were washed with glutathione to remove the disulfide linked biotin from cell surface accessible E-cadherin at intervals to monitor the rate of internalization. The cells were processed to determine total levels of biotinylated E-cadherin by western blotting. The results of the blotting were quantified (right) by densitometry using NIH ImageJ. The expression of active PIPKIγ661 clearly enhances the rapid endocytosis of E-cadherin while the kinase dead PIPKIγ661 does not.
Fig. 2.
Expression of PIPKIγ661 wild-type, but not kinase dead, accelerates E-cadherin recycling to the plasma membrane (11). MDCK cells expressing PIPKIγ661 wild type and kinase dead were induced to internalize E-cadherin by EGTA treatment. E-cadherin recycling was then stimulated with calcium (return to normal medium) followed by cell surface biotinylation at discrete time points. The cells were then processed to determine total levels of biotin labeled E-cadherin by western blotting. The results of the blotting were quantified using scanning densitometry and NIH Image J. These results, taken with those in Fig. 1, support a model for PIPKIγ661 playing a key role in E-cadherin endosomal trafficking to and from the plasma membrane.
3.4. Immunofluorescent Staining of MDCK Cells for E-Cadherin
Culture MDCK cells on 22-mm cover slips in a 6-well plate. Throughout this experiment, coverslips should always be handled with very fine-tipped forceps to avoid altering the cells on the slide. To seed the cells in the 6-well plate, first sterilize the cover slips by submerging them in ethanol, which should be flamed before placing the slip on the plate. The cells should be grown to confluence before transfection.
Treat the cells according to your protocol and then proceed with the fixation and staining steps detailed here.
Rinse the cover slips by transferring them with tweezers to coplin jars containing 37°C PBS. Rinse the cover slips a second time in a second coplin jar containing fresh 37°C PBS.
Transfer the cover slips to another coplin jar containing a 4% PMF/PBS solution for 15 min at room temperature.
Rinse the cover slips twice as before with room temperature PBS.
Extract the cover slips by transferring them to a coplin jar with PBS plus 0.2% Triton X-100 for 15 min.
Rinse the cover slips twice in room temperature PBS as before, then incubate them in a third coplin jar of PBS for 5 min.
Block the cells in 3% BSA/PBS (blocking solution) solution for 1 h at room temperature in a coplin jar.
Dilute the mouse anti-E-cadherin antibody (250 µg/mL from BD Transduction Labs) 1:400 to a final concentration of 625 ng/mL in blocking solution. If you are also staining for other proteins, add additional primary antibodies to the same tube. Place the cover slips face-up on a hydrophobic surface in a humidity chamber and add 200 µL of the primary antibody solution to each cover slip. Incubate at 37°C for 1 h.
Remove the cover slips from the humidifier and wash them in room temperature PBS with 0.1% Triton X-100 three times for 5 min.
Dilute the Alexa Fluor 488 goat anti-mouse IgG (2 mg/mL stock) 1:400 in blocking solution to 50 µg/mL. Place the cover slips face-up in the humidifier and add 200 µL of the secondary antibody solution to each cover slip. Incubate the cover slips in the humidifier for 30 min at 37°C (see Note 11).
After 30 min add 50 µL of 10 µg/mL DAPI to the secondary antibody solution on each cover slip for 2 min (optional).
Remove the cover slips from the humidifier and wash them in room temperature PBS with 0.1% Triton X-100 three times for 5 min.
Dry the cover slips by tapping the edges on a Kimwipe. Place a drop of vectashield mounting media (around 20 µL) on the slide and gently lower the cover slip onto the slide, making sure there are no bubbles between the two.
Place the slide face down on a large Kimwipe and apply firm, even pressure to the back of the slide, using a second Kimwipe to prevent your gloves from smudging the glass. This will press off any excess mounting media between the cover slip and the slide.
Using clear nail polish, seal the edges of the cover slip to the slide.
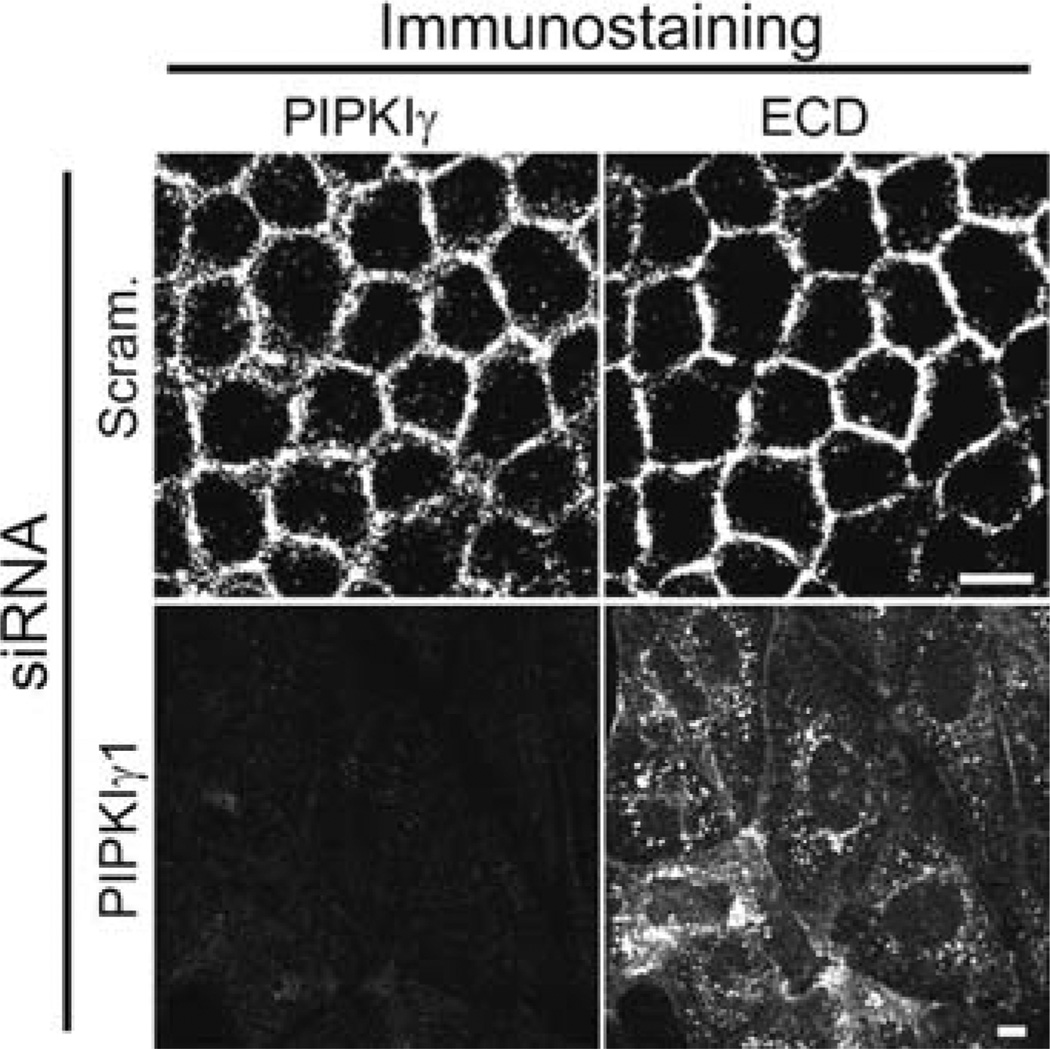
The slides may then be viewed by indirect immunofluorescence with emission and absorption spectra appropriate for the utilized fluorophores. Examples of the observed staining phenotype may be seen in Fig. 3.
Fig. 3.
PIPKIγ knockdown results in the mislocalization of E-cadherin in MDCK cells (11). PIPKIγ was knocked down by the addition of PIPKIγ siRNA via the calcium phosphate precipitation method. Immunofluorescent staining for E-cadherin (ECD) indicates that it colocalizes with PIPKIγ. Staining of the knockdown cells indicate that loss of the kinase results in the mistargeting of E-cadherin. These data support the hypothesis that PIPKIγ is an important mediator of E-cadherin trafficking to the plasma membrane in epithelial cells.
Acknowledgments
The authors would like to thank Nick Schill and Professor Richard Anderson for their editing, advice, and encouragement. This work was supported by R01 GM057549-08 and R01 CA104708-03.
Footnotes
It is recommended that only distilled, deionized water be used as a reagent in the assays described herein. Water that is to be used with cell culture work must be autoclaved for 40 min at 121°C 15 psi. All other reagents to be used in cell culture must be either autoclaved or sterile filtered through a 0.2-µm filter (Nalgene).
Keeping the cells at 18°C prevents the endocytosed E-cadherin from entering the lysozomal degradative pathway.
Sulfo-NHS SS-biotin is impermeable to cells and will therefore only label the proteins on the apical surface of the cell. Because of the disulfide bond linking the biotin to the labeled proteins, the biotin will be released from any proteins at the cell surface upon exposure to a reducing agent, such as the glutathione.
The glutathione solution should be made fresh before each use. The NaOH, NaCl, and BSA may all be added from concentrated stocks, but the glutathione itself should be resuspended before each use to ensure its effectiveness.
The binding affinity of EZview Red Streptavidin affinity gel is approx 10 µg of biotin per milliliter of settled gel. The 10 µL of affinity gel is therefore sufficient to bind 100 ng of biotin, which should be adequate to precipitate all of the biotin-labeled protein.
Beads should be pipeted using a pipet tip that is either of a naturally wide orifice or one that has had its most distal 5 mm cut off. This will allow the beads to flow more easily in and out of the tip. Because the beads tend to stick to the pipet tip, care should be taken to minimize the amount of contact they have with the tips.
Layering ethanol onto the freshly poured resolving gel creates a smooth interface between the resolving and stacking phases. This also serves to protect the gel from ambient oxygen, which inhibits efficient polymerization of acrylamide.
To avoid the occurrence of bubbles beneath and between the teeth of the gel comb, it should be washed and rinsed similar to the way the gel plates were. When inserting the comb, it should be lowered between the plates at an angle so that the rightmost tooth is dragged across the length of the unpolymerized gel as it is displacing it. This will also help to prevent the occurrence of bubbles.
To ensure the wells are properly formed, more than enough unpolymerized stacking gel should be added to the gel plate. Adding the comb may cause the excess gel to overflow the plates. Care should be taken when inserting the comb so that this excess unpolymerized acrylamide does not squirt out of the plates onto bare skin, as unpolymerized acrylamide is a skin-permeable neurotoxin.
For more information on casting gels of various volumes and densities ref. 13) is an excellent resource for this and other areas requiring technical expertise.
Keeping the transfer cool lowers the resistance to current provided by the buffer. The resistance can be a problem as the buffer heats up and the amperage demands on the power supply increase to maintain the same voltage. If a cold room is not available, transfers may be performed on a bench-top provided they are kept cool by being packed in ice, or by some other means.
We have found the Alexa flurophore-conjugated secondary antibodies to be exceptionally effective and photostable (14).
References
- 1.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen HC, Chu RY, Hsu PN, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003;201:97–106. doi: 10.1016/j.canlet.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Lawson G, Delos M, et al. Prognostic value of cell proliferation markers, tumour suppressor proteins and cell adhesion molecules in primary squamous cell carcinoma of the larynx and hypopharynx. Eur. Arch. Otorhinolaryngol. 2003;260:28–34. doi: 10.1007/s00405-002-0512-8. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev. Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell. Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.D’ Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 2005;15:19–26. doi: 10.1016/j.tcb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alphacatenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling K, Bairstow S, Carbonara C, Turbin D, Huntsman D, Anderson R. Type Iγ phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with µ1B adaptin. J. Cell. Biol. 2007;176 doi: 10.1083/jcb.200606023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell. Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 13.Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. 3 ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 14.Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]