Abstract
Although infection with hepatitis C virus (HCV) has become a leading cause of hepatocellular carcinoma, the mechanisms by which it results in carcinogenesis remain a subject of debate. Here, we explore the possibility that HCV replication impairs cellular DNA damage responses, thereby promoting instability of the infected host cell genome, and that HCV exerts a direct cancer-promoting effect in addition to eliciting immune-mediated inflammation and apoptosis of hepatocytes contributing to hepatocellular carcinogenesis.
No one would argue with the notion that chronic infection with hepatitis C virus (HCV) causes hepatocellular carcinoma (HCC). Early observations of the association between post-transfusion “non-A non-B” hepatitis and HCC in Japan from the 1980s1 have, unfortunately, proved to be all too true, and in many industrialized countries (including the U.S. and Japan), HCV infection is now the leading risk factor for HCC. The age-adjusted incidence rate of HCC has tripled in the U.S. over the past 30 years2, reflecting the spread of HCV among Americans decades earlier. Most cases occur in patients with well-established cirrhosis, by itself a very strong risk factor for liver cancer. However, this is not always the case. Eight percent of patients developing HCC in the prospective HALT-C study lacked any evidence of cirrhosis, although all had an Ishak fibrosis score of at least 3 when enrolled in the study3. Adjusting for other risk factors, such as alcohol intake, active HCV infection increases the risk of HCC about 18-fold4. Thus, the question is not whether HCV infection causes liver cancer, but rather how it does this. Is HCV directly carcinogenic? Or does infection simply set in motion a brisk inflammatory and pro-fibrotic response that causes cancer?
Human viruses fall along a continuum in terms of their potential to cause cancer, and how they go about doing this. Some, like high-risk papillomaviruses or the gamma herpesviruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus, could be considered directly carcinogenic, because the expression of particular viral gene products can overwhelm normal controls and directly drive unbridled cellular proliferation. At the other end of the spectrum, liver cancer associated with hepatitis B virus (HBV) may arise primarily as a result of the inflammatory response it evokes, despite a clear ability to integrate parts of its genome into chromosomal DNA. HCV appears to fall in the middle of this continuum - both eliciting indirect effects in the guise of inflammatory and pro-fibrotic host responses that contribute substantially to carcinogenesis, but also exerting direct effects upon the infected cell that may promote its malignant transformation.
The role played by inflammation in liver cancer is well documented. Elegant studies have shown that chronic immune-mediated liver damage is both necessary and sufficient for the development of HCC in HBV transgenic mice, and that other potential mechanisms of carcinogenesis such as insertional mutagenesis or viral transactivation are not required5. Inflammation may also be the root cause of the increased risk of primary liver cancer associated with obesity and type 2 diabetes6, 7. Fibrosis is a key feature of the wound healing response initiated by inflammation within the liver, and it is tightly associated with the development of HCC due to all causes. Exactly how inflammation, fibrosis and liver cancer are linked remains unsettled, although NF-κB, a master regulator of inflammation, may play a central role through its influence on the life and death of both parenchymal and nonparenchymal cells8. Given the strongly pro-fibrotic nature of chronic hepatitis C, these links are undoubtedly active in the development of HCV-associated HCC.
Multiple studies suggest that cirrhotic patients who achieve a sustained antiviral response (SVR) to therapy have approximately a 3-fold reduction in the risk of HCC9, 10. This demonstrates the importance of the role played by the virus, but it doesn't distinguish between direct effects of viral protein expression versus the immune response to viral antigens. The continued occurrence of HCC following elimination of the virus may reflect residual pro-carcinogenic effects of cirrhosis, or alternatively, the length of time required for newly developing HCC to become clinically apparent3. Other evidence comes from a longitudinal, community-based cohort study in Asia that found that the risk of HCC among seropositive individuals to be associated independently with both the serum viral RNA level and serum alanine aminotransferase (ALT)11. These data support a role for both direct and indirect (i.e. inflammation-related) mechanisms of carcinogenesis.
One way to look at the potential contribution of direct vs. indirect mechanisms to HCV-associated HCC is to ask whether there are significant differences in the cancers that arise in patients with HCV vs. HBV infection. If in both cases, cancer results from the effects of chronic immune-mediated liver damage and unresolved wound healing responses, one would anticipate few differences in the underlying genetics of these cancers. While we are poised to learn much more about this from whole cancer genome sequencing efforts, there are tantalizing clues that suggest significant differences. One difference appears to be in the expression of the liver-specific microRNA, miR-122, an indispensable host cell factor that is essential for HCV replication. Conserved in sequence from zebrafish to humans, miR-122 is abundantly expressed in hepatocytes where it comprises over 50% of mature miRNAs and represses numerous liver-specific genes12. Its role in HCV replication is independent of its regulation of hepatic genes and requires its binding near the 5’ end of the HCV RNA genome13. Recent work in our laboratory indicates that miR-122 recruits argonaute 2 to the viral RNA, protecting it from cellular RNA decay machinery14. miR-122 is essential to the HCV life-cycle and its therapeutic silencing with an antisense locked nucleic acid (LNA) oligonucleotide has potent antiviral effects in HCV-infected chimpanzees15.
miR-122 also has important tumor suppressor properties in the liver. Loss of miR-122 expression appears to contribute to the malignant phenotypes of tumor cells, as reconstituting its expression may reverse anchorage-independent growth, migration, invasion, and tumor formation in nude mice16, 17. miR-122 also regulates cyclin G1, and thus influences the stability and transcriptional activity of p5318. While several studies indicate that miR-122 expression is typically reduced in liver cancer (reviewed in 19), two reports suggest that its expression is preserved in HCV-associated cancers17, 20. Why should this be so, if HCV-associated cancer, like HCC due to many other causes, results from chronic inflammation and unresolved liver injury? One speculative possibility is that the replication of HCV is in some way intimately involved, directly, in carcinogenesis.
A quantitative analysis of nonmalignant liver tissues collected during surgical resection of HCV-related HCC indicated that only a small fraction of hepatocytes express detectable HCV antigens21. Such studies are plagued by technical difficulties inherent in detecting the low abundance of viral antigens expressed in the liver, but they raise a fundamental question that has yet to be answered: does cancer arise in an HCV-infected hepatocyte, or in uninfected bystander cells that are present in much greater numbers? Although more cancers need to be studied, the apparent preservation of miR-122 expression in HCV-associated HCC, despite its loss in HCC due to other causes, may be a clue that cancer arises within HCV-infected hepatocytes. Hepatocellular carcinogenesis is a multistep process22. Early loss of miR-122 expression during the progression toward cancer would restrict viral replication and prevent any subsequent direct contributions from viral protein expression. On the other hand, cells maintaining miR-122 expression would be at risk for continued direct effects of HCV, and would be selected during the progression toward cancer.
This speculative hypothesis is buttressed by the fact that some lineages of transgenic mice expressing HCV proteins are at risk for HCC despite the absence of immune-mediated inflammation and fibrosis23, 24. These lineages include mice with high-level expression of the viral core protein, as well as mice expressing a very low abundance of the complete viral polyprotein. The development of HCC in these transgenic mice in the absence of immune-mediated inflammation suggests that the expression of viral proteins may have a direct carcinogenic effect. However, steatosis is prominent in these mice23, 24. Thus, alternative inflammatory mechanisms operative in the development of HCC associated with metabolic syndrome, as mentioned above, may be at work here as well.
How could the expression of HCV proteins contribute directly to cancer? There is no evidence that HCC results from continued expression of a viral oncogene, such as occurs with the oncogenic papillomaviruses and gamma herpesviruses. Nor does it result from integration of viral sequences into the host cell genome, as HCV is an RNA virus that exploits an RNA lifecycle confined exclusively to the cytoplasm. However, one aspect of HCV biology that is increasingly evident is the manner in which it has evolved to hijack the functions of numerous cellular proteins (even miRNAs, as described above) to promote its survival in the liver. Viral proteins interact with signaling pathways to disable innate immune responses, and with a host of cellular proteins to facilitate viral entry, translation, RNA synthesis and the assembly and release of infectious virus. Some of these interactions involve cellular proteins that have significant roles in controlling cell proliferation or that have tumor suppressor properties19. One good example is the interaction of the viral RNA-dependent RNA polymerase, NS5B, with the retinoblastoma protein, Rb.
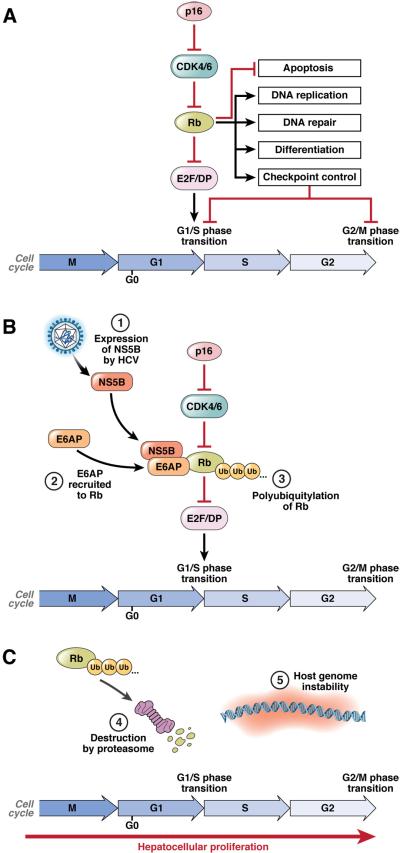
Rb is typically a nuclear protein, but it is synthesized in the cytoplasm where it is bound specifically by NS5B25, 26. E6-associated protein (E6AP, or UBE3A), a HECT-type E3 ubiquitin ligase that mediates papillomavirus degradation of p53, is recruited to this complex and directs ubiquitylation of Rb, targeting it for degradation by the proteasome and reducing Rb abundance in HCV-infected cells26 (Fig. 1). Only Rb is affected, not other pocket-binding proteins, but the net effect is an increase in activity of E2F-responsive promoters25. As Rb controls the G1 to S-phase transition, this mechanism may have evolved to overcome infection-induced blocks to cell cycle progression27, 28. Acute loss of Rb also impairs immune responses, and thus may facilitate virus escape from immunity29. However, Rb also regulates DNA damage responses, the G2 to M transition, and the mitotic spindle checkpoint30 (Fig. 1). These are critical controls in prevention of cancer, directing cells to repair DNA damage or chromosome mis-segregration or to induce apoptosis before cell division. The risk of oxidative DNA damage is high in the infected liver, not only due to inflammation as discussed above, but also possibly due to direct effects of HCV proteins31. NS5B-mediated loss of Rb expression, if it occurs in vivo as it does in infected cell cultures26, likely renders the infected hepatocyte unable to mount a normal DNA damage response and can be expected to promote genomic instability and increase the risk of HCC. Loss of hepatic Rb expression enhances genomic instability and diethyl-nitrosamine (DEN)-induced tumorigenesis in mice32. Several studies suggest that HCV may also disrupt the function of p53, a second major tumor suppressor in the liver (reviewed in19). The loss of p53 function could be synergistic with loss of Rb, but is not as well documented.
Figure 1. Disruption of the Rb pathway by HCV NS5B.
(A) Rb regulates multiple cellular processes, including cell cycle progression, apoptosis, DNA replication and repair, cellular differentiation and senescence. Rb binds to and represses E2F/DP transcription factor complexes. During the G1/S phase transition, Rb phosphorylation by cyclin-dependent kinases (CDKs) results in dissociation of Rb from E2F/DP complexes. The derepressed E2F/DP complexes are free to initiate transcription of S phase-specific genes, thus allowing cell cycle progression. (B) NS5B interacts with Rb in the cytoplasm of HCV-infected cells, and recruits the E3-ubiquitin ligase E6AP to facilitate ubiquitylation of Rb. (C) Ubiquitylation targets Rb for proteasome mediated degradation. Loss of Rb results in dysregulation of the many processes that Rb controls. For example, the unscheduled activation of E2F/DP-dependent gene expression can compromise cell cycle checkpoints that guard against chromosomal instability as well as induce p53-dependent apoptosis.
While it might seem paradoxical that HCV would directly disrupt the function of one key tumor suppressor (Rb), while the expression of another (miR-122) is preserved during the progression to cancer, such a scenario can be explained by how these host cell components influence the replication of the virus. The virus gains no evolutionary advantage from causing cancer. It regulates Rb, presumably, because this makes the host cell a more hospitable environment for replication33. miR-122 expression is preserved, because without it there is no further virus replication15, and no possible direct oncogenic consequences of virus infection.
Apoptosis likely plays a key role in HCV pathogenesis. A fraction of infected cells undergo apoptosis in cell culture34 and in mice with chimeric human livers35. This may occur via cell autonomous pathways induced by the presence of the virus, or result from sensitization of infected cells to extrinsic signals elicited as a result of the immune response to the virus36. The balance of pro- vs. anti-apoptotic signals determines whether an infected cell undergoes apoptosis or survives to divide. Virus-specific pro-survival effects, mediated in part by Rb loss, represent a mechanism by which HCV could promote cancer directly by enhancing survival of infected cells in the face of the pro-apoptotic effects of oxidative stress or DNA damage. On the other hand, pro-apoptotic signals induced by virus replication represent an alternative mechanism by which HCV infection could promote cancer by causing the death of hepatocytes, thereby resulting in compensatory proliferation in an environment of inflammation and associated oxidative stress. Such a mechanism has been suggested for DEN-mediated carcinogenesis, where apoptosis was found to drive hepatocellular carcinogenesis in mice37. Also supporting this idea are recent studies showing that stimulation of hepatocyte turnover can promote HCC in mice38.
So, is HCV a “carcinogenic” virus? There are strong arguments for both direct and indirect effects of the virus contributing to hepatocellular carcinogenesis (Fig. 2), and we will likely never know which if either predominates. Is this an important question? Regardless of the mechanisms involved, direct acting antiviral drugs are changing our expectations for hepatitis C therapy and one would hope that they will lessen the future incidence of HCV-associated liver cancer. Sustained antiviral responses should reduce if not ultimately eliminate the risk of HCC in infected persons. Some would argue that this would make the question moot. However, for those patients who fail DAA therapy, or those who for whatever reason never have access to it, a better understanding of the mechanisms of carcinogenesis and how HCV-associated HCC differs from HCC due to other causes may provide new avenues for prevention, and possibly even treatment, of this devastating cancer.
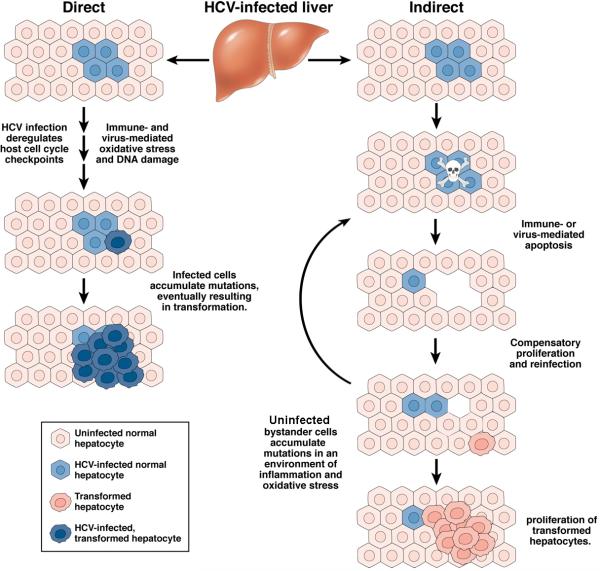
Figure 2. Direct versus indirect mechanisms of carcinogenesis in the HCV-infected liver.
Competing scenarios depicting how liver cancer might arise within an infected cell as the direct result of viral protein expression (left), or in uninfected hepatocytes proliferating in response to the apoptotic death of infected cells (right). Direct vs. indirect mechanisms of carcinogenesis are not mutually exclusive and are subject to considerable overlap. The risk of cancer arising via either scenario is likely to be enhanced by the presence of cirrhosis, immune-mediated inflammation, and oxidative stress.
Acknowledgments
Grant support: Supported in part by the University Cancer Research Fund and by grants from the National Institutes of Health: P20-CA150343 and RO1-AI095690.
Abbreviations
- CDK
cyclin-dependent kinase
- DEN
diethyl-nitrosamine
- E6AP
E6-associated protein
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HECT
homologous to E6AP carboxyl terminus
- NF-κB
nuclear factor-κB
- NS
nonstructural protein
- Rb
retinoblastoma protein
Footnotes
Disclosures: SML has served as a consultant for Abbott, Hoffman-LaRoche, Juvaris Biotherapeutics, GSK, Merck, Novartis, and Pfizer. Research in his laboratory is supported by grants from Merck and Tibotec.
Author Contributions: SML and DRM wrote the manuscript.
References
- 1.Kiyosawa K, Akahane Y, Nagata A, et al. Hepatocellular carcinoma after non-A, non-B hepatitis. American Journal of Gastroenterology. 1984;79:777–781. [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Everhart JE, Wright EC, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–9. doi: 10.1053/j.gastro.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 5.Nakamoto Y, Guidotti LG, Kuhlen CV, et al. Immune pathogenesis of hepatocellular carcinoma. J Exp.Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2011 doi: 10.1002/ijc.26338. Published on-line ahead of print, doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 7.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–18. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AK, Singh A, Jaganmohan S, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192–9. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–93. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 12.Chang J, Nicolas E, Marks D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 13.Jopling CL, Yi M, Lancaster AM, et al. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 14.Shimakami T, Yamane D, Jangra RK, et al. Stabilization of hepatitis C RNA by an Ago2-miR-122 complex. 2011. Submitted. [DOI] [PMC free article] [PubMed]
- 15.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai S, Nasser MW, Wang B, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015–27. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulouarn C, Factor VM, Andersen JB, et al. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–36. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornari F, Gramantieri L, Giovannini C, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–7. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 19.McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969–83. doi: 10.1038/onc.2010.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varnholt H, Drebber U, Schulze F, et al. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology. 2008;47:1223–32. doi: 10.1002/hep.22158. [DOI] [PubMed] [Google Scholar]
- 21.Liang Y, Shilagard T, Xiao SY, et al. Visualizing hepatitis C virus infections in human liver by two-photon microscopy. Gastroenterology. 2009;137:1448–58. doi: 10.1053/j.gastro.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 22.Kaposi-Novak P, Libbrecht L, Woo HG, et al. Central role of c-Myc during malignant conversion in human hepatocarcinogenesis. Cancer Res. 2009;69:2775–82. doi: 10.1158/0008-5472.CAN-08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriya K, Fujie H, Shintani Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat.Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 24.Lerat H, Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–365. doi: 10.1053/gast.2002.31001. [DOI] [PubMed] [Google Scholar]
- 25.Munakata T, Nakamura M, Liang Y, et al. Down-regulation of the retinoblastoma tumor suppressor by the hepatitis C virus NS5B RNA-dependent RNA polymerase. Proc.Natl.Acad.Sci.U.S.A. 2005;102:18159–18164. doi: 10.1073/pnas.0505605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munakata T, Liang Y, Kim S, et al. Hepatitis C virus induces E6AP-dependent degradation of the retinoblastoma protein. PLoS.Pathog. 2007;3:1335–1347. doi: 10.1371/journal.ppat.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannan RP, Hensley LL, Evers L, et al. Hepatitis C virus infection causes cell cycle arrest at the level of entry to mitosis. J Virol. 2011;85:7989–8001. doi: 10.1128/JVI.00280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters KA, Syder AJ, Lederer SL, et al. Genomic analysis reveals a potential role for cell cycle perturbation in HCV-mediated apoptosis of cultured hepatocytes. PLoS Pathog. 2009;5:e1000269. doi: 10.1371/journal.ppat.1000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markey MP, Bergseid J, Bosco EE, et al. Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene. 2007;26:6307–6318. doi: 10.1038/sj.onc.1210450. [DOI] [PubMed] [Google Scholar]
- 30.Hernando E, Nahle Z, Juan G, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 31.Korenaga M, Wang T, Li Y, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol.Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 32.Mayhew CN, Carter SL, Fox SR, et al. RB loss abrogates cell cycle control and genome integrity to promote liver tumorigenesis. Gastroenterology. 2007;133:976–84. doi: 10.1053/j.gastro.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 33.McGivern DR, Villanueva RA, Chinnaswamy S, et al. Impaired replication of hepatitis C virus containing mutations in a conserved NS5B retinoblastoma protein-binding motif. J Virol. 2009;83:7422–33. doi: 10.1128/JVI.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannan RP, Hensley LL, Evers LE, et al. Hepatitis C virus infection causes cell cycle arrest at the level of initiation of mitosis. J Virol. 85:7989–8001. doi: 10.1128/JVI.00280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joyce MA, Walters KA, Lamb SE, et al. HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice. PLoS Pathog. 2009;5:e1000291. doi: 10.1371/journal.ppat.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan L, Gorke S, Rau SJ, et al. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008;181:4926–35. doi: 10.4049/jimmunol.181.7.4926. [DOI] [PubMed] [Google Scholar]
- 37.Qiu W, Wang X, Leibowitz B, et al. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology. 2011;54:1249–58. doi: 10.1002/hep.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaji S, Zhang M, Zhang J, et al. Hepatocyte-specific deletion of DDB1 induces liver regeneration and tumorigenesis. Proc Natl Acad Sci U S A. 107:22237–42. doi: 10.1073/pnas.1015793108. [DOI] [PMC free article] [PubMed] [Google Scholar]