Abstract
Type 1 diabetes (T1D) occurs through a breakdown of self-tolerance resulting in the autoimmune destruction of the insulin producing β-islets of the pancreas. A numerical and functional waning of CD4+Foxp3+ regulatory T (Treg) cells, prompted by a pancreatic IL-2 deficiency, accompanies Th1 autoimmunity and T1D progression in non-obese diabetic (NOD) mice. Recently, we identified a dominant subset of intra-islet Treg cells that expresses the ICOS costimulatory receptor and promotes self-tolerance delaying the onset of T1D. ICOS co-stimulation potently enhances IL-2 induced survival and proliferation, and suppressive activity of Treg cells in situ. Here, we propose an ICOS-dependent mechanism of Treg cell homing to the β-islets during pre-diabetes in the NOD model via upregulation of the CXCR3 chemokine receptor. The islet-specific ICOS+ Treg cell subset preferentially expresses CXCR3 in the pancreatic lymph nodes (pLN) in response to Teff cell-mediated pancreatic inflammation, an expression correlating with the onset and magnitude of IFN-γ production by Teff cells in pancreatic sites. We also reveal that intra-pancreatic APC populations and insulin-producing β, but not α nor δ, islet cells secrete the CXCR3 chemokines, CXCL9, 10 and 11, and selectively promote ICOS+CXCR3+ Treg cell chemotaxis in vitro. Strikingly, islet-derived Treg cells also produce these chemokines suggesting an auto-regulation of homing by this subset. Unlike ICOS- cells, ICOS+ Treg cells adopt a Th1-like Treg phenotype while maintaining their suppressive capacity, characterized by expression of T-bet and CXCR3 and production of IFN-γ in the draining pLNs. Finally, in vivo neutralization of IFN-γ blocked Treg cell CXCR3 upregulation evincing its role in regulating expression of this chemokine receptor by Treg cells. Thus, CXCR3-mediated trafficking of Treg cells could represent a mechanism of homeostatic immunoregulation during diabetogeneesis.
Introduction
Mechanisms of peripheral immune self-tolerance prevent the onset and progression of pathological autoimmune responses. Immunosuppressive CD4+Foxp3+ T regulatory (Treg) cells, constitutively expressing CD25 (IL-2Rα), develop in the thymus (tTreg) or differentiate from non-regulatory CD4+Foxp3- T effector (Teff) cells in vitro (iTreg) or in the periphery (pTreg) [1], [2]. In order to establish and maintain dominant self-tolerance, Treg cells employ a plethora of immunosuppressive mechanisms including production of anti-inflammatory cytokines like TGF-β and IL-10, thereby inhibiting Teff cell expansion and effector functions. Developmental blockade of this lineage in mice via day 3 thymectomy provokes lympho-proliferative and multi-organ autoimmune disease [1]. Similarly, loss-of-function mutations in the Treg cell lineage-specifying transcription factor Foxp3 abrogate Treg cell development, resulting in severe autoimmunity in scurfy mice and immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) in humans [3].
NOD mice succumb to autoimmune diabetes resulting from a T-cell dependent destruction of the insulin producing β-islets of Langerhans [4]. Diabetogenesis in the NOD model shares many features with human T1D including insulin-responsive hyperglycemia, common risk loci, and the development of pancreas-specific auto-antibodies [4]. Inflammatory infiltrates are observed in the islets at 3–4 weeks of age however outright insulitis does not occur until 4–8 weeks later, suggesting immunoregulatory mechanisms are at least partially intact during this period. BDC2.5-NOD mice carry a transgenic TCR specific to a β-islet antigen, facilitating reliable, synchronous diabetes onset and transfer of diabetes via infusion of cells into lymphopenic hosts. Progression to insulitis in NOD and BDC2.5 mice results from the failure of multiple central and peripheral immune checkpoints. This includes a progressive waning in function and number of intra-islet Treg cell populations [5, 6, 7]. Recently, we and others have implicated an islet-specific deficiency in IL-2, a cytokine critical for Treg cell homeostasis, in the functional waning of Treg cells at the onset of insulitis [8, 9, 10]. Conversely, low dose IL-2 therapy both maintains pancreatic Treg cell populations and protects NOD mice from T1D [9, 7]. In addition, we recently demonstrated a critical dependence on the ICOS co-stimulatory pathway for the survival and function of intra-pancreatic Treg cells [7]. Specifically, the ICOS+ Treg cell subset, predominates in the islets of pre-diabetic NOD mice, and preferentially survives, proliferates and suppresses effector responses in situ. A decline in ICOS expression by Treg cells accompanies the progression to insulitis whereas IL-2 therapy selectively maintains the intra-islet ICOS+ Treg cell pool and protects from T1D. Furthermore, co-transfer of ICOS+ Treg cells, but not their ICOS- or icos deficient (ICOS-/-) counterparts, protects lymphopenic NOD recipients from Teff cell-induced insulitis and disease. Thus, a local network of cytokine and co-stimulatory mechanisms can co-ordinate Treg cell homeostasis in the pre-diabetic islets of autoimmune-prone mice.
While ICOS+ Treg cells preferentially accumulate in the pre-diabetic islets, the distinct mechanisms that mediate homing are unclear. Thus, we hypothesized that ICOS could also play a role in their preferential homing and accumulation of Treg cells in pancreatic sites of BDC2.5 TCR transgenic NOD mice. Recent studies have identified Treg sub-populations that express Teff cell-associated genes critical for their trafficking to sites of inflammation [11]. Notably, IFN-γ was shown to induce T-bet and CXCR3 expression in Treg cells, thus promoting their homing to sites of type-1 inflammation [12]. Similarly, IRF4 and STAT3 expression by Treg cells is necessary for suppression of pathogenic Th2 and Th17 responses, respectively [13, 14]. Given that ICOS+ Treg cells represent a dominant, suppressive subset that accumulates in a Th1-polarized environment, we hypothesized that ICOS+ Treg cells preferentially home to the inflamed islets using CXCR3.
Here, we show that ICOS+ Treg cells preferentially express CXCR3 in pLN of pre-diabetic NOD mice, a process dependent on expression of ICOS. IFN-γ produced by islet-reactive Teff cells in vivo induces expression of CXCR3 by ICOS+ Treg cells and correlates with the onset and magnitude of inflammation in pancreatic sites. Furthermore, unlike ICOS- cells, CXCR3-expressing ICOS+ Treg cell from pLN adopt a Th1-like phenotype, characterized by production of IFN-γ, and expression T-bet and IFN-γR, and preferentially respond to IFN-γ in vitro through activation of STAT1. Moreover, CXCR3 chemokines are specifically expressed by intra-pancreatic macrophages and dendritic cells, and surprisingly by insulin-producing β but not α or δ islet cells. Intriguingly, intra-pancreatic Treg cells were also found to express CXCR3 ligands. Finally, in vivo neutralization of IFN-γ significantly blunted CXCR3 upregulation by ICOS+ Treg cells. Collectively, this data indicates trafficking of Treg cells represents a mechanism of homeostatic regulation of autoimmunity in T1D.
Materials and Methods
Mice
NOD.TCRα-/- mice were a generous gift from C. Benoist (Harvard University). BDC2.5.Foxp3GFP ICOS-/-, BDC2.5.Foxp3GFP and BDC2.5. Foxp3GFP Thy1.1 mice were generated in house. All mouse strains were maintained in SPF conditions at RI-MUHC.
Antibodies and flow cytometry
Single cell suspension prepared from different organs were stained and acquired on FACSCanto (BD) or BD Fortessa and analyzed with FlowJo software (Tree Star). Surface phenotype staining was done with the following fluorochrome-conjugated or biotinylated mAbs: anti-CD3 (145-2c11), anti-CD4 (RM5), anti-ICOS (7E.17G9), anti-Thy1.1 (HIS51), anti-CD11c (HL3), anti-F4/80 (BM8), anti-CXCR3 (cxcr3-173), and anti-IFN-γR (2E2). The expression of Foxp3 (FJK-16s), T-bet (ebio4B10), CXCL9 (MIG-2F5.5) (eBioscience, San Diego, CA), CXCL10 (CL9177B) (Cedarlane, Burlington, ON), CXCL11 (rmCXCL11) (R&D systems, Burlington, ON), Ki-67 (B56) (BD Bioscience, Mississauga, Ontario) was determined by intracellular staining performed according to manufacturers’ protocols. The STAT1 phosphorylation assay was done following BD recommendations. To evaluate cytokine production, T cells were re-stimulated for 4hrs at 37°C with PMA (20ng/ml), ionomycin (1nM) (Sigma-Aldrich, Oakville, Canada) and BD GolgiStopTM (1:1000 dilution), and then stained intracellularly with anti-IFN-γ (XMG1.2) (eBioscience, San Diego, CA). T-bet fluorescence minus one (FMO) control and antibody titration is displayed in S1E Fig.
Cell purification
For some in vivo adoptive transfer studies, Treg and Teff cells from respective mouse strains were purified from splenocytes and LN cells either based on CD4 and CD25 expression using the autoMACS cell sorter, using BDC2.5.Foxp3GFP reporter mice (Miltenyi Biotech, San Diego, CA). Specifically, CD4+CD25+ (>90% purity) and CD4+CD25− (>93% purity) T cell fractions were obtained by positive selection. For some in vivo adoptive transfer studies and in vivo suppression assays, T cell subsets were isolated using a FACSAria Cell Sorter with a purity >98%. CD4+Foxp3+ Treg, CD4+Foxp3- Teff or CXCR3+ or CXCR3- ICOS+ Treg cells were sorted from BDC2.5Foxp3GFP reporter mice.
Adoptive T cell transfers
MACS purified CD4+CD25- (Teff,) or CD4+CD25+ (Treg) T cells (7.5X105 cells) from BDC2.5Foxp3GFP donor mice were transferred intravenously (i.v.) into NOD.TCRα-/- recipient mice at the indicated ratios. In some instances, T cell populations were FACS-purified from the above-described mouse strains. Blood glucose levels were determined every 2–3 days with Hemoglucotest kits (Roche Diagnostics, Montreal, Canada) and T1D was diagnosed at values >300 mg/dl.
In vivo antibody treatment
NOD.TCRα-/- mice were reconstituted with MACS-sorted CD4+CD25+ (Treg) or CD4+CD25- (Teff) cells either alone or at the indicated ratios. Mice also received i.p injections of either vehicle (PBS) or a neutralizing IFN-γ mAb (clone XMG1.2, 1.6 mg per kg), one day before transfer and every two days post-adoptive transfer, for a total of 18 days.
ELISA
DuoSet ELISA kits (R&D Systems) were used to analyze IFN-γ (DY485) and CXCL10 (DY466) in whole-pancreas protein samples.
Migration assay
CD11c+ dendritic cells and F4/80+ macrophages from NOD.TCRα-/- mice and CD4+ cells from BDC2.5 mice were isolated via MACS. Cells were resuspended in GIBCO RPMI media (10% FBS) and incubated at 2 x 106 cells/ml. CD11c+ cells and F4/80+ cells were plated at 106 cells/well in the bottom compartments of a 24-well Transwell with 5.μm pore polycarbonate membrane (Corning). CD4+ T cells were plated in the top of the wells at 105 cells/well and allowed to migrate for 3 hours at 37°C. Migrated cells were assessed via flow cytometry as described above.
Statistical analysis
Results are expressed as means ± SD. Analyses were performed with a Student’s t test, values of p < 0.05 were considered significant. For p values: * = <.05, ** = <.01, *** = <.001
Results
ICOS-dependent expression of CXCR3 by ICOS+ Treg cells in the pancreatic LN
We previously showed that ICOS+ Treg cells, compared to ICOS- Treg cells, display an increased functionally fit phenotype and preferentially accumulate in pre-diabetic islets of BDC2.5 NOD mice [7]. In vivo ICOS blockade induces changes in the gene expression profile of intra-pancreatic Treg cells, including genes encoding various chemokine receptors, such as CCR2, CCR5 and CXCR3 [15]. This prompted us to examine whether specific chemokine receptors, regulated in part by inflammation, could promote ICOS+ Treg trafficking to the β-islets.
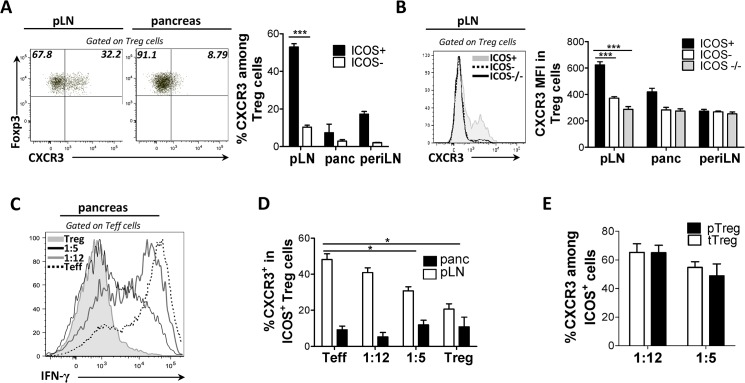
We initially screened ICOS+ or ICOS- Treg cells for expression of chemokine receptors including CXCR3, CCR2, CCR7, CCR4, CCR9, CXCR5 and CCR6 (data not shown). We found that in contrast to other T cell subsets, ICOS+ Treg cells displayed marked CXCR3 expression in the draining pLNs of pre-diabetic BDC2.5 NOD mice. Specifically, CXCR3 was highly expressed by ICOS+ Treg cells, relative to ICOS- Treg cells in terms of both frequency (52.9% vs. 10.31%) and level of expression (MFI 624 vs. 371), in the draining pLN but not in other lymphoid tissues (Fig 1A). Though ICOS+ Treg cells did not express CXCR3 in the pancreas, CXCR3 is known to undergo rapid internalization and degradation upon encounter with its ligands at the site of inflammation [16]. Moreover, Treg cells from BDC2.5 ICOS-/- mice expressed low CXCR3 at all sites examined, indicating that CXCR3 expression in Treg cells is regulated by ICOS (Fig 1B). Unexpectedly, only a low fraction Teff cells expressed CXCR3 in BDC2.5 mice in all sites examined (S1A Fig). Overall, ICOS-dependent CXCR3 expression coincided with specific accumulation of ICOS+ Treg cells within the target organ, suggesting this subset may use CXCR3 to home to pancreatic sites.
Fig 1. Autoreactive T cell-mediated inflammation induces CXCR3 expression by ICOS+ Treg cells prior to T1D onset.
Cell suspensions of pancreas and draining LN from 4 week old (A, B) WT and (B) ICOS-/- BDC2.5 mice were assessed for the frequency of CXCR3+ cells and levels of CXCR3 expression (MFI) between the ICOS+ and ICOS- subsets of Treg cells. (C and D) NOD.TCRα-/- mice received MACS sorted BDC2.5 CD4+CD25+ (Treg, 0.75X105) or CD4+CD25- (Teff, 7.5X105) cells alone or at the indicated Treg/Teff cell ratios. When the Teff cell recipient mice displayed hyperglycemia (>33mmol/L), mice were sacrificed and expression of IFN-γ by Teff cells (C) and CXCR3+ percent cells among the ICOS+ Treg cell subset were assessed. (E) NOD.TCRα-/- mice received the indicated ratios of FACS-sorted Thy1.2+ Treg cells to 7.5X105 BDC2.5 Thy1.1+ CD4+Foxp3- Teff cells. After 14 days, the Thy1.2 (tTreg) and Thy1.1+ (pTreg) subsets of ICOS+ Treg cells were compared for percent CXCR3+ cells. (n = 4, peri LN = pooled axial, brachial and inguinal peripheral lymph nodes).
Autoreactive T cell-mediated inflammation induces CXCR3 expression by ICOS+ Treg cells prior to T1D onset
Pro-inflammatory cytokines modulate expression of chemokine receptors to induce immune infiltration of the inflammatory lesion. Thus, we hypothesized that expression of CXCR3 is induced on Treg cells in response to intra-pancreatic inflammatory events. Most notably, intra-islet IFN-γ production, primarily by Teff cells, was of particular interest considering its various inflammatory actions on immune and islet cells in NOD mice. To determine if inflammation influenced ICOS+ Treg cell homing to pancreatic sites, we reconstituted NOD.TCRα-/- mice with CD4+CD25- Teff (almost exclusively GFP-) or CD4+CD25+ Treg cells from BDC2.5.Foxp3GFP reporter mice either alone or at different Teff/Treg cell ratios to mimic different inflammation levels. The transfer of BDC2.5 T cells in NOD.TCRα-/- mice reliably and predictably induces different levels of pancreatic inflammation, and allows for the tracking of T cell functional dynamics at T1D onset and progression. As expected, mice receiving only Teff cells succumbed to rapid T1D onset by day 12 (data not shown), and this correlated with increased production of IFN-γ by auto-reactive Teff cells in the pLN (S1B Fig) and pancreas (Fig 1C). Interestingly, CXCR3 was readily expressed on ICOS+ Treg cells in all mice co-transferred with Treg cells. The frequency of CXCR3+ Treg cells declined with decreased Teff cell-induced inflammation suggesting that pancreatic inflammation promotes the induction of CXCR3 on Treg cells (40% at 1:12 ratio, 30% at 1:5 ratios, and 21% with Treg cells alone) (Fig 1D). Intriguingly, our results show that mice reconstituted with Teff cells alone contained the highest frequency of CXCR3+ Treg cells in the pLN on day 12 (49%) suggesting that CXCR3 can be readily upregulated amongst pTreg cell populations induced from transferred Teff cells, very few of which express CXCR3 (Fig 1D). In order to directly determine whether CXCR3 is differentially expressed by tTreg or pTreg cells, we assessed CXCR3 expression relative to Foxp3 expression in transferred FACS sorted Thy 1.1+ (Teff GFP-)/Thy 1.2+ (tTreg GFP+) congenic cells. We found both pools of Treg cells were equally capable of expressing CXCR3 (Fig 1E), demonstrating that tTreg or pTreg cells upregulate CXCR3, in contrast to Teff cells, as pancreatic inflammation increases.
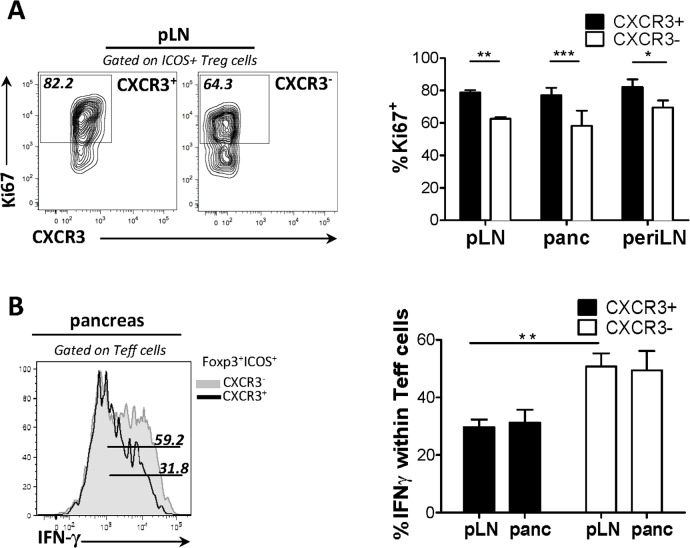
CXCR3 expression defines a functionally fit subpopulation of ICOS+ Treg cells
Next, we examined the phenotype and suppressive function of CXCR3+ICOS+ Treg cells, compared to their CXCR3- counterparts. In all sites examined, CXCR3+ Treg cells were more proliferative than CXCR3- Treg cells, as indicated by the frequency of Ki-67 positive cells (Fig 2A). In order to determine the relative suppressive capacity of CXCR3+ ICOS+ Treg cells in our T1D transfer model, we reconstituted NOD.TCRα-/- mice with FACS-sorted Teff cells alone or co-transferred with either CXCR3+ICOS+ Treg cells or CXCR3-ICOS+ Treg cells. We observed that CXCR3+ICOS+ Treg cells were endowed with a more potent suppressive potential in situ than CXCR3- ICOS+ Treg cells, as the former suppressed IFN-γ production by Teff cells, in the pLN and pancreas more efficiently (Fig 2B). Thus, CXCR3-expressing ICOS+ Treg cells represent a functionally fit subset with potent suppressive activity in situ.
Fig 2. CXCR3 expression delineates a functionally fit subpopulation of ICOS+ Treg cells.
(A) Cell suspensions of pancreatic draining LN from 4-week-old mice were isolated and percent cycle cells, determined by Ki-67 expression, were compared between the CXCR3+ and CXCR3- subsets of ICOS+ Treg cells. (B) NOD.TCRα-/- mice received Teff (7.5X105) cells and either CXCR3+ or CXCR3- ICOS+ Treg cells (7.5X104) cells isolated from pooled LN and spleen of BDC2.5 mice. After 14 days suppression was assessed via percent IFN-γ+ cells among Teff cells in the pancreas and draining LN. (n = 4).
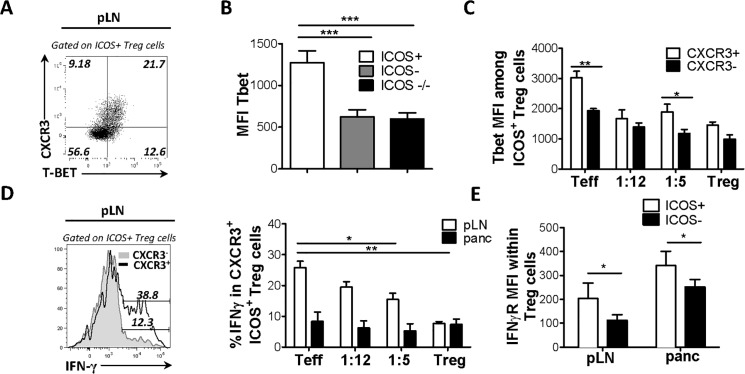
CXCR3-expressing ICOS+ Treg cells adopt a Th1-like phenotype in the pancreatic draining lymph nodes
We investigated whether ICOS+ Treg cells expressed markers that would functionally specify them for suppression at sites of type 1 inflammation. Notably, expression of CXCR3 by Treg cells depends on co-expression of Foxp3 and the Th1 lineage specifying transcription factor T-bet [12]. Thus, we predicted T-bet would be co-expressed with CXCR3 by ICOS+ Treg cells. We observed a marked co-expression of CXCR3 and T-bet by ex vivo Treg cells in pLN of BDC2.5 NOD mice (Fig 3A). Moreover, robust T-bet expression was confined to the ICOS+ subset of Treg cells in the pLN of BDC2.5 mice and was significantly reduced in ICOS-/- Treg cells suggesting that T-bet expression is partly ICOS-dependent (Fig 3B). Concordant with CXCR3 expression, there was also a trend of a positive correlation between T-bet expression in ICOS+ pTreg/tTreg cells and IFN-γ, which was mostly produced by pancreatic Teff cells, in the adoptive transfer model (Fig 3C and Fig 1C). Strikingly, following adoptive transfer of T1D, BDC2.5 CXCR3+ ICOS+ Treg cells expressed IFN-γ in the pLN, despite their enhanced suppressive potential in the pancreas. The frequency of IFN-γ-expressing Treg cells in the pLN correlated with the degree of pancreatic inflammation (Fig 3D). Furthermore, we show that ICOS+ Treg cells express IFN-γR at higher levels than ICOS- Treg cells at each site examined, consistent with a Th1-like phenotype (Fig 3E). Thus, unlike ICOS- Treg subset, ICOS+ Treg cells adopt a Th1-like phenotype in the draining pancreatic LN that correlates with Th1-polarized inflammation in the pancreas.
Fig 3. CXCR3-expressing ICOS+ Treg cells adopt a Th1-like phenotype in draining LN.
T-bet and CXCR3 co-expression was assessed in ICOS+ Treg cells from 4 week old WT BDC2.5 mice (A). T-bet expression (MFI) between ICOS+ and ICOS- subsets within Treg cells (B). NOD.TCRα-/- mice received BDC2.5 CD4+CD25+ Treg or CD4+CD25- Teff cells (0.75X105) at the indicated Treg/Teff cell ratios. When mice receiving Teff cells alone became hyperglycemic (>33mmol/L), mice were sacrificed and the frequency of (C) T-bet+ and (D) IFN-γ+ cells within the ICOS+ Treg subset was assessed. (E) NOD.TCRα-/- mice received WT Teff (7.5X105) cells and ICOS-/+ Treg (7.5X105) cells isolated from pooled LN and spleen. 10 days post-transfer, cell suspensions were obtained from the pancreas and draining pLN and IFN-γR expression (MFI) was compared between ICOS+ and ICOS- Treg cells. (3B-3D n = 4, 3E n = 5)
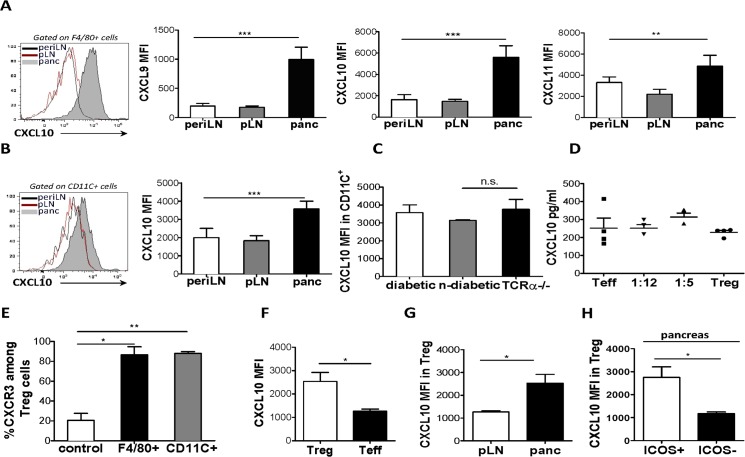
Pancreatic antigen presenting cells produce CXCR3 ligands
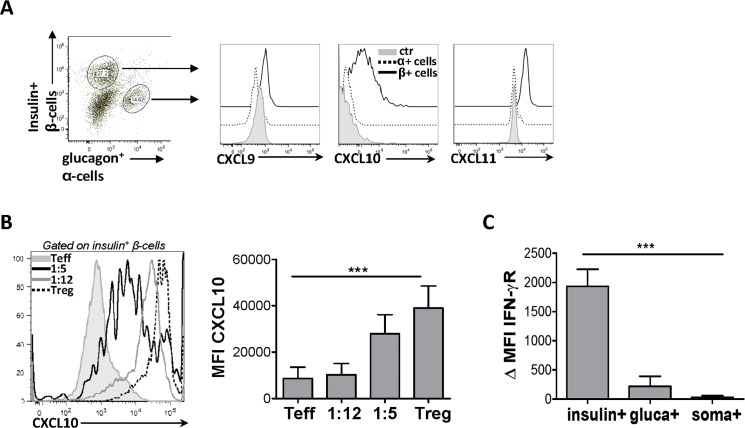
We then examined the expression of the three CXCR3-activating chemokines, CXCL9, CXCL10 and CXCL11, in the pancreas, which we hypothesized could account for the influx of ICOS+ Treg cells from the pLN. Furthermore, as IFN-γ has been shown to induce expression of these chemokines in numerous settings [17, 18], we predicted that chemokine expression should increase with pancreatic inflammation, along with CXCR3. In 4-week-old BDC2.5 mice, we observed expression of CXCL9, CXCL10, and CXCL11 (Fig 4A and 4B; data not shown), by APC populations resident to the pancreas, specifically F4/80+ macrophages (Fig 4A) and CD11c+ DCs (Fig 4B). This level of expression was not observed by lymphoid tissue-derived APC subsets (Fig 4A and 4B; data not shown). To determine whether APC chemokine production could induce specific Treg homing, Transwell assays were performed. To this end, MACS-sorted F4/80+ macrophages or CD11c+ DC cells were seeded in the lower chamber while CD4+ T cells were seeded in the upper chamber. The number of migrating cell types in the lower chamber was then evaluated following a 3-hour incubation period. CXCR3+ Treg cells were markedly enriched in the bottom chamber that contained APCs but not those containing media alone (Fig 4E). Thus, CXCR3+ Treg cells specifically migrated towards the CXCR3 chemokine-producing APC populations in vitro.
Fig 4. Resident leukocyte subsets in the pancreas express CXCR3-activating chemokines.
NOD.TCRα-/- mice initially received BDC2.5 CD4+ T cell (7.5X105) cells, and then cell suspensions from pancreas, draining pLN and peripheral LN of diabetic mice were examined for expression of CXCL9, CXCL10 and CXCL11 in F4/80+ (A) and CXCL10 in CD11c+ (B) cells was assessed by flow cytometry. (C) NOD.TCRα-/- mice were transferred with CD4+ T cells in order to induce T1D. Following adoptive transfer, glucose levels were measured daily in order to assess diabetes onset. Pancreatic cell suspensions from recipients were obtained and CXCL10 expression (MFI) was compared among CD11c+ cells from diabetic or non-diabetic recipients, as well as un-transferred (without T cell transfer) NOD.TCRα-/- mice. (D) NOD.TCRα-/- mice received Treg or Teff cells either alone or at the indicated Treg/Teff cell ratios. When mice receiving Teff cells alone became hyperglycemic (>33mmol/L), CXCL10 levels were assessed by ELISA in supernatants from pancreatic suspensions. (E), F4/80+ and CD11c+ (1X106/well) cells were seeded in triplicate in lower chambers, and CD4+ (1X106/well) cells in the upper chambers of 24 well Transwell plate. Following a 3 hour incubation period, the percent migrated CXCR3+ cells among ICOS+ Treg cells was compared between wells containing APCs and media alone (control). Cell suspensions of pancreas and draining LN of 4-week-old BDC2.5 mice were obtained and CXCL10 expression (MFI) was compared (F) between pancreatic Treg cells and Teff cells (G), between Trec cells at sites indicated, and between ICOS+ and ICOS+ Treg cells within pancreas (H). (4A-4C n = 6, 4D n = 4, 4E n = 2, 4F-4H n = 5).
In order to examine the effects of different levels of inflammation on chemokine expression NOD.TCRα-/- mice either received CD4+ T cells in order to induce T1D (Fig 4C) or the adoptive transfer experiments were performed as described in Fig 1 (Fig 4D). Unexpectedly, chemokine expression by APCs did not increase in the presence of BDC2.5 T cell-mediated inflammation in NOD.TCRα-/- recipients, irrespective of T1D outcome (Fig 4C and S1C Fig). Furthermore, we found no change in CXCL10 concentrations in pancreatic supernatants or frequency of CXCL9/10 or 11+APCs in the presence of BDC2.5 T cell-mediated inflammation in NOD.TCRα-/- recipients, irrespective of the transferred BDC2.5 Teff /Treg cell ratio (Fig 4D and data not shown). Thus, APC expression of CXCL9, CXCL10 and CXCL11 was confined to the pancreas of BDC2.5 NOD mice but not upregulated in response to inflammation.
Intra-pancreatic Foxp3+Treg cells express CXCR3 chemokines
Recent evidence indicates that in addition to a range of cytokines, Treg cells can also produce chemokines in certain inflammatory contexts [19]. Thus, we predicted that pancreatic Treg cells upregulate CXCR3 chemokines to augment recruitment of Treg cells to the inflammatory lesion. We found that Treg cells in the pancreas, compared to Teff cells, expressed significantly higher levels of CXCL9, CXCL10 and CXCL11 (Fig 4F and data not shown). This was specific to pancreatic Treg cells, as Treg cells from other sites did not express as high levels of the ligands (Fig 4G). Intriguingly, the ICOS+ subset expressed significantly higher levels of CXCR3 ligands than ICOS- Treg cells (Fig 4H). Thus, we observed chemokine expression by Foxp3+ Treg cells, which could represent a mechanism of intra-subset regulation of homing into inflamed target organs.
Intra-islet Treg cells reverse Teff cell-mediated abrogation of chemokine secretion by ß-islet cells
It has recently been established that endocrine β-cells regulate various immune cell subsets during NOD mouse diabetes progression [20]. Therefore, we sought to determine whether endocrine cells in BDC2.5 mice could express CXCR3 chemokines and contribute to the recruitment of Treg cells to pancreatic sites. Using our method recently developed to assess the phenotype and function of pancreatic islet cell populations by flow cytometry [21] we observed substantial expression of CXCL9, CXCL10 and CXCL11 specifically by insulin+ β-cells compared to the glucagon+ α-cells (Fig 5A) and somatostatin+ δ-cells (data not shown). While all three CXCR3 chemokines are expressed in the pancreas, they may not contribute equally to CXCR3+ Treg cell recruitment. The expression of CXCL9 by APCs and β-islet cells was much lower than CXCL10 and CXCL11. Considering CXCL9 is also the lowest affinity ligand and weakest inducer of migration of the three, it may prove less relevant to ICOS+ Treg cell trafficking in vivo. Furthermore, as immune-endocrine interactions in hte islets shapediabetogenesis in NOD mice, we evaluated chemokine expression by islet cells during diabetes development. We found that T1D progression induced by Teff cell transfer in NOD.TCRα-/- hosts resulted in impaired CXCL9 and CXCL10 expression by insulin+ β-cells (ie live, non-destroyed) (Fig 5B and data not shown). Intriguingly, co-transfer of Treg cells conferred dose-dependent protection from Teff cell-mediated abrogation of chemokine production (Fig 5B). Treg-derived mediators could directly act on β-cells to induce chemokine expression, or enhance production of chemokines through protection of the islets from Teff mediated cell death. Direct activation of apoptotic pathways by Teff-derived IFN-γ principally drives islet cell death in NOD mice, and indeed we found β cells expressed high levels of IFN-γR in our model compared to α and δ cells (Fig 5C) [26]. Furthermore, co-transfer of Treg cells both reduced intra-pancreatic IFN-γ production by Teff cells and protected β islet cells from cell death as measured by presence of insulin+ cells (S1C Fig). Thus, while Teff cell transfer abolishes chemokine expression and drives autoimmunity, Treg cells preserve CXCR3 ligand expression in the islets, perhaps through suppression of Teff cell functions in situ. Overall, our findings indicate that APCs, Treg cells and insulin-producing endocrine cells regulate CXCR3+ Treg cell recruitment from the draining LN to Th1 inflamed pancreatic sites. These findings support the notion that insulin producing β-cells play an active role in modulating Treg cell function in pancreatic sites.
Fig 5. Intra-islet Treg cells reverse Teff cell-mediated abrogation of chemokine secretion by ß-islet cells.
β-islet cells were isolated from 4-week-old BDC2.5 mice. (A) Expression of CXCR3 chemokines was compared between insulin+ cells and glucagon+ cells, and unstained controls. (B), NOD.TCRα-/- mice received Treg or Teff cells either alone or at the indicated Treg/Teff cell ratios. When mice receiving Teff cells alone became hyperglycemic (>33mmol/L), CXCL10 expression (MFI) in β-islet cells was compared between groups. (C) IFN-γR ΔMFI (ΔMFI was calculated by subtracting the isotype control MFI from IFN-γR antibody MFI) and was compared between β (insulin+), α (glucagon+) and δ (somatostatin+) cells isolated from 4-week-old BDC2.5 mice. (5B, 5C n = 5).
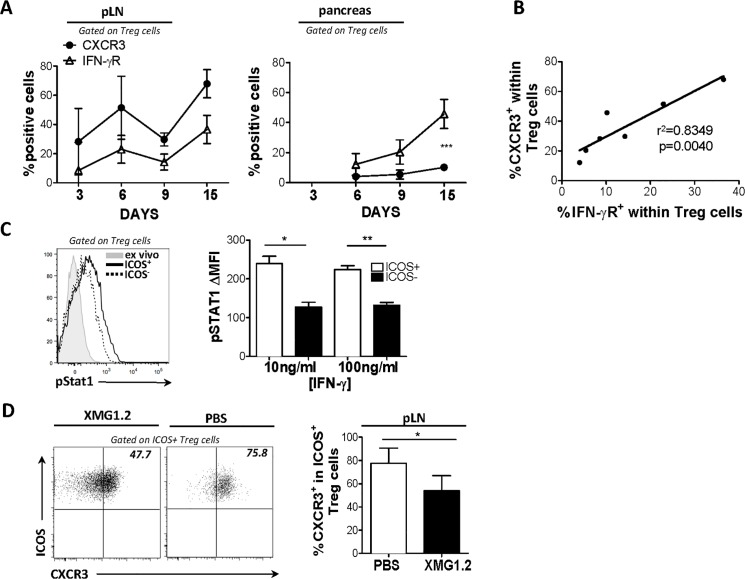
IFN-γ regulates CXCR3 expression and homing of ICOS+ Treg cells to islets
We further investigated a role for IFN-γ in the upregulation of CXCR3 on Treg cells. We assessed the expression of IFN-γ receptor (R) by CXCR3+ Treg cells and found that CXCR3+ Treg cells expressed higher levels of IFN-γR than CXCR3- Treg cells, suggesting that Treg cells that express CXCR3 are more responsive to IFN-γ signaling. To elucidate the association between IFN-γR and CXCR3 expression over the course of T1D development, we examined the co-expression of these markers on donor CD4+ T cells following transfer into NOD.TCRα-/- recipients. Mice were sacrificed on days 3, 6, 9 and 15 and CXCR3, IFN-γR and IFN-γ expression was assessed by flow cytometry on cell suspensions from pLN and pancreas. IFN-γ expression by pancreatic Teff cells increased with T1D progression and peaked at day 9 (data not shown). CXCR3 and IFN-γR expression by ICOS+ Treg cells correlated during the time course in all compartments evaluated (Fig 6A and 6B). Overall, this suggests increased IFN-γR signaling may selectively stimulate ICOS+ Treg cells to upregulate CXCR3.
Fig 6. IFN-γ regulates CXCR3 expression by ICOS+ Treg cells and their homing to islets.
NOD.TCRα-/- mice received BDC2.5 CD4+ T cells (7.5X105) and, (A) and the percent CXCR3+ and IFN-γR+ cells among total Treg cells in pancreas and draining LNs was assessed at the indicated times post-transfer. (B) Correlation between percent CXCR3+ and IFN-γR+ in pancreas and draining LN at all points examined. (C) BDC2.5 CD4+ T cells were stimulated with various concentrations of recombinant IFN-γ, and STAT1 phosphorylation was assessed by flow cytometry and compared between ICOS+ and ICOS- subsets of Treg cells. (D) NOD.TCRα-/- mice received Teff (7.5X105) cells and were injected i.p. with either PBS or anti-IFNγ Ab (XMG1.2) on days -1, 1, 3, 5 and 7 post transfer. Mice were sacrificed when the PBS group displayed hyperglycemia (>33mmol/L). Cell suspensions of the pancreatic draining LN were obtained and the percent CXCR3+ among pTreg cells was compared between groups. (6A, 6B, 6D n = 5. 6C n = 2).
As CXCR3 expression was largely confined to ICOS+ Treg cells, we hypothesized that a preferential responsiveness to IFN-γ resulted in upregulation of CXCR3 by this subset. Accordingly, we stimulated T cells isolated from pooled LN and spleen with recombinant IFN-γ, and examined the activity of IFN-γ signaling by assessing STAT1 activation in vitro using flow cytometry. ICOS+ Treg cells, not only expressed higher levels of IFN-γR, but demonstrated higher STAT1 phosphorylation upon stimulation, indicating a more sensitive and activated IFN-γR signaling pathway than their ICOS- counterparts, (Fig 6C). Overall, these results suggested to us that IFN-γ played a role in selective upregulation of CXCR3 by ICOS+ Treg cells due to their enhanced sensitivity to this cytokine.
We next sought to confirm that IFN-γ regulated CXCR3 expression on ICOS+ Treg in vivo. We predicted that anti-IFN-γ mAb treatment would impair expression of CXCR3 by ICOS+ Treg cells upon transfer into NOD.TCRα-/- recipients. To achieve this, we specifically neutralized IFN-γ activity in our transfer system using an anti-IFN-γ monoclonal antibody (mAb, XMG1.2), which potently inhibits IFN-γ in vivo. Our results show that anti-IFN-γ mAb treatment significantly blunted CXCR3 upregulation by ICOS+ pTreg cells (Fig 6D) indicating that IFN-γ positively regulates expression of CXCR3 by ICOS+ Treg cells during diabetogenesis. Thus, these results indicate that ICOS+ Treg cells detect intra-islet IFN-γ produced by Teff cells during T1D progression, and upregulate CXCR3 to home to the pancreas.
Discussion
Chemokines and chemokine receptors are critical regulators of leukocyte trafficking during the immune response. Selective use of chemokine receptors by Treg cells enables their homing to specific target tissues where they deploy their effector mechanisms to control inflammatory responses, as evidenced in models of infection and cancer. We previously demonstrated that during chronic Leishmania major infection, Treg cells preferentially express CCR5 and home to cutaneous lesions in which they suppress Teff cells and favor pathogen persistence [22]. Similarly, in human chronic liver disease and mouse models of hepatitis, CXCR3+ Treg cells accumulate in the liver, where they dampen immune-mediated tissue damage [23, 24]. Further, Koch et al., demonstrated that during Mycobacterium tuberculosis infection, CXCR3+ Treg cells accumulate and limit immune responses in the mediastinal LN [12]. Likewise, CXCR3+ Treg cells were isolated from human ovarian carcinomas and shown to suppress Teff proliferation and IFN-γ production ex vivo [25].
Studies of Treg cell homing in NOD mice have implicated CXCR3 therein. Indeed, Yamada [26] and colleagues observed accelerated onset of T1D, and decrease pancreatic accumulation of Treg cells in Cxcr3-/- NOD mice. A more recent study in NOD proinsulin 2-/- mice indicated that Treg cells upregulate CXCR3 in the pLN and accumulate in the pancreas [27]. The results described here go further in defining a subpopulation of potently suppressive ICOS+ Treg cells, which expresses CXCR3, the factors and cell types that may regulate Treg cell CXCR3 expression and Treg cell migration to the islets, and the functional and phenotypic characteristics of CXCR3-expressing Treg cells. Specifically, this is the first demonstration, to our knowledge, of ICOS-dependent Treg cell upregulation of trafficking receptors. However, these key findings were also corroborated in the non-TCR transgenic NOD mice, indicating that our proposed model may represent a mechanism of CXCR3 homing of Treg cells to inflamed, non-lymphoid tissues that is conserved across strains (S1D Fig). Finally, this study makes use of a novel, multi-parametric flow cytometry approach for the assessment of endocrine islet cell function and thereby identifies a previously unrecognized role for β-cells in modulating Treg cell responses
Diabetes induction by islet-reactive Teff cells severely inhibited chemokine production by β-islet cells; however, their active insulin-secreting status suggests that the loss of chemokine expression by the islet cells is not merely a consequence of their destruction by Teff cells alone. Strikingly, infusion of Treg cells reverses Teff cell-mediated abrogation of chemokine expression by ß-islet cells. This effect could be the result of suppression of intra-islet Teff production of inflammatory cytokines by Treg cells. For instance, Teff-derived IFN-γ can directly promote the apoptosis of islet cells as stat1 -/- islets are resistant to BDC2.5 Teff-mediated killing in vivo [28]. Consistently, our results show that insulin+ ß-islet cells express IFN-γR indicating their capacity to respond to IFN-γ in our model. Thus, Treg cell suppression of IFN-γ production in situ may both prevent Teff-mediated destruction of the islets and maintains β-islet production of CXCR3 chemokines that act to recruit Treg cells. Intruigingly, the sole presence of Treg cells in recipient mice augments islet production of CXCL10 suggesting that a direct Treg-islet cell interaction cannot be excluded.
An increase in Th1-mediated pancreatic inflammation leads to a dose-dependent upregulation of CXCR3 expression on Treg cells, suggesting that Teff cell-related signals or products, such as IFN-γ, induce mechanisms of pancreatic homing in Treg cells. In other models, CXCR3+ Treg cells co-express Foxp3 along with the IFN-γ-inducible Th1 lineage specifying transcription factor T-bet, which directly activates transcription of Cxcr3 in addition to promoting responsiveness to IFN-γ through induction of IFN-γR expression [12]. This indicated that the IFN-γ-T-bet pathway is the basis of the robust upregulation of CXCR3 by ICOS+ Treg cells. T-bet expression by ICOS+ Treg cells correlated with CXCR3 expression ex vivo throughout T1D progression. Furthermore, the ICOS-expressing subset of Treg cells preferentially expressed IFN-γ and IFN-γR, and displayed prominent STAT1 activation in response to IFN-γ in vitro. Moreover, neutralization of IFN-γ significantly blunted CXCR3 expression on ICOS+ Treg cells in response to activation of islet-reactive Teff cells in vivo. Taken as a whole, our results evince a model of ICOS+ Treg cell homing to the islets in which IFN-γ acts directly on Foxp3+ Treg cells, and through IFN-γR and STAT1 signalling induces T-bet expression and CXCR3 upregulation. Equipped with CXCR3, Treg cells then home towards inflamed islets in which immune and insulin-producing endocrine cells express CXCL9, CXCL10 and CXCL11.
While the expression of CXCR3 has been previously described in Teff cells in murine models and patients with T1D, this is the first demonstration, to our knowledge, of ICOS-regulated CXCR3 expression by Foxp3+ Treg cells [17, 31, 32]. In support of this, we show that CXCR3 and T-bet expression by Treg cells is significantly diminished in ICOS-/- mice. However, ICOS signaling has not been shown to induce T-bet expression by CD4+ T cells. Indeed, ICOS is well known to promote Th2 responses and more recently T-follicular helper cell differentiation, but shown to be unnecessary for Th1 differentiation [33]. However, the factors that govern T-bet expression in Treg cells and Th1 cells may differ. Indeed, IL-12Rβ2 expression, which is indispensible for stable expression of T-bet by Th1 cells, was found to be abrogated during differentiation of CXCR3+ Treg cells [30]. Therefore, in conjunction with IFN-γ signaling, induction of T-bet in Treg cells may require additional stimuli involving ICOS. Previously, we showed that ICOS signals endow Treg cells with an enhanced responsiveness to IL-2. Moreover, IL-2 can induce expression of T-bet in human CD4+ cells in vitro [34]. Therefore, responsiveness to IL-2 could potentially account for differential expression of T-bet between ICOS+ and ICOS- Treg cells.
In certain settings such as inflammation or lymphophenia, Treg cells can lose Foxp3 expression, fully reprogram into a Teff cell lineage and contribute to inflammatory processes [29]. Alternatively, recent studies have described transient forms of functional plasticity in Treg cells expressing Teff cell-associated markers critical for their trafficking and the survival at sites of Th1, Th2 or Th17 mediated pathogenesis [30, 13, 14]. Here, we show that CXCR3+ ICOS+ Treg cells, the predominant and most immunosuppressive Treg cell subset in the pancreas, express Th1-associated markers including the T-bet transcription factor. Thus ICOS+ Treg cells do not completely reprogram into Teff cells but acquire some Th1-like features, which may facilitate their preferential tissue-trafficking and functional fitness. Overall, this indicates that IFN-γ may not solely play a diabetogenic role but could also support tolerogenic mechanisms in the pancreas through regulation of Treg cell homing.
Tissue homing of leukocytes profoundly affects immunity, as spatio-temporal coordination of immune cell subsets can dictate the nature of immune response. As homing is increasingly recognized as critical for Treg cell-mediated suppression, it may represent a novel approach to enhancing Treg cell functions. Notably, CXCR3+Treg cells were recently shown to indispensably suppress Th1 responses, demonstrating they could be important in type 1 immune-mediated disease [12]. Concordantly, we show in a T1D model that CXCR3 is predominantly expressed by ICOS+ Treg cells, presenting an avenue for selective enhancement of this suppressive subset of Treg cells whilst minimizing recruitment of less suppressive ICOS- Treg cells. Though a very low percentage of Teff cells express CXCR3, their use of this receptor cannot be entirely excluded. Thus, further elucidation of the factors that specifically modulate ICOS+ Treg cell trafficking is required. If these are amenable to therapeutic modulation, their enhancement could potently dampen inflammation and control autoimmunity.
Supporting Information
NOD.TCRα-/- mice received BDC2.5 CD4+CD25+ (Treg) or CD4+CD25- (Teff, 7.5X105) cells alone or at the indicated Treg/Teff cell ratios. When the Teff cell recipient mice displayed hyperglycemia (>33mmol/L), mice were sacrificed, and expression of IFN-γ by Teff cells in pancLN was examined (B). Cell suspensions of the pancreas from 4 week old BDC2.5NOD mice were prepared and examined for CXCR3-ligand expression among Foxp3+ and Foxp3- T cells (C). NOD.TCRα-/- mice were left intact or received Treg or Teff cells at the indicated Treg/Teff cell ratios. When mice receiving Teff cells alone became hyperglycemic (>33mmol/L), insulin expression in β-islet cells was compared between groups (% insulin refers to % insulin+ cells out of total islet cells). (D). Cell suspensions from draining pLN of 4 week old BDC2.5 and NOD mice were obtained and the level of CXCR3 expression (MFI) between the ICOS+ and ICOS- subsets of Foxp3+ Treg cells was assessed. CXCR3 expression on Foxp3+ Treg cells from BDC2.5 ICOS-/- or NOD ICOS-/- mice are also shown (E). Cell suspensions of draining LN from 4 week old BDC2.5 mice were obtained and assessed for T-bet expression (MFI). A representative plot showing the T-bet antibody stain relative to FMO control (on left) are shown. A T-bet antibody titration was performed using the indicated concentrations of antibody (right panel) (F).
(TIFF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported from the grants of CIHR (CAP: MOP67211; SQ: MOP-102494). C.A.P. holds the Canada Research Chair. MK has received a doctoral fellowship from the Fonds de la recherche en santé du Québec (FRSQ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14: 307–308. 10.1038/ni.2554 [DOI] [PubMed] [Google Scholar]
- 3. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27: 18–20. [DOI] [PubMed] [Google Scholar]
- 4. Anderson MS, Bluestone JA. THE NOD MOUSE: a model of immune dysregulation. Annu Rev Immunol. 2004;23: 447–485. [DOI] [PubMed] [Google Scholar]
- 5. Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFβ coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201: 1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57: 113–123. [DOI] [PubMed] [Google Scholar]
- 7. Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188: 1064–1074. 10.4049/jimmunol.1101303 [DOI] [PubMed] [Google Scholar]
- 8. Sgouroudis E, Albanese A, Piccirillo CA. Impact of protective IL-2 allelic variants on CD4+Foxp3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice. J Immunol. 2008;181: 6283–6292. [DOI] [PubMed] [Google Scholar]
- 9. Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28: 687–697. 10.1016/j.immuni.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sgouroudis E, Kornete M, Piccirillo CA. IL-2 production by dendritic cells promotes Foxp3+ regulatory T-cell expansion in autoimmune-resistant NOD congenic mice. Autoimmunity. 2011;44: 406–414. 10.3109/08916934.2010.536795 [DOI] [PubMed] [Google Scholar]
- 11. Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11: 119–130. 10.1038/nri2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10: 595–602. 10.1038/ni.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458: 351–356. 10.1038/nature07674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326: 986–991. 10.1126/science.1172702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199: 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meiser A, Mueller A, Wise EL, McDonagh EM, Petit SJ, Saran N et al. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J Immunol. 2008;180: 6713–6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Höllander GA et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 18. Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MBA. Among CXCR3 chemokines, IFN-γ-Inducible Protein of 10 kDa (CXC Chemokine Ligand (CXCL) 10) but not Monokine Induced by IFN-γ (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol. 2003;171: 6838–6845. [DOI] [PubMed] [Google Scholar]
- 19. Himmel ME, Crome SQ, Ivison S, Piccirillo CA, Steiner TS, Levings MK. Human CD4+FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol. 2011;41: 306–312. 10.1002/eji.201040459 [DOI] [PubMed] [Google Scholar]
- 20. Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, et al. How does type 1 diabetes develop?: The notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60: 1370–1379. 10.2337/db10-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kornete M, Beauchemin H, Polychronakos C, Piccirillo CA. Pancreatic islet cell phenotype and endocrine function throughout diabetes development in non-obese diabetic mice. Autoimmunity. 2013;46: 259–268. 10.3109/08916934.2012.752462 [DOI] [PubMed] [Google Scholar]
- 22. Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203: 2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184: 2886–2898. 10.4049/jimmunol.0901216 [DOI] [PubMed] [Google Scholar]
- 24. Erhardt A, Wegscheid C, Claass B, Carambia A, Herkel J, Mittrücker HW, et al. CXCR3 deficiency exacerbates liver disease and abrogates tolerance in a mouse model of immune-mediated hepatitis. J Immunol. 2011;186: 5284–5293. 10.4049/jimmunol.1003750 [DOI] [PubMed] [Google Scholar]
- 25. Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, et al. CXCR3+ T regulatory cells Selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 2012;72: 4351–4360. 10.1158/0008-5472.CAN-12-0579 [DOI] [PubMed] [Google Scholar]
- 26. Yamada Y, Okubo Y, Shimada A, Oikawa Y, Yamada S, Narumi S, et al. Acceleration of diabetes development in CXC chemokine receptor 3 (CXCR3)-deficient NOD mice. Diabetologia. 2012;55: 2238–2245. 10.1007/s00125-012-2547-8 [DOI] [PubMed] [Google Scholar]
- 27. Beaudoin L, Diana J, Ghazarian L, Simoni Y, Boitard C, Lehuen A. Plasmacytoid dendritic cells license regulatory T cells, upon iNKT-cell stimulation, to prevent autoimmune diabetes. Eur J Immunol. 2014;44: 1454–1466. 10.1002/eji.201343910 [DOI] [PubMed] [Google Scholar]
- 28. Kim S, Kim HS, Chung KW, Oh SH, Yun JW, Im SH, et al. Essential Role for Signal Transducer and Activator of Transcription-1 in Pancreatic Beta-Cell Death and Autoimmune Type 1 Diabetes of Nonobese Diabetic Mice. Diabetes. 2007;56: 2561–2568. [DOI] [PubMed] [Google Scholar]
- 29. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10: 1000–1007. 10.1038/ni.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37: 501–510. 10.1016/j.immuni.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317: 620–631. 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159: 338–343. 10.1111/j.1365-2249.2009.04087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409: 97–101. [DOI] [PubMed] [Google Scholar]
- 34. Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12: 551–559. 10.1038/ni.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NOD.TCRα-/- mice received BDC2.5 CD4+CD25+ (Treg) or CD4+CD25- (Teff, 7.5X105) cells alone or at the indicated Treg/Teff cell ratios. When the Teff cell recipient mice displayed hyperglycemia (>33mmol/L), mice were sacrificed, and expression of IFN-γ by Teff cells in pancLN was examined (B). Cell suspensions of the pancreas from 4 week old BDC2.5NOD mice were prepared and examined for CXCR3-ligand expression among Foxp3+ and Foxp3- T cells (C). NOD.TCRα-/- mice were left intact or received Treg or Teff cells at the indicated Treg/Teff cell ratios. When mice receiving Teff cells alone became hyperglycemic (>33mmol/L), insulin expression in β-islet cells was compared between groups (% insulin refers to % insulin+ cells out of total islet cells). (D). Cell suspensions from draining pLN of 4 week old BDC2.5 and NOD mice were obtained and the level of CXCR3 expression (MFI) between the ICOS+ and ICOS- subsets of Foxp3+ Treg cells was assessed. CXCR3 expression on Foxp3+ Treg cells from BDC2.5 ICOS-/- or NOD ICOS-/- mice are also shown (E). Cell suspensions of draining LN from 4 week old BDC2.5 mice were obtained and assessed for T-bet expression (MFI). A representative plot showing the T-bet antibody stain relative to FMO control (on left) are shown. A T-bet antibody titration was performed using the indicated concentrations of antibody (right panel) (F).
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.