Abstract
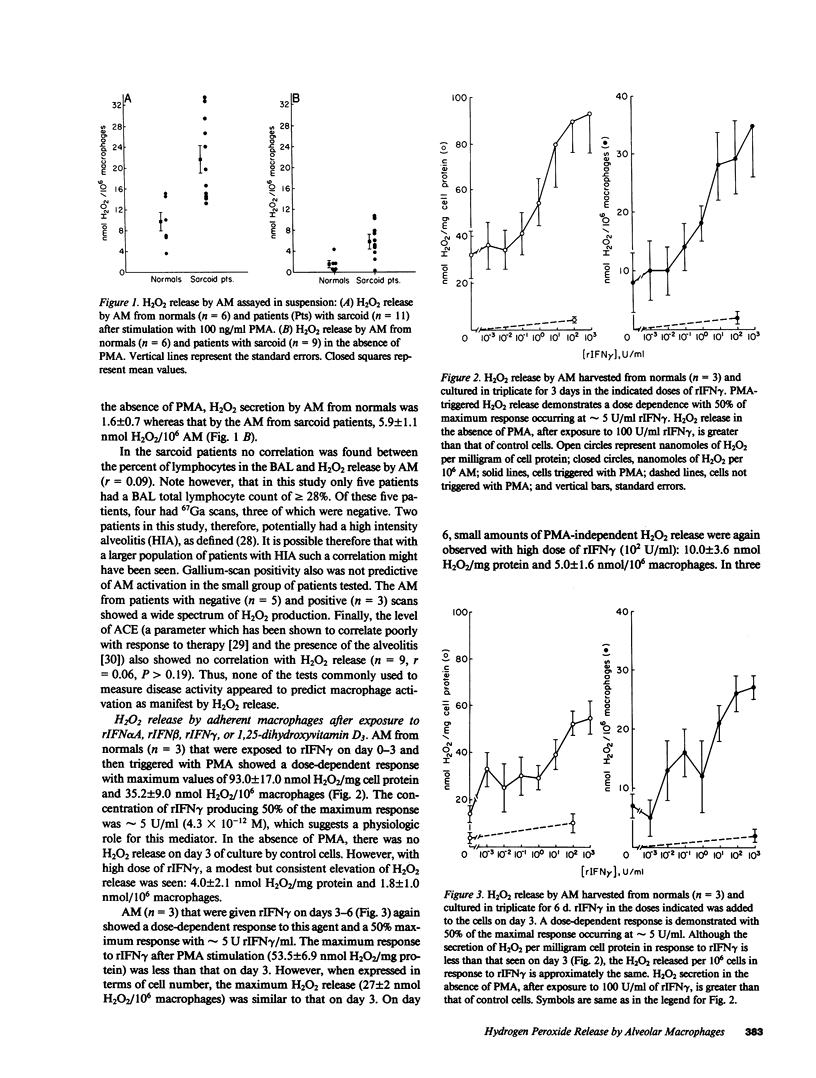
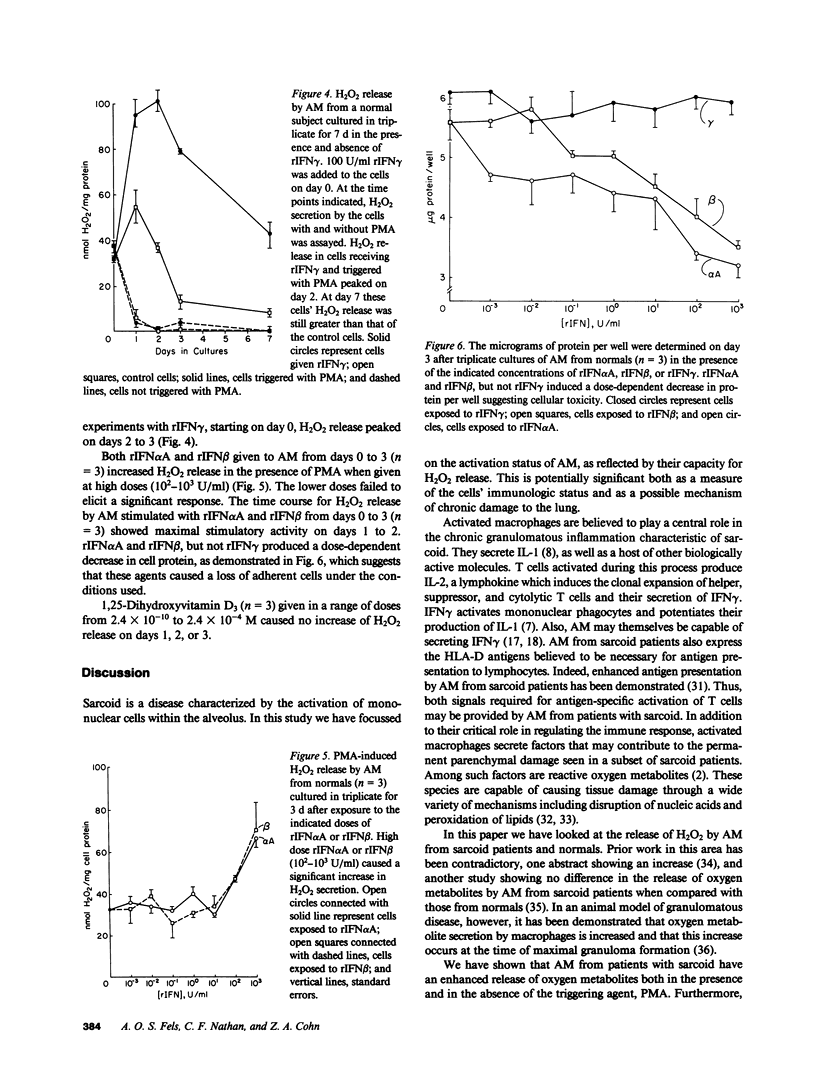
We measured H2O2 release by human alveolar macrophages (AM) from normals and sarcoid patients in suspension immediately after bronchoalveolar lavage in the presence and absence of the triggering agent, phorbol myristate acetate (PMA). AM from 11 sarcoid patients produced a mean (+/- SE) of 21.7 +/- 2.3 and 5.9 +/- 3.4 nmol H2O2/10(6) macrophages in the presence and absence of PMA, respectively. By contrast, AM from normals (n = 6) produced 9.8 +/- 1.7 and 1.6 +/- 0.7 nmol H2O2/10(6) macrophages with and without PMA, respectively. Macrophage activation, as monitored by H2O2 production, did not correlate with the angiotensin-converting enzyme levels, the result of gallium-67 scans, or the percent of lymphocytes in the bronchoalveolar lavage. To determine whether AM from normals could be stimulated to increase their H2O2 production to the level seen in patients with sarcoid, we measured H2O2 released by adherent AM after incubation in each of four potential activating agents: recombinant interferons alpha A, beta, gamma (rIFN alpha A, rIFN beta, and rIFN gamma, respectively), and 1,25-dihydroxyvitamin D3. H2O2 release in the range seen in sarcoid patients could be induced in PMA-triggered AM from normals by rIFN gamma in a time- (t1/2 approximately 1 d) and dose-dependent fashion (threefold increase, EC50 5 antiviral U/ml) and by rIFN alpha A and rIFN beta at higher concentrations, but not by 1,25-dihydroxyvitamin D3.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Munakata M., Nishimura M., Tsuneta Y., Terai T., Nakano I., Ohsaki Y., Kawakami Y. Gallium-67 scintigraphy, bronchoalveolar lavage, and pathologic changes in patients with pulmonary sarcoidosis. Chest. 1984 May;85(5):650–655. doi: 10.1378/chest.85.5.650. [DOI] [PubMed] [Google Scholar]
- Adams J. S., Singer F. R., Gacad M. A., Sharma O. P., Hayes M. J., Vouros P., Holick M. F. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 1985 May;60(5):960–966. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J. L., Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985 Apr;134(4):2444–2448. [PubMed] [Google Scholar]
- Baughman R. P., Fernandez M., Bosken C. H., Mantil J., Hurtubise P. Comparison of gallium-67 scanning, bronchoalveolar lavage, and serum angiotensin-converting enzyme levels in pulmonary sarcoidosis. Predicting response to therapy. Am Rev Respir Dis. 1984 May;129(5):676–681. doi: 10.1164/arrd.1984.129.5.676. [DOI] [PubMed] [Google Scholar]
- Beaumont D., Herry J. Y., Sapene M., Bourguet P., Larzul J. J., de Labarthe B. Gallium-67 in the evaluation of sarcoidosis: correlations with serum angiotensin-converting enzyme and bronchoalveolar lavage. Thorax. 1982 Jan;37(1):11–18. doi: 10.1136/thx.37.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell N. H. Vitamin D-endocrine system. J Clin Invest. 1985 Jul;76(1):1–6. doi: 10.1172/JCI111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Kunkel S. L., Higashi G. I., Ward P. A., Boros D. L. Production of superoxide anion, prostaglandins, and hydroxyeicosatetraenoic acids by macrophages from hypersensitivity-type (Schistosoma mansoni egg) and foreign body-type granulomas. Infect Immun. 1983 Dec;42(3):1116–1125. doi: 10.1128/iai.42.3.1116-1125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Mesler D. E., Snipes R. G., Gray T. K. 1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. J Immunol. 1986 Feb 1;136(3):1049–1053. [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- DeRemee R. A. The roentgenographic staging of sarcoidosis. Historic and contemporary perspectives. Chest. 1983 Jan;83(1):128–133. doi: 10.1378/chest.83.1.128. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening A. P., Lowrie D. B. Extracellular release of hydrogen peroxide by human alveolar macrophages: the relationship to cigarette smoking and lower respiratory tract infections. Clin Sci (Lond) 1983 Dec;65(6):661–664. doi: 10.1042/cs0650661. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hillerdal G., Nöu E., Osterman K., Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984 Jul;130(1):29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Bedell G. N., Zavala D. C., Monick M., Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983 Oct;128(4):634–638. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Lymphokine regulation of macrophage-derived growth factor secretion following pulmonary injury. Am J Pathol. 1985 Nov;121(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Merz T., Malmud L., McKusick K., Wagner H. N., Jr The mechanism of 67 Ga association with lymphocytes. Cancer Res. 1974 Oct;34(10):2495–2499. [PubMed] [Google Scholar]
- Murray H. W., Gellene R. A., Libby D. M., Rothermel C. D., Rubin B. Y. Activation of tissue macrophages from AIDS patients: in vitro response of AIDS alveolar macrophages to lymphokines and interferon-gamma. J Immunol. 1985 Oct;135(4):2374–2377. [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Horowitz C. R., de la Harpe J., Vadhan-Raj S., Sherwin S. A., Oettgen H. F., Krown S. E. Administration of recombinant interferon gamma to cancer patients enhances monocyte secretion of hydrogen peroxide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8686–8690. doi: 10.1073/pnas.82.24.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Glazier J., Monick M. M., Hunninghake G. W. Stimulated human alveolar macrophages secrete interferon. Am Rev Respir Dis. 1985 May;131(5):714–718. doi: 10.1164/arrd.1985.131.5.714. [DOI] [PubMed] [Google Scholar]
- Pantalone R., Page R. C. Enzyme production and secretion by lymphokine-activated macrophages. J Reticuloendothel Soc. 1977 May;21(5):343–357. [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Lazarus R., Fanning V., Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983 Oct 1;158(4):1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer F. K. Angiotensin-converting enzyme activity in sarcoidosis and other disorders. Sarcoidosis. 1985 Mar;2(1):25–34. [PubMed] [Google Scholar]
- Rossman M. D., Dauber J. H., Cardillo M. E., Daniele R. P. Pulmonary sarcoidosis: correlation of serum angiotensin-converting enzyme with blood and bronchoalveolar lymphocytes. Am Rev Respir Dis. 1982 Mar;125(3):366–369. doi: 10.1164/arrd.1982.125.3.366. [DOI] [PubMed] [Google Scholar]
- Smiéjan J. M., Cosnes J., Chollet-Martin S., Soler P., Basset F. M., Le Quintrec Y., Hance A. J. Sarcoid-like lymphocytosis of the lower respiratory tract in patients with active Crohn's disease. Ann Intern Med. 1986 Jan;104(1):17–21. doi: 10.7326/0003-4819-104-1-17. [DOI] [PubMed] [Google Scholar]
- Strunk R. C., Cole F. S., Perlmutter D. H., Colten H. R. gamma-Interferon increases expression of class III complement genes C2 and factor B in human monocytes and in murine fibroblasts transfected with human C2 and factor B genes. J Biol Chem. 1985 Dec 5;260(28):15280–15285. [PubMed] [Google Scholar]
- Valeyre D., Saumon G., Bladier D., Amouroux J., Pré J., Battesti J. P., Georges R. The relationships between noninvasive explorations in pulmonary sarcoidosis of recent origin, as shown in bronchoalveolar lavage, serum, and pulmonary function tests. Am Rev Respir Dis. 1982 Jul;126(1):41–45. doi: 10.1164/arrd.1982.126.1.41. [DOI] [PubMed] [Google Scholar]
- Venet A., Hance A. J., Saltini C., Robinson B. W., Crystal R. G. Enhanced alveolar macrophage-mediated antigen-induced T-lymphocyte proliferation in sarcoidosis. J Clin Invest. 1985 Jan;75(1):293–301. doi: 10.1172/JCI111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]



