Abstract
Background
Depression involves decreased positive affect. Whether this is due to a failure to achieve or maintain positive emotion in response to discrete stimuli is unclear. Understanding the nature of decreased positive affect could help to address how to intervene on the phenomenon, e.g., how to structure interventions using positive and rewarding stimuli in depression. Thus, we examined the time course of affect following exposure to positive stimuli in depressed and healthy individuals.
Methods
Seventy-one adults with major depressive disorder and 34 never-depressed controls read a self-generated highly positive script and continuously rated their affect for seven minutes.
Results
Both groups quickly achieved increased positive affect, however, compared to controls, depressed participants did not achieve the same level of positive affect, did not maintain their positive affect, spent less time rating their affect as happy, and demonstrated larger drops in mood.
Conclusions
These data indicate that depressed and non-depressed individuals can generate positive reactions to happy scripts, but depressed individuals cannot achieve or sustain equivalent levels of positive affect. Interventions for depression might fruitfully focus on increasing depressed individuals’ ability to maintain initial engagement with positive stimuli over a sustained period of time.
Keywords: depression, dynamics, mood induction, positive psychology, affect processing
Like healthy individuals, depressed individuals experience increases in affect for at least a short time following positive stimuli. Yet, they often return to a diminished positive or negative affective state[e.g., 1,2-6]. Despite the wealth of theories regarding how otherwise positive moods could become negative in depression[e.g., 7,8], few studies have documented the process of decreasing positive affect in the minutes following exposure to positive stimuli, a process that is highly relevant to both real-world functioning and therapeutic approaches. Documenting the time-course of positive affect, particularly in response to highly self-relevant, personal information, could have strong implications for both the basic science of depression as well as provide better specification of targets for depression interventions, many of which primarily focus on negative thinking (e.g., Cognitive Therapy[9], a popular psychotherapy, addresses over-evaluation of negative information to the near exclusion of positive information processing). To address this issue, this study examined the temporal dynamics of affect in depressed and healthy individuals for several minutes after reading a self-generated script about an “extremely happy” personal experience. Our primary question was whether we could observe depressed individuals’ affect naturalistically drop following induction into a positive affective state.
There are a number of reasons to expect that depressed individuals would display reduced and diminishing affective responses to positive stimuli. Inherent biases toward negative information and thoughts in individuals with depression[see 10 for reviews,11,12] result in decreased subjective positive affect[13-16], negative automatic thoughts[17,18], and negative dialogues[19], especially in those who ruminate[17]. Decreased positive affect in depression may also derive from altered responses specifically to positive information[20-24]. For example, depressed persons may perceive positive information as negative[25,26] or relatively less positive[27-30]. Tendencies to shift attention away from positive stimuli in depression have also been reported[6,31]. Consistent with this formulation, depressed individuals demonstrate decreased activity in a variety of regions associated with perception (lingual and fusiform gyri) as well as those associated with reward (ventral striatum) following presentation of happy faces[29,32]. Decreased activity in regions associated with reward such as the nucleus acumbens is also observed during upregulation of positive mood[33]. These findings are in contrast to increased activity in response to negative emotional stimuli in regions associated with emotion such as the amygdala in the same studies, arguing for a valence-specific phenomenon, rather than decreased arousal more generally. There are many possible explanations for decreased sustained processing of positive experiences (or savoring) given this formulation. For example, if neural activity above some threshold is required for the representation of positive emotional stimuli to be maintained[34], this threshold could be increased in depressed individuals, or associated activities or connectivities within a network necessary for a self-maintaining feedback loop could be decreased.
To capture the emergence of negative thinking from a nominally positive state we examined variations in affect, rated continuously([35]) for seven minutes. This approach is different from most research describing blunted responses to positive stimuli in depression which employs briefly presented normative stimuli[see 5 for summary] or retrospective questionnaire ratings following positive film clips[23,36]. We hypothesized that individuals with depression would initially engage positive affect (e.g., at early perceptual stages), yielding high ratings, but would not maintain positive affect after subsequent elaborative processing[34] yielding low ratings that might dip into the range of negative affect. Others have observed continuous affect ratings to drop in individuals with dysphoria when negative stimuli follow positive stimuli [37]. Frontal striatal network function has also been observed to decrease over time in depressed individuals [33]. Here we uniquely examined naturalistic drops in subjective affect using exclusively positive idiosyncratic information.
For reference, we quantitatively compared observed ratings with the States of Mind model’s[38] empirically-derived affective set points for healthy, and depressed individuals (see Supplement-S3).
Methods
Overview
Depressed and healthy participants viewed a self-generated positive script for seven minutes while listening to music they had judged to be happy, and used a mouse to continuously rate their affect.
Participants
Participants included unmedicated adults with major depressive disorder and healthy adult controls with no history of depression or other Axis I disorders, as diagnosed by the Structured Clinical Interview for DSM-IV Disorders, Patient edition (SCID[SCID; 39; Table 1]). The sample who consented to be scanned included 87 depressed and 34 healthy participants. Due to technical errors (e.g., the mouse did not work) and time constraints, or subjects who elected to end the experiment before this task, data from 23 depressed and 1 control was not useable from the scanner; 8 depressed and the control completed the task in a behavioral lab outside the scanner yielding a final sample of 71 depressed and 34 healthy participants (see Table 1 and Supplement-S1.1 for further information on included and non-included participants). The study was approved by the Internal Review Board of the University of Pittsburgh.
Table 1.
Demographics and Measures
| Controls (n=34) |
Depressed (n=71) |
Statistic testing group differences |
Statistical significance (P) |
|
|---|---|---|---|---|
| Mean Age (SD) | 35.32 (9.94) | 34.56(10.99) | t(104)=.35 | .73 |
| Sex (% Female) | 67.6% (n=23) | 70.8.1% (n=51) |
Fisher’s exact | .82 |
| Ethnicity (% Caucasian) |
82.4% (n=28) | 76.3% (n=55) |
Fisher’s exact | .62 |
| Mean Education (SD) |
16.03 (2.20)c | 14.93 (2,48) | t(103)=−2.20 | .03 |
| BDI (SD)a | 2.33 (4.44) | 30.54(9.31) | t(99.99)=−20.70b | <.0001 |
| HAM-D (SD)c | --- | 19.79 (5.61) | --- | --- |
| Median #
depressive episodes |
0 | 3 |
day of testing, N=33 Controls, N=69 patients (1 BDI from a week prior to testing)
equal variances not assumed
5 participants’ data was taken from a rating done in the weeks after, rather than before testing.
Positive affect induction
Preparation
Similar to previous affect induction studies, a combination of idiographic scripts and positive music was used to elicit positive affect[40-42]. Participants selected happy music from a list of non-linguistic pieces (e.g., Claude Bolling’s Suite for Flute and Jazz Piano; full list available on request). To generate scripts, participants were asked to compose a short paragraph (i.e., which fit on about a quarter page of lines) about a vivid, extremely positive personal experience, one of the best times in their lives when they felt happy or exuberant and that they could re-experience during the task, and which they would rate at least a 7 on a scale of 1-9 (one being neutral, and 9 being the happiest they had ever been). To provide objective evaluation, independent raters blind to diagnoses also rated 18 consecutive controls’ and 19 depressed participants’ scripts on the same 1- 9 script rating scale used by participants yielding no remarkable differences between the groups on either mean ratings or their variance (rater 1: control M(SD)= 3.83(1.2), depressed: 4.32(1.49), Mean: t(35)=−1.08, p=.29, Levine’s test of variances F=2.73, p=.11; rater 2: control M(SD)= 3.39(1.3), depressed: 3.89(1.70), Mean: t(35)=−1.02, p=.31, Levine’s test of variances F=.63, p=.43).
Continuous affect rating task
The majority of participants completed the affect rating task in a Magnetic Resonance Imaging (MRI) scanner. Subjective affect data are reported here; neuroimaging data will be reported on separately. Just before task administration, participants were instructed as follows, modeled after [43]:
“Now I would like you to get into a happy mood. You will listen to the music piece you selected, and think about the event you described. I would like you to read the event description and try to re-experience it. Once you have a clear image of the event, focus on the happiness of it and try to feel that happiness as strongly as you did when it occurred.”
During the next seven minutes, participants listened, through earphones, to the happy music they had selected. They concurrently viewed their script and rated their affect by moving a mouse left for more negative and right for more positive. To anchor affect ratings, a visual scale with a green tracking ball in a fixed horizontal plane was located above the participant’s typed script. Visual cues on the scale were indicated, from left to right in equidistant intervals: “very sad”, “somewhat sad”, “neutral”, “somewhat happy”, “very happy”..This type of in the moment measure continuously samples rapid changes in affect, minimizes response biases, is relatively simple to use and requires little training[35,44], and does not appear to decrease either self-reported affect or neural activity compared to passive viewing[45]. In similar studies, online ratings are correlated with post-viewing ratings (r=.5-.8 for ratings during an amusing film) and pertinent facial behavioral ratings (r=.73). Ratings during fMRI are highly correlated with ratings of the same film clips repeated outside the scanner [44] (r=.88-.98 for positive, negative and neutral clips). Software for the task was implemented in the E-prime presentation environment.[46]. Though the scripts varied in length, we did not control for length above and beyond giving participants a fixed space to write in. Participants’ experience of the task could vary on both the intensity of positivity of the task as well as script length.
Procedure
Study details were fully provided to participants, after which written informed consent was obtained. Prior to the task, participants completed their scripts, made their musical selections and were trained on the affect ratings described previously (without a script).
On the day of testing, participants completed the BDI-II (see Supplement-S1.2) and a series of tasks (not germane to the present study; list available upon request from the authors) ending with the positive affect task described here, during concurrent neuroimaging. As noted previously, data from 8 depressed and 1 control participants were acquired in a behavioral lab outside the scanner. (see Supplement-S1.1 and S5). Additional self-report ratings for “sad” and “happy” affect (rated 1-5 using a 5-button response glove) were completed before and after the task.
Analysis Plan
Group differences in demographics were analyzed via t-tests subject to familywise error correction (Bonferroni).
Affect rating data gathered using the mouse were resampled to 20Hz (from a variable sampling rate across participants up to 166Hz) and the mouse position was scaled using a range of 0 to 1 corresponding to the visual analog scale rating cues as: Very Sad=0-0.125; Somewhat Sad=0.125-0.375; Neutral=0.375-0.625; Somewhat Happy=0.625-0.875; Very Happy=0.875-1.0.
To evaluate the time course of affect in depressed individuals compared to controls, we conducted planned contrasts as well as exploratory analyses. For planned contrasts type I error was controlled within families of tests using a Bonferroni correction. Specifically examined parameters included tests of group differences in early affective reactivity (average affect rating; velocity to peak affect within the first minute), peak affect (maximum rated affect throughout seven minutes), sustained affect (average affect after one minute, lowest affect rating after three minutes and change in affect rating from peak to subsequent lowest affect rating), categorical differences in affective experience (percentage of time rating within different affect label cues on the visual analog scale; number of ratings that dropped below the “somewhat happy” visual cue), along with overall change in affect and variability.
Exploratory analyses involved two sample t-tests examining group differences at each sample along affect-rating waveforms to identify time-regions over the seven minutes in which there were reliable differences between groups. As in previous reports, Type I error was controlled across these non-independent tests using Guthrie and Buchwald’s [47] technique (see Supplement-S1.3).
Results
The groups did not differ significantly on measured demographic variables including age, gender, and ethnicity (Table 1). Controls had one year more education than depressed participants (p=.03) which was not statistically significant when type I error was controlled for the family of demographic tests. Depressive severity was higher in depressed individuals than controls.
Planned Contrasts
Mean ratings
As shown in the first sections of Table 2, compared to healthy controls, depressed participants’ affect ratings were significantly lower on the majority of planned analyses. Their early reactivity was significantly decreased as measured in the first 1 or 3 minutes as was their peak affect ratings and ratings throughout the waveform in each a priori interval. Their minimum affect was lower than controls as well. Thus, regardless of how it was measured, depressed participants reported decreased positive affect on the task; this was particularly true for the majority of depressed participants, whose affect dropped below the “somewhat happy” rating (Supplement S2). Consistent with inability to maintain a happy mood, depressed participants showed a pronounced decrease in affect from their peak and a greater change from peak affect to their mean during the last minute. Group differences in variability were not uniform but the standard deviation of the affect ratings after three minutes was approximately twice as large for the depressed group as for the controls.
Table 2.
Affect rating parameters x1000 (i.e., whereas ratings are reported as 0-1 throughout the manuscript, here they are reported as 0-1000 to allow examination of integer measures of change). =Significant within family using Bonferroni correction.
| Family of tests |
Measure | Statistic | Control Mean(Std) |
Depressed Mean(Std) |
|---|---|---|---|---|
| Early reactivity |
Mean Before 1 Minute |
t(103)=−2.64, p=0.01*, D(s)=− 60.56(110.05), d=−0.55 |
715.439(100.700) | 654.881(114.186) |
| Max in First 3 Minutes |
t(103)=−2.90, p<.005*, D(s)=− 69.78(115.38), d=−0.60 |
914.882(64.427) | 845.099(132.778) | |
| Peak and sustained affect |
Max | t(103)=−2.61, p=0.01*, D(s)=− 55.79(102.40), d=−0.54 |
923.706(63.565) | 867.915(116.299) |
| Mean After 1 Minutes |
t(103)=−6.13, p<.005*, D(s)=− 156.32(122.23), d=−1.28 |
839.790(72.396) | 683.465(139.690) | |
| Mean Last Minute |
t(103)=−6.17, p<.005*, D(s)=− 177.28(137.75), d=−1.29 |
823.378(101.183) | 646.096(151.964) | |
| Min After 3 Min |
t(103)=−6.17, p<.005*, D(s)=− 260.88(202.58), d=−1.29 |
747.206(149.751) | 486.324(223.185) | |
| Variability | Velocity to Peak in First Minute |
t(103)=−1.36, p=0.18, D(s)=−0.06(0.22), d=−0.28 |
0.375(0.246) | 0.312(0.211) |
| Std Dev After 3 Minutes |
t(103)=3.86,p<.005*, D(s)=41.95(52.05), d=0.81 |
35.662(43.713) | 77.608(55.550) | |
| Change From Peak to Last Minute |
t(103)=3.33, p<.005*, D(s)=107.50(154.83), d=0.69 | 91.504(104.853) | 199.002(173.471) | |
| Change After Max |
t(103)=3.84,p<.005*, D(s)=191.15(238.89), d=0.80 |
178.206(158.387) | 369.352(268.597) | |
| Slope After 3 Minutes |
t(103)=−0.83, p=0.41, D(s)=−0.00(0.03), d=−0.17 |
−0.004(0.019) | −0.009(0.032) | |
| Sadness | Percent Lower than Happy after 1Minute |
t(103)=6.69, p<.005*, D(s)=47.31(33.90), d=1.40 |
16.356(28.124) | 63.661(36.309) |
| Percent Lower than Happy after 3Minutes |
t(103)=6.31,p<.005*, D(s)=48.23(36.65), d=1.32 |
17.872(31.683) | 66.101(38.776) | |
| Affect categories | Very Sad | t(103)=1.24, p=0.22, D(s)=0.84(3.25), d=0.26 |
0.000(0.000) | 0.839(3.948) |
| Somewhat Sad |
t(103)=1.86, p=0.07, D(s)=4.86(12.57), d=0.39 |
0.000(0.000) | 4.861(15.242) | |
| Neutral | t(103)=5.13,p<.005*, D(s)=25.18(23.53), d=1.07 |
2.875(7.892) | 28.059(28.029) | |
| Somewhat Happy |
t(103)=0.50, p=0.62, D(s)=3.64(35.06), d=0.10 |
47.793(41.231) | 51.430(31.733) | |
| Very Happy | t(103)=−5.03, p<.005*, D(s)=− 34.61(32.97), d=−1.05 |
49.268(42.493) | 14.660(27.349) | |
| Somewhat or Very Sad |
t(103)=1.87, p=0.06, D(s)=5.70(14.64), d=0.39 |
0.000(0.000) | 5.703(17.757) | |
| Somewhat or Very Happy |
t(103)=−5.57, p<.005*, D(s)=− 30.96(26.64), d=−1.16 |
97.123(7.898) | 66.160(31.860) | |
| Proportions of participants |
#Went Below Somewhat Happy |
ChiSq=18.07, p<0.005* | 20 (59%) | 66 (93%) |
| #Went Below Neutral |
ChiSq=14.16,p<0.005* | 3 (9%) | 34 (48%) |
Time spent in rating categories
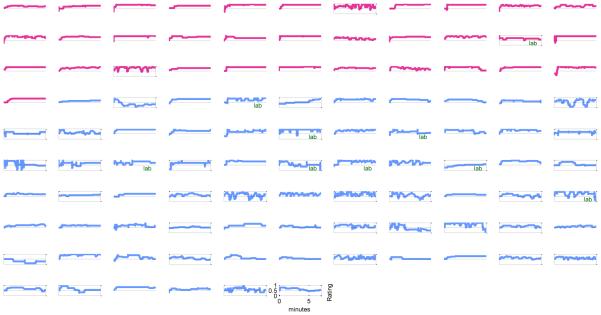
As shown in the “Affect Categories” section of Table 2 and Figure 1, healthy controls spent 97% of their time within the somewhat happy and very happy visual cues, compared to depressed participants who spent on average 66% of their time in these zones. Control participants spent more time in the very happy rating zone compared to depressed participants and depressed subjects spent more time in the neutral rating zone, with no significant differences between groups for time spent in the bands with few participants including Very Sad, Sad, and Somewhat Happy. Observed differences were not a result of just a few participants. Rather, 93% of depressed participants spent time below the “somewhat happy” mark compared to 59% of controls, and 48% spent time below the neutral mark compared to just 9% of controls. Figure 2 shows individual rating trajectories for each individual, with individuals whose ratings dropped below the neutral mark highlighted for illustration.
Figure 1.
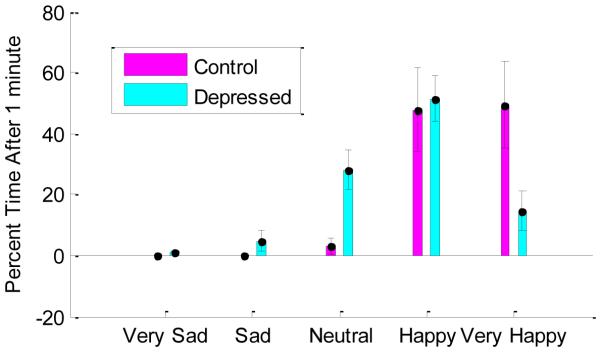
Mean percentage of time spent within each emotion rating cue for healthy and depressed participants. Statistically significant differences between groups p < 0.01 are marked with asterisks. Error bars represent 95% confidence intervals around the mean for each bar, computed separately within each affect label within each group.
Figure 2.
Continuous affect ratings, over seven minutes, for each participant. Control participants are shown in pink. Depressed participants are shown in blue. Dotted lines represent the “Neutral affect” anchor at the 0.5 rating. Participants whose affect after one minute feel below this anchor are highlighted. Participants assessed in the behavioral lab rather than the scanner are marked with the word “lab”.
Contrasts across the Waveform
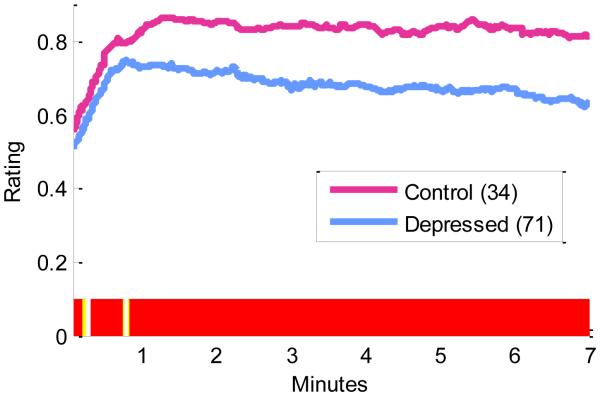
Figure 3 displays the mean rating trajectories for each group throughout the rating period. Regions of statistically significant differences across the waveforms are highlighted on the x-axis. Depressed participants displayed lower mean affect ratings compared to controls throughout nearly the entire time course with brief interruptions yielding nonsignificantly long windows from 1.8 to 14.4 seconds t(103)=3.56, p<.005, D=0.05, d=0.74, and 18.6 to 46.2s: t(103)=2.68, p=0.01, D=0.07, d=0.56, and a significant differences throughout the remainder of the waveform from 48.6 seconds to 7 minutes: t(103)=6.42, p<.005, D=0.15, d=1.34. Results were nearly identical with and without per-participant sample-wise outlier-rescaling. Observed differences remained when participants tested in the behavioral lab were removed from the sample (Supplement-S5).
Figure 3.
Mean continuous affect ratings for control participants (pink) and depressed participants (blue). Regions of statistically significant differences between the waveforms are highlighted below the x-axis. Yellow indicates differences significant at p < 0.1. Red indicates differences significant at p < 0.05.
Mood ratings before and after the task
Consistent with ratings during the task, as shown in Table 3, depressed participants rated their mood as more sad and less happy than controls before the task and after the task. Depressed participants did not become less sad (change in sadness was slightly negative) whereas controls did become less sad following the task. The groups did not differ significantly on their change in happiness.
Table 3.
Peri-task mood ratings for “sad” and “happy” for control and depressed participants who rated their mood before and after the task. Ratings were on a scale of 1 (low) to 5 (high).*=Significant within family using Bonferroni correction.
| Family of tests |
Measure | Statistic | Control (N=24) Mean(Std) |
Depressed (N=65) Mean(Std) |
|---|---|---|---|---|
| Pre-task affect ratings |
Sad | t(87)=5.02, p<.005*
D(s)=1.10(0.91), d=1.20 |
1.042(0.204) | 2.138(1.059) |
| Happy | t(87)=−3.69, p<.005*
D(s)=−0.99(1.13), d=−0.88 |
2.792(1.503) | 1.800(0.955) | |
| Post-task affect ratings |
Sad | t(87)=1.50, p=0.14, D(s)=0.39(1.09), d=0.36 |
1.500(1.251) | 1.892(1.033) |
| Happy | t(87)=−5.13, p<.005*, D(s)=−1.39(1.13), d=−1.23 |
3.833(1.129) | 2.446(1.132) | |
| Pre- to post- task changes in affect ratings |
Sad | t(87)=−2.84, p=0.01*, D(s)=−0.70(1.04), d=−0.68 |
0.458(1.285) | −0.246(0.936) |
| Happy | t(87)=−1.29, p=0.20, D(s)=−0.40(1.29), d=−0.31 |
1.042(1.488) | 0.646(1.205) |
Individual Differences
Supplement-S4 describes associations of multiple self-report measures with affect ratings. Among depressed participants, low affect ratings were associated with self-reported suicidality, state anxiety, rumination, loss of interest, and state negative affect. Higher positive affect was associated with self-reported reflection, sociability, positive automatic thoughts, reappraisal of negative thoughts, and emotion- and self-focus in response to positive information.
Discussion
This study examined differences in continuous affect ratings for depressed and never-depressed participants while viewing a highly positive, personally relevant script of an extremely happy experience over seven minutes. Depressed individuals neither achieved nor maintained the same level of positive affect ratings as controls. Depressed subjects had less positive initial reactions, more extreme drops from their peak affect rating and greater variability compared to healthy subjects. Despite being asked to relive one of the happiest moments of their life, depressed participants’ maximum ratings fell short of the “very happy” cue and they spent only 66% of their time within the somewhat happy or very happy zone compared to 97% for healthy controls. Decreased mood was associated with a variety of self-reported indicators of ruminative coping and inversely with indicators of more positive emotion-focused thinking.
Implications for the Basic Science of Depression
These data support the idea, described by cognitive theorists, that depression involves a spiral of negative thinking in which spontaneously emerging negative thoughts dampen positive affect recollections and states [e.g., 7,8]. They are consistent with studies reporting less intense responses to positive stimuli in depressed individuals [see 5 for review]. According to empirically derived “set-points” [38], depressed individuals’ positive affect began in the normal range and dropped to the subnormal range, in contrast to controls’ levels which remained in the optimal or superoptimal range throughout the mood induction (see Supplement-S3).
This study extends the literature by indicating that despite similar initial reactions to a positive personal memory (i.e., rising affect), the temporal pattern of affect over minutes distinguished depressed from healthy adults. For example, despite initially engaging in positive affect for the first minute with similar speed to peak rating, maximum happiness ratings were lower for depressed individuals, suggesting reduced capacity for, or estimates of, engaging the extremes of positive affect in depression. This result is consistent with literature indicating depressed and dysphoric individuals are capable of initially engaging in an increased, yet sub-normal, level of positive affect[28,48,49] but may demonstrate decreased sustained positive affect[33,37].
Drops in positive affect in depressed participants may have occurred for a variety of reasons including concurrent and competing increases in negative affect, an inability to fully engage or sustain positive emotions[34], comparison with unrealistic standards for judging high levels of positive affect. Associations of negative affect with self-reported rumination could also suggest that positive information can be “spun negative” in the context of increased rumination or intrusion of negative thoughts in depressed individuals [see 50 for review]. On discussion, many patients reported that indeed, their mood had fallen following negative thoughts, with stories such as “My script was about when I was happily in love. But then she left me. And I thought about how I will never have that again.” Mood congruent memory biases[13,51] could have shifted attention away from the positive script due to intrusive recall of negative memories. Additional potential explanations include not engaging brain mechanisms associated with increasing or maintaining affect[e.g., 33,52], not savoring positive affect[34], or conversely, engaging cognitive or brain mechanisms that regulate or dampen positive affect[53]. For instance, if positive experiences engender a sense of discomfort or guilt in individuals with depression, participants may have down-regulated their positive affect.
Clinical Implications
These data suggest a disruption in sustained processing of personally relevant positive information in depression. Coping styles such as rumination were associated with decreases in subjective affect. Thus, it may be helpful to expose depressed individuals to thinking styles geared towards maintaining affect towards positive information. These could be added to interventions such as Cognitive Therapy that traditionally emphasize negative cognitions or could be used alone based on patient needs. Emerging interventions that focus on savoring[54] may be particularly appropriate in this regard. This focus may be a relatively unexamined key to the success of time-tested protocols that explicitly help individuals to balance positive with negative thinking in response to positive events such as the efficacious Coping With Depression course[55]. Allowing patients to dynamically measure their affect, as in this protocol, may give them insight into the time course of their affective information processing, and thus into aspects of affect and regulation useful to focus on in treatment.
Limitations
The study has several limitations. Affect was assessed using a bipolar scale (happy or sad) that did not allow assessment of simultaneously occurring negative and positive affect[35]. Self-reported state sadness and anxiety on questionnaires measures was associated with ratings, which could mean that results were a function of state, rather than trait features. As with any experiment involving mood induction, either group may have exaggerated or limited their reported affect due to factors such as demand characteristics, e.g., depressed participants may have reported sad mood to “live up to” their depression diagnosis [56,57]. Task demands, involving attending to affect, could have altered ratings for depressed individuals, though they likely did not affect controls.[45] Other important unmeasured causes could be reduced emotional intensity, reduced task engagement, distraction by ambient noise in the scanner, differential time spent re-reading and associated habituation to the personally relevant script, overall personal relevance for depressed subjects, and/or diminished recall for autobiographical memories [e.g., 58,59,60].
Summary
In summary, data suggest that although individuals with depression could, with prompting, achieve positive affect consistent with euthymic individuals’ everyday experience, they did not rise to the same level of positive affect as healthy controls. Depressed participants then experienced larger decreases in affect from their peak and showed more variability in affect over time suggesting difficulty maintaining positive affect. These findings have potential importance in the clinical setting, particularly in supporting the utility of interventions geared towards enhancing positive affect [e.g., 34,61,62,63]. The employed paradigm may specifically be useful in future examinations of blunted positive affect in depression, particularly, for capturing and monitoring the spiraling negative affect and “emotional roller-coaster” that characterizes the lives of many depressed individuals, even in the context of nominally positive events.
Supplementary Material
Acknowledgements
Acknowledgements. We gratefully acknowledge the contributions of Mauri Cesare, and the staff of the Mood Disorders Treatment and Research Program at Western Psychiatric Institute and Clinic.
Supported by This research was supported by the National Institutes of Health MH074807, MH082998, MH58356, MH58397, MH69618, K12DA000357, and the Pittsburgh Foundation, Emmerling Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. ClinicalTrials.gov, identifiers: NCT00183664, NCT00787501
Footnotes
Previous Presentation. Portions of this manuscript were presented at the meeting of the Society for Psychophysiological Research, 2009, and the meeting of the American Academy of Child and Adolescent Psychiatry, 2009.
Disclosures: No authors had conflicts relevant to this manuscript. Greg Siegle is an unpaid consultant for Trial IQ and Neural Impact. Dr. Friedman has provided Speaker Bureaus or Advisory Boards: AstraZeneca, Eli Lilly GlaxoSmithKline, Pfizer Wyeth-Ayerst, and has obtained Grant /Research support from Aspect Medical Systems, Indevus, AstraZeneca, Bristol-Myers Squibb, Pfizer, Sanofi-Aventis, Wyeth-Ayerst, Cyberonics, Novartis, NorthStar/St. Jude Medical, Medtronics, Respironics
References
- 1.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 2.Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- 3.Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: Evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology. 2003;71:465–481. doi: 10.1037/0022-006x.71.3.465. [DOI] [PubMed] [Google Scholar]
- 4.Feldman GC, Joormann J, Johnson SL. Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research. 2006;32:507–525. doi: 10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Teasdale JD. Negative thinking in depression: Cause effect or reciprocal relationship. Advances in Behavior Research and Therapy. 1983;5:3–25. [Google Scholar]
- 8.Ingram RE. Toward an information processing analysis of depression Cognitive Therapy and Research. 1984;8:443–478. [Google Scholar]
- 9.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guilford Press; New York: 1979. p. 425. [vii] [Google Scholar]
- 10.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 11.Mor N, Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychol Bull. 2002;128:638–662. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- 12.Mogg K, Bradley BP. Attentional Bias in Generalized Anxiety Disorder Versus Depressive Disorder. Cognitive Therapy and Research. 2005;29:29–45. [Google Scholar]
- 13.Matt GE, Vazquez C, Campbell W. Mood congruent recall of affectively toned stimuli: A meta analytic review. Clinical Psychology Review. 1992;12:227–255. [Google Scholar]
- 14.Beck AT. Depression Clinical experimental and theoretical aspects. Hoeber; New York: 1967. [Google Scholar]
- 15.Bower G. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- 16.Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive Psychology and Emotional Disorders. Second John Wiley and Sons; Chichester: 1997. [Google Scholar]
- 17.Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behav Res Ther. 2007;45:2664–2678. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Hollon SD, Kendall PC. Cognitive self statements in depression Development of an automatic thoughts questionnaire. Cognitive Therapy Research. 1980;4:383–395. [Google Scholar]
- 19.Schwartz RM, Reynolds CF, Thase ME, Frank E, Fasiczka AL, Haaga DA. Optimal and normal affect balance in psychotherapy of major depression: Evaluation of the balanced states of mind model. Behavioural & Cognitive Psychotherapy. 2002;30:439–450. [Google Scholar]
- 20.Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- 21.Wood JV, Heimpel SA, Michela JL. Savoring versus dampening: self-esteem differences in regulating positive affect. J Pers Soc Psychol. 2003;85:566–580. doi: 10.1037/0022-3514.85.3.566. [DOI] [PubMed] [Google Scholar]
- 22.Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology. 2003;112:203–211. doi: 10.1037/0021-843x.112.2.203. [DOI] [PubMed] [Google Scholar]
- 23.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 24.Ingram RE, Wisnicki KS. Assessment of positive automatic cognition. J Consult Clin Psychol. 1988;56:898–902. doi: 10.1037//0022-006x.56.6.898. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton EW, Abramson LY. Cognitive patterns and major depressive disorder: a longitudinal study in a hospital setting. J Abnorm Psychol. 1983;92:173–184. doi: 10.1037//0021-843x.92.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Norman W, Miller I, Dow M. Characteristics of depressed patients with elevated levels of dysfunctional cognitions. Cognitive Therapy and Research. 1988;12:39–52. [Google Scholar]
- 27.Sloan DM, Bradley MM, Dimoulas E, Lang PJ. Looking at facial expressions: dysphoria and facial EMG. Biological Psychology. 2002;60:79–90. doi: 10.1016/s0301-0511(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 28.Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. J Affect Disord. 1997;46:135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- 29.Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- 30.Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- 31.Derry PA, Kuiper NA. Schematic processing and self-reference in clinical depression. J Abnorm Psychol. 1981;90:286–297. doi: 10.1037//0021-843x.90.4.286. [DOI] [PubMed] [Google Scholar]
- 32.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 33.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryant FB, Veroff J. Types of Savoring: An Integrative View. In, Savoring: A New Model of Positive Experience. Lawrence Erlbaum Associates, Inc., Publishers; 2007. [Google Scholar]
- 35.Ruef AM, Levenson RW. Continuous measurement of emotion. In: Coan JA, Allen JJ, editors. The Handbook of Emotion Elicitation and Assessment. Oxford University Press; New York: 2007. pp. 286–297. [Google Scholar]
- 36.Rottenberg J, Gross JJ, Wilhelm FH, Najmi S, Gotlib IH. Crying threshold and intensity in major depressive disorder. J Abnorm Psychol. 2002;111:302–312. [PubMed] [Google Scholar]
- 37.McMakin DL, Santiago CD, Shirk SR. The Time Course of Positive and Negative Emotion in Dysphoria. Journal of Positive Psychology. 2009;4:182–192. doi: 10.1080/17439760802650600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz RM. Consider the simple screw: cognitive science, quality improvement, and psychotherapy. J Consult Clin Psychol. 1997;65:970–983. doi: 10.1037//0022-006x.65.6.970. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM IV Axis I Disorders Patient Edition. Vol. 20. Biometrics Research Department New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 40.Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. European Journal of Social Psychology. 1996;26:557–580. [Google Scholar]
- 41.Gerrards-Hesse A, Spies K, Hesse FW. Experimental inductions of emotional states and their effectiveness: a review. British journal of psychology. 1995;85:55–78. [Google Scholar]
- 42.Kenealy PM. The velten mood induction procedure: A methodological review. Motivation and Emotion. 1986;10:315–335. [Google Scholar]
- 43.Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: A comparison of block contrast and subject-specific emotion intensity regression approaches. NeuroImage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? NeuroImage. 2005;27:656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 46.Schneider W, Eschmann A, Zuccolotto A. E-Prime user’s guide. Psychology Software Tools, Inc.; Pittsburgh, PA: 2002. [Google Scholar]
- 47.Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 48.Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: preliminary findings. Biol Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 49.Brenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depressoin. Journal of Abnormal Psychology. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegle GJ, Moore P, Thase ME. Rumination: One construct, many features in healthy individuals, depressed individuals, and individuals with Lupus. Cognitive Therapy & Research. 2004;28:645–668. [Google Scholar]
- 51.Blaney P. Affect and memory: A review Psychological Bulletin. 1986;99:229–246. [PubMed] [Google Scholar]
- 52.Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. Journal of Clinical Child and Adolescent Psychology. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman GC, Joormann J, Johnson SL. Responses to Positive Affect: A Self-Report Measure of Rumination and Dampening. Cogn Ther Res. 2006 doi: 10.1007/s10608-006-9083-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMakin DL, Siegle GJ, Shirck SR. Positive Affect Stimulation and Sustainment (PASS) module for depressed mood: A preliminary test of treatment-related effects. Cognitive Therapy & Research. doi: 10.1007/s10608-010-9311-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuijpers P, Munoz RF, Clarke GN, Lewinsohn PM. Psychoeducational treatment and prevention of depression: the "Coping with Depression" course thirty years later. Clin Psychol Rev. 2009;29:449–458. doi: 10.1016/j.cpr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 56.King MF, Bruner GC. Social desirability bias: A neglected aspect of validity testing. Psychology & Marketing. 2000;17:79–103. [Google Scholar]
- 57.Fisher RJ, Katz JE. Social-desirability bias and the validity of self-reported values. Psychology & Marketing. 2000;17:105–120. [Google Scholar]
- 58.Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- 59.Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- 60.Van Vreeswijk MF, De Wilde EJ. Autobiographical memory specificity, psychopathology, depressed mood and the use of the Autobiographical Memory Test: a meta-analysis. Behav Res Ther. 2004;42:731–743. doi: 10.1016/S0005-7967(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 61.Seligman ME, Csikszentmihalyi M. Positive psychology. An introduction. Am Psychol. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. [DOI] [PubMed] [Google Scholar]
- 62.Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karwoski L, Garratt GM, Ilardi SS. On the Integration of Cognitive-Behavioral Therapy for Depression and Positive Psychology. Journal of Cognitive Psychotherapy. 2006;20:159–170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.