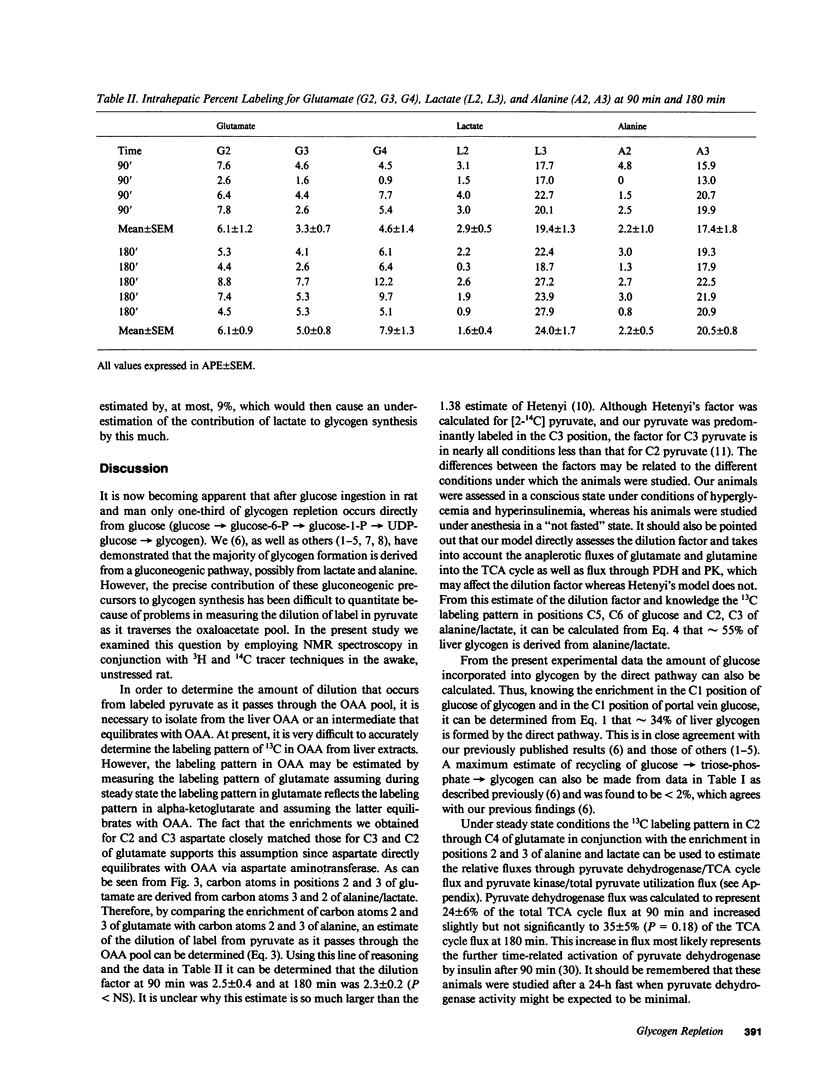
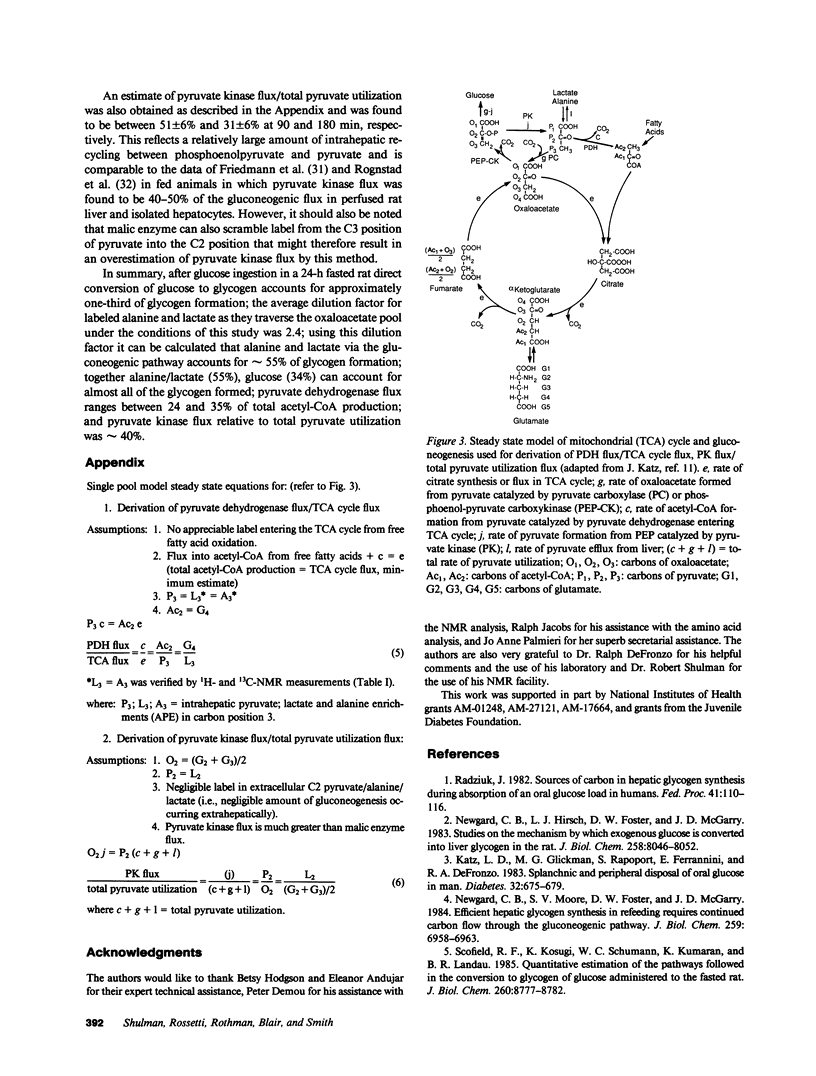
Abstract
In order to directly determine the amount of label exchange that occurs in the tricarboxylic cycle from labeled alanine and lactate after the ingestion of a glucose load [1-13C]glucose was administered by continuous intraduodenal infusion to awake catheterized rats to achieve steady state jugular venous glycemia (160 mg/dl) for 180 min. Liver was freeze-clamped at 90 and 180 min, and perchloric acid extracts of the liver were subjected to 13C and 1H nuclear magnetic resonance analysis. Dilution in the oxaloacetate pool was determined by comparing the intrahepatic 13C enrichments of C2, C3 positions of glutamate with the C2, C3 positions of alanine and lactate. In addition steady state flux equations were derived for calculation of relative fluxes through pyruvate dehydrogenase/TCA cycle flux and pyruvate kinase flux/total pyruvate utilization. After glucose ingestion in a 24-h fasted rat direct conversion of glucose was responsible for 34% of glycogen. The intrahepatic dilution factor for labeled pyruvate in the oxaloacetate pool was 2.4. Using this factor, alanine and lactate contributed approximately 55% to glycogen formation. Pyruvate dehydrogenase flux ranged between 24 and 35% of total acetyl-coenzyme A (CoA) production and pyruvate kinase flux relative to total pyruvate utilization was approximately 40%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP J. S., STEELE R., ALTSZULER N., DUNN A., BJERKNES C., DEBODO R. C. EFFECTS OF INSULIN ON LIVER GLYCOGEN SYNTHESIS AND BREAKDOWN IN THE DOG. Am J Physiol. 1965 Feb;208:307–316. doi: 10.1152/ajplegacy.1965.208.2.307. [DOI] [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK M., LORBER V. Conversion of 1-C14-Mannose and 1-C14-glucose to liver and muscle glycogen in the intact rat. J Biol Chem. 1952 Nov;199(1):1–8. [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Jeminet G., Williams R. J. Pulsed NMR methods for the observation and assignment of exchangeable hydrogens: application to bacitracin. FEBS Lett. 1974 Dec 1;49(1):115–119. doi: 10.1016/0014-5793(74)80645-9. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Blum J. J. Quantitative analysis of flux along the gluconeogenic, glycolytic and pentose phosphate pathways under reducing conditions in hepatocytes isolated from fed rats. Biochem J. 1983 Jun 15;212(3):585–598. doi: 10.1042/bj2120585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidmann B., Goodman E. H., Jr, Saunders H. L., Kostos V., Weinhouse S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys. 1971 Apr;143(2):566–578. doi: 10.1016/0003-9861(71)90241-4. [DOI] [PubMed] [Google Scholar]
- HERS H. G. The conversion of fructose-1-C14 and sorbitol-1-C14 to liver and muscle glycogen in the rat. J Biol Chem. 1955 May;214(1):373–381. [PubMed] [Google Scholar]
- Hetenyi G., Jr, Ferrarotto C. Correction for metabolic exchange in the calculation of the rate of gluconeogenesis in rats. Biochem Med. 1983 Jun;29(3):372–378. doi: 10.1016/0006-2944(83)90073-x. [DOI] [PubMed] [Google Scholar]
- Katz J. Determination of gluconeogenesis in vivo with 14C-labeled substrates. Am J Physiol. 1985 Apr;248(4 Pt 2):R391–R399. doi: 10.1152/ajpregu.1985.248.4.R391. [DOI] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGDON R. G., TAYLOR W. R. Intestinal absorption of glucose in the rat. Biochim Biophys Acta. 1956 Aug;21(2):384–385. doi: 10.1016/0006-3002(56)90027-0. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Chromatography of amino acids on sulfonated polystyrene resins. J Biol Chem. 1951 Oct;192(2):663–681. [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Radziuk J. Sources of carbon in hepatic glycogen synthesis during absorption of an oral glucose load in humans. Fed Proc. 1982 Jan;41(1):110–116. [PubMed] [Google Scholar]
- Randle P. J., Sugden P. H., Kerbey A. L., Radcliffe P. M., Hutson N. J. Regulation of pyruvate oxidation and the conservation of glucose. Biochem Soc Symp. 1978;(43):47–67. [PubMed] [Google Scholar]
- Reo N. V., Siegfried B. A., Ackerman J. J. Direct observation of glycogenesis and glucagon-stimulated glycogenolysis in the rat liver in vivo by high-field carbon-13 surface coil NMR. J Biol Chem. 1984 Nov 25;259(22):13664–13667. [PubMed] [Google Scholar]
- Rognstad R., Katz J. Role of pyruvate kinase in the regulation of gluconeogenesis from L-lactate. J Biol Chem. 1977 Mar 25;252(6):1831–1833. [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hetherington H. P., Shulman R. G. Homonuclear 1H double-resonance difference spectroscopy of the rat brain in vivo. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6330–6334. doi: 10.1073/pnas.81.20.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield R. F., Kosugi K., Schumann W. C., Kumaran K., Landau B. R. Quantitative estimation of the pathways followed in the conversion to glycogen of glucose administered to the fasted rat. J Biol Chem. 1985 Jul 25;260(15):8777–8782. [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Smith D., Johnson C. M., Blair J. B., Shulman R. G., DeFronzo R. A. Mechanism of liver glycogen repletion in vivo by nuclear magnetic resonance spectroscopy. J Clin Invest. 1985 Sep;76(3):1229–1236. doi: 10.1172/JCI112078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. E., Kelly P. J. Kinetic models of metabolism in intact cells, tissues, and organisms. Curr Top Cell Regul. 1981;19:103–158. doi: 10.1016/b978-0-12-152819-5.50021-x. [DOI] [PubMed] [Google Scholar]



