Abstract
Objective
To describe an electronic, telephone-delivered, suicide risk management protocol (SRMP) that is designed to guide research staff and safely triage study participants who are at risk for self-harm.
Methods
We tested the SRMP in the context of the NIH-funded randomized clinical trial “Bypassing the Blues” in which 302 patients who had undergone coronary artery bypass graft surgery (CABG) were screened for depression and assessed by telephone 2-weeks following hospital discharge and at 2-, 4-, and 8-month follow-up. We programmed the SRMP to assign different risk levels based on patients' answers from none to imminent with action items for research staff keyed to each of them. We describe frequency of suicidal ideation, SRMP use, and completion of specific steps in the SRMP management process over the 8-month follow-up period.
Results
Suicidal ideation was expressed by 74 (25%) of the 302 study participants in 139 (13%) of the 1,069 blinded telephone assessments performed by research staff. The SRMP was launched in 103 (10%) of assessments, and the suicidal risk level was classified as moderate or high in 10 (1%) of these assessments, thereby necessitating an immediate evaluation by a study psychiatrist. However, no hospitalizations, emergency room visits, or deaths ascribed to suicidal ideation were discovered during the study period.
Conclusion
The SRMP was successful in systematically and safely guiding research staff lacking specialty mental health training through the standardized risk assessment and triage of research participants at risk for self-harm.
Keywords: clinical trial, coronary artery bypass graft, depression, suicidal ideation, electronic research protocol
Introduction
Suicide ranks as the 10th leading cause of death in the USA. In 2011, there were nearly 38,000 deaths and approximately 600,000 emergency department visits and 250,000 hospitalizations related to suicide [(1-4)]. Suicidal ideation is even more ubiquitous, with prevalence ranging from 9-30% in the general populations and approaching 56% in primary care patients with depression [(5-10)]. Numerous studies and professional bodies have addressed the issue of suicide risks in psychiatric and primary care populations [(3, 7, 11-22)], but no gold standards to assess self-harm behavior have been established [(23)].
Studies involving participants with psychiatric conditions use stringent assessment batteries that probe for suicidal ideation, but only studies that explicitly focus on suicidal patients tend to assess the extent of suicidal plans and to follow at-risks patients [(24, 25)]. Institutional review boards and data safety monitoring boards generally require that research protocols for recruitment of any at-risk population explicitly stipulate how investigators plan to identify and safely manage patients expressing suicidal ideation [(26-29)]. However, a major challenge that is intrinsic to suicidal ideation assessment within the context of research trials is that investigators must often rely on their study staff to screen, detect, and sometimes even initially manage participants with suicidal ideation. Since many staff members lack advanced training and experience with addressing suicidality, they could benefit from a protocol that provides clear instructions about actions they should take to ensure the participant's safety while they simultaneously document pertinent information for immediate and subsequent analysis by study investigators, and monitoring bodies.
Because no validated standards and structured procedures have been developed to routinely assess and manage at-risk research cohorts [(30)], we developed a suicide risk management protocol (SRMP) for use in our clinical trials. Our SRMP is an electronic tool that is designed to systematically elicit pertinent information concerning suicidal ideation (e.g., risk factors, plans for self harm, access to means) and to triage potentially suicidal study participants to the appropriate level of care [(31)]. Many components of this tool are based on our prior experiences with protocols to assess suicidal ideation and to prevent self-harm behavior [(7, 11, 13, 25, 32)]. In this article, we describe the SRMP and discuss its use in a randomized clinical trial funded by the National Institutes of Health.
Methods
Overview
The University of Pittsburgh Institutional Review Board approved the SRMP as part of the protocol for the Bypassing the Blues (BtB) Trial, which examined the effectiveness of a telephone-delivered intervention for treating depression in patients who had undergone coronary artery bypass graft (CABG) surgery. The methods and main outcomes of this trial were previously published [(33, 34)].
Between 2004 and 2007, study nurse-recruiters described the trial to medically-stable post-CABG patients at 7 Pittsburgh-area hospitals and elicited their informed consent for participation. The nurse-recruiters used the 2-item Patient Health Questionnaire (PHQ) to screen patients for depression [(35)]. Research assessors (RA) telephoned the screen-positive participants 2 weeks after hospital discharge (baseline), and administered the 9-item PHQ (PHQ-9) to ascertain their depressive status [(36)]. Of those participants who had a score of ≥10 (signifying at least a moderate level of depressive distress), who met all other study eligibility criteria, and completed the remainder of the baseline assessment, 150 were randomly assigned to the study's collaborative care intervention and 152 to their physicians' usual care (UC) for depression [(34)].
The RAs, blinded to the participants' study assignment, telephoned the participants to collect sociodemographic characteristics and assessed their clinical and functional status at baseline and at 2-, 4-, and 8-month follow-up. These assessment tools included the Medical Outcomes Study Short Form (MOS SF-36) [(37)], MOS Social Support Scale (SSS) [(38)], and Hamilton Rating Scale for Depression (HRS-D) [(39)]. The RAs also documented data about participants' emergency room visits, hospitalizations, and causes of deaths during their study enrollment. All calls were digitally audiotaped for later review.
Study nurse care managers telephoned participants in the intervention group to deliver collaborative care. This care included proactively monitoring depressive symptoms with the PHQ-9, imparting self-management skills, and making treatment recommendations to participants' primary care physician or cardiologist, based upon consensus of the clinical team (internist, psychiatrist, psychologist) [(34)]. However, no medication or mental health treatment was provided to participants under study auspices.
Suicide Risk Management Protocol
Functions and Components of the Protocol
The SRMP is an electronic tool designed to objectively and consistently rate suicide related behavior with the validated Scale for Suicide Ideation (SSI) and to identify risk factors for self-harm behavior [(40, 41)] as used in our previous suicide protocols [(13, 25)]. In addition, the SRMP was to evaluate the potential for self-harm behavior on a probability spectrum [(42)], and provide information regarding the intensity and type of immediate and long-term follow-up care while not requiring that the study team member who elicited and recorded the information to personally decide upon or provide the needed care.
We enhanced the SRMP by the following features: automatic prompts when any suicidal ideation was endorsed; electronic documentation integrated into data collection procedures; skip patterns to streamline protocol administration; customized triage instructions; and readily available telephone access to the study's consulting psychiatrists.
This combination of functions and components distinguishes the SRMP from other suicide risk protocols.
Use of the Protocol
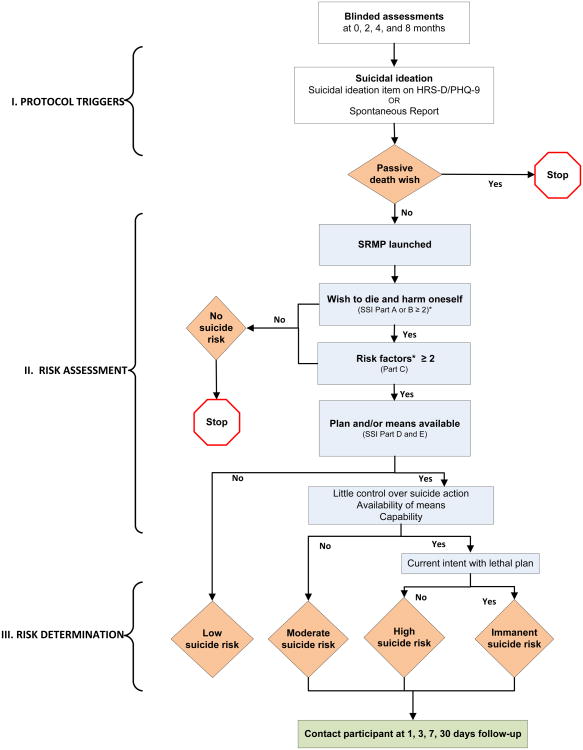
In the BtB Trial, the RAs had access to the electronic SRMP during all blinded telephone assessments of participants, and Figure 1 shows protocol triggers, risk assessment, and risk determination.
Figure 1.
Suicide risk management protocol (SRMP). Rhombuses show points at which risk evaluations occur, and rectangles show components of the SRMP.
*Eight risk factors for self-harm are assessed: previous attempt, moderate or greater level of depression, current alcohol abuse, current perceived stress, psychiatric comorbidity (depression, anxiety), lack of social support, poor impulse control, and access to weapons.
Abbreviations: HRS-D, Hamilton Rating Scale for Depression; PHQ-9, 9-item Patient Health Questionnaire; SSI, Scale for Suicide Ideation.
There were 3 ways that the electronic SRMP could be triggered and launched on the RA's computer screen. If the RA entered a participant's positive response to the suicidal question on the HRS-D (“This past week, have you been thinking about death or dying?”), or on the PHQ-9 (“Over the last 2 weeks, have you had thoughts that you would be better off dead or of hurting yourself in some way?”) the SRMP launched automatically. If a participant expressed suicidal thoughts spontaneously, the RA could trigger the SRMP manually. If the RA suspected that a suicide attempt was imminent at any time during the assessment, the RA could by a click of a red “emergency” button on top of the computer screen launch pop-up instructions for an immediate intervention.
In some cases, rather than expressing an active self-harm intention, a participant expressed a passive death wish (e.g., “I wish I would not wake up in the morning.”). This would terminate the SRMP.
Once the SRMP was launched on the computer screen, it called up the four-part 18-item SSI [(38)] to measure the intensity, pervasiveness, and characteristics of the patient's suicidal ideation and risk for self-harm. Each item was scored on a 3-point scale ranging from 0 to 2 (none to moderate/strong), and skip patterns were programmed to advance the assessment to the SRMP's subsequent part only if the participant' score was sufficiently high.
Parts A and B of the SSI included 5 items to assess the risk of self-harm, for a total score ranging from 0 to 10. If the participant's score was ≥2 in both Parts A and B, the RA asked about 8 established risk factors, including access to firearms [(31, 43)]. If the participant reported ≥2 risk factors, the RA then assessed the duration and acuteness of self-harm ideation (SSI Part D and E). This SRMP assessment required 5-30 minutes to administer, depending on the nature, extent, and rapidity of a participant's responses.
After this risk assessment, the SRMP automatically assigned a risk level for self-harm behavior, ranging from none to imminent, and outlined specific actions to be undertaken at each level. Regardless of the risk level, the RA instructed the participant to contact his or her physician or call 911 if the desire for self-harm intensified at any time; relinquish access to means of self-harm (e.g., firearms, pills); confide in a trusted person; seek 24/7 help from a crisis hotline; or pursue a combination of these strategies.
If the risk for self-harm was deemed moderate or high, the RA provided above instructions, asked the participant to enter a “contract for safety,” and scheduled a follow-up call within 2 hours [(44)]. During this interim, the RA would contact a study psychiatrist to review the SRMP responses and solicit a management recommendation. At the agreed upon time, the RA called back the participant to discussed the psychiatrist's recommendations. These recommendations could entail a change in dosage or type of antidepressant medication, a referral to a mental health specialist, or a direct telephone call from the study psychiatrist to elicit further clinical information. After the participant agreed to the treatment plan, the RA informed the study's clinical team and the treating physician and documented the instructions and actions taken.
In cases of imminent risk, the RA would keep the participant on the telephone line, dialed 911 on a second phone line, and follow the 911 respondent's instructions. Again, the RA immediately notified the study team and the treating physician, and recorded all instructions and actions taken in the electronic document.
The RA contacted each participant who experienced a moderate or higher suicide risk at 1, 3, 7, and 30 days following the incident to ascertain whether the suicidal ideation had diminished or resolved and whether treatment recommendations were being followed.
Initial and Continued Training for Use of the Protocol
BtB employed four RAs lacking both specialty mental health training and experience interacting with research participants at elevated risk for self-harm. This staffing strategy could produce inadequate assessments of a participant's suicidal risk, increase insecurity among assessment personnel, and then further stress the at-risk participant [(45)]. Therefore, our study psychologist conducted a half-day training program in which study staff learned about suicidal ideation and then engage in role-playing while they practiced administering the SRMP. The training session helped the staff learn methods for creating a non-threatening interpersonal environment when they were interviewing participants and for accurately assessing risks for self-harm. It also provided study staff with an opportunity to discuss personal fears and experiences associated with suicide [(46)].
During the BtB trial, the staff discussed periodically their experiences with the study psychologist. This gave the RAs a chance to discuss their personal emotional experience and receive feedback that was useful for subsequent such encounters with participants.
Data Collection and Analysis
We collected data on sociodemographic and clinical baseline characteristics of participants and on frequency of suicidal thinking, SRMP launches, and levels of self-harm risk over an 8-month period.
To compare results in the different treatment groups, we used t-tests for continuous variables and with Fisher's exact test or chi-square test for categorical variables. For all analyses, we used SAS software (SAS Institute, Inc., Cary NC) and considered a P value of <05 to be significant.
Results
During the 8-month follow-up period, RAs completed 1069 blinded telephone-delivered assessments of the 302 participants. During baseline calls participants voiced suicidal thinking more frequently (22%, 65/302), than during assessments at 2 months (11%; 29/257), 4 months (8%; 20/258) or 8 months (10%; 25/252) follow-up.
Thoughts of death or self-harm were expressed in a total of 139 of the 1069 assessments (13%) by 74 of the 302 participants (25%) (Table 1).
Table 1. Frequency of Factors Evaluated during Blinded Telephone-Delivered Assessments over 8 Months of Follow-up.
| Total Sample N (%) | Usual Care N (%) | Intervention N (%) | P | |
|---|---|---|---|---|
|
| ||||
| Total assessments | 1069 | 549 | 520 | |
|
| ||||
| Voiced suicidal ideation | 139 (13) | 87 (16) | 52 (10) | |
| Endorsed suicidal item* | 120 | 75 | 45 | 0.01 |
| Spontaneous ideation report | 19 | 12 | 7 | 0.36 |
|
| ||||
| Passive death wish | 36 (26) | 22 (25) | 14 (27) | 0.24 |
|
| ||||
| SRMP launches | 103 (74) | 65 (75) | 38 (73) | 0.02 |
|
| ||||
| Score on SRMP Parts A and B ≥ 2 | 10 (10)† | 6 (9) | 4 (11) | NA‡ |
|
| ||||
| Number of risk factors ≥ 2 | 10 (10) | 5 (8) | 5 (13) | NA‡ |
|
| ||||
| Completed all SRMP parts | 10 (10) | 5 (8) | 5 (13) | NA‡ |
|
| ||||
| Suicide risk | ||||
| Low | 2 (20) | 0 | 2 (40) | NA‡ |
| Moderate | 6 (60) | 4 (80) | 2 (40) | NA‡ |
| High | 2 (20) | 1 (20) | 1 (20) | NA‡ |
| Imminent | 0 | 0 | 0 | |
|
| ||||
| Study psychiatrist involved | 6 | 4 | 2 | NA‡ |
|
| ||||
| Total study participants | 302 | 152 | 150 | |
|
| ||||
| SRMP launches/participant | ||||
| Once | 54 (18) | 31 (20) | 23 (15) | 0.25 |
| Twice and more | 20 (7) | 14 (9) | 6 (4) | 0.07 |
Endorsed Item #9 on the 9-item Patient Health Questionnaire (PHQ-9) or #3 on Hamilton Rating Scale for Depression (HRS-D).
In one additional instance, the research assessor (RA) thought that the research participant, although screening negative at this point in the assessment, might still be at risk. The RA therefore overrode the SRMP and continued with the risk assessment, preferring to err on the side of safety. In 10 of these 11 preceding events, participants endorsed ≥ 2 risk factors.
Numbers are too small to calculate P values
Abbreviation: SRMP – suicide risk management protocol
Of the 139 expressions of thoughts of death or self-harm, 120 (86%) were identified via responses to the PHQ-9 or HRS-D question, and 19 (14%) were identified via spontaneous reporting of suicidal thoughts. Because 36 incidents (26%) involved feelings of passive death wish, the SRMP was terminated. The remaining 103 incidents (74%) launched the SRMP.
Of these 103 incidents, only 10 involved participants who had scores ≥2 in both Parts A and B, had ≥2 risk factors, and completed the last parts of the SRMP. This means that during the 8-month follow-up period, the RAs administered the entire SRMP only 10 times (1%; 10/1,069). In these 10 instances, the risk of self-harm was rated as low, moderate, high, and imminent in 2, 6, 2, and 0 cases, respectively. In 6 of these cases, a study psychiatrist formulated management recommendations. In the remaining cases, the psychiatrist was not contacted because thorough probing of participants by the RAs failed to reveal self-harm intent or because participants were identified as individuals who repeatedly reported suicidal ideation during follow-up and treatment recommendations had been initiated previously.
The SRMP was launched on a single occasion for 54 (18%) of the study participants, and on 2 or more occasions for 20 (7%) (Table 1).
Compared to those in the study's intervention arm, participants in the UC group were more likely to voice thoughts of death or self-harm in response to the PHQ-9 or HRS-D (75 vs. 45; P=0.01), but the groups did not differ in spontaneous expression of suicidal thoughts (Table 1). Consequently, launches of the SRMP were more likely in the UC group (65 vs. 38; p=0.02).
A comparison of the 74 participants for whom the SRMP was launched and the 228 participants for whom it was not launched showed no significant differences in sociodemographic characteristics (Table 2). However, those for whom the SRMP was launched, had significantly higher rates of co-morbid anxiety, higher levels of mood symptoms, and lower levels of social support and mental health-related quality of life.
Table 2. Baseline Characteristics of Participants Endorsing Suicidal Ideation.
| SRMP Launched (N=74) | SRMP Not Launched (N=228) | P | |
|---|---|---|---|
| Age, mean (SD) | 62.4 (11.0) | 64.4 (11.0) | .17 |
| Male, % | 54 | 60 | .36 |
| White, % | 88 | 92 | .32 |
| Married, % | 64 | 69 | .35 |
| SSS, mean (SD) | 65.7 (12.3) | 70.6 (10.1) | .002 |
| Depression treatment past 2 years, % | 27 | 13 | .005 |
| Comorbid anxiety, % | 54 | 21 | <.0001 |
| HRS-D score, mean (SD) | 20.4 (7.5) | 14.9 (6.3) | <.0001 |
| SF36 MCS score, mean (SD) | 36.6 (10.3) | 45.1 (11.1) | <.0001 |
| SF 36 PCS score, mean (SD) | 30.9 (6.5) | 30.6 (7.2) | .76 |
Abbreviations: HRS-D, Hamilton Rating Scale for Depression; SSS Medical Outcome Study Social Support Scale; SF-36 MSC, Medical Outcome Study Mental Health Component Scale; SF-36 PCS, Medical Outcome Study Physical Health Component Scale; SRMP, suicide risk management protocol
During the study period we did not uncover evidence of any deaths, psychiatric hospitalizations, or emergency room visits that could be ascribed to self-harm behavior.
Discussion
We developed a brief, stand-alone SRMP and tested it within the context of a randomized clinical trial with medically ill, depressed patients. Several important features of the SRMP allowed it to systematically guide study staff who lacked specialty mental health training through a standardized risk assessment and triage protocol for participants who expressed suicidal thinking.
First, integration of the SRMP into an electronic data entry system made the systematic assessment reliable and efficient. The SRMP was triggered automatically when participants endorsed any “thoughts of dying” on the HRS-D or PHQ-9 [(47)], and it could be launched manually upon spontaneous reports of suicidal thinking. The use of skip patterns readily and rapidly excluded low-risk participants from further assessment (84%). Therefore, using only 5 questions, we were able to promptly identify participants who posed a clinically meaningful risk for self-harm and warranted more detailed assessment.
Second, clear instructions based on the systematic, comprehensive risk assessment and its automatic evaluation facilitated prompt triage and elicited mental health specialist involvement in the rare cases identified as posing a moderate or higher risk.
Third, electronic integration of the documentation facilitated communication between study team members and participants' physicians. In addition, the study staff's brief training and ready access to a study psychiatrist for consultation regarding incidents of moderate to high risk of suicidality made the SRMP easy and comfortable to use by staff lacking mental health training.
Fourth, the SRMP facilitated serial follow-ups conducted by study staff over 30 days, a strategy found to decrease suicidal ideation or behavior [(13, 48-50)]. This combination of functions and components distinguishes our strategy from other suicide risk protocols [(30)].
Our finding that 13% of routine assessments revealed suicidal thoughts was similar to findings in other studies of cardiac populations [(51, 52)]. Suicidal thinking was voiced most frequently at the baseline assessment which could be attributed to the natural course of depressive symptoms [(53)], or to participants' feelings of “connectedness” developed through repeated contacts with study staff [(54)].
Numerous trials, including ours, have demonstrated the effectiveness of collaborative care in treating depression [(33, 55),(56)], which in turn can reduce suicidal ideation [(25, 53, 57)], and may explain our finding that the SRMP was launched less frequently in follow-up assessments of the intervention group than the UC group. In addition, the SRMP's integration into routine care management monitoring could also have resulted in additional clinical attention which then contributed to the lower incidence of suicidal ideation reported during the blinded assessments for participants in the intervention group. Indeed, suicidal thinking was expressed in only 4% (54/1,300) of the intervention care management calls versus 13% during blinded assessments, and all of these events were classified as low risk.
Since the PHQ-9 and HRS-D are relatively non-specific questionnaires to identify suicidal thinking, we found few participants at risk for self- harm (1%). Therefore, use of these measures to screen for self-harm risk could place an unnecessary burden on patients and clinical investigators [(52, 58)]. Since evaluating suicidal ideation is an integral part of suicide risk assessment, and suicidal ideation is a strong indication of a risk for future suicide acts [(59)], we believe that especially in vulnerable populations, routine screening and assessing of suicidal thoughts is a necessary safety measure. Thus, our pragmatic risk assessment protocol to follow-up on a positive answer to a question regarding suicidal thinking includes skip pattern to keep the assessment burden low others [(60)], and checklists to ensure our protocol is reliably and systematically administered.
Our study cohort consisted of participants with at least a moderate level of depressive symptoms at baseline (PHQ-9≥10), but who were not actively suicidal at recruitment, so that inpatient mental health services were necessary. While our risk management algorithm would work for more severely depressed participants, it might be less effective for study participants with severe mental disorders (e.g., schizophrenia) where mental health specialty care is needed.
We acknowledge that despite SRMP implementation, not all research participants at risk for suicidal behavior will be detected. Even when they are, studies have yet to address the overarching question of whether screening for suicidal ideation and risk factors for self-harm improves clinical outcomes or prevents suicidal behavior [(3, 61-63)]. We advised our staff to “contract for safety” with participants exhibiting any risk for suicidal behavior. While the effectiveness of these agreements remains controversial [(64)], we had no cases in which a participant with a contract engaged in suicidal behavior. Yet, we acknowledge that the follow-up period of 8 months after the first screening may have been too brief, as a large observational study found that endorsement of suicidal ideation results an increased risk of suicide attempt over a one year period of time [(65)].
Within the research setting, our staff had training and continued supervision by the study psychologist and psychiatrist. Although this may not be replicable in other study settings, an initial training and the availability of on-call psychiatrist should be sufficient for the implementation of the SRMP in other research studies.
Future Developments
The utility and efficiency of our SRMP could be enhanced without affecting its overall function by shortening it in places. For example, study staff and participants identified Parts C (risk factors), D, and E (suicide plans and means) as lengthy and repetitive. Moreover, recent studies suggest suicidal risk assessment can be reduced from 8 questions to 4 questions (previous attempt, depression severity, alcohol abuse, and access to weapons) [(9, 66)]. Indeed, since we commenced participant enrollment in 2004, the 4-item P4 has emerged as a reasonable alternative [(58)]. Still, its utility and acceptability versus a revised SRMP needs to be tested within the context of a new clinical trial. Additionally, since most risk factors are non-specific and non-modifiable, it may be best to be more inclusive in assessing contributing factors until carefully designed studies identify more patient-specific moderators of risk for suicidal behavior [(67)]. Furthermore, to identify potential buffers against suicidal behavior it may be helpful to systematically assess for protective factors [(9, 31, 49, 66, 68)].
Conclusions
Although several suicide assessment tools have recently been developed for routine clinical care [(58, 68-70)], our SRMP addresses a major gap in the availability of suicide risk management tools specifically designed for research settings. We have tested the SRMP in telephone encounters between research participants and study staff in two clinical trials [(33, 71)]. We used a stepped-care approach to maximize efficient use of resources and feasibility, as well as to minimize the respondents' burden [(33)]. The protocol is algorithm-based, with clear, succinct instructions to study staff for each triage step that have been found effective in suicide prevention studies [(72)]. Because answers during the SRMP are directly entered into an electronic data entry system, all information is readily accessible, thereby reducing the burden of additional questioning and facilitating expeditious triage and documentation. Research studies often exclude individuals with suicidal thoughts or do not probe for suicidal ideation [(52)], despite the fact that suicidal ideation is associated both with psychiatric and with somatic comorbidities [(6, 65, 73-76)]. We believe that the use of our SRMP, which is designed specifically for non-mental health specialty research settings, will encourage investigators to include participants expressing suicidal thoughts into their studies and thereby enhance the generalizability of their study findings [(21, 77)].
Highlights.
We describe an electronic suicide risk management protocol (SRMP) in context of clinical trial
SRMP guides research staff and safely triage study participants who are at risk for self-harm
25% of study participants expressed suicidal thoughts, and SRMP was launched in 10% of assessments
Suicidal risk level was classified as moderate to high in 1% of incidences of suicidal thinking
No hospitalizations, emergency room visits, or deaths ascribed to suicidal ideation were discovered
Acknowledgments
This work was supported by NIH Grant R01 HL70000 (Rollman) at http://www.bypassingtheblues.pitt.edu; NIH Grant P30 MH71944 (Reynolds); and by the UPMC Endowed Chair in Geriatric Psychiatry (Reynolds).
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT00091962 (http://clinicaltrials.gov/ct2/show/NCT00091962?term=rollman+cabg&rank=1)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy SL, Xiu J, Kochanek KD. Deaths: Priliminary Data for 2010. National Vital Statistics Reports. 2012;60(4) [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Suicide: Facts at a glance. [cited 2014 Nov 7];2012 Available from: http://www.cdc.gov/violenceprevention/pdf/Suicide-DataSheet-a.pdf.
- 3.Gaynes BN, West SL, Ford CA, Frame P, Klein J, Lohr KN. Screening for suicide risk in adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(10):822–35. doi: 10.7326/0003-4819-140-10-200405180-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ting SA, Sullivan AF, Boudreaux ED, Miller I, Camargo C. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. General Hospital Psychiatry. 2012;34:557–65. doi: 10.1016/j.genhosppsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschfeld RM, Russell JM. Assessment and treatment of suicidal patients. The New England journal of medicine. 1997;337(13):910–5. doi: 10.1056/NEJM199709253371307. Epub 1997/09/26. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin RD, Kroenke K, Hoven CW, Spitzer RL. Major depression, physical illness, and suicidal ideation in primary care. Psychosom Med. 2003;65(4):501–5. doi: 10.1097/01.psy.0000041544.14277.ec. Epub 2003/07/29. [DOI] [PubMed] [Google Scholar]
- 7.Schulberg HC, Lee PW, Bruce ML, Raue PJ, Lefever JJ, Williams JW, et al. Suicidal ideation and risk levels among primary care patients with uncomplicated depression. Annals of family medicine. 2005;3(6):523–28. doi: 10.1370/afm.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. The British journal of psychiatry: the journal of mental science. 2008;192(2):98–105. doi: 10.1192/bjp.bp.107.040113. Epub 2008/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gensichen J, Seising A, Konig J, Gerlach FM, Peterson JJ. Predictors of suicidal ideation in depressive primary care patients. Journal of Affective Disorders. 2010;125:124–27. doi: 10.1016/j.jad.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Howard LM, Flach C, Mehay A, Sharp D, Tylee A. The prevalence of suicidal ideation identified by the Edinburgh Postnatal Depression Scale in postpartum women in primary care: findings from the RESPOND trial. BMC pregnancy and childbirth. 2011;11:57. doi: 10.1186/1471-2393-11-57. Epub 2011/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexopoulos GS, Reynolds CF, Bruce ML, Katz IR, Raue PJ, Mulsant BH, et al. Reducing suicidal ideation and depression in older primary care patients:24-month outcomes of the PROSPECT study. American Journal of Psychiatry. 2009;166(8):882–90. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unützer J, Tang L, Oishi S, Katon W, Williams JW, Hunkeler E, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatric Soc. 2006;54:1550–56. doi: 10.1111/j.1532-5415.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 13.Schulberg HC, Bruce ML, Lee PW, Williams JW, Dietrich AJ. Preventing suicide in primary care patients: The primary care physician's role. General Hospital Psychiatry. 2004;26:337–45. doi: 10.1016/j.genhosppsych.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Juurlink DN, Herrmann N, Szalai JP, Kopp A, Redelmeier DA. Medical illness and the risk of suicide in the elderly. Archives of Internal Medicine. 2004;164:1179–84. doi: 10.1001/archinte.164.11.1179. [DOI] [PubMed] [Google Scholar]
- 15.Dombrowski AY, Szanto K, Siegle GJ, Wallace ML, Forman SD, Sahakian B, et al. Lethal forethought: Delayed reward discounting differentiates high- and low-lethality suicide attempts in old age. Biological Psychiatry. 2011;70:138–44. doi: 10.1016/j.biopsych.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szanto K, Dombrovski AY, Sahakian BJ, Mulsant BH, Houck PR, Reynolds CF, et al. Social emotion recognition, social functioning, and attempted suicide in late-life depression. American Journal of Geriatric Psychiatry. 2011:1–9. doi: 10.1097/JGP.0b013e31820eea0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlangsen A, Jeune B, Bille-Brahe U, Vaupel JW. Loss of partner and suicide risks among oldest old: A population-based register study. Age and Aging. 2004;33(4):378–83. doi: 10.1093/ageing/afh128. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. News Release. 2010. DSM-5 Proposed Revisions Includes New Risk Syndromes and Suicide Risk Assessment Tool Goals Include Earlier Identification and Treatment of Mental Disorders. 2/10/2010. [Google Scholar]
- 19.Erlangsen A, Nordentoft M, Conwell Y, Waern M, DeLeo D, Lindner R, et al. Key considerations for presenting suicide in older adults:Consensus opinions of an expert panel. Crisis. 2011;32(2):106–9. doi: 10.1027/0227-5910/a000053. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Preventing Suicide: Program Activities Guide. [cited 2014 Nov 7];2010 Available from: http://www.cdc.gov/violenceprevention/pub/preventingsuicide.html.
- 21.Institute of Medicine. Reducing suicide. A national imperative. [cited 2014 Nov 7];2002 Available from: http://www.iom.edu/reports/2002/reducing-suicide-a-national-imperative.aspx.
- 22.Joiner T, Kalafat J, Draper J, Stokes H, Knudsen M, Berman A, et al. Establishing standards for the assessment of suicide risk among callers to the National Suicide Prevention Lifeline. Suicide and Life-Threatening Behavior. 2007;37(3):353–65. doi: 10.1521/suli.2007.37.3.353. [DOI] [PubMed] [Google Scholar]
- 23.Bostwick JM, Rackley S. Addressing suicidality in primary care settings. Current psychiatry reports. 2012;14(4):353–9. doi: 10.1007/s11920-012-0286-7. Epub 2012/05/31. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003;60(1):82–91. doi: 10.1001/archpsyc.60.1.82. Epub 2003/01/07. [DOI] [PubMed] [Google Scholar]
- 25.Bruce ML, Ten Have T, Reynolds CF, Katz IR, Schulberg HC, Mulsant BH, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: A randomized controlled trial. JAMA: the Journal of the American Medical Association. 2004;291(9):1081–91. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 26.Oquendo MA, Stanley B, Ellis SP, Mann JJ. Protection of human subjects in intervention research for suicidal behavior. The American journal of psychiatry. 2005;161(9):1558–63. doi: 10.1176/appi.ajp.161.9.1558. [DOI] [PubMed] [Google Scholar]
- 27.Cukrowicz K, Smith P, Poindexter E. The effect of participating in suicide research: Does participating in a research protocol on suicide and psychiatric symptoms increase suicide ideation and attempts? Suicide and Life-Threatening Behavior. 2010;40(6):535–43. doi: 10.1521/suli.2010.40.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer RE, Salzman C, Youngstrom EA, Clayton PJ, Goodwin FK, Mann JJ, et al. Suicidality and risk of suicide - definition, drug safety concerns, and a necessary target for drug development: a consensus statement. Journal of Clinical Psychiatry. 2010;7:e1–e21. doi: 10.4088/JCP.10cs06070blu. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Health & Human Services. Code of Federal Regulations: Human Subjects Research (45 CFR 46 111) [cited 2014 Nov 7];2009 Available from: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.111.
- 30.Vannoy S, Whiteside U, Unützer J. Current practices of suicide risk management protocols in research. Crisis. 2010;31(1):7–11. doi: 10.1027/0227-5910/a000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beghi M, Rosenbaum JF. Risk factors for fatal and nonfatal repetition of suicide attempt: A critical appraisal. Current Opinion in Psychiatry. 2010;23:349–55. doi: 10.1097/YCO.0b013e32833ad783. [DOI] [PubMed] [Google Scholar]
- 32.Raue PJ, Alexopoulos GS, Bruce ML, Klimstra S, Mulsant BH, Gallo JJ, et al. The systematic assessment of depressed elderly primary care patients. International Journal of Geriatric Psychiatry. 2001;16:560–9. doi: 10.1002/gps.469. [DOI] [PubMed] [Google Scholar]
- 33.Rollman BL, Herbeck Belnap B, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone-delivered collaborative care for treating post-CABG depression: A randomized controlled trial. JAMA: the journal of the American Medical Association. 2009;302(19):2095–103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rollman BL, Herbeck Belnap B, LeMenager MS, Mazumdar S, Schulberg HC, Reynolds CF. The Bypassing the Blues treatment protocol: Stepped collaborative care for treating post-CABG depression. Psychosomatic Medicine. 2009;71(2):217–30. doi: 10.1097/PSY.0b013e3181970c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, W JB. The Patient Health Questionnaire-2. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL, W JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A User's Manual. 2nd. New England Medical Center; Boston, MA: 1994. [Google Scholar]
- 38.Blumenthal J, Burg M, Barefoot J, Williams R, Haney T, Zimet G. Social support, type A behavior, and coronary artery disease. Psychosomatic Medicine. 1987;49:331–40. doi: 10.1097/00006842-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes de Leon CF, et al. The Depression Interview and Structured Hamilton (DISH): Rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64(6):897–905. doi: 10.1097/01.psy.0000028826.64279.29. Epub 2002/12/04. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT, Kovacs M, Weissman A. Assessment of Suicidal Intention: The Scale for Suicide Ideation. Journal of consulting and clinical psychology. 1979;47(2):343–52. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Steer RA, Ranieri WF. Scale for suicide ideation: Psychometric properties of a self-report version. Journal of Clinical Psychology. 1988;44(4):499–505. doi: 10.1002/1097-4679(198807)44:4<499::aid-jclp2270440404>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Raue PJ, Brown EL, Meyers BS, Schulberg HC, Bruce ML. Does every allusion to possible suicide require the same response? A structured method for assessing and managing risk. The Journal of Family Practice. 2006;55(7):605–12. [PubMed] [Google Scholar]
- 43.Brown GK, Bruce ML, Pearson JL. High-risk management guidelines for elderly suicidal patients in primary care settings. International Journal of Geriatric Psychiatry. 2001;16(6):593–601. doi: 10.1002/gps.468. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs D, Brewer M. APA practice guidline provides recommendations for assessment and treating patients with suicidal behaviors. Psychiatric Annals. 2004;34(5):373–80. [Google Scholar]
- 45.Bisconer SW, Gross DM. Assessment of suicide risk in a psychiatric hopital. Professional Psychiatry: Research and Practice. 2007;38(2):143–49. [Google Scholar]
- 46.Takahashi C, Chida F, Nakamura H, Akasaka H, Yagi J, Koeda A, et al. The impact of inpatient suicide on psychiatric nurses and their need for support. BMC Psychiatry. 2011;11(38):1–8. doi: 10.1186/1471-244X-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uebelacker LA, German NM, Gaudiano BA, Miller IW. Patient Health Questionnaire Depression Scale as a suicide screening instrument in depressed primary care patients: A cross-sectional study. Prim Care Companion CNS Disord. 2011;13(1):e1–6. doi: 10.4088/PCC.10m01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaiva G, Walter M, Al Arab AS, Courtet P, Bellivier F, Demarty AL, et al. ALGOS: The development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry. 2011;11(1) doi: 10.1186/1471-244X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez HG. Risk factor model for suicide assessment and intervention. Professional Psychology: Research and Practice. 2001;32(4):351–58. [Google Scholar]
- 50.Conwell Y. Suicide prevention in later life: A glass half full, or half empty? The American journal of psychiatry. 2009;166:845–8. doi: 10.1176/appi.ajp.2009.09060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shemesh E, Annunziato RA, Rubinstein D, Sultan S, Malhotra J, Santra M, et al. Screening for depression and suicidality in patients with cardiovascular illnesses. The American Journal of Cardiology. 2009;104(9):1194–7. doi: 10.1016/j.amjcard.2009.06.033. Epub 2009/10/21. [DOI] [PubMed] [Google Scholar]
- 52.Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The PHQ-9 versus the PHQ-8--is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. Journal of Psychosomatic Research. 2012;73(3):163–8. doi: 10.1016/j.jpsychores.2012.06.001. Epub 2012/08/02. [DOI] [PubMed] [Google Scholar]
- 53.Gibbons RD, Brown H, Hur K, Davis JM, Mann JJ. Suicidal thoughts and behavior with antidepressant treatment. Arch Gen Psychiatry. 2012;69:580–7. doi: 10.1001/archgenpsychiatry.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motto JA, Bostrom AG. Letter to the Editor. Response to RG Liberman. Psychiatric Services. 2001;52:1254. [Google Scholar]
- 55.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression. Arch Intern Med. 2006;166(21):2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 56.Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. The Cochrane database of systematic reviews. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2. Epub 2012/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vannoy SD, Duberstein P, Cukrowicz K, Lin E, Fan MY, Unutzer J. The relationship between suicidal ideation and late-life depression. American Journal of Geriatric Psychiatry. 2007;15:1024–33. doi: 10.1097/JGP.0b013e3180cc2bf1. [DOI] [PubMed] [Google Scholar]
- 58.Dube P, Kroenke K, Bair MJ, Theobald D, Williams LS. The P4 Screener: Evaluation of a brief measure for assessing potential risk in 2 randomzied effectiveness trials of primary care and oncology patients. Prim Care Companion J Clin Psychiatry. 2010;12:e1–8. doi: 10.4088/PCC.10m00978blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenzel A, Berchick ER, Tenhave T, Halberstadt S, Brown GK, Beck AT. Predictors of suicide relative to other deaths in patients with suicide attempts and suicide ideation: a 30-year prospective study. J Affect Disord. 2011;132:375–82. doi: 10.1016/j.jad.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Pratt LA, Brody DJ. Implications of two-stage depression screening for identifying persons with thoughts of self-harm. Gen Hosp Psychiatry. 2014;36:119–23. doi: 10.1016/j.genhosppsych.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int'l J Psychiatry in Medicine. 2006;36:183–98. doi: 10.2190/3QXD-UR0H-K8FH-2CU8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oquendo MA, Currier D, Lamm JJ. Prospective studies of suicidal behavior in major depressive and bipolar disorders: What is the evidence for predictive risk factors? Acta Psychiatrica Scandinavica. 2006;114:151–58. doi: 10.1111/j.1600-0447.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 63.Yip PSF. Towards evidence-based suicide prevention programs. Crisis. 2011;32(3):117–20. doi: 10.1027/0227-5910/a000100. [DOI] [PubMed] [Google Scholar]
- 64.Edwards SJ, Sachmann MD. No-suicide contracts, no-suicide agreements, and no-suicide assurances. A study of the nature, utilizaiton, perceived effectiveness, and potential to cause harm. Crisis. 2010;31(6):290–302. doi: 10.1027/0227-5910/a000048. [DOI] [PubMed] [Google Scholar]
- 65.Simon GE, Rutter CM, Peterson D, Oliver M, Whiteside U, Operskalski B, et al. Does Response on the PHQ-9 Depression Questionnaire Predict Subsequent Suicide Attempt or Suicide Death? Psychiatr Serv. 2013 doi: 10.1176/appi.ps.201200587. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Judd F, Jackson H, Komiti A, Bell R, Fraser C. The profile of suicide: Changing or changeable? Soc Psychiat Psychiatr Epidemiol. 2012;47:1–9. doi: 10.1007/s00127-010-0306-z. [DOI] [PubMed] [Google Scholar]
- 67.Pearson JL, Stanley B, King CA, Fisher CB. Intervention research with persons at high risk for suicidality: Safety and ethical considerations. The Journal of clinical psychiatry. 2001;62(Suppl. 25):17–26. [PubMed] [Google Scholar]
- 68.McDowell AK, Lineberry TW, Bostwick JM. Practical suicide-risk management for the busy primary care physician. Mayo Clinic proceedings Mayo Clinic. 2011;86(8):792–800. doi: 10.4065/mcp.2011.0076. Epub 2011/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobs D. Suicide assessment five-step evaluation and triage (SAFE-T) [cited 2014 Nov 7];2009 Available from: http://www.sprc.org/libraryresources/sprc/listing?page=3&order=title&sort=asc.
- 70.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rollman BL, Mazumdar S, Herbeck Belnap B, Houck PR, Lenze E, Schulberg HC. Main outcomes from the Relax trial of telephone delivered collaborative care for panic and generalized anxiety disorder. J Gen Intern Med. 2010;25:S326. [Google Scholar]
- 72.Lapierre S, Erlangsen A, Waern M, DeLeo D, Oyama H, Scocco P, et al. A systematic review of elderly suicide prevention programs. Crisis. 2011;32(2):88–98. doi: 10.1027/0227-5910/a000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dutton GR, Bodell LP, Smith AR, Joiner TE. Examination of the relationship between obesity and suicidal ideation. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2012.224. Epub 2013/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodwin RD, Prescott MR, Tamburrino M, Calabrese JR, Liberzon I, Galea S. Cigarette smoking and subsequent risk of suicidal ideation among National Guard Soldiers. J Affect Disord. 2013;145(1):111–4. doi: 10.1016/j.jad.2012.05.003. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nadorff MR, Fiske A, Sperry JA, Petts R, Gregg JJ. Insomnia symptoms, nightmares, and suicidal ideation in older adults. The journals of gerontology Series B, Psychological sciences and social sciences. 2013;68(2):145–52. doi: 10.1093/geronb/gbs061. Epub 2012/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pompili M, Venturini P, Campi S, Seretti ME, Montebovi F, Lamis DA, et al. Do stroke patients have an increased risk of developing suicidal ideation or dying by suicide? An overview of the current literature. CNS neuroscience & therapeutics. 2012;18(9):711–21. doi: 10.1111/j.1755-5949.2012.00364.x. Epub 2012/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes CW. Objective assessment of suicide risk: Significant improvements in assessment, classification, and prediction. American Journal of Psychiatry. 2011;168:1233–4. doi: 10.1176/appi.ajp.2011.11091362. [DOI] [PubMed] [Google Scholar]