Abstract
Background
Hypothesizing that changing hospitals between diagnosis and definitive therapy (care transition) may delay timely treatment, our objective was to identify the association between care transitions and treatment delay ≥3 months in patients with muscle invasive bladder cancer (MIBC).
Methods
Using the National Cancer Database, all patients with stage ≥II urothelial carcinoma treated from 2003–2010 were identified. A care transition was defined as a change in hospital from diagnosis to definitive course of treatment (diagnosis to RC or start of neoadjuvant chemotherapy). Logistic regression models were used to test the association between care transition and treatment delay.
Results
Of 22,251 patients, 14.2% experienced a treatment delay of ≥3 months, and this proportion increased over time (13.5% [2003–2006] versus 14.8% [2007–2010], p=0.01). 19.4% of patients undergoing a care transition experienced a delay to definitive treatment compared to 10.7% of patients diagnosed and treated at the same hospital (p<0.001). The proportion of patients experiencing a care transition increased over the study period (37.4% [2003–2006] versus 42.3% [2007–2010], p<0.001). Following adjustment, patients were more likely to experience a treatment delay when undergoing a care transition (OR 2.0 [CI 1.8–2.2]).
Conclusions
Patients with MIBC who underwent a care transition were more likely to experience a treatment delay of ≥3 months. Strategies to expedite care transitions at the time of hospital referral may be a means to improve quality of care.
Keywords: bladder cancer, radical cystectomy, care transition, quality, treatment delay
Introduction
Patients often travel great distances for centralized surgical care1 and are at risk to be temporarily taken out of their usual healthcare system. Improvement of provider care coordination at the time of “care transitions”, loosely defined as movement between health care practitioners and care settings as needs change during the course of a chronic or acute illness,2 are a priority of contemporary health care reform. While the majority of current research and interventions have focused on the transition between inpatient and outpatient care for longitudinal management of chronic illnesses, the impact of interruptions in care coordination when patients change providers and hospitals for complex surgical care has been inadequately studied.
In Medicare beneficiaries, high surgical volume is associated with reduced mortality and improved outcomes for numerous cancers at the national level,3 and has been proposed as a surrogate for care quality. Regionalization of complex cancer operations and high-risk surgical procedures to high volume providers may provide one mechanism to curtail potentially avoidable expenses which has been championed by the media and advocacy organizations such as a the Leap Frog Group.4 However, widespread centralization of surgical care could result in a large proportion of patients changing hospitals and healthcare systems, exacerbating existing access disparities, and overwhelming the resources of tertiary and quaternary referral centers.5 Confirming these concerns, as caseloads at specialized centers have increased over the past decade, wait times for cancer treatment in 8 common solid organ malignancies have increased.6
Bladder cancer, the second most common genitourinary malignancy in the United States and one of the most expensive from diagnosis to death,7 represents a targetable area for quality improvement. Radical cystectomy (RC) with urinary diversion in conjunction with administration of neoadjuvant or adjuvant chemotherapy,8 is the gold standard for treatment of muscle invasive bladder cancer (MIBC) with 5-year survival rate of 62%–80%.9 The time from cancer diagnosis to treatment reflects availability of hospital resources and efficiency of overall care,6, 10 and greater than 3 months delay in the receipt of RC has been associated with decreased disease specific and overall survival.11, 12 Hypothesizing that care transitions at the time of referral for RC may delay timely treatment in patients with muscle invasive bladder cancer, our objective was to examine the association between care transitions and treatment delay ≥3 months using a large national tumor registry.
Patients and Methods
Cohort Definition
A program of the American College of Surgeons, Commission on Cancer, and American Cancer Society, the National Cancer Data Base (NCDB), a national cancer registry established in 1989, serves as a comprehensive clinical surveillance resource for cancer care in the United States. The NCDB compiles data from more than 1,500 commission-accredited cancer programs in the United States and Puerto Rico and captures approximately 70% of all newly diagnosed cancer cases.
All patients with urothelial carcinoma of the bladder were identified based on International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) site codes (8120, 8121, 8122, 8123, 8124, 8130, 8131, 8132). Our analytic cohort was restricted to adults 18 to 90 years of age undergoing RC for analytic stage II–IV disease during 2003–2010. Patients with non-urothelial histologic type, stage ≤ I or unknown stage, or second primary cancers were excluded. Patient socioeconomic characteristics were provided using census tract data. Co-morbidity burden was determined using the Charlson-Deyo classification and categorized as 0, 1, or ≥2.
Based on case volume and access to cancer-related services and specialists, the NCDB classifies hospitals as unknown, community (100–500 new cancer cases per year), comprehensive community (>500 cases per year), and teaching/research (academic) centers defined by either National Cancer Institute designation or medical school affiliation. Using previously described methods,3, 13, 14 annual RC hospital volume status (by tercile) was determined by dividing the total number of RC’s performed at each hospital over the study period by the number of years the hospital reported any bladder cancer cases. Distance between the patient’s residence and the hospital of record was defined by mile quintiles using zip code centroid location to determine residence and hospital latitude and longitude. The NCDB requires reporting of dates of initial cancer diagnosis (defined by the first clinical or histologic confirmation), as well as treatment initiation and treatment completion dates for the index surgery and neoadjuvant chemotherapy. Neoadjuvant chemotherapy was defined as systemic treatment received prior to RC using initiation of therapy date. Using these data time to treatment was defined as time from diagnosis to either index surgery or initiation of neoadjuvant chemotherapy to avoid penalizing hospitals in which pre-operative chemotherapy is preferentially administered.15 Treatment delay was defined as ≥3 months from diagnosis to treatment. While the facility reporting each case to the NCDB is the hospital in which a patient receives the first course of definitive therapy, the NCDB also requires reporting if diagnosis and definitive treatment were performed at differing hospitals. Using these data, a care transition was defined as a change in hospital from diagnosis to definitive treatment.6
Statistical Analyses
Trends in care transition and delay to definitive treatment were assessed during the period 2003–2010 using Chi-square tests. Patient demographic and clinical characteristics were compared between those experiencing a care transition and those who did not by using Chi-square tests. Adjusting for year, age, gender, race, ethnicity, volume, distance, payer group, Charlson-Deyo score, income, education, tumor grade, analytic stage, urban/rural status, and facility type and location, we examined the association between care transition and delay in receipt of definitive therapy using multivariable logistic regression. To account for clustering within hospitals, we calculated robust standard errors using Generalized Estimating Equations. All statistical analyses were performed using SAS software (version 9.3).
Results
We identified 22,251 patients (mean age 67.6 ± 10.7 years, 74.0% male) with stage ≥II urothelial carcinoma undergoing RC from 2003–2010. The majority of patients had Medicare (56.1%) or private insurance (33.4%), and were treated at comprehensive community (43.7%) or academic health centers (44%). On final pathology, 38% of patients had stage II disease, 32% had stage III disease, and 30% had stage IV disease. A minority (12%) of the sample underwent neoadjuvant chemotherapy, and this proportion increased from 6% to 23% over the length of the study period.
Forty percent of the cohort experienced a care transition between diagnosis and treatment hospital. Characteristics of patients with and without care transitions, differed with respect to gender, ethnicity, race, age, income, education, co-morbidity, tumor stage, tumor grade, facility location, and hospital volume status (all p values<0.001); there were no significant differences in payor group/insurance status (p=0.069) (Table 1). Following adjustment, male gender (OR 1.07 [CI 1.03–1.11]), African American race (OR 0.86 [CI 0.75–0.99]), Medicare (OR 0.83 [CI 0.69–0.99] or unknown/no (OR 0.75 [CI 0.64–0.88]) insurance status, and treatment at an academic center (OR 2.28 [CI 1.8–2.8]) were associated with care transitions (Table 2). The proportion of patients experiencing a care transition increased over the study period from 37.4% (2003–2006) to 42.3% (2007–2010), (p<0.001) (Figure 1) (+1.1% per year, p<0.001). Moreover, high volume hospitals more often treated patients who experienced a care transition (71.2%) compared to both low (18.2%) and intermediate (31.3%) volume hospitals respectively (p<0.001) (Figure 2).
Table 1.
Characteristics of the Sample
| Overall | No Care Transition | Care Transition | P-value | |
|---|---|---|---|---|
| 22,251 | 13,370 (60.0) | 8881 (40.0) | ||
| N (%) | Proportion | |||
| Age (years) | <0.001 | |||
| <50 | 1343 (6.0) | 752 (5.6) | 591 (6.7) | |
| ≥50 to 60 | 3943 (17.7) | 2296 (17.2) | 1647 (18.5) | |
| ≥61 to 70 | 6808 (30.6) | 4015 (30.0) | 2793 (31.4) | |
| ≥71 | 10,157 (45.6) | 6307 (47.2) | 3850 (43.4) | |
| Charlson Deyo Score | <0.001 | |||
| 0 | 15,925 (71.6) | 9381 (70.2) | 6544 (73.7) | |
| 1 | 4879 (21.9) | 3056 (22.9) | 1823 (20.5) | |
| ≥2 | 1447 (6.5) | 933 (6.9) | 514 (5.8) | |
| Gender | <0.001 | |||
| Male | 16,457 (74.0) | 9693 (72.5) | 6764 (76.2) | |
| Female | 5794 (26.0) | 3677 (27.5) | 2117 (23.8) | |
| Race | <0.001 | |||
| White | 20,297 (91.2) | 12,135 (90.8) | 8162 (91.9) | |
| African American | 1287 (5.8) | 859 (6.4) | 428 (4.8) | |
| Other | 667 (3.0) | 376 (2.8) | 291 (3.3) | |
| Hispanic | <0.001 | |||
| No | 19,778 (88.9) | 11,779 (88.1) | 7999 (90.1) | |
| Yes | 629 (2.8) | 338 (2.5) | 291 (3.3) | |
| Unknown | 1844 (8.3) | 1253 (9.4) | 591 (6.7) | |
| Median income | <0.001 | |||
| <$30K | 2719 (12.3) | 1579 (11.8) | 1140 (12.8) | |
| $30–34.9K | 4154 (18.7) | 569 (4.3) | 3585 (40.4) | |
| $35–45.9K | 6287 (28.3) | 911 (6.8) | 5376 (60.5) | |
| ≥$46K | 7894 (35.5) | 1086 (8.1) | 6808 (76.7) | |
| Unknown | 1197 (5.4) | 161 (1.2) | 1036 (11.7) | |
| * Education | <0.001 | |||
| ≥29% | 3308 (14.9) | 1896 (14.2) | 1412 (15.9) | |
| 20–28.9% | 5075 (22.7) | 2920 (21.8) | 2155 (24.3) | |
| 14–19.9% | 5461 (24.5) | 3248 (24.3) | 2213 (24.9) | |
| <14% | 7210 (32.4) | 4613 (34.5) | 2597 (29.2) | |
| Unknown | 1197 (5.4) | 693 (5.2) | 504 (5.7) | |
| Payor Group | 0.069 | |||
| Private/HMO | 7432 (33.4) | 4405 (3.3) | 3027 (34.1) | |
| Medicaid | 920 (4.1) | 572 (4.3) | 348 (3.9) | |
| Medicare | 12,479 (56.1) | 7565 (56.6) | 4914 (55.3) | |
| None/other | 1420 (6.4) | 828 (6.2) | 592 (6.7) | |
| Urban/Rural | <0.001 | |||
| Rural | 1768 (7.9) | 863 (6.5) | 905 (10.2) | |
| Suburban | 2754 (12.4) | 1364 (10.2) | 1390 (15.7) | |
| Small metropolitan | 6628 (29.8) | 4328 (32.4) | 2300 (25.9) | |
| Large metropolitan | 9766 (43.9) | 6026 (45.1) | 3740 (42.1) | |
| Unknown | 1335 (6.0) | 789 (5.9) | 546 (6.1) | |
| Tumor Stage | <0.001 | |||
| II | 8483 (38.0) | 5435 (40.7) | 3048 (34.3) | |
| III | 7112 (32.0) | 4251 (31.8) | 2861 (32.2) | |
| IV | 6656 (30.0) | 3684 (27.6) | 2972 (33.5) | |
| Tumor Grade | <0.001 | |||
| Low Grade (1/2) | 1012 (4.5) | 656 (4.9) | 356 (4.0) | |
| High Grade (3/4) | 20,051 (90.1) | 12,052 (90.1) | 7999 (90.1) | |
| Unknown | 1188 (5.4) | 662 (5.0) | 526 (5.9) | |
| Facility Location | <0.001 | |||
| Northeast | 1389 (6.2) | 1013 (7.6) | 376 (4.2) | |
| Atlantic | 3118 (14.0) | 1717 (12.8) | 1401 (15.8) | |
| Southeast | 4449 (20.0) | 2517 (18.8) | 1932 (21.8) | |
| Great Lakes | 4328 (19.5) | 2803 (21.0) | 1525 (17.2) | |
| South | 1573 (7.1) | 883 (6.6) | 690 (7.8) | |
| Midwest | 2202 (9.9) | 1377 (10.3) | 825 (9.3) | |
| West | 1677 (7.5) | 947 (7.1) | 730 (8.2) | |
| Mountain | 1042 (4.7) | 663 (5.0) | 379 (4.3) | |
| Pacific | 2473 (11.1) | 1450 (10.8) | 1023 (11.5) | |
| Year of Diagnosis | <0.001 | |||
| 2003 | 2759 (12.4) | 1755 (13.1) | 1004 (11.3) | |
| 2004 | 2690 (12.1) | 1718 (12.8) | 972 (10.9) | |
| 2005 | 2784 (12.5) | 1744 (13.0) | 1040 (11.7) | |
| 2006 | 2728 (12.3) | 1641 (12.3) | 1087 (12.2) | |
| 2007 | 2765 (12.4) | 1595 (11.9) | 1170 (13.2) | |
| 2008 | 2819 (12.7) | 1661 (12.4) | 1158 (13.0) | |
| 2009 | 2771 (12.5) | 1595 (11.9) | 1176 (13.2) | |
| 2010 | 2935 (13.2) | 1661 (12.4) | 1274 (14.3) | |
| Distance (Mile Quintiles) | <0.001 | |||
| 0–4.2 | 4259 (19.1) | 3632 (27.2) | 627 (7.1) | |
| 4.3–9.2 | 4266 (19.2) | 3256 (24.3) | 1010 (11.4) | |
| 9.3–18.8 | 4216 (18.9) | 2804 (21.0) | 1412 (15.9) | |
| 18.9–48.2 | 4248 (19.1) | 2085 (15.6) | 2163 (24.4) | |
| 48.3–3217 | 4248 (19.1) | 1004 (7.5) | 3244 (36.5) | |
| Missing | 1014 (4.6) | 589 (4.4) | 425 (4.8) | |
| Facility Type | <0.001 | |||
| Community | 2754 (12.4) | 2279 (17.0) | 475 (5.3) | |
| Comp community | 9715 (43.7) | 7356 (55.0) | 2359 (26.6) | |
| Academic | 9782 (44.0) | 3735 (27.9) | 6047 (68.1) | |
| Volume (Tertiles) | <0.001 | |||
| T1 | 7444 (33.5) | 6091 (45.6) | 1353 (15.2) | |
| T2 | 7561 (34.0) | 5194 (38.8) | 2367 (26.7) | |
| T3 | 7246 (32.6) | 2085 (15.6) | 5161 (58.1) | |
| Mean (median) Time to Definitive Treatment (days) | 66.8 (50.5) | 56 (42) | 77.6 (59) | |
| Community | 56.5 (42) | – | – | |
| Comprehensive Community | 57.9 (43) | – | – | |
| Academic | 73.2 (56) | – | – | |
| T1 | 59 (43) | – | – | |
| T2 | 59.2 (45) | – | – | |
| T3 | 75.7 (58) | – | – | |
Education reported as the % of adults in the patient’s zip code who did not receive a high school diploma
Table 2.
Characteristics associated with undergoing a care transition in receipt of definitive therapy (RC or neoadjuvant chemotherapy)
| Characteristic | aOR [CI] | P Value | Characteristic | aOR [CI] | P Value |
|---|---|---|---|---|---|
| Age (years) | Hospital Category | ||||
| ≤50 | 1.0 | 0.4851 | Comp community | 1.1 [0.92–1.3] | 0.2939 |
| 51 to 60 | 1.02 [0.89–1.17] | 0.8033 | Academic | 2.28 [1.8–2.8] | <0.001 |
| 61 to 70 | 0.97 [0.85–1.1] | 0.696 | Payor Group | ||
| ≥71 | 0.94 [0.81–1.1] | 0.399 | Private/HMO | 1.0 | |
| Gender | Medicaid | 0.83 [0.69–0.99] | 0.0376 | ||
| Male | 1.07 [1.03–1.11] | <0.001 | Medicare | 1.07 [0.99–1.16] | 0.1063 |
| Race | None/Unknown | 0.75[0.64–0.88] | <0.001 | ||
| White | 1.0 | Geographic Location | |||
| AA | 0.86 [0.75–0.99] | 0.0419 | Rural | 0.98 [0.86–1.1] | 0.8043 |
| Other | 1.09 [0.88–1.4] | 0.4302 | Suburban | 0.99 [0.89–1.1] | 0.7694 |
| Charlson-Deyo Score | Small metropolitan | 0.97 [0.89–1.1] | 0.541 | ||
| 0 | 1.0 | Large metropolitan | 1.08 [0.98–1.2] | 0.1401 | |
| 1 | 0.94 [0.87–1.0] | 0.0703 | Unknown | Ref | |
| ≥2 | 0.89 [0.79–1.0] | 0.072 | Facility Location | ||
| Analytic Stage | Northeast | 0.75 [0.59–0.96] | 0.0208 | ||
| II | 0.88 [0.84–0.92] | <0.001 | Atlantic | 1.1 [0.93–1.3] | 0.2406 |
| III | 1.03 [0.99–1.08] | 0.1837 | Southeast | 1.2 [1.0–1.4] | 0.0246 |
| IV | 1.0 | Great Lakes | 0.99 [0.85–1.2] | 0.9219 | |
| Tumor Grade | South | 0.89 [0.72–1.1] | 0.2743 | ||
| Low Grade (1/2) | 0.95 [0.86–1.1] | 0.3186 | Midwest | 0.82 [0.68–0.99] | 0.0428 |
| High Grade (3/4) | 0.88 [0.82–0.94] | <0.001 | West | 0.71 [0.59–0.87] | 0.0006 |
| Unknown | 1.0 | Mountain | 1.16 [0.93–1.4] | 0.195 | |
| Median Income | Pacific | 1.0 | |||
| <$30K | 1.0 | Year of Diagnosis | |||
| $30–34.9K | 1.16 [1.02–1.33] | 0.0245 | 2003 | 0.92 [0.84–1.0] | 0.0607 |
| $35–45.9K | 1.13 [0.98–1.3] | 0.0916 | 2004 | 0.95 [0.88–1.04] | 0.2575 |
| ≥$46K | 1.04 [0.88–1.2] | 0.6454 | 2005 | 0.93 [0.86–1.0] | 0.128 |
| Unknown | 1.14 [0.87–1.5] | 0.3475 | 2006 | 0.97 [0.90–1.1] | 0.5375 |
| Percent less than high school education | 2007 | 1.1 [1.0–1.19] | 0.0187 | ||
| ≥29% | 1.0 | 2008 | 1.0 [0.93–1.1] | 0.7451 | |
| 20–28.9% | 1.0 [0.90–1.1] | 0.9016 | 2009 | 1.1 [0.98–1.2] | 0.1629 |
| 14–19.9% | 1.1 [0.92–1.2] | 0.4304 | 2010 | 1.0 | |
| <14% | 1.0 [0.87–1.2] | 0.9886 | Volume (Tertiles) | ||
| Distance (Mile Quintiles) | T1 | 1.0 | |||
| Q1 | 1.0 | T2 | 1.47 [1.3–1.7] | <0.001 | |
| Q2 | 1.5 [1.3–1.7] | <0.001 | T3 | 3.9 [3.0–5.0] | <0.001 |
| Q3 | 2.1 [1.8–2.3] | <0.001 | |||
| Q4 | 3.7 [3.2–4.2] | <0.001 | |||
| Q5 | 6.9 [5.8–8.2] | <0.001 | |||
| Unknown | 2.4 [1.7–3.5] | <0.001 | |||
Controlling for age, gender, race, Charlson-Deyo score, analytic stage, tumor grade, hospital category, payor group, geographic location, median income, proportion with less than high school education, year, region
Figure 1.
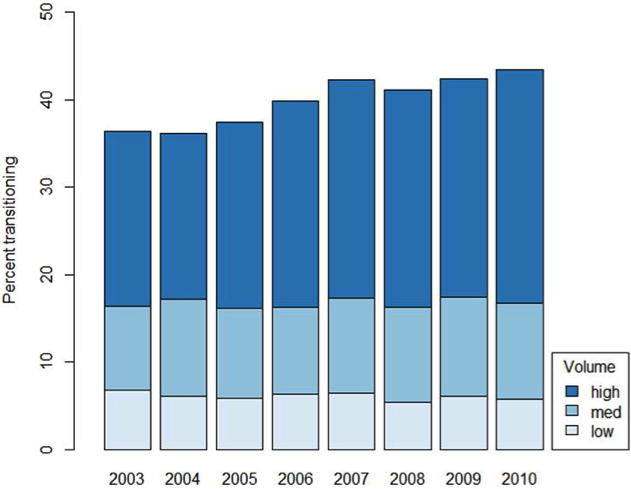
Proportion of patients undergoing a care transition to low, medium, and high volume hospitals for RC
Figure 2.
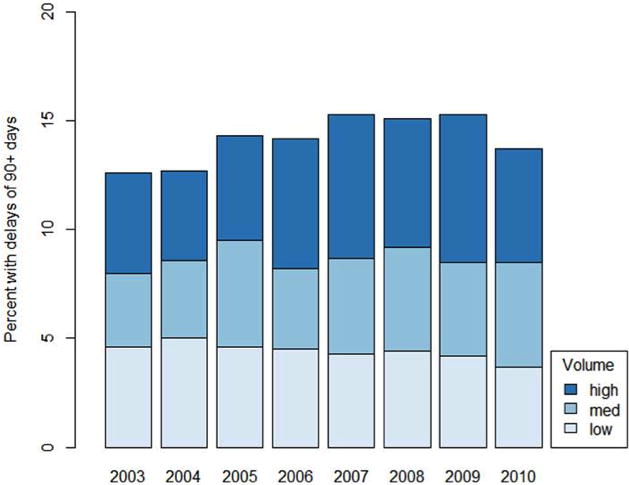
Proportion of patients experiencing a significant delay (≥90 days) in receipt of RC stratified by hospital volume
Of the cohort, 3156 patients (14.2%) experienced a treatment delay of ≥3 months. Evaluating unadjusted trends over time, the proportion of patients experiencing a treatment delay increased from 12.6% to 15.3% over the length of the study period (p=0.01) (Figure 1) (+0.3% per year, p=0.007). Delay to definitive treatment occurred more frequently for those experiencing a care transition (19.4%) compared to patients diagnosed and treated at the same hospital (10.7%) (p<0.001). Following adjustment for potential confounders, patients with care transitions were two times more likely to experience a treatment delay (OR 2.0 [95% CI 1.8–2.2]). Additional covariates associated with treatment delay included male gender (OR 1.1 [CI 1.1–1.2]), African American race (OR 1.5 [CI 1.3–1.7]), Hispanic ethnicity (OR 1.6 [CI 1.3–1.9]), insurance status (Medicaid OR 1.4 [CI 1.1–1.7], Medicare OR 1.2 [CI 1.08–1.34], unknown or no insurance OR 1.3 [CI 1.07–1.54]), treatment facility located in the Northeast (OR 1.52 [CI 1.25–1.85]) or Atlantic (OR 1.36 [CI 1.20–1.54]) regions, and Charlson-Deyo classification ≥2 (OR 1.3 [CI 1.08–1.45]). There was a trend towards treatment delay in patients treated at an academic center (OR 1.20 [CI 1.0–1.5]; p=0.0536) (Table 3).
Table 3.
Characteristics associated with delay ≥90 days in receipt of definitive therapy (RC or neoadjuvant chemotherapy)
| Characteristic | aOR [CI] | P Value | Characteristic | aOR [CI] | P Value |
|---|---|---|---|---|---|
| Care Transition | 2.0 [1.8–2.2] | <0.001 | Hospital Category | ||
| Age (years) | Community | 1.0 | |||
| ≤50 | 1.0 | 0.4851 | Comp community | 1.01 [0.86–1.18] | 0.9437 |
| 51 to 60 | 1.07 [0.89–1.29] | 0.4851 | Academic | 1.20 [1.00–1.45] | 0.0536 |
| 61 to 70 | 1.13 [0.95–1.35] | 0.1734 | Payor Group | ||
| ≥71 | 1.19 [1.00–1.43] | 0.0563 | Private/HMO | 1.0 | |
| Gender | Medicaid | 1.37 [1.13–1.66] | 0.0014 | ||
| Male | 1.11 [1.07–1.16] | <.0001 | Medicare | 1.20 [1.08–1.34] | 0.0012 |
| Race | None/Unknown | 1.28 [1.07–1.54] | 0.0077 | ||
| White | 1.0 | Geographic Location | |||
| AA | 1.48 [1.26–1.73] | <.0001 | Rural | 1.0 | |
| Other | 0.82 [0.63–1.06] | 0.1367 | Suburban | 1.00 [0.89–1.12] | 0.9773 |
| Charlson-Deyo Score | Small metropolitan | 1.01 [0.92–1.10] | 0.8749 | ||
| 0 | 1.0 | Large metropolitan | 0.99 [0.89–1.10] | 0.8256 | |
| 1 | 1.09 [1.00–1.19] | 0.0599 | Unknown | Ref | |
| ≥2 | 1.25 [1.08–1.45] | 0.0034 | Facility Location | ||
| Analytic Stage | Northeast | 1.52 [1.25–1.85] | <.0001 | ||
| II | 1.02 [0.96–1.07] | 0.5819 | Atlantic | 1.36 [1.20–1.54] | <.0001 |
| III | 1.02 [0.96–1.07] | 0.5298 | Southeast | 1.00 [0.88–1.12] | 0.948 |
| IV | 1.0 | Great Lakes | 0.86 [0.76–0.97] | 0.018 | |
| Tumor Grade | South | 0.72 [0.59–0.87] | 0.0008 | ||
| Low Grade (1/2) | 1.05 [0.93–1.20] | 0.4101 | Midwest | 0.80 [0.64–0.99] | 0.0432 |
| High Grade (3/4) | 0.95 [0.87–1.04] | 0.2595 | West | 0.71 [0.59–0.87] | 0.0006 |
| Unknown | 1.0 | Mountain | 1.01 [0.80–1.28] | 0.9195 | |
| Median Income | Pacific | 1.0 | |||
| <$30K | 1.0 | Year of Diagnosis | |||
| $30–34.9K | 0.93 [0.80–1.09] | 0.3853 | 2003 | 0.88 [0.78–0.99] | 0.0307 |
| $35–45.9K | 1.07 [0.92–1.24] | 0.3651 | 2004 | 0.91 [0.81–1.02] | 0.09 |
| ≥$46K | 1.11 [0.94–1.32] | 0.2225 | 2005 | 1.04 [0.95–1.15] | 0.4153 |
| Unknown | 0.81 [0.57–1.14] | 0.2204 | 2006 | 1.02 [0.92–1.12] | 0.7672 |
| Percent less than high school education | 2007 | 1.09 [0.96–1.23] | 0.1974 | ||
| ≥29% | 1.0 | 2008 | 1.07 [0.97–1.18] | 0.162 | |
| 20–28.9% | 0.94 [0.83–1.07] | 0.3262 | 2009 | 1.08 [0.97–1.21] | 0.1434 |
| 14–19.9% | 0.84 [0.73–0.96] | 0.0119 | 2010 | 1.0 | |
| <14% | 0.69 [0.59–0.82] | <.0001 | Volume (Tertiles) | ||
| Unknown | 1.0 [1.0–1.0] | 0.546 | T1 | 1.0 | |
| T2 | 0.86 [0.75–0.99] | 0.0369 | |||
| T3 | 0.87 [0.74–1.02] | 0.0939 | |||
Controlling for age, gender, race, Charlson-Deyo score, analytic stage, tumor grade, hospital category, payor group, geographic location, median income, proportion with less than high school education, year, region
Discussion
Timeliness of care is one of 6 domains of quality health care defined by the Institute of Medicine and is a proxy for unmeasured aspects of health care efficiency, resource utilization, and handling of excess case volume.6 In this large all payer sample of hospitals reporting to the NCDB, we observed an increase in the proportion of patients undergoing care transitions over the length of the study period even after accounting for the increased use of neoadjuvant chemotherapy. Our finding that patients with MIBC who transitioned between diagnosis and treatment hospitals were twice as likely to experience a treatment delay of ≥ 3 months (OR 2.0 [1.8–2.2]) may provide an actionable target for improving the quality of care coordination at the time of referral for complex oncologic care.
Oncologic treatment is most effective when delivered expeditiously, and recent data support that the timing of RC is critical in the treatment of MIBC.11, 16–18 Delays in definitive therapy have been associated with pathologic upstaging at the time of RC,17 and have been shown to adversely impact disease specific16, 18 and overall11, 12, 18 mortality. While a number of other characteristics of our cohort such as race, socioeconomic status, payor group, and burden of co-morbidity likely influence treatment delays, we feel that findings of this study highlight how interruptions in care coordination at the time of care transition between hospitals and providers may result in adverse consequences.
Over the past decade, an expanding body of evidence has demonstrated dramatic differences in short term (30 day) peri-operative mortality in patients treated at high versus low volume centers3. As a result, experts, policy makers, politicians, and the media champion regionalization of complex procedures to experienced centers.4 In the UK, the National Institute for Clinical Excellence (NICE), has established the precedent at the national level for regionalizing urologic cancer care (most notably radical prostatectomy and radical cystectomy) to teams who serve populations of one million or more and carry out a cumulative total of at least 50 procedures per year. Further, it is recommended that surgeons with very low procedural volumes (<5/year) transfer surgical care to more experienced high volume colleagues19. While similar procedure thresholds and mandates do not currently exist in the United States, recent studies performed at the state and national level have clearly demonstrated that the proportion of patients treated at high and very high volume centers has markedly increased over the past decade.20 This may be due, in part, to the increasing sub-specialization of providers, changing referral patterns, improved information dissemination, and changes in procedure reimbursement.21 Further, 47.1% of patients in our sample who received treatment at a high volume hospital underwent a care transition. While the short-term benefits of regionalization of surgical care are indisputable, an untoward effect of regionalization of care may be exacerbation of existing access disparities for the disadvantaged, increased travel burden for patients from rural areas, and overwhelming the existing workforce capacity of referrent centers.21, 22
Central to current healthcare reform initiatives is the improvement of coordination between providers, and patients who traverse hospitals and healthcare systems when regionalized for surgical care may represent a population at risk for adverse consequences. Evidence has accumulated that quality and patient safety are compromised during vulnerable care transition periods due to high rates of medication errors, incomplete or inaccurate information transfer, and lack of appropriate follow-up care.23 While emerging reimbursement mechanisms such as bundled payment programs, accountable care organizations, and patient centered medical homes that create “episodes of care” through incorporation of pre- and post- hospital care periods demonstrate the potential for improving healthcare delivery, there are concerns that “fractured care” due to poor coordination at the time of care transitions may result in over utilization or duplication of services, increased costs, and preventable hospital readmissions.24 Compared to patients with chronic illnesses who are commonly treated longitudinally within a single health system25, elderly patients undergoing complex cancer surgery may be more vulnerable to the negative consequences of care transitions, particularly during the immediate post operative period in which the provision of timely and effective outpatient care can help to reduce the risks of re-hospitalization.26
While the treatment delays demonstrated in our study may be a downstream result of disruptions in care coordination, there are mechanisms for improvement inherent to contemporary healthcare reform efforts.27 Implementation of Health Information Technology, the use of “virtual care teams” through remote consultation, and creation of dedicated screening centers have been proposed as means to reduce delays associated with care transitions.27 Patient navigator programs have demonstrated promise to improve communication between providers and patients as they negotiate handoffs during the pre-treatment transition period28. Similarly, several models for improving care transitions post hospitalization have been implemented and tested29. While to date these have focused on chronic health conditions in the elderly such as congestive heart failure and chronic obstructive pulmonary disease, efforts to improve medication reconciliation, scheduling of follow up and primary care visits, taking ownership of personal health information, and recognizing “red flags” that could indicate a worsening health condition are certainly applicable to surgical patients as well.30
Inherent limitations to use of the NCDB database include its retrospective nature, lack of specific information regarding patient and surgeon preferences, and incomplete mortality data. Given the constraints of data availability, we were unable to evaluate individual surgeon performance, and relied on hospital self-report for quality assurance. Further, any comparison of treatment groups derived from nonrandomized cohorts is prone to bias from unmeasured confounders; as a result, we were unable to completely adjust for selection bias. While an association between treatment delay of ≥90 days and poor survival outcomes has been reported in patients with MIBC,11, 12, 16, 17 we were unable to measure the impact of treatment delay on overall survival in our cohort as these data were available for only a small subset of patients (pre-2005). Regarding data reliability, information for patients who received some of their treatment at a hospital that does not report to the NCDB may be limited, and differentiating between non-primary treatment services between diagnosis and treatment hospitals is not possible. Finally, while the exposure of interest in our study is the transition between diagnosis and treatment hospital, lack of diagnosis hospital specific information only allows evaluation of hospital characteristics and discrimination of volume status of the definitive treatment hospital, which may be a threat to inference.
Conclusions
In the NCDB, patients with MIBC who experienced a care transition between diagnosis and treatment were more likely to experience a treatment delay of ≥3 months. Strategies to improve provider care coordination at the time of care transitions may be a means to improve quality of care. Implementation of proposed strategies to reduce the adverse consequences of care transitions, including incorporation of health information technology, nursing navigation, and post hospitalization interventions, should be prioritized for patients undergoing cancer surgery.
Acknowledgments
Source of Funding: This publication was supported by the National Cancer Institute at the National Institutes of Health (grant number: P30 CA006927, RU) and the Department of Defense, Physician Research Training Award (AK).
Footnotes
No financial disclosures.
References
- 1.Stitzenberg KB, Sigurdson ER, Egleston BL, et al. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27:4671. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock J, Mitchell J, Irby K, et al. Association between quality improvement for care transitions in communities and rehospitalizations among Medicare beneficiaries. JAMA. 2013;309:381. doi: 10.1001/jama.2012.216607. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135:569. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbeck BK, Dunn RL, Miller DC, et al. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol. 2007;25:91. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]
- 6.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253:779. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 7.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 8.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 9.Gore JL, Litwin MS, Lai J, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst. 2013;105:104. doi: 10.1093/jnci/djs506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CT, Madii R, Daignault S, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. 2006;175:1262. doi: 10.1016/S0022-5347(05)00644-0. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni GS, Urbach DR, Austin PC, et al. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. 2009;182:1318. doi: 10.1016/j.juro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Elting LS, Pettaway C, Bekele BN, et al. Correlation between annual volume of cystectomy, professional staffing, and outcomes: a statewide, population-based study. Cancer. 2005;104:975. doi: 10.1002/cncr.21273. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthony T, Corcoran EH, Daniel Canter Jeffrey J, Tomaszewski Justin E, Bekelman Simon P, Kim Robert G, Uzzo Alexander Kutikov, Marc C Smaldone. Variation in performance of candidate surgical quality measures for muscle invasive bladder cancer by hospital type. BJU Int. 2013 doi: 10.1111/bju.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez-Ortiz RF, Huang WC, Mick R, et al. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110. doi: 10.1016/S0022-5347(05)64047-5. [DOI] [PubMed] [Google Scholar]
- 17.Chang SS, Hassan JM, Cookson MS, et al. Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol. 2003;170:1085. doi: 10.1097/01.ju.0000086828.26001.ca. [DOI] [PubMed] [Google Scholar]
- 18.Gore JL, Lai J, Setodji CM, et al. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115:988. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Clinical Excellence. Guideline on Cancer Services. Improving Outcomes in Urological Cancers. 2002 http://www.nice.org.uk accessed December 12, 2012.
- 20.Cooperberg MR, Modak S, Konety BR. Trends in regionalization of inpatient care for urological malignancies, 1988 to 2002. J Urol. 2007;178:2103. doi: 10.1016/j.juro.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Stitzenberg KB, Sheldon GF. Progressive specialization within general surgery: adding to the complexity of workforce planning. J Am Coll Surg. 2005;201:925. doi: 10.1016/j.jamcollsurg.2005.06.253. [DOI] [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 23.Parry C, Mahoney E, Chalmers SA, et al. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317. doi: 10.1097/MLR.0b013e3181589bdc. [DOI] [PubMed] [Google Scholar]
- 24.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 25.Peikes D, Chen A, Schore J, et al. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 26.Vest JR, Gamm LD, Oxford BA, et al. Determinants of preventable readmissions in the United States: a systematic review. Implement Sci. 2010;5:88. doi: 10.1186/1748-5908-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aiello Bowles EJ, Tuzzio L, Wiese CJ, et al. Understanding high-quality cancer care: a summary of expert perspectives. Cancer. 2008;112:934. doi: 10.1002/cncr.23250. [DOI] [PubMed] [Google Scholar]
- 28.Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104:848. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- 29.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695. doi: 10.1001/jamainternmed.2013.171. [DOI] [PubMed] [Google Scholar]


