Abstract
Pontine respiratory nuclei provide synaptic input to medullary rhythmogenic circuits to shape and adapt the breathing pattern. An understanding of this statement depends on appreciating breathing as a behavior, rather than a stereotypic rhythm. In this review, we focus on the pontine-mediated inspiratory off-switch (IOS) associated with postinspiratory glottal constriction. Further, IOS is examined in the context of pontine regulation of glottal resistance in response to multimodal sensory inputs and higher commands, which in turn rules timing, duration, and patterning of respiratory airflow. In addition, network plasticity in respiratory control emerges during the development of the pons. Synaptic plasticity is required for dynamic and efficient modulation of the expiratory breathing pattern to cope with rapid changes from eupneic to adaptive breathing linked to exploratory (foraging and sniffing) and expulsive (vocalizing, coughing, sneezing, and retching) behaviors, as well as conveyance of basic emotions. The speed and complexity of changes in the breathing pattern of behaving animals implies that “learning to breathe” is necessary to adjust to changing internal and external states to maintain homeostasis and survival.
Preface
Breathing is a motor behavior, generated and controlled by the central nervous system. The neurons that generate breathing are arranged as a column in the lateral pons and ventrolateral (vl) medulla, and will be henceforth referred to as the lateral respiratory column (LRC). The LRC specifically extends from the caudal medulla to the rostral pons, aligning dorsally in the rostral pons (8). The LRC forms the principle anatomical substrate for the respiratory central pattern generator (rCPG) because anatomical integrity of the bilateral pontomedullary columns is essential to produce the basic respiratory rhythm, in other words eupnea (65, 245, 312, 322, 343, 395). By the definition proposed by Grillner and Wallen (147), the terminology CPG applies to the neuronal networks involved in appropriating a specific motor function. This would also include breathing. Therefore, it is important to note that the rCPG may involve additional nuclei apart from the LRC, particularly the nucleus tractus solitarius (NTS), which forms the dorsal respiratory group (235, 394) and the brainstem raphé neurons (262, 273, 274). In the following section, we discuss the complexity of the respiratory motor pattern generated by the rCPG as it is expressed by various cranial and spinal motor nerves that coordinate ventilation via pump muscles and adjust airflow resistance via valvular muscles. Following a general introduction in Section “Pontine Control of Breathing: An Overview,” the anatomical connectivity and physiological significance of the pontine nuclei of the LRC will form the core of this article.
Motor Activity Generating the Breathing Pattern Controls Airway Pressure and Resistance
Rhythmic motor behaviors such as walking, running, or flying are mediated by the contraction of antagonistic skeletal muscle pairs (e.g., extensor and flexor muscles) driven by CPGs (147, 367). Understanding the kinesiology of the movements has lead to identifying features necessary in the control of these rhythmic behaviors. For instance, the fundamental concept of “half-center oscillators,” which has evolved into CPGs, was derived from the finding that antagonistic muscle contract and relax in opposing phases independently of rhythmic afferent input (147, 367). The essential feature of half-centers is reciprocal inhibition interconnecting the two half-centers whose constituent neurons are not intrinsically rhythmic. This approach to understand motor rhythm, based on kinesiology, is common to both locomotion and respiration. The breathing pattern implies further complexity in that in addition to the opposing directions of airflow (inhalation and exhalation), both active and passive forces act on airflow.
In contrast to locomotion, the eupneic motor pattern of breathing is defined by not two but three distinct phases: inspiration (I), postinspiration (post-I), or passive expiration (termed stage 1 expiration, E1), and stage 2 (E2, late or active expiration; see Fig. 1). To understand the apparent complexity of the respiratory motor pattern, one has to consider that the rCPG controls two functionally distinct groups of muscles: those controlling thoracic pressure and those controlling airway resistance. The motoneurons for the primary pump muscles are located in the spinal cord (186, 260), while those for the muscles controlling airflow (via upper airway caliber) are located in the brainstem (26). The coordinated activities of both cranial (cranial nerves: V, IX, X, and XII) and spinal (cervical and thoracic) motor pools define the breathing pattern (102, 312, 343, 344, 356). The pattern associated with eupnea, which is “normal” breathing at rest is commonly marked by only the phrenic nerve activity that defines inspiration. However, the generation of a coordinated cranial and spinal motor output integral to eupnea depends on the integrity of the entire LRC in mammals, including man (34,102,245,290,310,312,343,344,355,356). Therefore, the definition and investigation of eupnea in anatomically reduced preparations in vitro presents significant conceptual barriers (312, 343, 344).
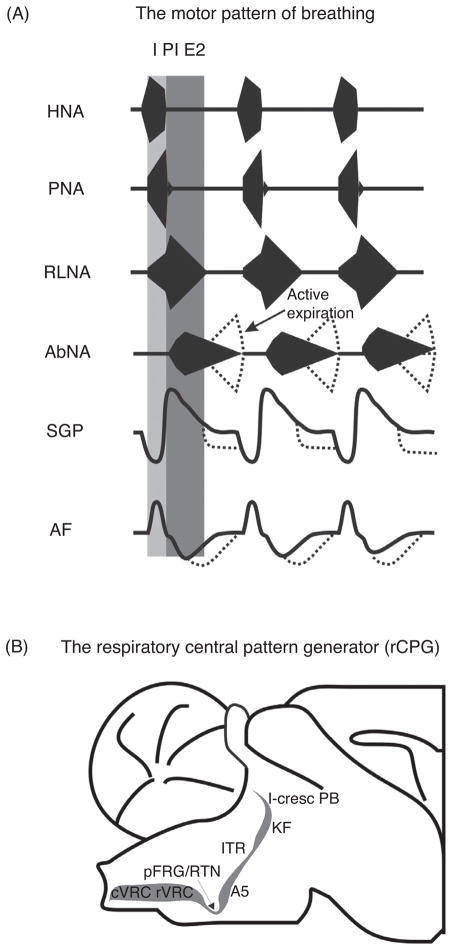
Figure 1.
(A) Respiratory motor outputs in relation to the three major phases of the respiratory cycle (I, inspiration, post-I, postinspiration, E2, late expiration). HNA, hypoglossal nerve activity; PNA, phrenic nerve activity; RLNA, recurrent laryngeal nerve activity; AbNA, abdominal nerve activity (lumbar segment 1). The lower two traces illustrate the dynamic changes in subglottal pressure (SGP) related to changes in upper airway resistance and airflow (AF). (B) Sagittal section of the anatomical organization of the lateral respiratory column (LRC) which includes the respiratory central pattern generator (rCPG) in the pontomedullary brainstem. Abbreviations: cVRC, caudal ventral respiratory column; rVRC, rostral ventral respiratory column; RTN/pFRG, retro-trapezoidal nucleus/parafacial respiratory group; A5, noradrenaline-containing neurons in the ventrolateral pons; ITR, intertrigeminal region; KF, Kölliker Fuse nucleus; l-cresc PB, lateral crescent nucleus of the parabrachial complex.
Spinal motor outputs that drive respiratory pump muscles are active only during a specific phase of the respiratory cycle (see Fig. 1). The exception is, “postinspiratory after discharge” in the crural diaphragm (370). Phrenic nerve activity in early expiration controls the relaxation of the diaphragm and helps slowing of airflow during early expiration (105). Postinspiratory activity of the diaphragm has been identified in humans, cats, dogs, rabbits, and recently in rodents (76, 105, 174, 282, 370). In contrast to spinal motor neurons, respiratory motor outputs from the cranial nerves often show biphasic discharge patterns. For example, the recurrent laryngeal nerve (RLN) is active during inspiration and post-I because it is a mixed motor nerve whose different branches innervate either ab- or ad-ductors of the larynx (see Fig. 1). Inspiratory activity in the RLN activates the laryngeal abductors (e.g., posterior cricoarytenoid) decreasing airway resistance during inspiration, while postinspiratory motor discharge activates laryngeal adductor muscles (e.g., thyroarytenoid muscle) (26,102,159). Laryngeal adductor activation narrows the upper airway at the glottis which then reduces expiratory air-flow and counteracts the intrinsic recoil forces exerted by the expanded lung (26, 102). As expiration progresses glottal constriction, via laryngeal adductors, maintains volume and prevents atelectasis (i.e., alveolar collapse). During eupnea, the mechanical acts of inspiration and expiration are controlled by the inspiratory pump (e.g., diaphragm) and laryngeal valvular muscles. Thus, under these conditions expiration is considered to be passive because exhalation is not driven by the activity of expiratory pump muscles. In contrast, active expiration emerges during exercise, during expulsive acts such coughing and sneezing in response to hypoxia and hypercapnia. Conditions of “forced breathing” involve the recruitment and contraction of abdominal and thoracic expiratory muscle groups. In active expiration, air is exhaled in a shorter interval (compared to eupnea), and/or below functional residual capacity of the lung often in combination with an increased tidal volume (see abdominal nerve, Fig. 1). Thus, activity in the expiratory muscles occurs under the conditions of high chemical drive for breathing (3). Depending on the species, drive to abdominal expiratory muscles is reported to be present in eupnea (Fig. 1); however this drive is most likely related to posture and maintenance of intra-abdominal pressure, and hence represents nonrespiratory input to spinal expiratory motor pools (186).
Pontine Control of Breathing: An Overview
The respiratory nuclei at the rostral end of the LRC in the dorsolateral pons are the Kölliker-Fuse nucleus (KF) and adjacent subnuclei of the parabrachial (PB) complex [see Section “The Parabrachial Complex and Kölliker-Fuse Nuclei of the Dorsolateral Pons”]. The second area is the intertrigeminal region (ITR, also termed peritrigeminal region) of the central pons. The connectivity and function of this area that is different to the KF is yet to be explored. Thus, the ITR is only discussed briefly in Section “Intertrigeminal Region of the Mediolateral Pons” of the article. The third area is the noradrenergic A5 (see Section “The A5 Cell Group in the Ventrolateral Pons”) of the caudal pons. Anatomically the most caudal pontine aspect of the LRC is the retrotrapezoidal nucleus at the pontomedullary transition. The current literature refers to a RTN/parafacial respiratory group (RTN/pFRG) that includes rhythmogenic neurons just ventral to facial motor neurons identified in neonates (278). The RTN/pFRG is hypothesized as the main area for generation of expiratory oscillations during eupnea (117, 189, 250) and is shown to have a very prominent role in central chemosensitivity (153, 155, 269, 280). The role of the RTN/pFRG in rhythmogenic mechanism and chemoreception is beyond the scope of this article and hence will be not discussed further. In addition to the nuclei of LRC, the pons also includes other brain nuclei such as the locus coeruleus (11,24,63,224,283,284), pedunculopontine tegmental nuclei which can contribute to adaptation of breathing. Detailing the physiology and anatomical connectivity of these nuclei including the Barrington’s nucleus (pontine micturition center) would require a separate publication and are reviewed elsewhere (11, 24, 63, 177, 211, 213, 224, 283, 284).
The Parabrachial Complex and Kölliker-Fuse Nuclei of the Dorsolateral Pons
The KF nuclei including adjacent areas of the PB complex are certainly the most investigated pontine part of the LRC. The KF nuclei and the following specific PB nuclei such as the lateral-crescent, extreme-lateral nucleus, external-medial subnuclei of the lateral, and medial PB are considered to be the main respiratory areas of the PB complex. For the reason of simplification, we will refer to the KF-area in the context of respiratory control (Fig. 2). Moreover, the role of KF-area nuclei could vary with species. For instance, the medial PB is seen as a vital component of the pontine “pneumotaxic center” in the cat (34, 66, 395), whereas in rat the medial PB receives major projections from the gustatory NTS (167) and is known for its important role in taste processing and taste aversion (88). The physiologically diverse functions of the KF-area in respiratory control are supported by a complex anatomical framework.
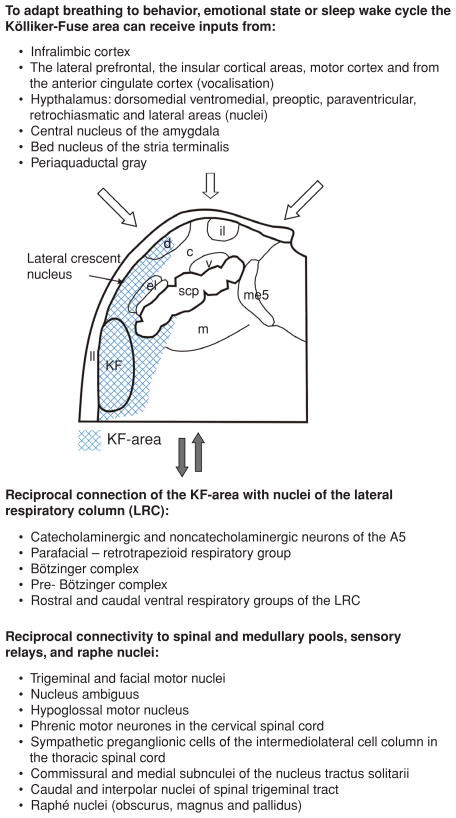
Figure 2.
The efferent and afferent connections of the primary respiratory nuclei (shaded): Kölliker-Fuse nucleus and adjacent subnuclei of the parabrachial complex of the dorsolateral pons (KF-area). The data supporting the KF-area projecting to the parafacial nucleus are unpublished and supplied by Bellintani, Herbert, and Dutschmann. Abbreviations: c, central subnucleus of the PB; d, dorsal subnucleus of the PB; el, external lateral subnucleus of the PB; il, internal lateral subnucleus of the PB; ll, lateral lemniscus; m, medial subnucleus of the PB; me5, mesencephalic trigeminal nucleus; scp, superior cerebellar peduncle; v, ventral subnucleus of the PB.
The hallmark respiratory function of the KF-area is the regulation of the inspiratory-expiratory (IE) phase transition and the dynamic control of upper airway patency during the respiratory cycle. The latter particularly involves the control of expiratory airflow patterns. The continuous motor activity that ventilates also integrates other behaviors that use chest wall respiratory muscle and the larynx for vocalizing, sniffing, and coughing. These behaviors are “respiratory” in that airflow is controlled by changing lung volume to alter elastic recoil of the lung or by changing airway resistance in the larynx. Nonrespiratory behaviors such as swallowing or emesis also need to be coordinated with the respiratory motor pattern because both ingestion/emesis and breathing share a common route through the oropharyngeal cavity. Timing mechanisms of respiratory phase resetting, synaptic plasticity, and memory in the KF are critical to behavioral adaptation of respiratory airflow.
Synaptic plasticity is a general property of the nervous system. Brainstem structures express plasticity, for example in response to intermittent hypoxic insults during various stages of development causing long-term facilitation of respiratory motor output. Thus, synaptic plasticity in chemosensory relays such as the NTS or at level of medullary or spinal respiratory motor neuron populations is well investigated (20, 225, 254, 299). However, plasticity in the KF-area circuits reflects plasticity of premotor circuits required for adaptive breathing in response to behavior and emotion. Synaptic plasticity in the pons emerges at time points when the behavioral and emotional repertoires of mammals develop and breathing becomes a behavior. The important role of the pons and also the midbrain in coordinative functions such as feeding, chewing, swallowing, vocalizing, and breathing, is conserved phylogenetically. In amphibians and reptiles, the lower vertebrates, the dorsal midbrain-pons transitional zone plays a key role in controlling breathing pattern (252) as well bird song as an example for a learned motor pattern (330).
The developmental changes observed in the pons and in other brainstem/spinal cord structures related to breathing are linked to genetic and epigenetic regulation of the formation and function of brain circuits. The combination of genetics and anatomy has helped identifying a variety of neurochemical markers for the specific nuclei of the LRC. The pre-Bötzinger complex, the purported locus for inspiratory rhythm generation, expresses the homeobox gene, Dbx1, as a marker for rhythmogenic neurons (49, 145). The primary expiratory oscillator is proposed to be located in RTN/pFRG and is characterized by the expression of the developmental transcription factor Phox2B. In turn, mutation of the Phox2B is linked to congenital central hypoventilation syndrome (CCHS) (Ondine’s curse) curse (9, 10). The Phox2B expressing neurons of the RTN/pFRG have significance for controlling abdominal expiratory activity and central chemosensitivity (1, 90, 246, 280, 366). Some of the Phox2b expressing neurons in the neonatal RTN/pFRG may migrate to the A5-area that also expresses Phox2b (for details see Section “The A5 Cell Group in the Ventrolateral Pons”) in the adult stage (366), and thus can serve as a genetic marker for caudal pontine LRC. For the other pontine nuclei of the LRC, preliminary data suggest that the developmental transcription factor FoxP2 could be an early developmental marker for the KF-area (144). More recently, the transcription factor Runx1 was suggested to be a selective marker for lateral PB nuclei (411). The potential significances of these transcription factors are further discussed later in the article. Finally, the genetic basis neurodevelopmental and neurodegenerative disease causing breathing disorders that occur after birth or later in children, adolescents, or adults receives special attention. Therefore, the recent evidence implication a role of the pontine respiratory nuclei in neurogenic breathing disorders is also discussed in the article. Finally, KF-area functions related to chemosensory modulation of the breathing pattern and for a variety of nonrespiratory functions are introduced. In particular, the lateral PB nuclei are associated with functions such as blood pressure and thermoregulation that affect breathing as well. The links of the PB nuclei to cardio respiratory coupling is also discussed. Besides its importance in homeostasis, a major role of the lateral PB nuclei relates to pain processing via descending regulation of the sensory gain and ascending projections, which relay pain information to the limbic system. The PB nucleus is also a pivotal center for the regulation of sodium balance (sodium appetite) and central taste pathway. However, these functions are only summarized in a few words at the end of the section.
Afferent and efferent connectivity of the KF-area
The heterogeneity of physiological functions mediated by the KF-area is reflected in its anatomy with topographical organization of specific afferent and efferent connections. Reciprocal tract tracing studies demonstrate that nuclei of the KF-area are major ascending targets of the NTS that receives inputs from various visceral sensory afferents via the vagal and glossopharyngeal nerves (167, 234, 309). The earlier studies of Norgren and Leonard (272) identified gustatory and visceral sensory relays in NTS that then targeted functionally identified PB subnuclei. Later studies of Saper and Loewy (124, 329), underpinned the subnuclear organization of the PB and KF nuclei in rat showing the topographical organization of its afferent and efferent connectivity. Several follow-up tract-tracing studies completed the picture of the complex topography of connections of the PB and KF nuclei with autonomic and limbic brain areas along the neuraxis (167, 257, 258).
The main afferent and efferent connections of the respiratory KF-area are schematically summarized in Figure 2. The KF nuclei have dense descending projections innervating the A5-area, the entire LRC in the medulla oblongata, the NTS, and the nucleus ambiguus (89, 107, 167). The distribution and density of KF descending fibers and terminals in the target nuclei within the caudal pons and medulla oblongata is depicted in Figure 3. The reciprocal anatomical connections of the KF with all other nuclei within the LRC support the KF’s role as an integral part of the pontomedullary LRC. In addition to prominent descending projections to the caudal pons and medulla, investigations have identified projections to brainstem motor nuclei such as trigeminal, facial, hypoglossal nuclei, and the nucleus ambiguus (113, 167, 313, 373). In the spinal cord, the KF targets the cervical phrenic motoneurons (124, 313) and spinal motoneurons that supply the intercostal and abdominal respiratory muscles (314). Less dense caudal projections innervate the sympathetic preganglionic cells of the intermediolateral cell column in the thoracic spinal cord (124). Ascending projections of the KF target the lateral hypothalamic area, the lateral preoptic area, and the central nucleus of the amygdala (124, 329), and in particular, the midbrain periaqueductal gray (PAG) (38, 210). In turn, the KF receives somato- and viscerosensory afferent information originating in the NTS, from the spinal trigeminal nuclei and from the upper cervical cord (31, 54, 115, 167). The afferent inputs convey sensory information arising from the upper and lower airways, emphasizing the general importance of the KF for processing afferent information relevant for the adaptation of the breathing pattern. The KF-area also shows strong connectivity with nuclei of the ascending recticular activating system (ARAS) such as the locus coeruleus and medullary raphé nuclei (15, 38, 129–131, 237).
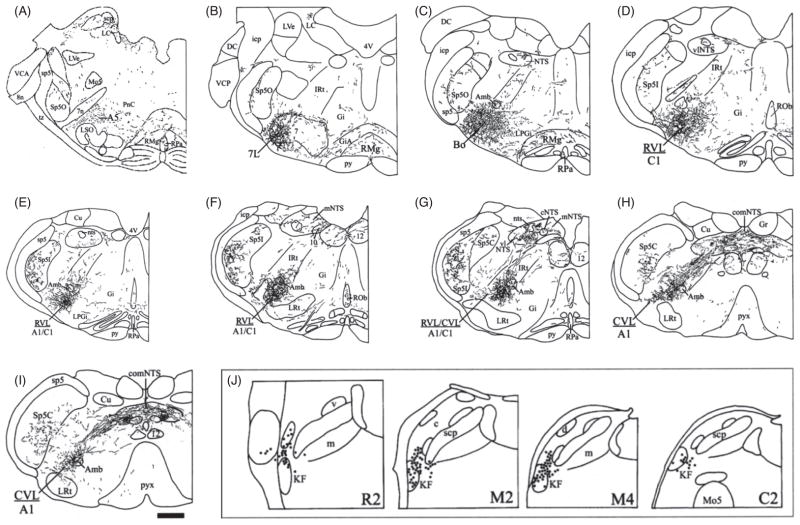
Figure 3.
Camera lucida drawings of coronal sections through the caudal pons and medulla oblongata from rostral to caudal (a–i) illustrating the pattern and distribution of anterogradely labeled descending fibers following PHA-L injection into the KF (see inset j, filled circles reflect neurons which have been filled with PHA-L, and therefore represent the probable neurons with projections). Figure published with permission of Horst Herbert, Tübingen, Germany. Orientation: Panels a–i are from rostral to caudal transverse sections through the pontomedullary brainstem. Abbreviations: 4V, 4th ventricle; 7, facial nucleus; 7L, lateral facial motor nucleus; 7n, facial nerve; 8n, vestibulocochlear nerve; 10, dorsal motor nucleus of vagus; 12, hypoglossal nucleus; A1, A1 noradrenergic cell group; A5, A5 noradrenergic cell group; Amb, nucleus ambiguus; AP, area postrema; Bo, Bötzinger complex; C1, C1 adrenergic cell group; CnF, cuneiform nucleus; Cu, cuneate nucleus; CVL, caudal ventrolateral reticular nucleus; DC, dorsal cochlear nucleus; DLL, dorsal nucleus of the lateral lemniscus; Gi, gigantocellular reticular nucleus; GiA, gigantocellular reticular nucleus, part alpha; Gr, gracile nucleus; icp, inferior cerebellar peduncle; IRt, intermediate reticular nucleus; KF, Kölliker-Fuse nucleus; LC, locus coeruleus; LPGi, lateral paragigantocellular nucleus; LRt, lateral reticular nucleus; LSO, lateral superior olive; LVe, lateral vestibular nucleus; me5, mesencephalic trigeminal tract; Mo5, trigeminal motor nucleus; NTS, nuclei of solitary tract; nts, solitary tract; PB, parabrachial nucleus; PnC, pontine reticular nucleus, caudal part; Pr5, principal sensory trigeminal nucleus; PrB, pre-Bötzinger complex; py, pyramidal tract; pyx, pyramidal decussation; RMg, raphé magnus nucleus; Rob, raphé obscurus nucleus; RPa, raphé pallidus nucleus; RVL, rostroventrolateral reticular nucleus; scp, superior cerebellar peduncle; sp5, spinal trigeminal tract; Sp5C, spinal trigeminal nucleus, caudal part; Sp5I, spinal trigeminal nucleus, interpolar part; Sp5O, spinal trigeminal nucleus, oral part; tz, trapezoid body; VCA, ventral cochlear nucleus, anterior part; VCP, ventral cochlear nucleus, posterior part subnuclei of the parabrachial complex (PB) (same as Fig. 3): c, central lateral; d, dorsal lateral; el, external lateral; eli, inner part of extern lateral; elo, outer part of extern lateral; exl, external lateral; exm, external medial; il, internal lateral; m, medial; s, superior; v, ventral; w, waist. Subnuclei of the nucleus of solitary tract (NTS): c, central; com, commissural; dm, dorsomedial; m, medial; vl, ventrolateral.
Descending forebrain projections to the KF-area are numerous. In the cat, the motor cortex, red nucleus, bed nucleus of stria terminals, and particularly the PAG are shown to project to the pontine lateral tegmental field that includes PB nuclei and KF (73, 176, 182). In the rat, descending forebrain projections to the KF-area is not well investigated, though reciprocal connectivity of the PB nuclei and KF-area with various cortical and limbic areas has been reported (328). In the rat, the KF-area clearly receives dense input from the PAG (209). Nevertheless, the topography of descending forebrain projections in the rat waits further detailing in the future. Although the role of these descending forebrain and midbrain projections are yet to be explored, they provide a platform for the concept that the KF-area is important for the gating of behavioral or emotional modulation of the breathing pattern within the rCPG circuits (97, 98). However, many of the forebrain-targeted areas in the lateral PB nuclei are largely devoid of projections toward the caudal LRC. Therefore, the unexplored intrinsic pontine connectivity between lateral, medial PB nuclei and the KF could provide a strong anatomical basis for gating higher brain input within the rCPG via intra-PB synaptic interaction.
In summary, retrograde and anterograde tract tracing provide evidence for an anatomical framework of the KF-area to function as an integral part of the LRC. The descending fore-brain inputs to the KF-area and its efferent connectivity with important sensory relay nuclei in brainstem and spinal cord supports the important role of rostral pons in the gating of adaptive breathing during behavior, emotion, and associated homeostasis.
KF-mediated control of inspiratory off-switch: A balance between afferent feedback and intrinsic synaptic mechanisms
The inspiratory off-switch (IOS), results from interplay among sensory feedback from slowly adapting pulmonary stretch receptors, and intrinsic synaptic mechanisms of the rCPG located in the KF-area (Fig. 4). Sensory feedback from the inflated lung arising from pulmonary stretch receptors is termed Breuer-Hering reflex (BHR) after the discoverers (50, 168, 212). The physiological significance of the BHR for IOS was demonstrated in numerous experiments examining the effect of diminishing the vagally mediated stretch receptor feedback with vagotomy (deafferation), cooling of the vagus nerve, preventing lung deflation, as well as mimicking vagal feedback using lung inflation and vagal stimulation. Absence of BHR feedback transforms the breathing pattern leading to a modest prolongation of inspiratory and expiratory phase durations, a significant increase in burst amplitude and a decrease in respiratory frequency (212). This obvious BHR impact on inspiratory phase duration has been confirmed in numerous studies and interpreted as BHR sensory feedback during lung inflation conveys a primary signal to initiate the IOS, and thus controls transition from inspiration to expiration. The role of the KF-area in IOS has almost as long a history as that of BHR. Pontine modulation of IOS was first demonstrated by Marckwald in 1887 (245). Marckwald showed that bilateral lesions of the dorsolateral pons and then subsequent cooling of the vagal nerves can transform the normal breathing pattern into apneusis—a breathing pattern that is characterized by severely prolonged inspiration. These results were later confirmed by Stella (352). Today apneusis after KF-area lesions or transections in vagotomized animals is seen as marker for lack of pontine control and timing of IOS (29,94,104,226,236,245,264,343,356,396). Ironically, Lumsden (236) who coined the term “pontine pneumotaxic center” challenged this interpretation holding that vagotomy was not necessary for apneusis. Other investigations have shown that chronic pneumotaxic lesion in vagi intact animals does not produce apneusis, but merely causes a small increase in the inspiratory duration (134, 362, 363). Nevertheless, a most recent investigation using spontaneously breathing, vagally intact, pontine lesioned goats concluded that KF-area is critical for the generation of eupnea (46). The dependence of the generation of apneusis on both vagotomy and inactivation of the KF-area indicates that vagal afferent input and rostral pontine input act through a common central IOS mechanism involved in the proper timing of IOS and inspiratory/expiratory phase transition (Fig. 4). The concept of vagal and pontine inputs mediating a common inspiratory inhibitory element in the rCPG has persisted through many models of the rCPG (4, 64, 235, 245, 310, 318, 322, 355, 396).
Figure 4.
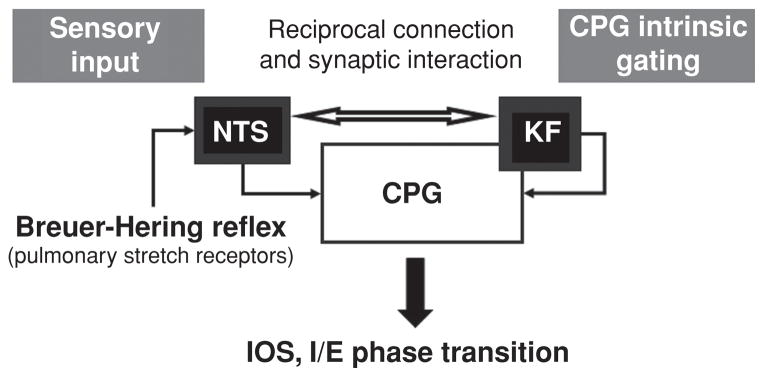
Schematic drawing illustrating the two convergent pathways involved in the mediation of inspiratory off-switch (IOS) and inspiratory/expiratory (I/E) phase transition. (A) An afferent pathway mediating Breuer-Hering reflex originates from pulmonary stretch receptor input via the vagus and terminates in the nuclei of the solitary tract (NTS). (B) A central pathway that requires the Kölliker-Fuse nucleus (KF) of the pons involves reciprocal interaction between the KF and central pattern generator (CPG). Plasticity in the expression of the breathing pattern depends on intrinsic gating mechanisms (for details see text) of the CPG attributed to the KF and results from interaction between reciprocal connections of the afferent and the network pathways.
The effect of the KF-area on the medullary rCPG could be either phasic or tonic. Numerous experiments performed during the latter half of the 20th century hypothesized that the KF-area tonically modulates the threshold for IOS mediated in the medullary nuclei of the LRC (395). Curt von Euler’s conceptual model of a pontine modulated IOS by was largely based on the finding that tidal volume required for IOS to reach threshold is decreasing during the progressing inspiratory phase (61). This shows a phasic relation between centrally generated inspiratory activity and BHR feedback. In a subsequent study, lesioning the KF-area in the vagi intact cat caused a significant increase in tidal volume threshold required for BHR reflex evoked IOS, albeit the phasic relationship between central inspiratory activity and BHR remained unchanged (396). From these elegant studies, von Euler concluded that KF-area provides important tonic excitatory drive for the gating of IOS; however he largely excluded pontine phasic descending inputs to the medullary rCPG as trigger for IOS. Around the same time, important investigations by Morton Cohen showed that phasic pontine activity can be suppressed by BHR acting to disfacilitate rhythmic input to the pons or inhibit pontine activity (66, 116, 118). Accepting these previous data, it appears that phasic BHR feedback is the primary mechanism for the initiation of IOS in an intact organism. However, recent evidence challenges this fundamental principle of respiratory physiology.
The workgroup of Chi-Sang Poon has shown that vagally mediated BHR feedback habituates (241, 342, 348). Habituation is a decrease in response strength to a repeated stimulus. Thus, if the supposedly essential BHR feedback can rapidly decrease in synaptic strength, the pontine mechanisms for IOS must provide phasic information to maintain rhythmic patterning of inspiration and expiration. The possibility of phasic inspiratory inhibitory mechanisms arising from the KF-area is further supported by stimulation experiments. Chemical or electrical stimulation of the KF-area evokes short latency phase resetting and IOS in animals whose vagi are intact (59, 60, 91, 93, 216, 217, 276, 277). A recent study expanded the previous findings of the KF-area and demonstrated that bilateral pharmacological lesion of the KF-area abolished all postinspiratory activity from upper airway motor output (94). According to current understanding, it is not feasible that the generation of a normal three phase motor pattern after vagotomy depends on tonic gating of the vital postinspiratory phase of the respiratory cycle. In all models that describe the formation of the respiratory motor pattern, the generation of the postinspiratory phase depends on phasic synaptic interaction of various types of respiratory neurons (see later). In addition, indirect evidence for phasic synaptic interaction is provided by the presence of phasic respiratory neurons in the KF-area. In 2006, Kazuhisa Ezure reported recording from 235 phasic respiratory neurons in the KF-area in vagi intact rat (111), also see references 239, 341, 359, and 374 for recordings in cats). In vagotomized animals, phasic respiratory activity appears to be even more pronounced (37,81,86,94,266). Direct evidence for an increasing phasic pontine activity after vagotomy was demonstrated in recent studies (86, 319). With the vagi intact pontine neurons express tonic activity with weak respiratory modulation, whereas after vagotomy or in the absence of lung inflation, the strength and consistency of phasic respiratory activities in the KF-area increase significantly. However, despite the existence of strong descending projections these and previous studies have been able to identify only a few monosynaptic connections between pontine and medullary neurons (36, 37, 331, 332). These data may seem to question the existence of strong phasic pontomedullary interaction. However, general experimental procedures, effect of anesthesia (e.g., ketamine as N-Methyl-D-aspartate (NMDA)-receptor antagonist) or methods of artificial ventilation impact significantly on respiratory neuron activity in KF-area. Because of these technical inadequacies, the physiological significance of identified phasic pontomedullary synaptic interactions still remains un-derappreciated. However, the role of KF-area-mediated IOS in reduced quietly breathing animals may not provide an insight into a role of the pons in behaving animals even with the vagi intact (68, 97, 98, 296).
In summary, the IOS is controlled by the interplay of the phasic sensory BHR feedback and phasic synaptic interaction of the KF-area with the rCPG. Nevertheless, other nuclei are involved in IOS. Injection of the inhibitory neuropeptide somatostatin in the Bötzinger complex (53), blockade of glutamatergic or GABAergic neurotransmission in the NTS (72, 399, 400), or lesion of the caudal vl pons (191) can trigger changes in the breathing pattern from subtle prolongation of inspiration to apneusis. These changes are indicative of disturbed IOS mechanism and identify processing of IOS beyond the sensory-pontine loop. Thus, these studies potentially relate to degeneracy within the respiratory network that describes the ability of structurally distinct elements to generate similar function (249). Network degeneracy also explains why apneusis after KF-area lesion and vagotomy is not permanent because structures outside the primary sensory-pontine loop compensate initial lack of function (375). This important network property for IOS requires further detailing regarding a potential hierarchical organization of brain structures involved in the control of IOS.
Phasic pontomedullary synaptic interaction during inspiratory off-switch and the role of NMDA-receptor-mediated neurotransmission
The electrophysiological analysis of IOS mechanism revealed that late-inspiratory (late-I) neurons initiate IOS and subsequent activation of the post-I neurons completes the transition from inspiration to post-I. The sequential activation of late-I and post-I neurons during IOS is the essence of models of respiratory rhythm and pattern generation (34, 266, 320–322, 343, 344). Although computational models have been developed to explain possible pontine mechanisms (4,318,319,322), very little is known about intrapontine synaptic interactions, biophysical properties of pontine respiratory neurons, and their control over the primary rhythm and pattern generating circuits in the medulla. Extracellular recordings of pontine IE phase spanning neurons, situated within the KF region, support their involvement in the regulation of IOS (111, 239, 341, 359, for review see 68). In particular, data from Cohen and Shaw (2004) indicated that medullary late-I neurons receive excitatory inputs from pontine IE neurons. This shows that pontine and medullary late-I neurons are coupled synaptically providing a framework for the KF-area to control IOS. Intracellular neuronal recordings from the KF-area reveal a variety of phasic respiratory activities (12, 81, 94, 266). The majority of KF respiratory neurons exhibit either early-I (early-I, also termed decrementing inspiratory, I-dec), IE or post-I activities. In particular, the high numbers of IE phase-spanning neurons in the pons is in agreement with data from the cat and the rat in vivo (68, 86, 111). Based on the analysis of the membrane trajectories of intracellularly recorded KF neurons, we propose a model for IOS that depends on an efference copy. To understand this model, it is important to note that most previous models are almost exclusively based on reciprocal synaptic inhibition and biophysical properties (expression of subsets of ion channels) generating the alternating bursting of respiratory interneurons (310). However, to contract the respiratory muscles the rhythmic bursts of the interneurons have to be transmitted via excitatory premotor neurons to the motoneuron pools. According to more recent models, these excitatory premotor neurons are activity involved in respiratory pattern formation (322). The model for pontine mediated and efference copy-based IOS considers two paradigms. First, the primary motor pattern and rhythm is generated within the medullary LRC. Second, the KF-area has no intrinsic capacity to generate respiratory rhythm. Therefore, the generation of phasic respiratory unit activity in the KF-area depends on the efference copy of synaptic activity of specific inspiratory units of the medullary rCPG (Fig. 5).
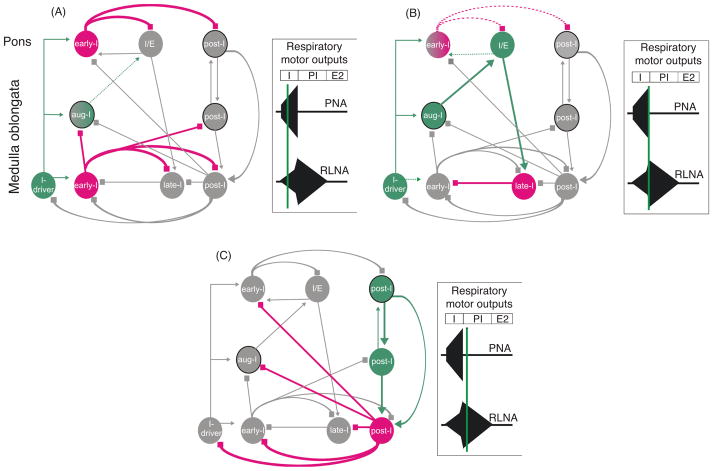
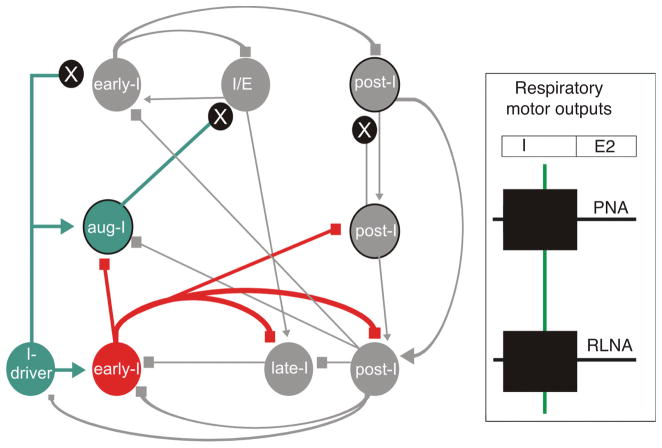
Figure 5.
Schematic illustration of the sequential phase of the pontine mediated inspiratory off-switch (IOS). The figure is adapted from Mörschel and Dutschmann 2009. Excitatory neurons and excitatory drive are highlighted in green; inhibitory neurons and interactions, red; and inactive cell populations and connections, gray. The model is based on the concept that ascending drive from the medullary respiratory neurons is required for the pontine mediated IOS. Panels on right show three time points in the cycle. (A) During the inspiratory phase, a pontine early-I population receives excitatory synaptic input (efference copy 1) from inspiratory driver neurons (I-driver) located in the pre-Bötzinger complex. The pontine early-I populations are inhibitory interneurons of the pons. The pontine early I neurons inhibit the pontine inspiratory/expiratory phase spanning neuron (I/E) and pontine postinspiratory premotoneurons (post-I) to prevent initiation of phase transition during early and mid-inspiration. With ongoing inspiration the pontine I/E neurons receive increasing excitatory drive (efference copy 2) from medullary augmenting-inspiratory premotor neurons that override the inhibition of early-I causing firing onset around late inspiration. (B) The pontine I/E neurons are excitatory pontobulbar neurons that activate the medullary late-I neurons to initiate the inspiratory/expiratory phase transition. (C) Finally, the inhibitory late-I neurons of the medulla terminate the activity of the medullary early-I neurons and release the medullary post-I population (inhibitory interneurons) from synaptic inhibition. These inhibitory post-I neurons inhibit the inspiratory populations (medullary and pontine). Consequently, the pontine and medullary postinspiratory premotor population innervating thyroarytenoid motoneurons starts firing.
In this model, pontine early-I neurons are inhibitory. Both pontine and medullary early-I neurons receive synaptic drive from inspiratory-driver (I-driver) neurons (Fig. 5). I-driver neurons are neurons with conditional pacemaker properties located within the medullary pre-Botzinger complex (117, 319, 345). The pre-I neurons in integrated network models are considered to be potential I-driver cells (319, 322, 343, 344). However, more likely, the I-driver population reflects neurons providing tonic excitatory drive from peripheral and central chemoreceptors or from the reticular arousal systems. The pontine early-I cells inhibit IE phase spanning neurons and post-I neurons of the pons via local connections during the early stages of inspiration. This connectivity prevents initiation of phase transition during the early inspiratory phase (Fig. 5). With ongoing inspiration, IE neurons receive increasing input drive from excitatory augmenting-inspiratory (aug-I) premotor populations of the medullary rCPG. Both decreasing inhibition from pontine early-I and increasing excitatory drive from aug-I neurons cause onset of firing of the pontine IE cells during mid or late expiration. In turn, IE neurons are excitatory and trigger activation of medullary late-I neurons that initiate the IE phase transition. Late-I neurons deactivate the early-I neurons via synaptic inhibition. This releases inhibitory (interneurons) and excitatory (premotoneurons) post-I neurons from synaptic inhibition provided by the early-I population, reflecting the transition from inspiration to expiration as described in various reviews (34, 110, 310, 311, 344). The pontine and medullary premotor post-I populations are reciprocally coupled via excitatory connectivity. This excitatory coupling of post-I premotor neurons allows for a dynamic regulation of strength and duration of the post-I phase. The present concept for pontine mediated IOS is based on the prediction that pontine neurons receive excitatory synaptic inputs from major neuron populations such as I-driver, augmenting-I, and post-I neurons. In our model, the drive from the medullary inspiratory premotor population is adjusted by a single intrapontine inhibitory synaptic interaction, which shapes the activity pattern of I/E neurons that initiate inspiratory termination (68, 319, 322). This intrapontine connectivity remains to be experimentally verified.
The crucial excitatory inputs to the KF-area required for control of IOS involve glutamatergic neurotransmission via NMDA receptors (NMDA-R). Numerous experiments illustrate that IOS is disrupted after local blockade of NMDA-R in the KF-area or following systemic application of NMDA-R antagonists (34,43,48,71,79,99,120,121,125,127,160,161, 226,266,291–293,355). These findings are consistent with the dense expression of NMDA-Rs in KF neurons (151,207,259). Because NMDA-Rs commonly expressed postsynaptically, it is most likely that local NMDA-R blockade is suppressing glutamatergic input to KF neurons. Thus, local pontine NMDA-R blockade disrupts the postsynaptic processing of phasic excitatory synaptic input provided via the efference copy of medullary inspiratory promoting neurons and aug-I premotor neurons. Without the critical excitatory drive from medullary inspiratory neurons, the pontine IE cells cannot start firing and thus IOS is delayed and consequently the motor pattern reflects apneusis (Fig. 6). Nevertheless, it is remarkable that pharmacological blockade of NMDA-R alone disrupts neurotransmission. Normally, an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainite receptor-mediated predepolarization is required to remove Mg2+ from the pore of NMDA-R to become active in many neural systems (e.g., hippocampus). NMDA-Rs are heteromeric containing at least two different receptor subunits (74, 261). Recently, it was shown that NMDA-Rs of KF neurons express NR2D subunits (207). The presence of the NR2D subunits allows for rapid membrane depolarization following NMDA-R-mediated glutamatergic neurotransmission without AMPA/kainate-receptor-dependent predepolarization (62). These data imply that neurotransmission involves specialized NMDA-R in the KF-area.
Figure 6.
Illustration of the effect of postsynaptic blockade of glutamatergic neurotransmission (e.g., NMDA-receptor antagonism) within the dorsolateral pons. Local blockade of excitatory synaptic interaction (black circles with white “x”) in the pons suppresses the efference copies 1–2 (see text and Fig. 5) causing blockade of the descending excitatory synaptic input from the pontine I/E neurons to the medullary late-I population. This abolishes the pontine mediated timing of the inspiratory/expiratory phase transition causing arrest in the inspiratory phase (apneusis). Figure adapted, with permission, from Mörschel and Dutschmann, 2009.
Naturally, NMDA-Rs are not the only receptors in the KF-area. In situ hybridization has revealed a multitude of neurotransmitters and receptors in the PB and KF nuclei. The KF has both GABAergic and glycinergic cell populations (166, 402, 408, 409). The noradrenergic A7 cell group also overlaps with the KF. In addition to GABA, glycine, and noradrenalin receptors, various glutamate (AMPA/kainic acid, NMDA, and metabotropic receptors (59, 149–152) and cholinergic and serotonergic (5-HT) receptors are expressed in the PB and KF nuclei (103, 165, 244). Moreover, large subset of neuropeptide transmitters such as neurotensin, cholecystokinin, substance P, somatostatin, and calcitonin gene-related peptide and their associated receptors are also expressed (324–327). However, their role in mediation of IOS is not explored.
Learning to breathe: Lessons from the postnatal maturation of rCPG
In the previous sections, we introduced the theory of a balance between the sensory BHR evoked and KF-area-mediated IOS. BHR feedback from the expanded lung during inspiration initiates IOS via exactly the same mechanism: excitation of late-I and (67, 158, 206, 310, 311) and post-I neurons (157, 163, 310, 311, 322). Consistent with the balance theory KF-area-mediated activation of late-I neurons can be suppressed by BHR feedback. A series of recent studies in the cat using multielectrode arrays to record pontine respiratory activity support this theory (86,319,331). Dependent on homeostasis and general arousal the IOS mechanisms may alternate between BHR and KF-area-regulated IOS in behaving animals. Such a mechanism requires plasticity within the two convergent pathways regulating IOS (Fig. 7). In this way, KF-area pathway would be released from afferent inhibition and mediate IOS when BHR feedback habituates (97,98,240,241,348). In such situation, the rCPG would operate under a pontine-dominated IOS based on the efference copy of inspiratory activity of the rCPG. This functional state of the rCPG would permit adaption of the breathing pattern required for behaviors such as vocalizing or sniffing, etc. (described later). In contrast, during increased synaptic pulmonary stretch receptors (PSR) feedback during high tidal volume (e.g., exercise, hypoxia, and hypercapnia), then the sensory feedback would dominate IOS. In this situation, the KF-area neurons are inhibited (66,116,118) and the IOS is rather determined by the afferent feedback (Fig. 7). In a state of high metabolic demands, reflected in high tidal volume and BHR feedback adapting breathing to suit behavior is decreased due to inhibition of the KF-area. This diminished effectiveness of the pons, for example, may explain the difficulty in talking during intense exercise.
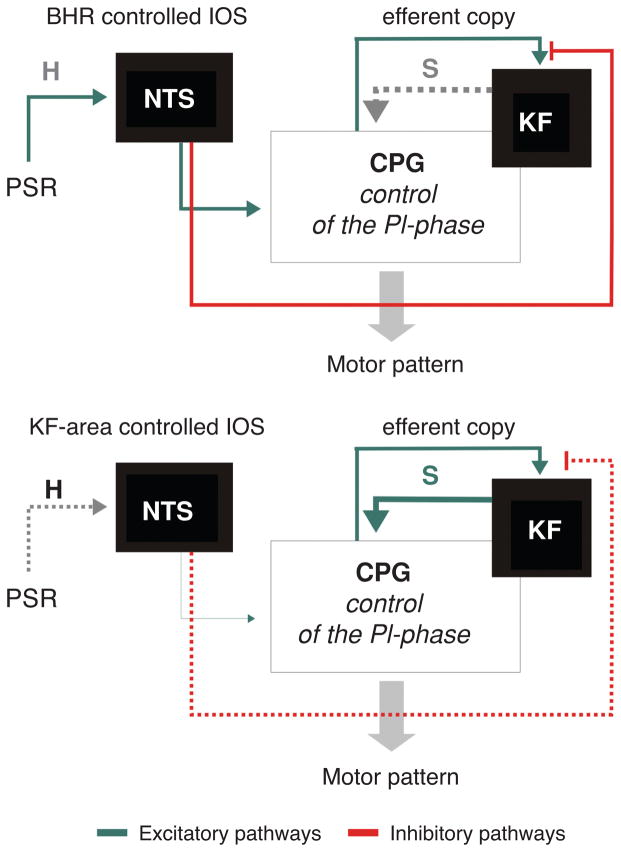
Figure 7.
Conceptual model of a dynamic switch between either Breuer-Hering reflex (BHR, pulmonary stretch receptor) or Kölliker-Fuse complex (KF) controlled inspiratory off-switch (IOS). This switch occurs naturally in the process of postnatal maturation. The concept is based on the dual processing theory and involves habituation of the PSR input at level of the nuclei of the solitary tract (NTS) and sensitization of synaptic activity of the KF-area. (A) During PSR dominance the second-order neurons that receive PSR input (pump cells) of the NTS inhibit the efference copy (see also Fig. 7) to the KF and the sensory feedback mediates the IOS. (B) During KF dominance the PSR input habituates (H) at level of the NTS and in turn sensitizes (S) the descending KF input to CPG to mediate IOS.
The synaptic mechanisms enabling transition from KF-area to BHR dominated IOS (or vice versa) may fulfill the dual processing theory (148). Donald Hebb in 1949 introduced the hypothesis that synaptic memory is based on either homosynaptic facilitation or depression. These two forms of synaptic learning were extended to heterosynaptic mechanisms of synaptic facilitation and depression via modulatory interneurons acting at the presynaptic terminals (18). The heterosynaptic strengthening is called sensitization while the decrease of a behavioral (motor) response to repeated stimuli is termed “habituation.” Chi-Sang Poon adapted the dual processing theory, which is broadly used in experimental and clinical physiology and social sciences to describe BHR plasticity (reviewed in reference 296). The dual processing for BHR plasticity is the waning and waxing of habituation and sensitization of IOS within the convergent pathways of BHR and KF-area-mediated IOS. Thus, the reported habituation of BHR input can causing sensitization of the KF-mediated IOS mechanism which then dominates the respiratory pattern formation during eupnea (Fig. 7 and reference 98). The application of the perfused brainstem preparation for investigation of postnatal maturation of the rCPG starts to illuminate the postnatal emergence of synaptic mechanisms underlying the dual processing and associated synaptic plasticity of IOS.
The respiratory network serves a vital function and errors in synaptic organization during development could have fatal consequences. Extensive studies utilizing the perfused brainstem preparation of newborn the rat clearly show the eupneic neuronal rCPG activity is very similar to adults. In particular, glycinergic synaptic inhibition required for network oscillation and respiratory pattern formation is functional immediately after birth (51, 100, 102, 104, 288). Thus, most of the synaptic changes observed during postnatal development of the rCPG are likely to be related to the processing of peripheral sensory information, and behavioral or emotional commands (98). With the first breaths, the neonatal respiratory network must cope immediately sensory feedback that was absent during the embryonic stages development (146). In particular, these include the mechanical feedback from the expanded lung after birth. Moreover, basic survival behavior linked to feeding, pain, and distress are operational as well (128). Beyond doubt, neonates are immediately able to process afferent inputs and many important afferent control mechanisms are fully functional at birth. However, several studies demonstrate changes in reflex responses during ontogeny (13, 97, 99, 141, 285, 377, 379, 381, 398) indicating the postnatal maturation of synaptic pathways integrating respiratory reflexes. Most of these reflex pathways involve the KF-area. The mechanisms of neuronal reorganization of respiratory reflex pathways during ontogeny as well as the underling synaptic mechanisms are not well understood. Presently, developmental changes have been identified in the biophysical properties, histochemical profile, and neurotransmission of with the rCPG circuits and in particular, the NTS. These changes impact the processing of afferent information in the intact rCPG. Predominantly, afferents carrying the arterial chemosensory information have been investigated (see references 28, 39, 55, 142, 201, and 306). Most neonatal breathing disorders, such as “apnea of prematurity” and “sudden infant death syndrome (SIDS),” appear to be associated with an altered chemical drive (202, 286).
A small number of experimental data address the postnatal maturation of the PB and KF-area. Lesion or transection experiments of the PB and KF in in vivo and in situ preparations suggest that basic pontine mechanisms influencing the respiratory motor pattern are already present at birth (104, 126). Even superfused, neonatal en bloc spinal-cord preparations have strong phasic bursting activity at the level of the KF-area that is modulated with burst of respiratory motor activity (12, 203, 279). The KF has projections to the phrenic motor nucleus and this pathway is established at birth (347) but its functional connectivity with the medullary rCPG has not been investigated. The cytoarchitecture and cell morphology of the PB and KF are substantially immature at birth (97, 205, 207, 220, 233, 308) indicating that the pontine modulation of the breathing pattern requires development in the early postnatal period. The PB complex develops its adult-like cytoarchitecture around postnatal days 12–15 in the rat (97, 208, 308). Approximately the same time as fundamental changes in the molecular composition of neurotransmitter receptors for gamma-aminobutyric acid (GABA), ##AMPA/Kainate (2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid), Glycine, 5-HT, and neurokinin 1 receptor are reported for the medullary parts of the rCPG (172,228–232,404,405). Detailed investigation of the physiology of these extensive cytochemical changes in rCPG arising naturally two weeks after birth, need to be undertaken. Nevertheless, evidence accumulates that synaptic plasticity associated with BHR and pontine IOS is absent in neonates and does not emerge before postnatal days 12–14 (97–99) (Subramanian et al., Respir Physiol Neurobiol in revision). Thus, in the following paragraph, we specifically focus on the developmental changes of cellular and network plasticity in the BHR pathways (e.g., NTS) and the KF-area controlling IOS.
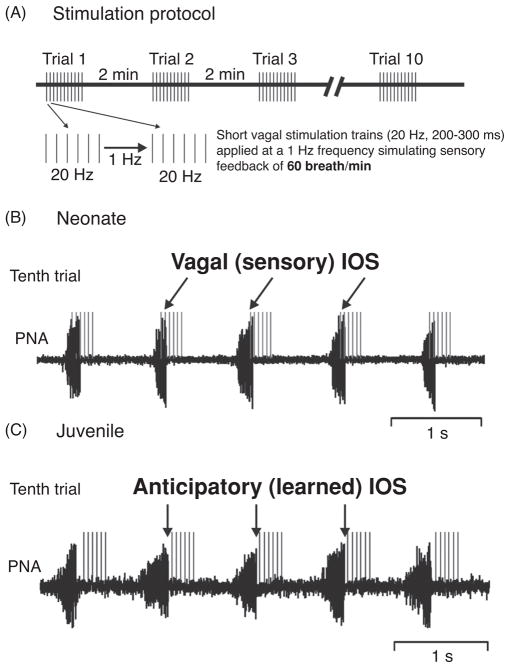
Several in vitro electrophysiological studies have shown that short- and long-term depression (STD and LTD), as a cellular bearing for habituation, were not present in the NTS of neonates (22, 23). These and other synaptic mechanisms obviously require postnatal development in the NTS (22, 23, 44, 77, 143, 275, 389, 390, 410). However, at early neonatal stages synaptic plasticity could be “unwarranted” developmentally, because the processing of unchanged sensory signals is required for physiological maturation of the developing circuits. For instance, BHR feedback is of major importance for the stabilization and maintenance of the eupneic respiratory motor pattern. Diminished or complete absence of BHR input has been shown to be life threatening for neonates (114,267,301). In a study using a NMDA-R subunit knockout mouse, an anomalous neonatal form of LTD was expressed as a cellular correlate for habituation. The neonatal form of LTD induced by the genetic manipulation triggered respiratory depression and premature death of the animals (297). Physiological studies on BHR development show that in maturing rats habituation of the BHR does not occur before postnatal days 13–15 (Subramanian et al., Respir Physiol Neurobiol in revision). HBR habituation in this study was investigated using stimulation of vagal nerve afferents to mimic the HBR inflation-withhold reflex causing a stimulus-dependent prolongation of the expiratory phase that diminished with repeated stimuli only in the juvenile animal. In accordance with the cellular findings of physiological absence of STD and LTD neonates displayed stereotyped processing of BHR feedback. Since BHR feedback is not restricted to a single breath, but is linked to rhythmic expansion of the lungs during each inspiration, a second study investigated the developmental changes in the motor response to rhythmic BHR feedback stimulation. This study showed that repetitive presentation of a rhythmic vagal stimuli or lung inflation in the perfused brainstem preparation can trigger learning and recall of respiratory patterns in juvenile, but not in neonatal rats (99). The stimulation protocol is illustrated in Figure 8A. In neonates, immediate and stereotyped 1:1 entrainment occurred during repetitive simulation trails. IOS was triggered stereotypically by onset of stimulation trains in the late-I phase. The finding that neonatal breathing activity spontaneously aligns to the vagal IOS during the late-I phase underpins the physiological significance of the BHR in neonates (Fig. 8B). The initial stimulation trials in the juvenile rat were different, and yielded irregular 1:1 entrainment. Many of the rhythmic stimulation trains completely suppressed phrenic activity and produced skipped breaths. With repeated presentation of the stimuli the 1:1 entrainment emerged. Different to neonates the 1:1 entrainment was characterized by anticipatory IOS before the onset of an individual stimulus train which indicates the sensory signal for IOS (Fig. 8C). The occurrence of the anticipatory IOS provides the evidence that a given pattern of afferent IOS signals can be learned because breathing still entrains to the pattern and frequency of the applied sensory stimulation. Consistent with previous data the observed learning of the vagal feedback pattern perfectly coincides with the postnatal emergence of BHR habituation that triggers the release of the KF-mediated IOS from BHR-mediated synaptic inhibition (66, 118). In subsequent experiments, this scenario was further verified by showing that bilateral blockade of NMDA-R in KF-area completely prevented anticipatory IOS. In addition, following NMDA-R blockade an immediate and stereotyped 1:1 entrainment was observed which remained unchanged during repetitive stimulation, as it was seen in neonates that have an immature KF-area (99). Therefore, our studies not only provides proof that the anticipatory IOS is mediated by the KF-area but also strongly suggests that glutamatergic neurotransmission via NMDA-R in the KF-area is important for synaptic mechanisms underlying learning and memory in breathing.
Figure 8.
(A) Illustration of the rhythmic vagal stimulation protocol. (B) Traces illustrating entrainment of phrenic nerve activity (PNA) to rhythmic vagal stimulation during the tenth stimulation trial in a neonatal perfused brainstem preparation. In the neonate, PNA entrains to stimulation trains (gray) occurring during the late inspiratory phase. Thus, the vagal stimuli provide the sensory trigger for IOS (details see reference 99). (C) Traces illustrating entrainment of phrenic nerve activity (PNA) to rhythmic vagal stimulation during the tenth stimulation trial in a juvenile rat. In juvenile rats, PNA is also entrained to the rhythmic vagal stimulation, but IOS occurs anticipatory before the onset of the stimulus train. This indicates that the KF-area has learned to pattern of vagal input and generates IOS prior to the sensory input (further details see text).
The analysis of the expression profile of NMDA-R subunits revealed a strong developmental upregulation of the NR2D subunit in the KF. The characteristics of NMDA-currents in the KF match the expression of the NR2D subunit (207, see previous section). The NR2D-dependent NMDA-currents subunit had a reduced Mg2+ block (14) accompanied by low baseline conductance, long deactivation kinetics, and a lack of desensitization (74). The long deactivation kinetics of NR2D-mediated NMDA-current can produce a continuous background current (385). Such a continuous background current could promote learning of breathing patterns associated with multiple respiratory cycles (207). Besides the general characteristics of NMDA-currents the associated Ca2+ influx is an essential trigger for various intracellular signaling cascades linked to short- and long-term changes in membrane excitability and conductance of a postsynaptic neuron. These changes are prerequisite for learning and memory. In various forms of synaptic plasticity, the NMDA-R-dependent Ca2+ conductance is modified by the release of neuromodulators. Amongst various potential neuromodulators potentially contributing to synaptic plasticity in the KF-area only brain-derived neurotrophic factor (BDNF) has been investigated (205–208). It has been demonstrated that BDNF-application provoked a progressive, protein-kinase-C-dependent potentiation of NMDA-current in KF neurons (207). BDNF induced short-term potentiation was present in juvenile animals and was absent in neonates. The BNDF-mediated NMDA-current potentiation correlates with the developmental time window for anticipatory IOS and HBR habituation. These cellular findings support the working hypothesis that NMDA-R-dependent neurotransmission in the KF-area is not only linked to IOS but the same synapses are also associated with learning and memory of breathing patterns.
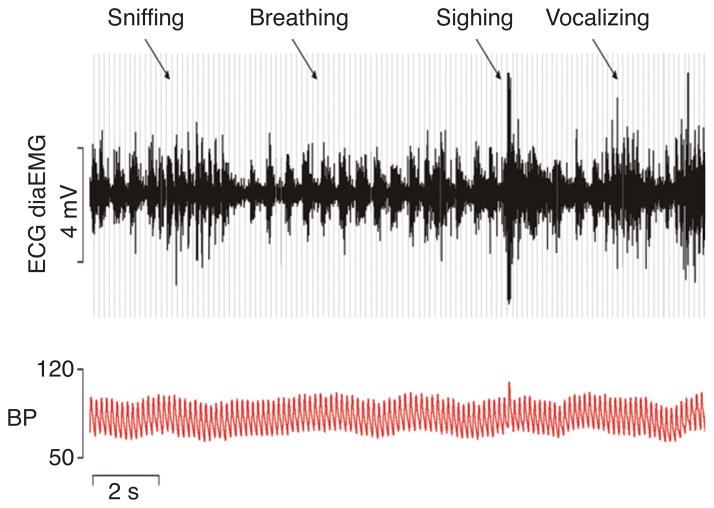
The course of the postnatal maturation of synaptic function in the KF-area is coincident with an increasing behavioral and emotional repertoire of the developing mammal. Because most developmental studies are performed in rodent models, it is important to remember that rats and mice do not see and hear until postnatal day 10–12. With these sensory systems functional, the exploratory behavior increases dramatically and subsequently the rCPG has to cope with rapid changes from eupneic to adaptive breathing linked to behaviors such as foraging and other explorative behaviors like sniffing (see Fig. 9) as well as basic emotions (e.g., fear and anxiety—flight or fight response). The dexterity of changes in the breathing patterns in behaving animals implies that learning is required to adjust respiratory control (98) to changing internal and external states to maintain homeostasis and survival (128, 371).
Figure 9.
Radiotelemetry recordings of electrocardiogram (ECG, gray lines), diaphragm electromyogram (DiaEMG, black) and arterial blood pressure (BP, red) in a conscious rat illustrating the continuous modulation of breathing in the context of behaviors such as sniffing, vocalizing, and sighing. Figure is published with permission of Julian Paton, Bristol.
KF-area mediation of protective airway reflexes and upper (non) respiratory behavior such as swallowing and vocalizing
Most protective reflexes are expiratory (e.g., sneeze, cough, sensory apnea, etc.) and at the least require initially postinspiratory mediated glottal closure. The physiological significance of this is twofold. First, glottal closure protects the lower airways from aspiration (42,101,287,307) and second abdominal muscle contraction against the closed upper airway valve generates high abdominal and thoracic pressure required for compulsory reflexes and behavior (27,300,333–335,338,339).
The KF-area mediates the post-I apnea elicited by noxious stimulation of the nasal or laryngeal mucosa (91–93, 255), as well as during upper airway reflex such as sneezing (215, 247, 397) and coughing (136, 188, 294, 336, 337). Nevertheless, the important role of the KF-area in orchestration of almost all airway reflexes is consistent with data showing that excitatory postinspiratory premotor neurons of the KF control the ambiguual motoneurons that mediate glottal constriction (94). In our model for the pontomedullary synaptic interaction, descending excitatory input to inhibitory post-I neurons allows the pons to directly switch to post-I phase and activate post-I motor activity irrespective of other pontomedullary interactions (Fig. 5C). Moreover, in the medulla oblongata, post-I laryngeal motoneurons have been classified as multifunctional neurons (338,339). In this context, specific activation patterns of the laryngeal adductor motor neurons could process input from multifunctional premotor neurons that initiate oral behavior and reflexes. Taken the physiological and anatomical evidence into consideration, it is likely that a major population of these multifunctional premotor neurons is located in the KF-area (see reference 97).
The anatomical connectivity and physiological significance of the KF-area in the control of upper airway muscles also provides the platform for the mediation of upper airway related nonrespiratory behavior such as swallowing and vocalizing. During swallowing, laryngeal adductor activation is important to prevent aspiration during ingestion (35, 137, 138, 289). In addition to the essential prevention of aspiration, swallowing is also linked to tongue movement. The hypoglossal motor pool, which controls tongue movement, receives a strong modulatory input from the KF-area (47, 95, 137, 214). Thus, pontine descending inputs to the medullary hypoglossal motor and premotor pools are also potentially involved in the coordination of swallowing and breathing (35, 137). A recent study using chronic lesion of the KF-area indeed reveal a strong modulatory influence of the KF-area for coordination of breathing and swallowing in the awake goat (47).
The biological significance of the KF-area in the mediation of multimodal afferent inputs involves the adaptation and modulation of breathing to behavior. Amongst various behaviors, vocalization is linked closely to primary motor circuits of breathing. However, most experimental data regarding vocal control circuits originated from the cat, in which the synaptic pathway for vocalization (25,175,178,368,413) and breathing (34, 310, 395) are fairly well characterized. Non-human primates have also been used for the understanding of vocalization generated in midbrain and forebrain areas (194, 196, 219, 382). In contrast, in the rat neural mechanisms of vocalization remain uncharacterized. Therefore, at the present stage, we are forced to make models based on experimental studies derived from the several species.
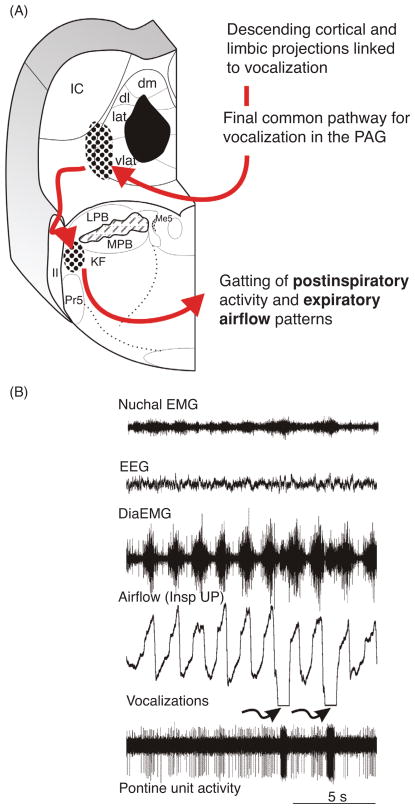
Vocalization in non-human primates and cats involves an innate vocal pattern attached to emotions and very elementary intraspecies communication. In cats, the innate vocal pattern can be elicited upon chemical stimulation of specific areas of the PAG (25,368,369,413). The PAG, which acts as a critical relay of the limbic system (176) represent the final common pathway for vocalization in mammals (175,369). Vocalization can be considered to be the most complex motor behavior in mammals since it requires a highly coordinated activation of laryngeal, thoracic, and abdominal muscles. Many of these muscles are accessory respiratory muscles and thus are arbitrated by the rCPG. However, it is not understood how synaptic signals arising from the PAG or other forebrain structures are integrated within the rCPG. It is known that laryngeal and abdominal pathways for vocalization are organized through the nucleus retroambiguus (NRA) (369,412). The NRA can be considered as the caudal part of the LRC as many respiratory related neurons (251) have been recorded and perturbation to the NRA causes disruption of eupnea (369). However, since post-I laryngeal adductor activation mediates vocal fold tensioning, the KF-area could be involved in adapting timing and duration of inspiration (via IOS) and expiration (via post-I regulation) for sound modulation. Thus, the KF-area could serve a critical role in reorganizing rCPG-derived eupnea into a vocal breathing pattern. The dense reciprocal connectivity of the vl column of the PAG and the KF-area provides the anatomical structure (38,209,210) to this hypothesis (Fig. 10). Physiological evidence is provided by a report that showed that during the conditioned vocalizations neurons in the KF-area in the cat change their activity (112). Recently, experiments on vocalization in the bat demonstrated a mandatory role for the KF-area in sound production (346). Bats like rodents have a similar vocal pattern in the ultrasonic spectrum, and thus it can be hypothesized that the KF in rat may be involved in vocalization too. In the cat and non-human primate experimental evidence suggests that the final conversion of the breathing pattern to a vocal pattern required laryngeal premotor populations of the NRA (195, 197, 238, 369). The NRA receives strong descending projections from the KF (135, 179) and in turn gets activated by KF stimulation (19). According to the final common pathway for vocalization (175, 369), the PAG-NRA pathway is critical for the production of sound. However, for conveyance of emotions, the sound itself needs to be modulated. The modulation of sound is dependent on the timing of inspiratory and expiratory activity, fine motor control of the upper airways and the diaphragm and sensorimotor integration of homeostatic and vagal feedbacks. In this regard, the PAG-KF pathway could play a vital role.
Figure 10.
(A) Schematic illustration of the proposed pathway for adaptation of the breathing pattern during vocalization. (B) Original recording of an inspiratory modulated respiratory pontine unit showing pronounced activation during vocalization. Recording was obtained from the KF-area of cat. The data are unpublished and supplied by Dick TE, Anderson C, and Orem, JM.
Interestingly, Foxp2, a transcription factor directly linked to the development of speech (119, 383), appeared to be a selective marker for KF neurons in the developing rCPG (144). Thus, the function of the KF-area within the rCPG is also seen in the coupling of respiration to vocal behavior. However, this role of the KF-area requires future investigation.
KF-area and neurogenic breathing disorders
Studies of breathing dysfunction and the underlying synaptic pathomechanisms in the rCPG can be investigated in a number of genetically engineered animal models (132). Here, we summarize the role of the KF-area and associated pontomedullary synaptic interactions in transgenic mouse strains designed to mimic human neurological diseases.
The KF-area is explicitly identified to contribute to breathing disorders in a mouse model for a neurodevelopmental disease called Rett-syndrome (RTT). The genetic cause and neurological disorder in RTT are reviewed elsewhere (56) and detailed information on synaptic mechanisms contributing to breathing disorders in RTT can be found in references 200 and 227. In brief, RTT is an X-linked neurologic disease associated with breathing abnormalities and severe mental retardation in females. Gene mutations or gene deletions in the MECP2 gene located on Xq28 have been identified as cause of the disease. The clinical course of RTT is characterized by a regression period, which occurs between 1 and 3 years of age accompanied by state-dependent breathing abnormalities. Respiratory disturbances during wakefulness comprise alternating periods of hyperventilation and apneusis, breath holding frequently terminated by Valsalva’s maneuvers and forced and deep breathing. Mecp2−/y knockout mice develop an RTT-like respiratory disorder approximately 3–4 weeks after birth (388). The breathing disorders observed after the equivalent regression period in mice involve, impaired responses to hypoxia, hypercapnia (392), altered postsigh breathing activity (393), and pronounced breath hold accompanied by glottal closure (391). In the perfused brainstem preparation, the control of post-I activity was impaired and this impairment involved both KF-area and BHR circuits for IOS (353). Indeed, the synaptic impairment of the major control circuitry for the post-I phase correlated highly to the upper airway related neurological deficits observed in RTT patients such as apneas with laryngeal closure, loss of vocalization, and weak coordination of swallowing with breathing (2, 200, 227, 354). A detailed investigation of the breathing dysfunction in RTT not only revealed a potential synaptic impairment within the KF but, more importantly, a lack of plasticity during NTS/KF dependent processing of BHR (353). The delayed onsets of breathing disorders in both, RTT patients and the Mecp2−/y KO mouse correlate with the developmental changes in the KF-area (and elsewhere in rCPG) related to synaptic plasticity particularly required for coordinating breathing and vocalization. Therefore, genetic factors specifically involved in the maturation of synaptic plasticity in the rCPG, and in particular the KF-area can be linked to neurogenic breathing disorders in RTT.
Besides RTT, the pathology within the KF-area has implications in SIDS. Postmortem examination of infants reveals significant changes in histology including alterations in the neurochemical profile of the KF-area (221–223, 253). The abnormality of KF-area to as a cause for SIDS remains unexplored. However, it is consistent with the mainstream hypothesis (202, 286) that, breathing failure in SIDS may involve pathophysiological changes in the serotonergic modulation of the pontine circuitry. This serotonergic modulation is provided by the interaction of medullary raphé and KF-area (172, 274).
Recent experimental evidence corroborates the KF-area to upper-airway dysfunction in neurodegenerative diseases associated with dementia. Neurodegenerative diseases, such as Alzheimer’s disease are inherently associated with tauopathy. In turn, tauopathy manifests postmortem as neurofibrillary tangles and neuropil treads. Hyperphosphorylation of the microtubule associated protein tau causes tauopathy and abnormal intracellular aggregation and is evident as accumulation of Tau protein. Investigation of the transgenic mouse model P.301L expressing tauopathy at 7–8 months of age, reveal reduced post-I discharge of laryngeal motor activity (96). With high chemical drive (hypercapnia), post-I discharge is nearly absent. In vivo data support the hypothesis that KF-area tauopathy shifts the post-I laryngeal constrictor activity toward inspiration. This potential post-I shift has caused a significant increase in inspiratory resistive load due to glottal constriction during inspiration. Although upper airway dysfunction is not directly linked to death in dementia patients, it would be a factor for the associated vocalization disorders [e.g., primary progressive aphasia (315)], impaired swallowing and aspiration reflexes in these patients (185). Histological analysis of postmortem brains of dementia patients revealed severe tauopathy in the KF-area (317) supporting synaptic dysfunction of the pontine control of upper airway activity. Thus, KF-area dysfunction contributes to neurodegenerative disorders that involve modulation of the breathing pattern during oropharyngeal behaviors. Similar, KF-area dysfunction was also observed in transgenic mouse model of tauopathy (96). The strong overlap of tauopathy in the KF-area and the PAG as the main output relay for the limbic system (178), implies that breathing dysfunction in neurodegenerative disease are linked to the emotional and behavioral respiratory control (see previous paragraph and Fig. 10).
In summary, we conclude that pathophysiological changes involving the KF-area and PAG circuits are closely associated with breathing dysfunctions. These sustained breathing disorders have been identified within dementia (neurodegenerative) and autism spectrum diseases (neurodevelopmental). Neuropharmacological therapy for such breathing dysfunction requires an increasing understanding of the importance of midbrain-pontine interactions.
The role of the KF-area in chemical control of breathing
The KF-area receives strong projection from the carotid body afferent relays within the NTS (167), and from the RTN/pFRG (316). This anatomical connection implies a physiological role of the KF-area in the adaptation of breathing in response to activation of central or arterial chemoreceptors. Lesioning and recording studies show direct evidence that the KF-area is involved in mediating the magnitude of the arterial chemoreceptor reflex (204, 256, 273, 323). Several investigations using the immediate early gene c-Fos as a marker of neural activity have revealed strong activation of the KF-area during hypoxia, hypercapnia or carotid sinus nerve stimulation (32, 40, 108, 173, 218, 378). In the cat and the rats, bilateral lesions of the KF-area attenuated increases in respiratory minute volume during hypoxic or hypercapnic challenges (256, 323, 364). But discrete lesions in the lateral PB nuclei within the KF-area prevent the shortening of the expiratory phase that is associated with the increase in respiratory frequency in response to hypoxia and hypercapnia (349, 350). Thus, perhaps the interaction between pontine and medullary networks contributes to respiratory frequency responses during hypoxia and hypercapnia. A potential interaction between dorsolateral pons and the caudal vl A5 region is discussed in section “The A5 Cell Group in the Ventrolateral Pons.” Finally, the processing of chemosensory information in the KF-area is also of importance during the mediation of adaptive breathing behavior to maintain and control homeostasis.
The role of the KF-area in the modulation of breathing across the sleep/wake cycle
Breathing displays distinct changes during sleep and wake-fulness (281, 282). Early studies report that chronic lesion of the KF-area in the cat change the respiratory pattern and rhythm during sleep states (21, 45, 134, 357, 360, 361). Subsequent vagotomy in these same cats exacerbated breathing instability and resulted in apneusis during rapid eye movement (REM)-sleep (21). Pontine respiratory-modulated neurons modulate their discharge pattern to arousal state alteration, specifically with a significant decrease in activity during REM-sleep (239,340). This general pattern also occurred in a Carbachol (mixed cholinergic agonist) induced REM-sleep-like state (139,140). Furthermore, the discharge frequency of expiratory modulated neuron activity was decreased considerably more compared to inspiratory unit activity (139, 140). However, tonic or weakly modulated respiratory neurons in the KF-area show heterogeneous effects, and are either activated or deactivated following Carbachol induced REM-sleep like states or during natural REM sleep (139, 140).
Unfortunately, the potential role of KF-area in sleep-related respiratory disorders, specifically obstructive or central sleep apnea is not clear. Considering that the KF-area excites upper airway motor neurons responsible for maintenance of upper airway patency (94,95,137,214,216), it would be likely that a decrease in discharge of the KF-area activity could contribute to development of upper airway atonia. Consequently, disfacilitation of upper airway activity could contribute to decreased upper airway muscle tone and obstructive sleep apnea. The reported decrease in discharge activity of expiratory units as well as the decrease in the activity of tonic KF-area units during REM-sleep could be effectively linked to the decrease in upper airway tone during REM-sleep. This may play a key role in the pathologically reduced airway patency contributing to obstructive REM-sleep apnea, in a variety of clinical settings.
The role of parabrachial and KF nuclei in the cardiovascular-respiratory coupling
Apart from their role in breathing control the PB and KF nuclei are implicated in cardiovascular regulation. This is consistent with the fact that cardiovascular and respiratory regulations are coupled (e.g., cardiac vagal, sympathetic, and phrenic activity patterns have common frequency components). This is also consistent with the strong anatomical supposition of respiratory and cardiovascular neural networks in the brainstem (75,156,268,376). In the following section, we briefly introduce the potential role of the PB and KF nuclei in cardiovascular-respiratory coupling.
Cardiovascular and respiratory activities are inherently rhythmic. Cardiovascular-respiratory coupling refers not only to the influence of respiratory activity on heartbeat (380) and sympathetic nerve activity (5) but also to the expression of the arterial-pulse pressure on respiratory activity (84, 85). Thus, cardiovascular-respiratory coupling has been shown to be reciprocal (75,80). Cardiovascular-respiratory coupling may serve systemic homeostasis by coordinating blood flow and blood gasses for optimal efficiency. Gas exchange as an integrated function is akin to studying effectors of heart rate and vascular resistance for the cardiovascular system or blood gasses and lung volume for the respiratory system.
In each respiratory cycle, the heart rate increases during inspiration, which is referred to as respiratory sinus arrhythmia, while sympathetic nerve activity increases during post-I. This coupling depends on both reflex and central mechanisms (106, 193, 198, 199), although the discrete mechanisms involved are still controversial. A central neural mechanism is evident that a sinus arrhythmia can be identified in the in situ preparation in which rhythmic sensory (including vagal, chemo-, and baroreceptors) inputs are blocked (17, 80). Furthermore, after dorsolateral pontine transection or bilateral lesion in the dorsolateral pons, the respiratory modulation of heart rate and of sympathetic nerve activity is attenuated (17, 263, 264). Thus, coupling, at the least, the respiratory modulated rhythm in the heart rate and in the sympathetic nerve is modulated by the pons.
Parabrachial complex-mediated modulation of breathing during pain
The lateral nuclei subnuclei of the PB complex receive both second- and higher order relay neurons for nociceptive sensory information (30, 52, 133, 190, 403). Nociceptive stimuli trigger the profound alterations in cardiorespiratory functions such as apnea/tachypnea, tachycardia/bradycardia, and rise or fall in arterial blood pressure associated with nociceptive stimuli. The specific cardiorespiratory responses depend on the strength and source of input. The PB complex plays a role in mediating these cardiorespiratory responses. Moreover, chemical stimulation of the lateral PB nuclei (59, 60, 93, 94, 216, 217, 276) may be associated directly with the processing of pain. However, so far, only a single study provides evidence that the lateral crescent PB nucleus links nociception and breathing (190). Thus, the lateral PB complex may be specifically involved in mediating the respiratory response to nociceptive stimuli.
Brief summary of additional physiological functions associated with the PB complex
The PB complex is an important relay of the central taste pathway. Particularly, nuclei of caudal PB complex relay gustatory information from the rostral nucleus of NTS to the ventrobasal nucleus of the thalamus (272). Another related role of PB nuclei is the regulation of water and sodium intake (248). In general, forebrain limbic and reward systems receive ingestion-taste-related information via the PB nuclei [for review see references 88 and 407)]. An elegant recent study showed that transgenic inactivation of GABA synthesis in the arcuate nucleus caused a loss of GABAergic signaling to the PB nucleus that resulted in voluntary starvation of the animals (406). Moreover, of particular importance is the role of PB complex in conditioned taste aversion as a propensity of learning and memory (401). The role in taste aversion underlines the role of PB complex in the expression of learned behavior (407). In addition to taste and ingestion, other related systems are influenced by PB nuclei. The lateral PB nuclei have been implicated in thermoregulation (265, 271). The potential role of lateral PB nuclei in respiratory-thermoregulatory interaction during exercise was recently discussed but still requires experimental evidence (295). Furthermore, neurons in the KF-area are involved in defecation (122, 123, 186). Stimulation of the KF-area can also elicit sustained and rhythmic straining behavior in the decerebrate dog (122, 123). The involvement of the PB complex in straining behavior is supported by dense projections to the Barrington’s nucleus, which is commonly referred to as the pontine micturition center, located in the dorsolateral pontine tegmentum (177). Finally, lesions of PB nuclei accelerate incisor growth in Long-Evans rats (270), while stimulation of the PB nuclei also evokes salivation in cats (83).
Intertrigeminal Region of the Mediolateral Pons
The ITR, also defined as peritrigeminal region, lies between the principal sensory and motor trigeminal nuclei and interconnects the rostral dorsolateral and the caudal vl pontine respiratory nuclei. Glutamate microinjected into the ITR consistently triggers brief apnea which indicates that this nucleus is an “expiratory-facilitatory” area (57, 60). Similar to the KF-area the ITR possesses reciprocal connectivity with the medullary LRC and brainstem motor nuclei (180, 181). In addition, the ITR receives synaptic input from the NTS and spinal trigeminal nuclei. A more recent study has identified anatomical connectivity connection between the ITR and the medullary raphé nuclei (384). All three of these sites, NTS, spinal trigeminal and raphé nuclei, are associated with sensory apnea; therefore, the ITR may be involved in mediating apnea. Apnea evoked by glutamate injections in the ITR could be blocked by injection of glutamate receptor antagonist (303–305). NMDA-R blockade completely abolished, while AMPA-receptor antagonism only partially attenuates the glutamate-evoked apnea (187). Furthermore, AMPA-receptor blockade did not affect vagally mediated reflex apnea induced by intravenous infusion of serotonin (187), while pharmacological blockade of metabotropic glutamate receptors exacerbated apneic responses to intravenous serotonin (365). Thus, functional apneic responses from the ITR may be mediated via NMDA-R, which are reported to be densely expressed in this pontine brain region (151). Yet, ironically unilateral lesioning of the ITR region increases the incidence of sleep apnea (302).
The ventral tip of the KF has also been identified as an apneic site (59, 60, 93, 94). Therefore, the close proximity of these structures hinders distinguishing the physiological exclusivity of the ventral parts of KF and ITR. Mapping the dorsolateral and mediolateral pons with glutamate injection provides some evidence that KF stimulation triggers a post-I protective apnea associated with strong laryngeal constriction, while the apnea evoked from the ITR region appears to occur without laryngeal constriction (94). If ITR and KF mediate functionally different types of apnea then this would provide insight into the mechanism of apnea.
The A5 Cell Group in the Ventrolateral Pons
The A5 cell group in the vl pons contains both noradrenergic and glutamatergic neurons that modulate cardiovascular and respiratory function. Anatomical studies revealed that vl pons project caudally to the medullary cardiorespiratory centers and to the sympathetic premotor neurons in the spinal cord (6–8). However, the role of the vl pons may be species specific, because of the known differences between the rat and the cat, the two primary models studied in cardiorespiratory control. Furthermore, the anatomy of the vl pons has not been well defined into subnuclei as in the case of the PB complex. The A5 neurons, the primary structure to which the actions of the vl pons are attributed is a distributed cluster group of neurons. For the purposes of this review, we focus on the A5-area because it projects directly to the rostral ventral respiratory group and that it was well integrated in the LRC (6–8). With regards to respiratory control, vl pons like the dl pons interacts with BHR inputs to determine respiratory phase duration. Thus, these pontine areas may have interconnections (351).
A role for the vl pons in cardiovascular regulation was identified prior to its potential role in respiratory control. Retrograde labeling studies demonstrate that the noradrenaline-containing neurons of the A5 cell group project directly to the preganglionic sympathetic neurons (54). Stimulation of A5 neurons with glutamate revealed differential control of blood flow in both anesthetized rat and dog and in the unanesthetized rabbit (87,183,243). Specifically, A5-area activation increases splanchnic and renal nerve activity but usually decreases lumbar sympathetic nerve activity (87,183,243). However, blocking A5 activity with injections of muscimol does not affect resting renal sympathetic nerve activity in the unanesthetized rabbit (242). Recording A5 neurons identified via antidromic activation of their axons in the spinal cord and by its complete inactivation following systemic injections of clonidine indicates that these cells have a respiratory (post-I)-modulated activity pattern (154,184). Although many bulbo-spinal sympathetic neurons express respiratory-modulated activity, this type of activity indicates a potential role for the vl pons in respiratory as well as sympathetic control.
Subsequent studies provided extensive evidence on the vl pons modulated respiration in the rat. While bilateral electrolytic lesions in vl pons cause apneusis, experiments that involve bilateral injections of muscimol (GABAA-receptor agonist) only slightly prolongs inspiration. It is to be noted that the prolongation of inspiratory duration (Ti) does not result in the profound apneusis that followed similar dl pontine intervention (191). However, as observed for the KF-area, stimulating the vagi reverses the apneusis (191) and in spontaneously breathing rats baseline-breathing patterns are also altered only slightly following vl pontine interventions in the vagi intact rat (69, 82). Activation of neurons in the vl pons with glutamate prolongs expiratory duration (Te) (192). It appears that like KF-area, ITR is also primarily an expiratory-facilitatory area. Thus, this vl pons may be interpreted as a ventrocaudal extension of the expiratory-facilitatory areas. Recording revealed respiratory modulated activity particularly post-I and E activity. These activities are responsive to hypoxia (82, 154). The expiratory-facilitatory effect evoked by stimulation of the vl pons is consistent with the recording of expiratory-modulated activity within.
The expiratory-facilitatory role of the vl pons becomes physiologically evident in the dynamic regulation of breathing during and following brief hypoxic episodes. The changes in the respiratory pattern during hypoxia depend on numerous factors including the age of the animals and the severity of the hypoxic episode, that is, the partial pressure of the oxygen and the duration of the exposure (298). Briefly, during severe hypoxia respiratory frequency increases rapidly and then begins to slow. If the exposure persists then breathing will stop and gasping occurs. If the hypoxic exposure is stopped, breathing frequency will drop below normal and slowly return to normal. The drop in breathing frequency is referred to as posthypoxic frequency decline. Interestingly, these changes in respiratory frequency originate from the neural network rather than a result of metabolic demands because they have been observed during and following carotid sinus nerve stimulation under hyperoxic conditions also (162). The amplitude of the motor activity however follows a different course. Amplitudes increase to an asymptote and after hypoxia decreases to baseline. In the rat, both the dl and vl pons have a role in mediating the changes in respiratory frequency during and following hypoxia.
As discussed in Section “Intertrigeminal Region of the Mediolateral Pons,” in the cat, a facilitatory role has been identified for the PB complex (358). In rat, the facilitatory role of the vl pons differs from that of the KF-area. Even though bilateral interventions in either KF-area or vl pons results in a significant decrease in baseline respiratory frequency, specific elements in response to hypoxia can be affected differentially. When rats with bilateral lesions of the lateral external nucleus of the PB complex are exposed to hypoxia, expiratory duration does not decrease during hypoxia (350). The change in Te was the only alteration in the hypoxic response; Ti decreased during hypoxia and Te prolonged following hypoxia just as before the interventions (350). In contrast, when rats with bilateral vl pontine interventions were exposed to hypoxia, Ti and Te decreased with no changes in peak respiratory frequency. However, Te does not decrease after hypoxia (69,82). Thus, even though normal breathing pattern was altered following vl pontine lesions, the acute response remains during hypoxia and only the posthypoxic pattern is altered (69, 82). These data raise the possibility that interaction between the KF-area and vl pons is critical for the mediation of breathing changes in hypoxia.
Recordings show that vl pontine neurons respond to hypoxia. Further, this activity may arise from A5 neurons (204). First, selective lesioning of the noradrenergic neurons with the toxin 6-hydroxydopamine (6-OHDA) in the vl pons attenuated the sympathetic chemoreflex to the same extent as muscimol in the vl pons. Thus, neurons in the vl pons are activated during hypoxia and continue to remain activated. Expiratory neurons in the A5 region fit this profile. Their activity correlates with prolongations in Te during and following hypoxia.
Noradrenergic neurotransmission may mediate the Te prolongation associated with post hypoxic frequency decline after brief hypoxia. Noradrenaline has been demonstrated to be produced and released by A5 neurons, and activation of α2-adrenergic receptors modulates Te (41, 164). In addition, with an en bloc in vitro neonatal rat brainstem-spinal cord preparation, bath application of α2-antagonists increased burst frequency (170, 171). The decrease in respiratory frequency following hypoxia was not consistently blocked after administration of α2-antagonists (16,70). These data although nonlinear, do support the general working hypothesis that the vl pons acting via A5 neurons modulate breathing.
Noradrenaline function and dysfunction in breathing
In the last 25 years, the use of preparations, which are tractable to experiments in neonatal rodent models and identifying specific gene deficits that are associated with aberrant respiratory patterns, have revealed the role f the vl pons in generating a stable breathing pattern.
The en bloc preparation developed in the 1980s is a super-fused in vitro preparation consisting of the brainstem spinal cord (372). The phrenic bursts produced in this preparation are dependent on the integrity of the pontomedullary circuitry. The bursting frequency increased when the pons was removed (109). The increased rate resulted from removing a noradrenergic input located in the pons, specifically the A5 neurons in the vl pons. However, even prior to that, it was evident that action of noradrenaline was complex, with the ability to increase or decrease respiratory rate depending on the site of action (pons or medulla, dorsal or ventral, and α1- or α2-receptors) (109, 386, 387). The en bloc preparation indicates that the breathing pattern was modulated by pontine noradrenergic neurons even in prenatal preparations.
Subsequent work on genetic “knockouts” shows the importance of pontine noradrenaline neurons in the development of the respiratory control network (169). The HASH-1-PHOX2A-PHOX2B developmental cascade differentially controls the development of neurons Locus Coeruleus (PHOX2A), A5-area (PHOX2B), and other brainstem catecholaminergic cells with a definitive or transient noradrenaline phenotype (10, 78). Initially, mutations in the PHOX2 and MECP2 (discussed earlier) genes were thought to primarily affect noradrenaline-expressing neurons. Thus, a noradrenaline connection was thought to underlie the disorderly breathing patterns associated with severe respiratory disturbances such as SIDS (169), CCHS, and Rett syndrome (78). However, recent studies showed that the RTN/pFRG is perhaps the more directly affected system. Indeed, ablation of catecholamine-containing neurons (with saporin conjugated to dopamine-beta-hydroxylase) decreases breathing frequency and chemoresponsiveness in adult animals after development of the respiratory control system, but in these animals breathing arrest did not take place during sleep (224).
CCHS results from polyalanine repeat expansion mutations in the paired-like homeobox (PHOX)2B gene in more than 90% of cases. CCHS is characterized by sleep apnea attributed to decreased chemoreflex excitatory drive (33). However, despite the association of Phox2b with catecholaminergic neurons, in the adult rat Phox2b is not expressed in pontine noradrenaline cell groups but rather in medullary neurons involved in chemosensation directly (366). Indeed, mice with mutations like the humans (with polyalanine repeat expansion (Phox2b27Ala/+) lack Phox2b-expressing glutamatergic neurons in the parafacial region of the ventral medulla and die soon after birth from central apnea (90).
Conclusion
Compared to the rhythmogenic medullary respiratory circuits, research on pontine nuclei that shape and modulate the eupneic respiratory rhythm and pattern, still remains largely uninvestigated. Here, we aimed to underline the physiological significance for a pontine mediated IOS for the generation of both the eupneic and adaptive breathing pattern. The IOS is not a simple termination of inspiratory motor activity, but is tightly linked to the motor act of laryngeal adduction (constriction). The dynamic regulation of the strength and duration of laryngeal adduction controls the expiratory airflow profile or primes breathing to be integrated with various oropharyn-geal motor acts, such as swallowing or vocalizing. The multimodal sensory influences, which include processing of higher behavioral and emotional commands, on the timing and dynamic of the IOS and laryngeal adduction, manifests within convergent and reciprocally connected pontomedullary rCPG circuits.
The descending inputs from the midbrain, limbic, and forebrain structures, and in turn its reciprocal connectivity with various brainstem sensory and motor nuclei illustrates that the pons function as the supramedullary control center for breathing. We coin this functionality of the pons as the “adaptive breathing center.” Adaptive breathing requires a high degree of synaptic plasticity that is linked to physiological aspects of learning and memory. The future is ripe to understand the microcircuitry and synaptic processing underlying adaptive breathing. For this breathing should be investigated as a quintessential behavior rather than stereotypic generation of rhythm.
Acknowledgments
Research in Mathias Dutschmann’s laboratory has been supported by grants from the Center of Molecular Physiology of the Brain and the Bernstein Center for Computational Neurosciences at Göttingen and in Thomas E. Dick’s laboratory by National Institutes Health (National Heart, Lung and Blood Institute) and American Lung Association. The authors would like to thank Dr. Hari Subramanian for helpful suggestions and corrections.
References
- 1.Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: Patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:19–25. doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adrian ED, Bronk DW. Discharges in mammalian sympathetic nerves. J Physiol. 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- 7.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: An overview. Respir Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: Lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol. 2009;168:125–132. doi: 10.1016/j.resp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- 11.Andrzejewski M, Muckenhoff K, Scheid P, Ballantyne D. Synchronized rhythms in chemosensitive neurons of the locus coeruleus in the absence of chemical synaptic transmission. Respir Physiol. 2001;129:123–140. doi: 10.1016/s0034-5687(01)00300-0. [DOI] [PubMed] [Google Scholar]
- 12.Arata A. Respiratory activity of the neonatal dorsolateral pons in vitro. Respir Physiol Neurobiol. 2009;168:144–152. doi: 10.1016/j.resp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Arsenault J, Moreau-Bussiere F, Reix P, Niyonsenga T, Praud JP. Postnatal maturation of vagal respiratory reflexes in preterm and full-term lambs. J Appl Physiol. 2003;94:1978–1986. doi: 10.1152/japplphysiol.00480.2002. [DOI] [PubMed] [Google Scholar]
- 14.Arvanian VL, Bowers WJ, Petruska JC, Motin V, Manuzon H, Narrow WC, Federoff HJ, Mendell LM. Viral delivery of NR2D subunits reduces Mg2+ block of NMDA receptor and restores NT-3-induced potentiation of AMPA-kainate responses in maturing rat motoneurons. J Neurophysiol. 2004;92:2394–2404. doi: 10.1152/jn.00278.2004. [DOI] [PubMed] [Google Scholar]
- 15.Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: Anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 16.Bach KB, Kinkead R, Mitchell GS. Post-hypoxia frequency decline in rats: Sensitivity to repeated hypoxia and alpha2-adrenoreceptor antagonism. Brain Res. 1999;817:25–33. doi: 10.1016/s0006-8993(98)01181-0. [DOI] [PubMed] [Google Scholar]
- 17.Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol. 2008;93:803–816. doi: 10.1113/expphysiol.2007.041400. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- 19.Baker JP, Jr, Remmers JE. Response of medullary respiratory neurons to rostral pontine stimulation. Respir Physiol. 1982;50:197–208. doi: 10.1016/0034-5687(82)90018-4. [DOI] [PubMed] [Google Scholar]
- 20.Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: Differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol. 2001;129:25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- 21.Baker TL, Netick A, Dement WC. Sleep-related apneic and apneustic breathing following pneumotaxic lesion and vagotomy. Respir Physiol. 1981;46:271–294. doi: 10.1016/0034-5687(81)90127-4. [DOI] [PubMed] [Google Scholar]
- 22.Balland B, Lachamp P, Kessler JP, Tell F. Silent synapses in developing rat nucleus tractus solitarii have AMPA receptors. J Neurosci. 2008;28:4624–4634. doi: 10.1523/JNEUROSCI.5355-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balland B, Lachamp P, Strube C, Kessler JP, Tell F. Glutamatergic synapses in the rat nucleus tractus solitarii develop by direct insertion of calcium-impermeable AMPA receptors and without activation of NMDA receptors. J Physiol. 2006;574:245–261. doi: 10.1113/jphysiol.2006.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballantyne D, Andrzejewski M, Muckenhoff K, Scheid P. Rhythms, synchrony and electrical coupling in the Locus coeruleus. Respir Physiol Neurobiol. 2004;143:199–214. doi: 10.1016/j.resp.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett D., Jr Respiratory functions of the larynx. Physiol Rev. 1989;69:33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Batsel HL, Lines AJ. Neural mechanisms of sneeze. Am J Physiol. 1975;229:770–776. doi: 10.1152/ajplegacy.1975.229.3.770. [DOI] [PubMed] [Google Scholar]
- 28.Bavis RW, Olson EB, Jr, Vidruk EH, Fuller DD, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J Physiol. 2004;557:645–660. doi: 10.1113/jphysiol.2004.061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger AJ, Herbert DA, Mitchell RA. Properties of apneusis produced by reversible cold block of the rostral pons. Respir Physiol. 1978;33:323–327. doi: 10.1016/0034-5687(78)90059-2. [DOI] [PubMed] [Google Scholar]
- 30.Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: Electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- 31.Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: A PHA-L study in the rat. J Comp Neurol. 1995;353:480–505. doi: 10.1002/cne.903530403. [DOI] [PubMed] [Google Scholar]
- 32.Berquin P, Cayetanot F, Gros F, Larnicol N. Postnatal changes in Fos-like immunoreactivity evoked by hypoxia in the rat brainstem and hypothalamus. Brain Res. 2000;877:149–159. doi: 10.1016/s0006-8993(00)02632-9. [DOI] [PubMed] [Google Scholar]
- 33.Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med. 2006;174:1139–1144. doi: 10.1164/rccm.200602-305OC. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: Neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi AL, Gestreau C. The brainstem respiratory network: An overview of a half century of research. Respir Physiol Neurobiol. 2009;168:4–12. doi: 10.1016/j.resp.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi AL, St John WM. Pontile axonal projections of medullary respiratory neurons. Respir Physiol. 1981;45:167–183. doi: 10.1016/0034-5687(81)90058-x. [DOI] [PubMed] [Google Scholar]
- 37.Bianchi AL, St John WM. Medullary axonal projections of respiratory neurons of pontile pneumotaxic center. Respir Physiol. 1982;48:357–373. doi: 10.1016/0034-5687(82)90039-1. [DOI] [PubMed] [Google Scholar]
- 38.Bianchi R, Corsetti G, Rodella L, Tredici G, Gioia M. Supraspinal connections and termination patterns of the parabrachial complex determined by the biocytin anterograde tract-tracing technique in the rat. J Anat. 1998;193(Pt 3):417–430. doi: 10.1046/j.1469-7580.1998.19330417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–R1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- 40.Bodineau L, Larnicol N. Brainstem and hypothalamic areas activated by tissue hypoxia: Fos-like immunoreactivity induced by carbon monoxide inhalation in the rat. Neuroscience. 2001;108:643–653. doi: 10.1016/s0306-4522(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 41.Bolme P, Fuxe K. Pharmacological studies on a possible role of central noradrenaline neurons in respiratory control. J Pharm Pharmacol. 1973;25:351–352. doi: 10.1111/j.2042-7158.1973.tb10027.x. [DOI] [PubMed] [Google Scholar]
- 42.Bongianni F, Mutolo D, Carfi M, Fontana GA, Pantaleo T. Respiratory neuronal activity during apnea and poststimulatory effects of laryngeal origin in the cat. J Appl Physiol. 2000;89:917–925. doi: 10.1152/jappl.2000.89.3.917. [DOI] [PubMed] [Google Scholar]
- 43.Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- 44.Bonham AC, Chen CY, Sekizawa S, Joad JP. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol. 2006;101:322–327. doi: 10.1152/japplphysiol.00143.2006. [DOI] [PubMed] [Google Scholar]
- 45.Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol. 2010;109:159–170. doi: 10.1152/japplphysiol.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. Site-specific effects on respiratory rhythm and pattern of ibotenic acid injections in the pontine respiratory group of goats. J Appl Physiol. 2010;109:171–188. doi: 10.1152/japplphysiol.00934.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonis JM, Neumueller SE, Marshall BD, Krause KL, Qian B, Pan LG, Hodges MR, Forster HV. The effects of lesions in the dorsolateral pons on the coordination of swallowing and breathing in awake goats. Respir Physiol Neurobiol. 2011;175:272–282. doi: 10.1016/j.resp.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borday V, Foutz AS, Nordholm L, Denavit-Saubie M. Respiratory effects of glutamate receptor antagonists in neonate and adult mammals. Eur J Pharmacol. 1998;348:235–246. doi: 10.1016/s0014-2999(98)00160-5. [DOI] [PubMed] [Google Scholar]
- 49.Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- 50.Breuer J. Akad Wiss. Wien: 1868. Die Selbststeurung der Athmung durch den Nervus Vagus; pp. 909–937. [Google Scholar]
- 51.Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 52.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 53.Burke PG, Abbott SB, McMullan S, Goodchild AK, Pilowsky PM. Somatostatin selectively ablates post-inspiratory activity after injection into the Botzinger complex. Neuroscience. 2010;167:528–539. doi: 10.1016/j.neuroscience.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 54.Byrum CE, Guyenet PG. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1987;261:529–542. doi: 10.1002/cne.902610406. [DOI] [PubMed] [Google Scholar]
- 55.Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- 56.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143:115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamberlin NL, Saper CB. Differential distribution of AMPA-selective glutamate receptor subunits in the parabrachial nucleus of the rat. Neuroscience. 1995;68:435–443. doi: 10.1016/0306-4522(95)00129-7. [DOI] [PubMed] [Google Scholar]
- 60.Chamberlin NL, Saper CB. A brainstem network mediating apneic reflexes in the rat. J Neurosci. 1998;18:6048–6056. doi: 10.1523/JNEUROSCI.18-15-06048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarke RJ, Johnson JW. NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J Neurosci. 2006;26:5825–5834. doi: 10.1523/JNEUROSCI.0577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- 64.Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol. 1971;217:133–158. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen MI. Central determinants of respiratory rhythm. Annu Rev Physiol. 1981;43:91–104. doi: 10.1146/annurev.ph.43.030181.000515. [DOI] [PubMed] [Google Scholar]
- 66.Cohen MI, Feldman JL. Models of respiratory phase-switching. Fed Proc. 1977;36:2367–2374. [PubMed] [Google Scholar]
- 67.Cohen MI, Huang WX, Barnhardt R, See WR. Timing of medullary late-inspiratory neuron discharges: Vagal afferent effects indicate possible off-switch function. J Neurophysiol. 1993;69:1784–1787. doi: 10.1152/jn.1993.69.5.1784. [DOI] [PubMed] [Google Scholar]
- 68.Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol. 2004;143:127–140. doi: 10.1016/j.resp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 69.Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J Physiol. 1996;497(Pt 1):79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coles SK, Ernsberger P, Dick TE. A role for NMDA receptors in posthypoxic frequency decline in the rat. Am J Physiol. 1998;274:R1546–R1555. doi: 10.1152/ajpregu.1998.274.6.R1546. [DOI] [PubMed] [Google Scholar]
- 71.Connelly CA, Otto-Smith MR, Feldman JL. Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res. 1992;596:99–110. doi: 10.1016/0006-8993(92)91537-o. [DOI] [PubMed] [Google Scholar]
- 72.Costa-Silva JH, Zoccal DB, Machado BH. Glutamatergic antagonism in the NTS decreases post-inspiratory drive and changes phrenic and sympathetic coupling during chemoreflex activation. J Neurophysiol. 2010;103:2095–2106. doi: 10.1152/jn.00802.2009. [DOI] [PubMed] [Google Scholar]
- 73.Cowie RJ, Holstege G. Dorsal mesencephalic projections to pons, medulla, and spinal cord in the cat: Limbic and non-limbic components. J Comp Neurol. 1992;319:536–559. doi: 10.1002/cne.903190406. [DOI] [PubMed] [Google Scholar]
- 74.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: Diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 75.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 76.D’Angelo E, Monaco A, Pecchiari M. Motor control of the diaphragm in anesthetized rabbits. Respir Physiol Neurobiol. 2010;170:141–149. doi: 10.1016/j.resp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Denavit-Saubie M, Kalia M, Pierrefiche O, Schweitzer P, Foutz AS, Champagnat J. Maturation of brain stem neurons involved in respiratory rhythmogenesis: Biochemical, bioelectrical and morphological properties. Biol Neonate. 1994;65:171–175. doi: 10.1159/000244048. [DOI] [PubMed] [Google Scholar]
- 78.de Pontual L, Nepote V, Attie-Bitach T, Al Halabiah H, Trang H, Elghouzzi V, Levacher B, Benihoud K, Auge J, Faure C, Laudier B, Vekemans M, Munnich A, Perricaudet M, Guillemot F, Gaultier C, Lyonnet S, Simonneau M, Amiel J. Noradrenergic neuronal development is impaired by mutation of the proneural HASH-1 gene in congenital central hypoventilation syndrome (Ondine’s curse) Hum Mol Genet. 2003;12:3173–3180. doi: 10.1093/hmg/ddg339. [DOI] [PubMed] [Google Scholar]
- 79.Dhingra RR, Jacono FJ, Fishman M, Loparo KA, Rybak IA, Dick TE. Vagal-Dependent Nonlinear Variability in the Respiratory Pattern of anesthetized, spontaneously breathing rats. J Appl Physiol. 2011;111:272–284. doi: 10.1152/japplphysiol.91196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol. 2009;168:76–85. doi: 10.1016/j.resp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res. 1994;636:259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- 82.Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- 83.Dick TE, Haxhiu MA, Cherniack NS. Salivary secretion elicited by activation of the parabrachial nuclei in the cat. J Auton Nerv Syst. 1992;39:19–27. doi: 10.1016/0165-1838(92)90247-e. [DOI] [PubMed] [Google Scholar]
- 84.Dick TE, Morris KF. Quantitative analysis of cardiovascular modulation in respiratory neural activity. J Physiol. 2004;556:959–970. doi: 10.1113/jphysiol.2003.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Arterial pulse modulated activity is expressed in respiratory neural output. J Appl Physiol. 2005;99:691–698. doi: 10.1152/japplphysiol.01124.2004. [DOI] [PubMed] [Google Scholar]
- 86.Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol. 2008;586:4265–4282. doi: 10.1113/jphysiol.2008.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dickerson LW, Panico WH, Kuhn FE, Willis AC, Fitzgerald JF, Meyer EL, Norman WP, Gillis RA. Stimulation of dog RVLM and A5 area changes sympathetic outflow to vascular beds without effect on the heart. Am J Physiol. 1997;272:R821–R839. doi: 10.1152/ajpregu.1997.272.3.R821. [DOI] [PubMed] [Google Scholar]
- 88.Di Lorenzo PM, Platt D, Victor JD. Information processing in the parabrachial nucleus of the pons. Ann N Y Acad Sci. 2009;1170:365–371. doi: 10.1111/j.1749-6632.2009.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 90.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dutschmann M, Herbert H. The Kolliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport. 1996;7:1432–1436. doi: 10.1097/00001756-199605310-00022. [DOI] [PubMed] [Google Scholar]
- 92.Dutschmann M, Herbert H. Fos expression in the rat parabrachial and Kolliker-Fuse nuclei after electrical stimulation of the trigeminal ethmoidal nerve and water stimulation of the nasal mucosa. Exp Brain Res. 1997;117:97–110. doi: 10.1007/s002210050203. [DOI] [PubMed] [Google Scholar]
- 93.Dutschmann M, Herbert H. NMDA and GABAA receptors in the rat Kolliker-Fuse area control cardiorespiratory responses evoked by trigeminal ethmoidal nerve stimulation. J Physiol. 1998;510(Pt 3):793–804. doi: 10.1111/j.1469-7793.1998.793bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dutschmann M, Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 95.Dutschmann M, Kron M, Morschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Dutschmann M, Menuet C, Stettner GM, Gestreau C, Borghgraef P, Devijver H, Gielis L, Hilaire G, Van Leuven F. Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei. J Neurosci. 2010;30:1810–1821. doi: 10.1523/JNEUROSCI.5261-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dutschmann M, Morschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: Implications for the pontine Kolliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 98.Dutschmann M, Morschel M, Reuter J, Zhang W, Gestreau C, Stettner GM, Kron M. Postnatal emergence of synaptic plasticity associated with dynamic adaptation of the respiratory motor pattern. Respir Physiol Neurobiol. 2008;164:72–79. doi: 10.1016/j.resp.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Dutschmann M, Morschel M, Rybak IA, Dick TE. Learning to breathe: Control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol. 2009;587:4931–4948. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dutschmann M, Paton JF. Glycinergic inhibition is essential for coordinating cranial and spinal respiratory motor outputs in the neonatal rat. J Physiol. 2002;543:643–653. doi: 10.1113/jphysiol.2001.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dutschmann M, Paton JF. Influence of nasotrigeminal afferents on medullary respiratory neurones and upper airway patency in the rat. Pflugers Arch. 2002;444:227–235. doi: 10.1007/s00424-002-0797-x. [DOI] [PubMed] [Google Scholar]
- 102.Dutschmann M, Paton JF. Inhibitory synaptic mechanisms regulating upper airway patency. Respir Physiol Neurobiol. 2002;131:57–63. doi: 10.1016/s1569-9048(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 103.Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JF. The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc Lond B Biol Sci. 2009;364:2611–2623. doi: 10.1098/rstb.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dutschmann M, Wilson RJ, Paton JF. Respiratory activity in neonatal rats. Auton Neurosci. 2000;84:19–29. doi: 10.1016/S1566-0702(00)00177-6. [DOI] [PubMed] [Google Scholar]
- 105.Easton PA, Katagiri M, Kieser TM, Platt RS. Postinspiratory activity of costal and crural diaphragm. J Appl Physiol. 1999;87:582–589. doi: 10.1152/jappl.1999.87.2.582. [DOI] [PubMed] [Google Scholar]
- 106.Eckberg DL. Point:counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1740–1742. doi: 10.1152/japplphysiol.91107.2008. discussion 1744. [DOI] [PubMed] [Google Scholar]
- 107.Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- 108.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within cate-cholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 109.Errchidi S, Monteau R, Hilaire G. Noradrenergic modulation of the medullary respiratory rhythm generator in the newborn rat: An in vitro study. J Physiol. 1991;443:477–498. doi: 10.1113/jphysiol.1991.sp018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- 111.Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience. 2006;141:1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 112.Farley GR, Barlow SM, Netsell R. Factors influencing neural activity in parabrachial regions during cat vocalizations. Exp Brain Res. 1992;89:341–351. doi: 10.1007/BF00228250. [DOI] [PubMed] [Google Scholar]
- 113.Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res Brain Res Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- 114.Fedorko L, Kelly EN, England SJ. Importance of vagal afferents in determining ventilation in newborn rats. J Appl Physiol. 1988;65:1033–1039. doi: 10.1152/jappl.1988.65.3.1033. [DOI] [PubMed] [Google Scholar]
- 115.Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker-Fuse nuclei. J Comp Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- 116.Feldman JL, Cohen MI, Wolotsky P. Powerful inhibition of pontine respiratory neurons by pulmonary afferent activity. Brain Res. 1976;104:341–346. doi: 10.1016/0006-8993(76)90629-6. [DOI] [PubMed] [Google Scholar]
- 117.Feldman JL, Del Negro CA. Looking for inspiration: New perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feldman JL, Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J Neurophysiol. 1976;39:31–44. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- 119.Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25:166–177. doi: 10.1016/j.tig.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 120.Foutz AS, Champagnat J, Denavit-Saubie M. N-methyl-D-aspartate (NMDA) receptors control respiratory off-switch in cat. Neurosci Lett. 1988;87:221–226. doi: 10.1016/0304-3940(88)90452-1. [DOI] [PubMed] [Google Scholar]
- 121.Foutz AS, Champagnat J, Denavit-Saubie M. Involvement of N-methyl-D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Res. 1989;500:199–208. doi: 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- 122.Fukuda H, Fukai K. Location of the reflex centre for straining elicited by activation of pelvic afferent fibres of decerebrate dogs. Brain Res. 1986a;380:287–296. doi: 10.1016/0006-8993(86)90224-6. [DOI] [PubMed] [Google Scholar]
- 123.Fukuda H, Fukai K. Postural change and straining induced by distension of the rectum, vagina and urinary bladder of decerebrate dogs. Brain Res. 1986b;380:276–286. doi: 10.1016/0006-8993(86)90223-4. [DOI] [PubMed] [Google Scholar]
- 124.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 125.Fung ML, St John WM. Neuronal activities underlying inspiratory termination by pneumotaxic mechanisms. Respir Physiol. 1994;98:267–281. doi: 10.1016/0034-5687(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 126.Fung ML, St John WM. The functional expression of a pontine pneumotaxic centre in neonatal rats. J Physiol. 1995;489(Pt 2):579–591. doi: 10.1113/jphysiol.1995.sp021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fung ML, Wang W, St John WM. Involvement of pontile NMDA receptors in inspiratory termination in rat. Respir Physiol. 1994;96:177–188. doi: 10.1016/0034-5687(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 128.Gallego J, Nsegbe E, Durand E. Learning in respiratory control. Behav Modif. 2001;25:495–512. doi: 10.1177/0145445501254002. [DOI] [PubMed] [Google Scholar]
- 129.Gang S, Mizuguchi A, Aoki M. Axonal projections from the pontine pneumotaxic region to the nucleus raphe magnus in cats. Respir Physiol. 1991;85:329–339. doi: 10.1016/0034-5687(91)90072-q. [DOI] [PubMed] [Google Scholar]
- 130.Gang S, Mizuguchi A, Kobayashi N, Aoki M. Descending axonal projections from the medial parabrachial and Kolliker-Fuse nuclear complex to the nucleus raphe magnus in cats. Neurosci Lett. 1990;118:273–275. doi: 10.1016/0304-3940(90)90645-p. [DOI] [PubMed] [Google Scholar]
- 131.Gang S, Nakazono Y, Aoki M, Aoki N. Differential projections to the raphe nuclei from the medial parabrachial-Kolliker-Fuse (NPBM-KF) nuclear complex and the retrofacial nucleus in cats: Retrograde WGA-HRP tracing. J Auton Nerv Syst. 1993;45:241–244. doi: 10.1016/0165-1838(93)90056-z. [DOI] [PubMed] [Google Scholar]
- 132.Gaultier C, Gallego J. Neural control of breathing: Insights from genetic mouse models. J Appl Physiol. 2008;104:1522–1530. doi: 10.1152/japplphysiol.01266.2007. [DOI] [PubMed] [Google Scholar]
- 133.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 134.Gautier H, Bertrand F. Respiratory effects of pneumotaxic center lesions and subsequent vagotomy in chronic cats. Respir Physiol. 1975;23:71–85. doi: 10.1016/0034-5687(75)90073-0. [DOI] [PubMed] [Google Scholar]
- 135.Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: A wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol. 1996;373:173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 136.Gestreau C, Bianchi AL, Grelot L. Differential brainstem Fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J Neurosci. 1997;17:9340–9352. doi: 10.1523/JNEUROSCI.17-23-09340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 138.Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp Brain Res. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- 139.Gilbert KA, Lydic R. Parabrachial neuron discharge in the cat is altered during the carbachol-induced REM sleep-like state (DCarb) Neurosci Lett. 1990;120:241–244. doi: 10.1016/0304-3940(90)90049-f. [DOI] [PubMed] [Google Scholar]
- 140.Gilbert KA, Lydic R. Pontine cholinergic reticular mechanisms cause state-dependent changes in the discharge of parabrachial neurons. Am J Physiol. 1994;266:R136–R150. doi: 10.1152/ajpregu.1994.266.1.R136. [DOI] [PubMed] [Google Scholar]
- 141.Goksor E, Rosengren L, Wennergren G. Bradycardic response during submersion in infant swimming. Acta Paediatr. 2002;91:307–312. doi: 10.1080/08035250252833978. [DOI] [PubMed] [Google Scholar]
- 142.Gozal D. New concepts in abnormalities of respiratory control in children. Curr Opin Pediatr. 2004;16:305–308. doi: 10.1097/01.mop.0000127159.74908.27. [DOI] [PubMed] [Google Scholar]
- 143.Grabauskas G, Bradley RM. Postnatal development of inhibitory synaptic transmission in the rostral nucleus of the solitary tract. J Neurophysiol. 2001;85:2203–2212. doi: 10.1152/jn.2001.85.5.2203. [DOI] [PubMed] [Google Scholar]
- 144.Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- 145.Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBotzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Greer JJ, Funk GD, Ballanyi K. Preparing for the first breath: Prenatal maturation of respiratory neural control. J Physiol. 2006;570:437–444. doi: 10.1113/jphysiol.2005.097238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grillner S, Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- 148.Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 149.Guthmann A, Fritschy JM, Ottersen OP, Torp R, Herbert H. GABA, GABA transporters, GABA(A) receptor subunits, and GAD mRNAs in the rat parabrachial and Kolliker-Fuse nuclei. J Comp Neurol. 1998;400:229–243. [PubMed] [Google Scholar]
- 150.Guthmann A, Herbert H. Distribution of metabotropic glutamate receptors in the parabrachial and Kolliker-Fuse nuclei of the rat. Neuroscience. 1999;89:873–881. doi: 10.1016/s0306-4522(98)00387-x. [DOI] [PubMed] [Google Scholar]
- 151.Guthmann A, Herbert H. Expression of N-methyl-D-aspartate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei and in selected pontomedullary brainstem nuclei. J Comp Neurol. 1999;415:501–517. [PubMed] [Google Scholar]
- 152.Guthmann A, Herbert H. In situ hybridization analysis of flip/flop splice variants of AMPA-type glutamate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei. Brain Res Mol Brain Res. 1999;74:145–157. doi: 10.1016/s0169-328x(99)00281-8. [DOI] [PubMed] [Google Scholar]
- 153.Guyenet PG. The 2008 Carl Ludwig Lecture: Retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol. 1993;264:R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- 155.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hade JS, Mifflin SW, Donta TS, Felder RB. Stimulation of parabrachial neurons elicits a sympathetically mediated pressor response in cats. Am J Physiol. 1988;255:H1349–H1358. doi: 10.1152/ajpheart.1988.255.6.H1349. [DOI] [PubMed] [Google Scholar]
- 157.Haji A, Okazaki M, Takeda R. Synaptic interactions between respiratory neurons during inspiratory on-switching evoked by vagal stimulation in decerebrate cats. Neurosci Res. 1999;35:85–93. doi: 10.1016/s0168-0102(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 158.Haji A, Okazaki M, Yamazaki H, Takeda R. Physiological properties of late inspiratory neurons and their possible involvement in inspiratory off-switching in cats. J Neurophysiol. 2002;87:1057–1067. doi: 10.1152/jn.00470.2001. [DOI] [PubMed] [Google Scholar]
- 159.Harding R. Perinatal development of laryngeal function. J Dev Physiol. 1984;6:249–258. [PubMed] [Google Scholar]
- 160.Harris MB, Milsom WK. The influence of NMDA receptor-mediated processes on breathing pattern in ground squirrels. Respir Physiol. 2001;125:181–197. doi: 10.1016/s0034-5687(00)00219-x. [DOI] [PubMed] [Google Scholar]
- 161.Harris MB, Milsom WK. Apneusis follows disruption of NMDA-type glutamate receptors in vagotomized ground squirrels. Respir Physiol Neurobiol. 2003;134:191–207. doi: 10.1016/s1569-9048(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 162.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: Intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- 163.Hayashi F, McCrimmon DR. Respiratory motor responses to cranial nerve afferent stimulation in rats. Am J Physiol. 1996;271:R1054–R1062. doi: 10.1152/ajpregu.1996.271.4.R1054. [DOI] [PubMed] [Google Scholar]
- 164.Hedrick MS, Ryan ML, Pizarro J, Bisgard GE. Modulation of respiratory rhythm by alpha 2-adrenoceptors in awake and anesthetized goats. J Appl Physiol. 1994;77:742–750. doi: 10.1152/jappl.1994.77.2.742. [DOI] [PubMed] [Google Scholar]
- 165.Herbert H, Flugge G. Distribution of alpha 2-adrenergic binding sites in the parabrachial complex of the rat. Anat Embryol (Berl) 1995;192:507–516. doi: 10.1007/BF00187181. [DOI] [PubMed] [Google Scholar]
- 166.Herbert H, Guthmann A, Zafra F, Ottersen OP. Glycine, glycine receptor subunit and glycine transporters in the rat parabrachial and Kolliker-Fuse nuclei. Anat Embryol (Berl) 2000;201:259–272. doi: 10.1007/s004290050316. [DOI] [PubMed] [Google Scholar]
- 167.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 168.Hering E. Akad Wiss. Wien: 1868. Die Selbststeurung der Athmung durch den Nervus Vagus; pp. 672–677. [Google Scholar]
- 169.Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: Possible implication for SIDS. Auton Neurosci. 2006;126–127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 170.Hilaire G, Monteau R, Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: An in vitro study in the newborn rat. Brain Res. 1989;485:325–332. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- 171.Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 172.Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol. 2010;174:76–88. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience. 1997;80:1209–1224. doi: 10.1016/s0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 174.Hodges PW, Butler JE, McKenzie DK, Gandevia SC. Contraction of the human diaphragm during rapid postural adjustments. J Physiol. 1997;505(Pt 2):539–548. doi: 10.1111/j.1469-7793.1997.539bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- 176.Holstege G. Descending motor pathways and the spinal motor system: Limbic and non-limbic components. Prog Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- 177.Holstege G. The emotional motor system and micturition control. Neurourol Urodyn. 29:42–48. doi: 10.1002/nau.20789. [DOI] [PubMed] [Google Scholar]
- 178.Holstege G, Bandler R, Saper CB. The emotional motor system. Prog Brain Res. 1996;107:3–6. doi: 10.1016/s0079-6123(08)61855-5. [DOI] [PubMed] [Google Scholar]
- 179.Holstege G, Kerstens L, Moes MC, Vanderhorst VG. Evidence for a periaqueductal gray-nucleus retroambiguus-spinal cord pathway in the rat. Neuroscience. 1997;80:587–598. doi: 10.1016/s0306-4522(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 180.Holstege G, Kuypers HG. Propriobulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. I. An anterograde degeneration study in the cat. Brain. 1977;100:239–264. doi: 10.1093/brain/100.2.239. [DOI] [PubMed] [Google Scholar]
- 181.Holstege G, Kuypers HG, Dekker JJ. The organization of the bulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. II. An autoradiographic tracing study in cat. Brain. 1977;100:264–286. [PubMed] [Google Scholar]
- 182.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- 183.Huangfu D, Hwang LJ, Riley TA, Guyenet PG. Splanchnic nerve response to A5 area stimulation in rats. Am J Physiol. 1992;263:R437–R446. doi: 10.1152/ajpregu.1992.263.2.R437. [DOI] [PubMed] [Google Scholar]
- 184.Huangfu DH, Koshiya N, Guyenet PG. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Physiol. 1991;261:R393–R402. doi: 10.1152/ajpregu.1991.261.2.R393. [DOI] [PubMed] [Google Scholar]
- 185.Humbert IA, McLaren DG, Kosmatka K, Fitzgerald M, Johnson S, Porcaro E, Kays S, Umoh EO, Robbins J. Early deficits in cortical control of swallowing in Alzheimer’s disease. J Alzheimers Dis. 2010;19:1185–1197. doi: 10.3233/JAD-2010-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 187.Isenovic ER, Radulovacki M, Carley DW. Impact of intertrigeminal region AMPA receptor blockade on respiratory responses in rats. Respir Physiol Neurobiol. 2007;158:39–44. doi: 10.1016/j.resp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 188.Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol. 2008;160:289–300. doi: 10.1016/j.resp.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol. 2004;143:215–233. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 191.Jodkowski JS, Coles SK, Dick TE. A ‘pneumotaxic centre’ in rats. Neurosci Lett. 1994;172:67–72. doi: 10.1016/0304-3940(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 192.Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol. 1997;82:377–381. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- 193.Julien C, Parkes MJ, Tzeng SY, Sin PY, Ainslie PN, van de Borne P, Fortrat JO, Custaud MA, Gharib C, Porta A, Vallais F, Baselli G, Pagani M, Lucini D, Hughson RL, Taylor JA, Tan CO, Baekey DM, Dick TE, Paton JF, Taha B. Comments on point:counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1745–1749. doi: 10.1152/japplphysiol.00196.2009. [DOI] [PubMed] [Google Scholar]
- 194.Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 195.Jurgens U. A study of the central control of vocalization using the squirrel monkey. Med Eng Phys. 2002;24:473–477. doi: 10.1016/s1350-4533(02)00051-6. [DOI] [PubMed] [Google Scholar]
- 196.Jurgens U. The neural control of vocalization in mammals: A review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 197.Jurgens U, Hage SR. On the role of the reticular formation in vocal pattern generation. Behav Brain Res. 2007;182:308–314. doi: 10.1016/j.bbr.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 198.Karemaker JM. Counterpoint: Respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1742–1743. doi: 10.1152/japplphysiol.91107.2008a. discussion 1744. [DOI] [PubMed] [Google Scholar]
- 199.Karemaker JM. Last word on point:counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1750. doi: 10.1152/japplphysiol.00225.2009. [DOI] [PubMed] [Google Scholar]
- 200.Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: Progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: Intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:207–218. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- 202.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kobayashi S, Onimaru H, Inoue M, Inoue T, Sasa R. Localization and properties of respiratory neurons in the rostral pons of the newborn rat. Neuroscience. 2005;134:317–325. doi: 10.1016/j.neuroscience.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 204.Koshiya N, Guyenet PG. Role of the pons in the carotid sympathetic chemoreflex. Am J Physiol. 1994;267:R508–R518. doi: 10.1152/ajpregu.1994.267.2.R508. [DOI] [PubMed] [Google Scholar]
- 205.Kron M, Morschel M, Reuter J, Zhang W, Dutschmann M. Developmental changes in brain-derived neurotrophic factor-mediated modulations of synaptic activities in the pontine Kolliker-Fuse nucleus of the rat. J Physiol. 2007;583:315–327. doi: 10.1113/jphysiol.2007.134726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Krolo M, Tonkovic-Capin V, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Subtype composition and responses of respiratory neurons in the pre-botzinger region to pulmonary afferent inputs in dogs. J Neurophysiol. 2005;93:2674–2687. doi: 10.1152/jn.01206.2003. [DOI] [PubMed] [Google Scholar]
- 207.Kron M, Reuter J, Gerhardt E, Manzke T, Zhang W, Dutschmann M. Emergence of brain-derived neurotrophic factor-induced postsynaptic potentiation of NMDA currents during the postnatal maturation of the Kolliker-Fuse nucleus of rat. J Physiol. 2008;586:2331–2343. doi: 10.1113/jphysiol.2007.148916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kron M, Zhang W, Dutschmann M. Developmental changes in the BDNF-induced modulation of inhibitory synaptic transmission in the Kolliker-Fuse nucleus of rat. Eur J Neurosci. 2007;26:3449–3457. doi: 10.1111/j.1460-9568.2007.05960.x. [DOI] [PubMed] [Google Scholar]
- 209.Krout KE, Jansen AS, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- 210.Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30:163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- 211.Kubin L. Carbachol models of REM sleep: Recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- 212.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004;143:235–249. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 214.Kuna ST, Remmers JE. Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir Physiol. 1999;117:85–95. doi: 10.1016/s0034-5687(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 215.Kunibe I, Nonaka S, Katada A, Adachi M, Enomoto K, Harabuchi Y. The neuronal circuit of augmenting effects on intrinsic laryngeal muscle activities induced by nasal air-jet stimulation in decerebrate cats. Brain Res. 2003;978:83–90. doi: 10.1016/s0006-8993(03)02770-7. [DOI] [PubMed] [Google Scholar]
- 216.Lara JP, Dawid-Milner MS, Lopez MV, Montes C, Spyer KM, Gonzalez-Baron S. Laryngeal effects of stimulation of rostral and ventral pons in the anaesthetized rat. Brain Res. 2002;934:97–106. doi: 10.1016/s0006-8993(02)02364-8. [DOI] [PubMed] [Google Scholar]
- 217.Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid-Milner MS, Spyer KM. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol. 1994;477(Pt 2):321–329. doi: 10.1113/jphysiol.1994.sp020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Larnicol N, Wallois F, Berquin P, Gros F, Rose D. c-fos-like immunoreactivity in the cat’s neuraxis following moderate hypoxia or hypercapnia. J Physiol Paris. 1994;88:81–88. doi: 10.1016/0928-4257(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 219.Larson CR, Kistler MK. The relationship of periaqueductal gray neurons to vocalization and laryngeal EMG in the behaving monkey. Exp Brain Res. 1986;63:596–606. doi: 10.1007/BF00237482. [DOI] [PubMed] [Google Scholar]
- 220.Lasiter PS, Kachele DL. Postnatal development of the parabrachial gustatory zone in rat: Dendritic morphology and mitochondrial enzyme activity. Brain Res Bull. 1988;21:79–94. doi: 10.1016/0361-9230(88)90122-0. [DOI] [PubMed] [Google Scholar]
- 221.Lavezzi AM, Ottaviani G, Ballabio G, Rossi L, Matturri L. Preliminary study on the cytoarchitecture of the human parabrachial/Kolliker-fuse complex, with reference to sudden infant death syndrome and sudden intrauterine unexplained death. Pediatr Dev Pathol. 2004;7:171–179. doi: 10.1007/s10024-003-1011-7. [DOI] [PubMed] [Google Scholar]
- 222.Lavezzi AM, Ottaviani G, Rossi L, Matturri L. Cytoarchitectural organization of the parabrachial/Kolliker-Fuse complex in man. Brain Dev. 2004;26:316–320. doi: 10.1016/j.braindev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 223.Lavezzi AM, Ottaviani G, Rossi L, Matturri L. Hypoplasia of the parabrachial/kolliker-fuse complex in perinatal death. Biol Neonate. 2004;86:92–97. doi: 10.1159/000078310. [DOI] [PubMed] [Google Scholar]
- 224.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ling L. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol. 2008;164:233–241. doi: 10.1016/j.resp.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ling L, Karius DR, Speck DF. Role of N-methyl-D-aspartate receptors in the pontine pneumotaxic mechanism in the cat. J Appl Physiol. 1994;76:1138–1143. doi: 10.1152/jappl.1994.76.3.1138. [DOI] [PubMed] [Google Scholar]
- 227.Lioy DT, Wu WW, Bissonnette JM. Autonomic dysfunction with mutations in the gene that encodes methyl-CpG-binding protein 2: Insights into Rett syndrome. Auton Neurosci. 2011;161:55–62. doi: 10.1016/j.autneu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 228.Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: Implication for a sensitive period. J Appl Physiol. 2009;106:1212–1222. doi: 10.1152/japplphysiol.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- 230.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 231.Liu Q, Wong-Riley MT. Postnatal changes in the expression of serotonin 2A receptors in various brain stem nuclei of the rat. J Appl Physiol. 2008;104:1801–1808. doi: 10.1152/japplphysiol.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: Implication for a sensitive period. Neuroscience. 2010;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu YY, Wong-Riley MT. Developmental study of cytochrome oxidase activity in the brain stem respiratory nuclei of postnatal rats. J Appl Physiol. 2001;90:685–694. doi: 10.1152/jappl.2001.90.2.685. [DOI] [PubMed] [Google Scholar]
- 234.Loewy AD, Burton H. Nuclei of the solitary tract: Efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978;181:421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- 235.Long S, Duffin J. The neuronal determinants of respiratory rhythm. Prog Neurobiol. 1986;27:101–182. doi: 10.1016/0301-0082(86)90007-9. [DOI] [PubMed] [Google Scholar]
- 236.Lumsden T. Observations on the respiratory centers in the cat. J Physiol. 1923:152–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- 238.Luthe L, Hausler U, Jurgens U. Neuronal activity in the medulla oblongata during vocalization. A single-unit recording study in the squirrel monkey. Behav Brain Res. 2000;116:197–210. doi: 10.1016/s0166-4328(00)00272-2. [DOI] [PubMed] [Google Scholar]
- 239.Lydic R, Orem J. Respiratory neurons of the pneumotaxic center during sleep and wakefulness. Neurosci Lett. 1979;15:187–192. doi: 10.1016/0304-3940(79)96111-1. [DOI] [PubMed] [Google Scholar]
- 240.MacDonald SM, Song G, Poon CS. Nonassociative learning promotes respiratory entrainment to mechanical ventilation. PLoS One. 2007;2:e865. doi: 10.1371/journal.pone.0000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.MacDonald SM, Tin C, Song G, Poon CS. Use-dependent learning and memory of the Hering-Breuer inflation reflex in rats. Exp Physiol. 2009;94:269–278. doi: 10.1113/expphysiol.2008.045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Maiorov DN, Malpas SC, Head GA. Influence of pontine A5 region on renal sympathetic nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol. 2000;278:R311–319. doi: 10.1152/ajpregu.2000.278.2.R311. [DOI] [PubMed] [Google Scholar]
- 243.Maiorov DN, Wilton ER, Badoer E, Petrie D, Head GA, Malpas SC. Sympathetic response to stimulation of the pontine A5 region in conscious rabbits. Brain Res. 1999;815:227–236. doi: 10.1016/s0006-8993(98)01150-0. [DOI] [PubMed] [Google Scholar]
- 244.Mallios VJ, Lydic R, Baghdoyan HA. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol. 1995;268:L941–949. doi: 10.1152/ajplung.1995.268.6.L941. [DOI] [PubMed] [Google Scholar]
- 245.Marckwald M. Die Athembewegungen und deren Innervation beim Kaninchen. Z Biol. 1887;23:149–283. [Google Scholar]
- 246.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Masmoudi K, Larnicol N, Wallois F, Gros F. Changes in Fos-like immunoreactivity evoked by maturation of the sneeze reflex triggered by nasal air puff stimulation in kittens. Brain Res. 1997;757:102–110. doi: 10.1016/s0006-8993(97)00167-4. [DOI] [PubMed] [Google Scholar]
- 248.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: Location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 249.Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol. 172:1–7. doi: 10.1016/j.resp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Merrill EG. The lateral respiratory neurones of the medulla: Their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970;24:11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- 252.Milsom WK, Abe AS, Andrade DV, Tattersall GJ. Evolutionary trends in airway CO2/H +chemoreception. Respir Physiol Neurobiol. 2004;144:191–202. doi: 10.1016/j.resp.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 253.Mingrone R, Fulcheri E, Lavezzi AM, Matturri L. Absolute shortness of the umbilical cord and hypoplasia of the arcuate nucleus and of the parabrachial/Kolliker-Fuse complex in a case of sudden intrauterine fetal death. Eur J Obstet Gynecol Reprod Biol. 2009;147:112–114. doi: 10.1016/j.ejogrb.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 254.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 255.Miyaoka Y, Takahashi Y, Sato S, Shimada K. Autonomic nervous reflexes in respiration elicited by mechanical stimulation of the velopharyngeal region in rabbits. J Auton Nerv Syst. 1989;26:177–180. doi: 10.1016/0165-1838(89)90166-5. [DOI] [PubMed] [Google Scholar]
- 256.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol. 1995;489(Pt 3):877–884. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- 258.Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: Cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- 259.Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- 261.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 262.Morris KF, Nuding SC, Segers LS, Baekey DM, Shannon R, Lindsey BG, Dick TE. Respiratory and Mayer wave-related discharge patterns of raphe and pontine neurons change with vagotomy. J Appl Physiol. 109:189–202. doi: 10.1152/japplphysiol.01324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Morrison SF. Respiratory modulation of sympathetic nerve activity: Effect of MK-801. Am J Physiol. 1996;270:R645–R651. doi: 10.1152/ajpregu.1996.270.3.R645. [DOI] [PubMed] [Google Scholar]
- 264.Morrison SF, Cravo SL, Wilfehrt HM. Pontine lesions produce apneusis in the rat. Brain Res. 1994;652:83–86. doi: 10.1016/0006-8993(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 265.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Morschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci. 2009;364:2517–2526. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mortola JP, Fisher JT, Sant’Ambrogio G. Vagal control of the breathing pattern and respiratory mechanics in the adult and newborn rabbit. Pflugers Arch. 1984;401:281–286. doi: 10.1007/BF00582597. [DOI] [PubMed] [Google Scholar]
- 268.Mraovitch S, Kumada M, Reis DJ. Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res. 1982;232:57–75. doi: 10.1016/0006-8993(82)90610-2. [DOI] [PubMed] [Google Scholar]
- 269.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 270.Muller G, Klingberg H, Klingberg F. Incisor growth acceleration after lesions in the lateral pontine reticular formation of rats. Biomed Biochim Acta. 1991;50:219–222. [PubMed] [Google Scholar]
- 271.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173:1136–1139. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- 273.Nuding SC, Segers LS, Baekey DM, Dick TE, Solomon IC, Shannon R, Morris KF, Lindsey BG. Pontine-ventral respiratory column interactions through raphe circuits detected using multi-array spike train recordings. J Neurophysiol. 2009;101:2943–2960. doi: 10.1152/jn.91305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci. 2009;364:2501–2516. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ohtake PJ, Simakajornboon N, Fehniger MD, Xue YD, Gozal D. N-Methyl-D-aspartate receptor expression in the nucleus tractus solitarii and maturation of hypoxic ventilatory response in the rat. Am J Respir Crit Care Med. 2000;162:1140–1147. doi: 10.1164/ajrccm.162.3.9903094. [DOI] [PubMed] [Google Scholar]
- 276.Okazaki M, Takeda R, Yamazaki H, Haji A. Synaptic mechanisms of inspiratory off-switching evoked by pontine pneumotaxic stimulation in cats. Neurosci Res. 2002;44:101–110. doi: 10.1016/s0168-0102(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 277.Oku Y, Dick TE. Phase resetting of the respiratory cycle before and after unilateral pontine lesion in cat. J Appl Physiol. 1992;72:721–730. doi: 10.1152/jappl.1992.72.2.721. [DOI] [PubMed] [Google Scholar]
- 278.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Onimaru H, Homma I. Optical imaging of respiratory neuron activity from the dorsal view of the lower brainstem. Clin Exp Pharmacol Physiol. 2005;32:297–301. doi: 10.1111/j.1440-1681.2005.04187.x. [DOI] [PubMed] [Google Scholar]
- 280.Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. discussion 31. [PubMed] [Google Scholar]
- 282.Orem J, Anderson CA. Diaphragmatic activity during REM sleep in the adult cat. J Appl Physiol. 1996;81:751–760. doi: 10.1152/jappl.1996.81.2.751. [DOI] [PubMed] [Google Scholar]
- 283.Oyamada Y, Andrzejewski M, Muckenhoff K, Scheid P, Ballantyne D. Locus coeruleus neurones in vitro: pH-sensitive oscillations of membrane potential in an electrically coupled network. Respir Physiol. 1999;118:131–147. doi: 10.1016/s0034-5687(99)00088-2. [DOI] [PubMed] [Google Scholar]
- 284.Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol. 1998;513(Pt 2):381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Parrish MD, Hill JM, Kaufman MP. Cardiovascular and respiratory response to static exercise in the newborn kitten. Pediatr Res. 1991;30:95–99. doi: 10.1203/00006450-199107000-00019. [DOI] [PubMed] [Google Scholar]
- 286.Paterson DS, Hilaire G, Weese-Mayer DE. Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome. Respir Physiol Neurobiol. 2009;168:133–143. doi: 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Paton JF. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. J Physiol. 1997;502(Pt 3):623–639. doi: 10.1111/j.1469-7793.1997.623bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Paton JF, Dutschmann M. Central control of upper airway resistance regulating respiratory airflow in mammals. J Anat. 2002;201:319–323. doi: 10.1046/j.1469-7580.2002.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Paton JF, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 290.Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, Rogers R, Tracey I, Wise RG. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage. 2009;44:295–305. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 291.Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. The bulbar network of respiratory neurons during apneusis induced by a blockade of NMDA receptors. Exp Brain Res. 1992;89:623–639. doi: 10.1007/BF00229887. [DOI] [PubMed] [Google Scholar]
- 292.Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. J Physiol. 1994;474:509–523. doi: 10.1113/jphysiol.1994.sp020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pierrefiche O, Foutz AS, Denavit-Saubie M. Pneumotaxic mechanisms in the non-human primate: Effect of the N-methyl-D-aspartate (NMDA) antagonist ketamine. Neurosci Lett. 1990;119:90–93. doi: 10.1016/0304-3940(90)90763-y. [DOI] [PubMed] [Google Scholar]
- 294.Poliacek I, Stransky A, Szereda-Przestaszewska M, Jakus J, Barani H, Tomori Z, Halasova E. Cough and laryngeal muscle discharges in brainstem lesioned anaesthetized cats. Physiol Res. 2005;54:645–654. [PubMed] [Google Scholar]
- 295.Poon CS. Optimal interaction of respiratory and thermal regulation at rest and during exercise: Role of a serotonin-gated spinoparabrachial thermoafferent pathway. Respir Physiol Neurobiol. 2009;169:234–242. doi: 10.1016/j.resp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Poon CS, Young DL. Nonassociative learning as gated neural integrator and differentiator in stimulus-response pathways. Behav Brain Funct. 2006;2:29. doi: 10.1186/1744-9081-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Poon CS, Zhou Z, Champagnat J. NMDA receptor activity in utero averts respiratory depression and anomalous long-term depression in newborn mice. J Neurosci. 2000;20:RC73. doi: 10.1523/JNEUROSCI.20-09-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 299.Prabhakar NR, Kline DD. Ventilatory changes during intermittent hypoxia: Importance of pattern and duration. High Alt Med Biol. 2002;3:195–204. doi: 10.1089/15270290260131920. [DOI] [PubMed] [Google Scholar]
- 300.Price WM, Batsel HL. Respiratory neurons participating in sneeze and in response to resistance to expiration. Exp Neurol. 1970;29:554–570. doi: 10.1016/0014-4886(70)90080-4. [DOI] [PubMed] [Google Scholar]
- 301.Rabbette PS, Stocks J. Influence of volume dependency and timing of airway occlusions on the Hering-Breuer reflex in infants. J Appl Physiol. 1998;85:2033–2039. doi: 10.1152/jappl.1998.85.6.2033. [DOI] [PubMed] [Google Scholar]
- 302.Radulovacki M, Pavlovic S, Carley DW. Pontine intertrigeminal region attenuates sleep apneas in rats. Sleep. 2004;27:383–387. doi: 10.1093/sleep/27.3.383. [DOI] [PubMed] [Google Scholar]
- 303.Radulovacki M, Pavlovic S, Saponjic J, Carley DW. Intertrigeminal region attenuates reflex apnea and stabilizes respiratory pattern in rats. Brain Res. 2003;975:66–72. doi: 10.1016/s0006-8993(03)02587-3. [DOI] [PubMed] [Google Scholar]
- 304.Radulovacki M, Pavlovic S, Saponjic J, Carley DW. Modulation of reflex and sleep related apnea by pedunculopontine tegmental and intertrigeminal neurons. Respir Physiol Neurobiol. 2004;143:293–306. doi: 10.1016/j.resp.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 305.Radulovacki M, Stoiljkovic M, Saponjic J, Carley DW. Effects of intertrigeminal region NMDA and non-NMDA receptors on respiratory responses in rats. Respir Physiol Neurobiol. 2007;156:40–46. doi: 10.1016/j.resp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 306.Reeves SR, Mitchell GS, Gozal D. Early postnatal chronic intermittent hypoxia modifies hypoxic respiratory responses and long-term phrenic facilitation in adult rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1664–R1671. doi: 10.1152/ajpregu.00851.2005. [DOI] [PubMed] [Google Scholar]
- 307.Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- 308.Reuter J, Kron M, Dutschmann M. Postnatal changes in morphology and dendritic organization of neurones located in the area of the Kolliker-Fuse nucleus of rat. Adv Exp Med Biol. 2010;669:37–41. doi: 10.1007/978-1-4419-5692-7_8. [DOI] [PubMed] [Google Scholar]
- 309.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 310.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- 311.Richter DW. Neural regulation of respiration: Rhythmogenesis and afferent control. In: Gregor RW, Windhorst U, editors. Comprehensive Human Physiology. Berlin: Springer-Verlag; 1996. pp. 2079–2095. [Google Scholar]
- 312.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: Comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 313.Rikard-Bell GC, Bystrzycka EK, Nail BS. Brainstem projections to the phrenic nucleus: A HRP study in the cat. Brain Res Bull. 1984;12:469–477. doi: 10.1016/0361-9230(84)90162-x. [DOI] [PubMed] [Google Scholar]
- 314.Rikard-Bell GC, Bystrzycka EK, Nail BS. The identification of brainstem neurones projecting to thoracic respiratory motoneurones in the cat as demonstrated by retrograde transport of HRP. Brain Res Bull. 1985;14:25–37. doi: 10.1016/0361-9230(85)90174-1. [DOI] [PubMed] [Google Scholar]
- 315.Rohrer JD, Schott JM. Primary progressive aphasia - defining genetic and pathological subtypes. Curr Alzheimer Res. 2011;8:266–272. doi: 10.2174/156720511795563728. [DOI] [PubMed] [Google Scholar]
- 316.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 317.Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The autonomic higher order processing nuclei of the lower brain stem are among the early targets of the Alzheimer’s disease-related cytoskeletal pathology. Acta Neuropathol. 2001;101:555–564. doi: 10.1007/s004010000320. [DOI] [PubMed] [Google Scholar]
- 318.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: A computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100:1770–1799. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. I. Models of respiratory neurons. J Neurophysiol. 1997;77:1994–2006. doi: 10.1152/jn.1997.77.4.1994. [DOI] [PubMed] [Google Scholar]
- 321.Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J Neurophysiol. 1997;77:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- 322.Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Morschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol. 2004;143:307–319. doi: 10.1016/j.resp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 323.Saint John WM. Differing responses to hypercapnia and hypoxia following pneumotaxic center ablation. Respir Physiol. 1975;23:1–9. doi: 10.1016/0034-5687(75)90066-3. [DOI] [PubMed] [Google Scholar]
- 324.Saleh TM, Cechetto DF. Neurotransmitters in the parabrachial nucleus mediating visceral input to the thalamus in rats. Am J Physiol. 1994;266:R1287–R1296. doi: 10.1152/ajpregu.1994.266.4.R1287. [DOI] [PubMed] [Google Scholar]
- 325.Saleh TM, Cechetto DF. Neurochemical interactions in the parabrachial nucleus mediating visceral inputs to visceral thalamic neurons. Am J Physiol. 1995;268:R786–R795. doi: 10.1152/ajpregu.1995.268.3.R786. [DOI] [PubMed] [Google Scholar]
- 326.Saleh TM, Cechetto DF. Peptide changes in the parabrachial nucleus following cervical vagal stimulation. J Comp Neurol. 1996;366:390–405. doi: 10.1002/(SICI)1096-9861(19960311)366:3<390::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 327.Saleh TM, Cechetto DF. Peptides in the parabrachial nucleus modulate visceral input to the thalamus. Am J Physiol. 1993;264:R668–R675. doi: 10.1152/ajpregu.1993.264.4.R668. [DOI] [PubMed] [Google Scholar]
- 328.Saper CB. Reciprocal parabrachial-cortical connections in the rat. Brain Res. 1982;242:33–40. doi: 10.1016/0006-8993(82)90493-0. [DOI] [PubMed] [Google Scholar]
- 329.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 330.Schmidt MF, Ashmore RC, Vu ET. Bilateral control and interhemispheric coordination in the avian song motor system. Ann N Y Acad Sci U S A. 2004;1016:171–186. doi: 10.1196/annals.1298.014. [DOI] [PubMed] [Google Scholar]
- 331.Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol. 2008;100:1749–1769. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Segers LS, Shannon R, Lindsey BG. Interactions between rostral pontine and ventral medullary respiratory neurons. J Neurophysiol. 1985;54:318–334. doi: 10.1152/jn.1985.54.2.318. [DOI] [PubMed] [Google Scholar]
- 333.Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol. 2000;525(Pt 1):207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulm Pharmacol. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 335.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 336.Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Pontine respiratory group neuron discharge is altered during fictive cough in the decerebrate cat. Respir Physiol Neurobiol. 2004;142:43–54. doi: 10.1016/j.resp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 337.Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 338.Shiba K, Nakazawa K, Ono K, Umezaki T. Multifunctional laryngeal premotor neurons: Their activities during breathing, coughing, sneezing, and swallowing. J Neurosci. 2007;27:5156–5162. doi: 10.1523/JNEUROSCI.0001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: An intracellular study in the cat. J Neurosci. 1999;19:2717–2727. doi: 10.1523/JNEUROSCI.19-07-02717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sieck GC, Harper RM. Discharge of neurons in the parabrachial pons related to the cardiac cycle: Changes during different sleep-waking states. Brain Res. 1980;199:385–399. doi: 10.1016/0006-8993(80)90696-4. [DOI] [PubMed] [Google Scholar]
- 341.Sieck GC, Harper RM. Pneumotaxic area neuronal discharge during sleep-waking states in the cat. Exp Neurol. 1980;67:79–102. doi: 10.1016/0014-4886(80)90162-4. [DOI] [PubMed] [Google Scholar]
- 342.Siniaia MS, Young DL, Poon CS. Habituation and desensitization of the Hering-Breuer reflex in rat. J Physiol. 2000;523(Pt 2):479–491. doi: 10.1111/j.1469-7793.2000.t01-1-00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: A hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Smotherman M, Kobayasi K, Ma J, Zhang S, Metzner W. A mechanism for vocal-respiratory coupling in the mammalian parabrachial nucleus. J Neurosci. 2006;26:4860–4869. doi: 10.1523/JNEUROSCI.4607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Song A, Ashwell KW, Tracey DJ. Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat Embryol (Berl) 2000;202:159–177. doi: 10.1007/s004290000096. [DOI] [PubMed] [Google Scholar]
- 348.Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 349.Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol. 2009;165:9–12. doi: 10.1016/j.resp.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol. 2009;165:1–8. doi: 10.1016/j.resp.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Stella G. On the mechanism of production and the physiological significance of apneusis. J Physiol. 1938;93:10–23. doi: 10.1113/jphysiol.1938.sp003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Stettner GM, Huppke P, Gartner J, Richter DW, Dutschmann M. Disturbances of breathing in Rett syndrome: Results from patients and animal models. Adv Exp Med Biol. 2008;605:503–507. doi: 10.1007/978-0-387-73693-8_88. [DOI] [PubMed] [Google Scholar]
- 355.St-John WM. Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol. 1998;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 356.St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol. 2000;123:201–213. doi: 10.1016/s0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 357.St John WM. Characterization of the tidal volume regulating function of the pneumotaxic center. Respir Physiol. 1973;18:64–79. doi: 10.1016/0034-5687(73)90022-4. [DOI] [PubMed] [Google Scholar]
- 358.St John WM. Differential alteration by hypercapnia and hypoxia of the apneustic respiratory pattern in decerebrate cats. J Physiol. 1979;287:467–491. doi: 10.1113/jphysiol.1979.sp012671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.St John WM. Influence of pulmonary inflations on discharge of pontile respiratory neurons. J Appl Physiol. 1987;63:2231–2239. doi: 10.1152/jappl.1987.63.6.2231. [DOI] [PubMed] [Google Scholar]
- 360.St John WM. Integration of peripheral and central chemoreceptor stimuli by pontine and medullary respiratory centers. Fed Proc. 1977;36:2421–2427. [PubMed] [Google Scholar]
- 361.St John WM. Respiratory tidal volume responses of cats with chronic pneumotaxic center lesions. Respir Physiol. 1972;16:92–108. doi: 10.1016/0034-5687(72)90091-6. [DOI] [PubMed] [Google Scholar]
- 362.St John WM, Glasser RL, King RA. Apneustic breathing after vagotomy in cats with chronic pneumotaxic center lesions. Respir Physiol. 1971;12:239–250. doi: 10.1016/0034-5687(71)90056-9. [DOI] [PubMed] [Google Scholar]
- 363.St John WM, Glasser RL, King RA. Rhythmic respiration in awake vagotomized cats with chronic pneumotaxic area lesions. Respir Physiol. 1972;15:233–244. doi: 10.1016/0034-5687(72)90100-4. [DOI] [PubMed] [Google Scholar]
- 364.St John WM, Wang SC. Integration of chemoreceptor stimuli by caudal pontile and rostral medullary sites. J Appl Physiol. 1976;41:612–622. doi: 10.1152/jappl.1976.41.5.612. [DOI] [PubMed] [Google Scholar]
- 365.Stoiljkovic M, Radulovacki M, Carley DW. Local antagonism of intertrigeminal region metabotropic glutamate receptors exacerbates apneic responses to intravenous serotonin. Respir Physiol Neurobiol. 2009;165:137–142. doi: 10.1016/j.resp.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Stuart DG, Hultborn H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res Rev. 2008;59:74–95. doi: 10.1016/j.brainresrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 368.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. J Neurosci. 2009;29:3824–3832. doi: 10.1523/JNEUROSCI.0607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Subramanian HH, Holstege G. The midbrain and medullary control of post-inspiratory activity of the crural and costal diaphragm in vivo. J Neurophysiol. 2011;105:2852–2862. doi: 10.1152/jn.00168.2011. [DOI] [PubMed] [Google Scholar]
- 371.Subramanian HH, Holstege G. Periaqueductal gray control of breathing. Adv Exp Med Biol. 2010;669:353–358. doi: 10.1007/978-1-4419-5692-7_72. [DOI] [PubMed] [Google Scholar]
- 372.Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Takada M, Itoh K, Yasui Y, Mitani A, Nomura S, Mizuno N. Distribution of premotor neurons for the hypoglossal nucleus in the cat. Neurosci Lett. 1984;52:141–146. doi: 10.1016/0304-3940(84)90364-1. [DOI] [PubMed] [Google Scholar]
- 374.Takagi K, Nakayama T. Respiratory discharge of the pons. Science. 1958;128:1206. doi: 10.1126/science.128.3333.1206. [DOI] [PubMed] [Google Scholar]
- 375.Tang PC. Localization of the pneumotaxic center in the cat. Am J Physiol. 1953;172:645–652. doi: 10.1152/ajplegacy.1953.172.3.645. [DOI] [PubMed] [Google Scholar]
- 376.Taylor EW, Jordan D, Coote JH. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev. 1999;79:855–916. doi: 10.1152/physrev.1999.79.3.855. [DOI] [PubMed] [Google Scholar]
- 377.Tchobroutsky C, Merlet C, Rey P. The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Respir Physiol. 1969;8:108–117. doi: 10.1016/0034-5687(69)90048-6. [DOI] [PubMed] [Google Scholar]
- 378.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 379.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;111(Suppl 8A):69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- 380.Traube L. Über periodische Thatigkeits-Äusserungen des vasomotorischen und Hemmungs-Nervencentrums. Zentr Med Wiss. 1865;56:881–885. [Google Scholar]
- 381.Trippenbach T. Pulmonary reflexes and control of breathing during development. Biol Neonate. 1994;65:205–210. doi: 10.1159/000244054. [DOI] [PubMed] [Google Scholar]
- 382.Vanderhorst VG, Mouton LJ, Blok BF, Holstege G. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. J Comp Neurol. 1996;376:361–385. doi: 10.1002/(SICI)1096-9861(19961216)376:3<361::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 383.Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- 384.Verner TA, Pilowsky PM, Goodchild AK. Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res. 2008;1208:128–136. doi: 10.1016/j.brainres.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 385.Vicini S, Rumbaugh G. A slow NMDA channel: In search of a role. J Physiol. 2000;525(Pt 2):283. doi: 10.1111/j.0021-3751.2000.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Viemari JC. Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol. 2008;164:123–130. doi: 10.1016/j.resp.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 387.Viemari JC, Burnet H, Bevengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: In vivo and in vitro studies. Eur J Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- 388.Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Vincent A, Tell F. Postnatal changes in electrophysiological properties of rat nucleus tractus solitarii neurons. Eur J Neurosci. 1997;9:1612–1624. doi: 10.1111/j.1460-9568.1997.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 390.Vincent A, Tell F. Postnatal development of rat nucleus tractus solitarius neurons: Morphological and electrophysiological evidence. Neuroscience. 1999;93:293–305. doi: 10.1016/s0306-4522(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 391.Voituron N, Menuet C, Dutschmann M, Hilaire G. Physiological definition of upper airway obstructions in mouse model for Rett syndrome. Respir Physiol Neurobiol. 2010;173:146–156. doi: 10.1016/j.resp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 392.Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2009;168:109–118. doi: 10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 393.Voituron N, Zanella S, Menuet C, Lajard AM, Dutschmann M, Hilaire G. Early abnormalities of post-sigh breathing in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2010;170:173–182. doi: 10.1016/j.resp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 394.von Baumgarten K, Kanzow E. The interaction of two types of inspiratory neurons in the region of the tractus solitarius of the cat. Arch Ital Biol. 1957;96:361–373. [Google Scholar]
- 395.von Euler C. On the central pattern generator for the basic breathing rhythmicity. J Appl Physiol. 1983;55:1647–1659. doi: 10.1152/jappl.1983.55.6.1647. [DOI] [PubMed] [Google Scholar]
- 396.von Euler C, Marttila I, Remmers JE, Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand. 1976;96:324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]
- 397.Wallois F, Gros F, Masmoudi K, Larnicol N. C-Fos-like immunoreactivity in the cat brainstem evoked by sneeze-inducing air puff stimulation of the nasal mucosa. Brain Res. 1995;687:143–154. doi: 10.1016/0006-8993(95)00487-b. [DOI] [PubMed] [Google Scholar]
- 398.Wallois F, Macron JM, Larnicol N, Rose D. The sneeze: Maturation of the reflex in kittens. Neuroreport. 1993;4:240–242. [PubMed] [Google Scholar]
- 399.Wasserman AM, Ferreira M, Jr, Sahibzada N, Hernandez YM, Gillis RA. GABA-mediated neurotransmission in the ventrolateral NTS plays a role in respiratory regulation in the rat. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1423–1441. doi: 10.1152/ajpregu.00488.2001. [DOI] [PubMed] [Google Scholar]
- 400.Wasserman AM, Sahibzada N, Hernandez YM, Gillis RA. Specific subnuclei of the nucleus tractus solitarius play a role in determining the duration of inspiration in the rat. Brain Res. 2000;880:118–130. doi: 10.1016/s0006-8993(00)02782-7. [DOI] [PubMed] [Google Scholar]
- 401.Welzl H, D’Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 402.Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- 403.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 405.Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol. 2008;164:28–37. doi: 10.1016/j.resp.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Yamamoto T, Takemura M, Inui T, Torii K, Maeda N, Ohmoto M, Matsumoto I, Abe K. Functional organization of the rodent parabrachial nucleus. Ann N Y Acad Sci U S A. 2009;1170:378–382. doi: 10.1111/j.1749-6632.2009.03883.x. [DOI] [PubMed] [Google Scholar]
- 408.Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: A combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 409.Yokota S, Tsumori T, Ono K, Yasui Y. Glutamatergic pathways from the Kolliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Res. 2004;995:118–130. doi: 10.1016/j.brainres.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 410.Yoshioka M, Kawai Y. Activity-dependent reorganization of local circuitry in the developing visceral sensory system. Neuroscience. 2007;150:905–914. doi: 10.1016/j.neuroscience.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 411.Zagami CJ, Stifani S. Molecular characterization of the mouse superior lateral parabrachial nucleus through expression of the transcription factor Runx1. PLoS One. 2010;5:e13944. doi: 10.1371/journal.pone.0013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhang SP, Bandler R, Davis PJ. Brain stem integration of vocalization: Role of the nucleus retroambigualis. J Neurophysiol. 1995;74:2500–2512. doi: 10.1152/jn.1995.74.6.2500. [DOI] [PubMed] [Google Scholar]
- 413.Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: Role of the midbrain periaqueductal gray. J Neurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]