Abstract
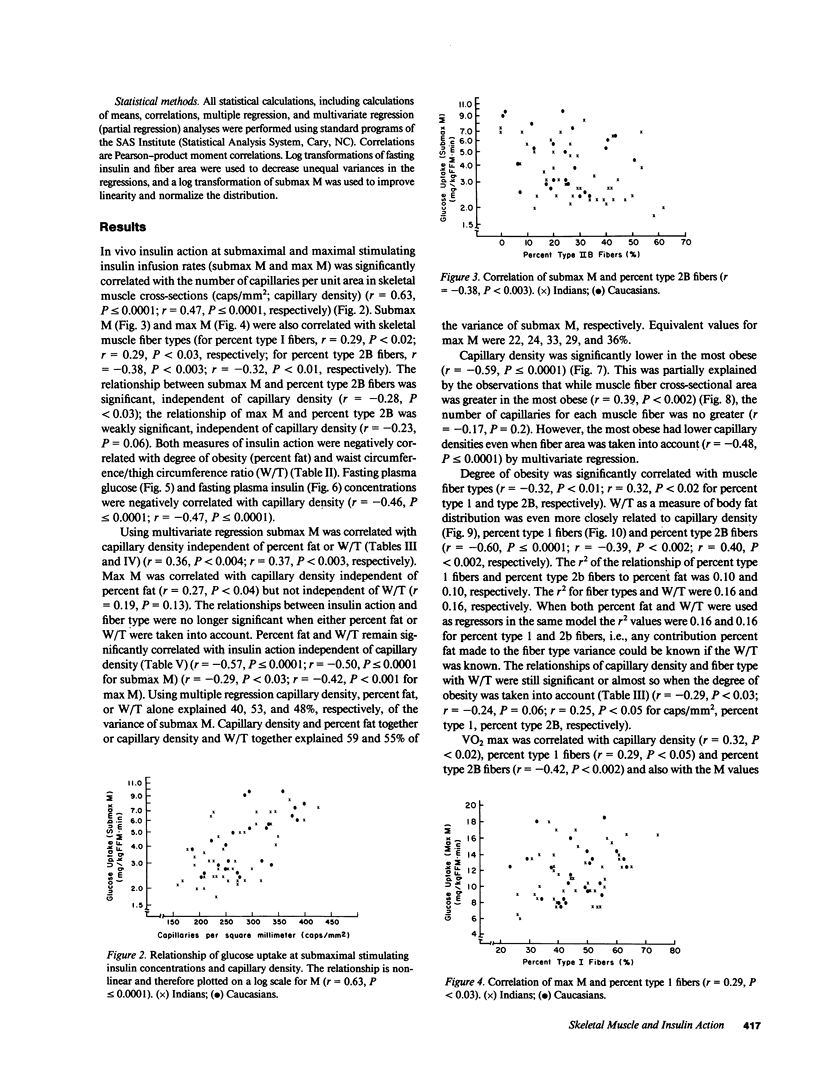
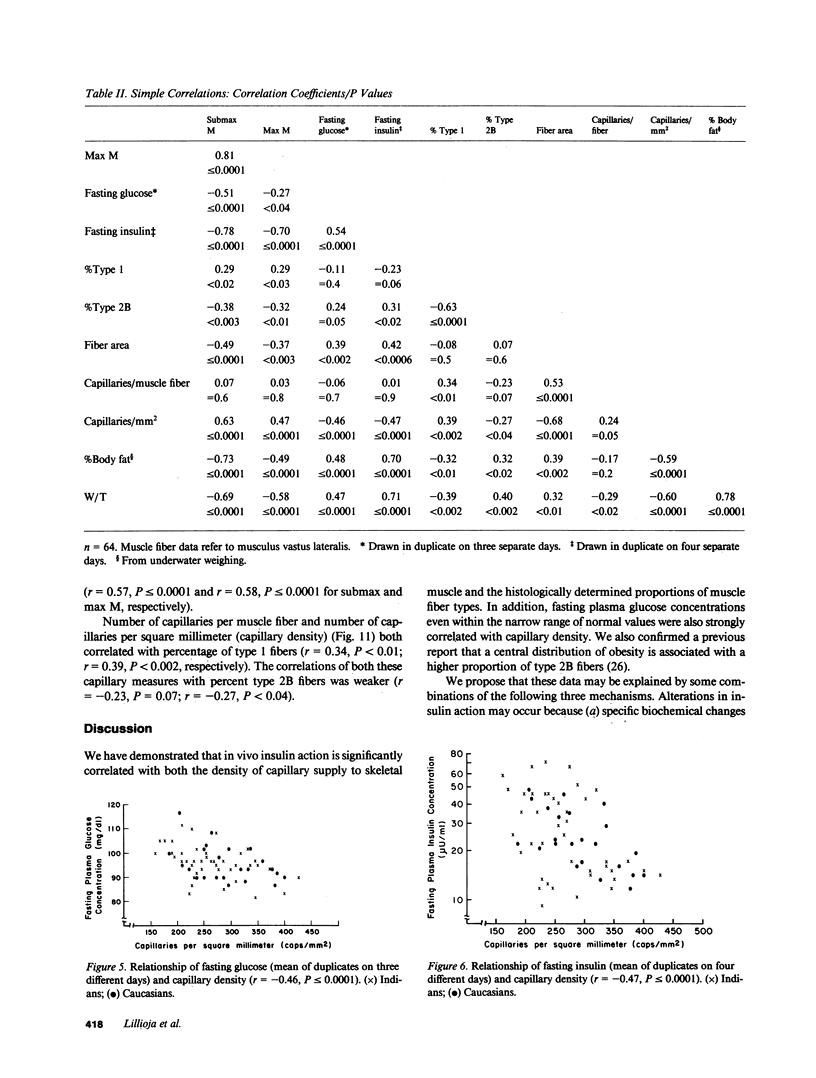
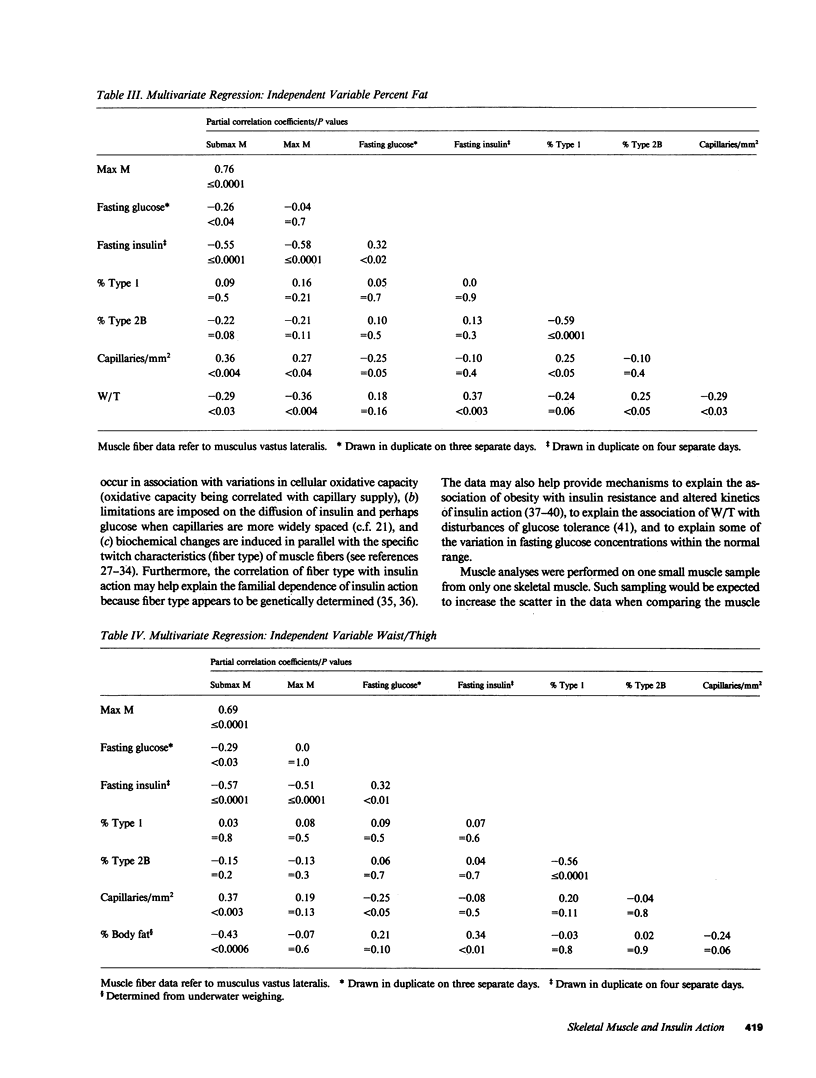
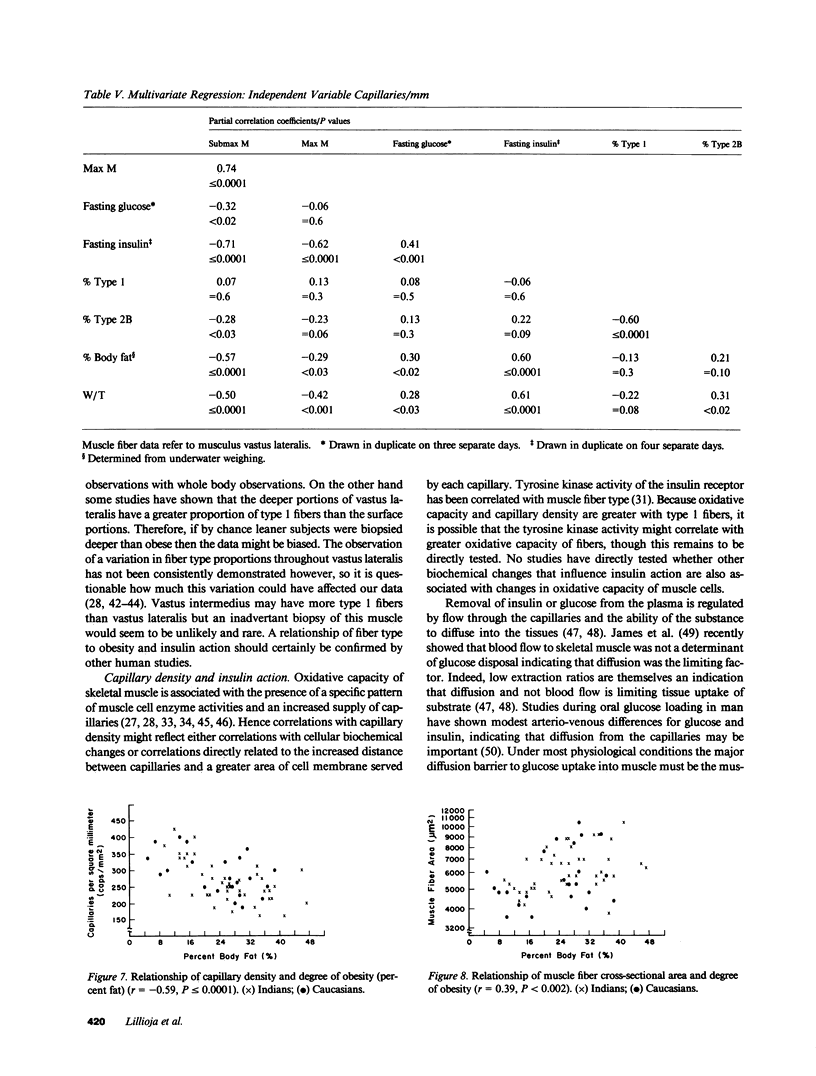
We have compared the capillary density and muscle fiber type of musculus vastus lateralis with in vivo insulin action determined by the euglycemic clamp (M value) in 23 Caucasians and 41 Pima Indian nondiabetic men. M value was significantly correlated with capillary density (r = 0.63; P less than or equal to 0.0001), percent type I fibers (r = 0.29; P less than 0.02), and percent type 2B fibers (r = -0.38; P less than 0.003). Fasting plasma glucose and insulin concentrations were significantly negatively correlated with capillary density (r = -0.46, P less than or equal to 0.0001; r = -0.47, P less than or equal to 0.0001, respectively). Waist circumference/thigh circumference ratio was correlated with percent type 1 fibers (r = -0.39; P less than 0.002). These results suggest that diffusion distance from capillary to muscle cells or some associated biochemical change, and fiber type, could play a role in determining in vivo insulin action. The association of muscle fiber type with body fat distribution may indicate that central obesity is only one aspect of a more generalized metabolic syndrome. The data may provide at least a partial explanation for the insulin resistance associated with obesity and for the altered kinetics of insulin action in the obese.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975 Oct;95(2):203–205. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Mott D. M., Hollenbeck C., Reaven G. Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol. 1985 Mar;248(3 Pt 1):E286–E291. doi: 10.1152/ajpendo.1985.248.3.E286. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Mott D., Reaven G. R., Kashiwagi A., Foley J. E. Relationship between obesity and maximal insulin-stimulated glucose uptake in vivo and in vitro in Pima Indians. J Clin Invest. 1984 Mar;73(3):800–805. doi: 10.1172/JCI111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaffi J. L., Heldt A., Lewis L. D., Grodsky G. M. The third phase of in vitro insulin secretion. Evidence for glucose insensitivity. Diabetes. 1986 Mar;35(3):370–373. doi: 10.2337/diab.35.3.370. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Smith J. L., Simpson D. R. Muscle fibre type populations of human leg muscles. Histochem J. 1975 May;7(3):259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Cohen I. S. The ultrastructure of the cardiac Purkinje strand in the dog: a morphometric analysis. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):191–213. doi: 10.1098/rspb.1983.0006. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Murray R., Kissebah A. H. Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal, and insulin binding. Effects of obesity and body fat topography. J Clin Invest. 1984 Oct;74(4):1515–1525. doi: 10.1172/JCI111565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes G. B., Welle S. L. Lean body mass in obesity. Int J Obes. 1983;7(2):99–107. [PubMed] [Google Scholar]
- Golay A., Chen Y. D., Reaven G. M. Effect of differences in glucose tolerance on insulin's ability to regulate carbohydrate and free fatty acid metabolism in obese individuals. J Clin Endocrinol Metab. 1986 Jun;62(6):1081–1088. doi: 10.1210/jcem-62-6-1081. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hom F. G., Goodner C. J. Insulin dose-response characteristics among individual muscle and adipose tissues measured in the rat in vivo with 3[H]2-deoxyglucose. Diabetes. 1984 Feb;33(2):153–159. doi: 10.2337/diab.33.2.153. [DOI] [PubMed] [Google Scholar]
- Ingjer F. Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J Physiol. 1979 Sep;294:419–432. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- James D. E., Burleigh K. M., Storlien L. H., Bennett S. P., Kraegen E. W. Heterogeneity of insulin action in muscle: influence of blood flow. Am J Physiol. 1986 Oct;251(4 Pt 1):E422–E430. doi: 10.1152/ajpendo.1986.251.4.E422. [DOI] [PubMed] [Google Scholar]
- James D. E., Jenkins A. B., Kraegen E. W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985 May;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- KEYS A., BROZEK J. Body fat in adult man. Physiol Rev. 1953 Jul;33(3):245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Komi P. V., Viitasalo J. H., Havu M., Thorstensson A., Sjödin B., Karlsson J. Skeletal muscle fibres and muscle enzyme activities in monozygous and dizygous twins of both sexes. Acta Physiol Scand. 1977 Aug;100(4):385–392. doi: 10.1111/j.1365-201X.1977.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J. L., Cooper H. E., Deal D. A., Weir G. C. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986 Mar;77(3):908–915. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- Lindgärde F., Eriksson K. F., Lithell H., Saltin B. Coupling between dietary changes, reduced body weight, muscle fibre size and improved glucose tolerance in middle-aged men with impaired glucose tolerance. Acta Med Scand. 1982;212(3):99–106. doi: 10.1111/j.0954-6820.1982.tb03179.x. [DOI] [PubMed] [Google Scholar]
- Lithell H., Lindgärde F., Hellsing K., Lundqvist G., Nygaard E., Vessby B., Saltin B. Body weight, skeletal muscle morphology, and enzyme activities in relation to fasting serum insulin concentration and glucose tolerance in 48-year-old men. Diabetes. 1981 Jan;30(1):19–25. doi: 10.2337/diab.30.1.19. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Kimmey J. S., Felder S., Chi M. M., Kaiser K. K., Passonneau P. N., Kirk K. A., Lowry O. H. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978 Nov 25;253(22):8269–8277. [PubMed] [Google Scholar]
- Mahon M., Toman A., Willan P. L., Bagnall K. M. Variability of histochemical and morphometric data from needle biopsy specimens of human quadriceps femoris muscle. J Neurol Sci. 1984 Jan;63(1):85–100. doi: 10.1016/0022-510x(84)90111-4. [DOI] [PubMed] [Google Scholar]
- Meistas M. T., Margolis S., Kowarski A. A. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol. 1983 Aug;245(2):E155–E159. doi: 10.1152/ajpendo.1983.245.2.E155. [DOI] [PubMed] [Google Scholar]
- Naeye R. L., Roode P. The sizes and numbers of cells in visceral organs in human obesity. Am J Clin Pathol. 1970 Aug;54(2):251–253. doi: 10.1093/ajcp/54.2.251. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Prager R., Wallace P., Olefsky J. M. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest. 1986 Aug;78(2):472–481. doi: 10.1172/JCI112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasio E. A., Mack E., Egdahl R. H., Herrera M. G. Passage of insulin and inulin across vascular membranes in the dog. Diabetes. 1968 Nov;17(11):668–672. doi: 10.2337/diab.17.11.668. [DOI] [PubMed] [Google Scholar]
- Rasio E. A. The displacement of insulin from blood capillaries. Diabetologia. 1969 Dec;5(6):416–419. doi: 10.1007/BF00427981. [DOI] [PubMed] [Google Scholar]
- Rasio E. The capillary barrier to circulating insulin. Diabetes Care. 1982 May-Jun;5(3):158–161. doi: 10.2337/diacare.5.3.158. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Enhanced muscle glucose metabolism after exercise: modulation by local factors. Am J Physiol. 1984 Jun;246(6 Pt 1):E476–E482. doi: 10.1152/ajpendo.1984.246.6.E476. [DOI] [PubMed] [Google Scholar]
- Rossell R., Gomis R., Casamitjana R., Segura R., Vilardell E., Rivera F. Reduced hepatic insulin extraction in obesity: relationship with plasma insulin levels. J Clin Endocrinol Metab. 1983 Mar;56(3):608–611. doi: 10.1210/jcem-56-3-608. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sjögren K., Damber J. E., Liliequist B. Sexuality after stroke with hemiplegia. I. Aspects of sexual function. Scand J Rehabil Med. 1983;15(2):55–61. [PubMed] [Google Scholar]
- Skalak T. C., Schmid-Schönbein G. W., Zweifach B. W. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res. 1984 Jul;28(1):95–112. doi: 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- Sonksen P. H., McCormick J. R., Egdahl R. H., Soeldner J. S. Distribution and binding of insulin in the dog hindlimb. Am J Physiol. 1971 Dec;221(6):1672–1680. doi: 10.1152/ajplegacy.1971.221.6.1672. [DOI] [PubMed] [Google Scholar]
- Stunkard A. J., Sørensen T. I., Hanis C., Teasdale T. W., Chakraborty R., Schull W. J., Schulsinger F. An adoption study of human obesity. N Engl J Med. 1986 Jan 23;314(4):193–198. doi: 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- Tesch P. A., Thorsson A., Kaiser P. Muscle capillary supply and fiber type characteristics in weight and power lifters. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):35–38. doi: 10.1152/jappl.1984.56.1.35. [DOI] [PubMed] [Google Scholar]
- Thomason D. B., Baldwin K. M., Herrick R. E. Myosin isozyme distribution in rodent hindlimb skeletal muscle. J Appl Physiol (1985) 1986 Jun;60(6):1923–1931. doi: 10.1152/jappl.1986.60.6.1923. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]



