Abstract
Abstract
Patients with the postural orthostatic tachycardia syndrome (POTS) are primarily premenopausal women, which may be attributed to female sex hormones. We tested the hypothesis that hormonal fluctuations of the menstrual cycle alter sympathetic neural activity and orthostatic tolerance in POTS women. Ten POTS women were studied during the early follicular (EF) and mid-luteal (ML) phases of the menstrual cycle. Haemodynamics and muscle sympathetic nerve activity (MSNA) were measured when supine, during 60 deg upright tilt for 45 min or until presyncope, and during the cold pressor test (CPT) and Valsalva manoeuvres. Blood pressure and total peripheral resistance were higher during rest and tilting in the ML than EF phase; however, heart rate, stroke volume and cardiac output were similar between phases. There were no mean ± SD differences in MSNA burst frequency (8 ± 8 EF phase vs. 10 ± 10 bursts min–1 ML phase at rest; 34 ± 15 EF phase vs. 36 ± 16 bursts min–1 ML phase at 5 min tilt), burst incidence or total activity, nor any differences in the cardiovagal and sympathetic baroreflex sensitivities between phases under any condition. The incidence of presyncope was also the same between phases. There were no differences in haemodynamic or sympathetic responses to CPT or Valsalva. These results suggest that the menstrual cycle does not affect sympathetic neural activity but modulates blood pressure and vasoconstriction in POTS women during tilting. Thus, factors other than sympathetic neural activity are probably responsible for the symptoms of orthostatic intolerance across the menstrual cycle in women with POTS.
Key points
Women with the postural orthostatic tachycardia syndrome (POTS) report fluctuations in orthostatic tolerance throughout the menstrual cycle.
The mechanism(s) underlying blood pressure control across the menstrual cycle in women with POTS are unknown.
The findings of the present study indicate that the menstrual cycle does not affect muscle sympathetic nerve activity but modulates blood pressure and vasoconstriction in POTS women during orthostatic stress.
Factors other than sympathetic neural activity are likely responsible for the symptoms of orthostatic intolerance across the menstrual cycle in women with POTS.
Introduction
It is estimated that the postural orthostatic tachycardia syndrome (POTS) affects approximately 170/100,000 people worldwide, the majority of whom are premenopausal women (Schondorf et al. 1999). As a group, POTS patients are often characterized by a small heart, reduced blood and plasma volume, and small stroke volume, which manifest as reflex tachycardia during orthostasis (Fu et al. 2010a). Medical reports, scientific investigations and anecdotal accounts suggest that as many as 80% of women with POTS experience symptomatic fluctuations with the menstrual cycle (Raj, 2006). Generally, worsening orthostatic intolerance in POTS women corresponds with either the premenstrual or early follicular (EF) phase, when both oestrogen and progesterone levels are dropping or low (Raj, 2006; Thieben et al. 2007; Fu et al. 2010b; Peggs et al. 2012).
Previously, we have reported a reduction in plasma renin activity and aldosterone (e.g. important modulators of plasma volume and blood pressure during orthostasis) during 2 h of standing, accompanied by an increased rate of presyncope, during the early follicular (EF) compared to mid-luteal (ML) phase in POTS patients (Fu et al. 2010b). Sympathetic neural activity also plays a critical role in arterial blood pressure maintenance, mainly through baroreflex-mediated vasoconstriction during short-term and sustained orthostasis (Wallin & Sundlof, 1982; Fu et al. 2004, 2006). Endogenous hormonal fluctuations characteristic of the menstrual cycle have been shown to affect this sympathetic neural activity at rest and during orthostasis in healthy euvolaemic women (Minson et al. 2000a; Carter et al. 2009, 2013; Fu et al. 2009; Yang et al. 2012). Specifically, sympathetic neural activity, measured via microneurography as muscle sympathetic nerve activity (MSNA), is increased in healthy women when the ratio of oestradiol to progesterone is lower (i.e. generally during the ML phase) (Carter et al. 2013). Similar results are seen in women using hormonal contraceptives, with the ‘high-hormone’ phase eliciting greater sympathetic neural activity in some studies (Usselman et al. 2013), although not in all (Minson et al. 2000b; Carter et al. 2010). Findings regarding sympathetic baroreflex sensitivity (BRS) changes throughout the menstrual cycle are also mixed, with both no difference (Fu et al. 2009; Yang et al. 2012) and increased sensitivity during ML (or the ‘high-hormone’ phase) (Minson et al. 2000a, b2000) being reported.
POTS patients sometimes present as hyperadrenergic, with an exaggerated MSNA response to baroreflex challenges compared to healthy individuals (Muenter Swift et al. 2005). However, as is seen in healthy women, whether or not there are alterations in sympathetic activity coincident with hormonal fluctuations of the menstrual cycle in POTS women is unknown. We hypothesized that an attenuated increase in sympathetic neural activity from the supine to upright position during EF compared to ML contributes to the greater orthostatic intolerance observed during EF in POTS patients.
Methods
Subjects
Ten normally menstruating female POTS patients who were referred to our tertiary Autonomic Function Clinic participated in both phases of the study. All women met the criteria for POTS and had a heart rate rise ≥ 30 b.p.m. or a rate that exceeded 120 b.p.m. after 10 min of standing without any evidence of orthostatic hypotension (Low, 1995; Raj, 2005). All had self-reported regular menstrual cycles of ∼28 days and had never taken or had not used hormonal contraceptives for ≥6 months. All subjects were non-smokers. Subjects stopped taking any medications that could affect the autonomic nervous system ≥2 weeks before testing. All subjects were informed of the study purpose and protocols, and they provided their written informed consent to a protocol approved by the institutional review boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. The subject characteristics have been described previously (Fu et al. 2010b). The study followed guidelines set forth in the Declaration of Helsinki.
Protocol
Subjects were studied twice: once during the EF phase (1–4 days after the onset of menstruation when both oestrogen and progesterone are low) and once during the ML phase (19–22 days after the onset of menstruation when both hormones are high), with the order being randomized and counterbalanced. The second testing session occurred within one or two menstrual cycles after the first (mean ± SD time between testing = 49 ± 22 days). Menstrual cycle phase was determined by (i) the onset of menstruation and (ii) the detection of the luteinizing hormone surge by an ovulation prediction kit (OvuQuick; Quidel Corp., San Diego, CA, USA) and verified by circulating oestradiol and progesterone concentrations on each study day. All women were on an isocaloric diet consisting of 200 mEq of sodium, 100 mEq of potassium and 1000 mg of calcium, with ad libitum fluid intake, for 3 days prior to testing. Fluid intake and dietary compliance were assessed by 24 h urine output the day immediately prior to testing. Women were also instructed to not exercise for at least 24 h before testing. A urine pregnancy test was taken on each study day to confirm that the women were not pregnant.
The experiment was performed in the morning ≥2 h after a light meal and ≥72 h after the last caffeinated or alcoholic beverage. The study laboratory was quiet and environmentally controlled with an ambient temperature of ∼25°C. The subject was placed in the supine position on a tilt bed and an i.v. catheter was inserted into an antecubital vein of the left arm for blood samples. Haemodynamic variables were measured after ≥30 min in the supine position. At least 10 min after an acceptable nerve recording site had been found, baseline MSNA data were collected during spontaneous breathing and controlled breathing (0.2 Hz, 12 breaths min–1) for 6 min each. Next, for the assessment of BRS, all subjects were asked to perform two Valsalva manoeuvres at 40 mmHg for 20 s after a normal inspiration, separated by a 3 min recovery period. After sufficient recovery, the subject performed a cold pressor test (CPT), where the hand was immersed in an ice water bath for 2 min, after which the subject's hand was immediately dried and warmed. The CPT was used to assess the central integration of vasomotor sympathetic processes and their efferent pathways (Victor et al. 1987; Seals, 1990). The subject was then tilted passively to 60 deg for 45 min or until presyncope. A belt was placed across the woman's waist to ensure that she would not fall. A bicycle saddle was used to support approximately two-thirds of the body weight during tilting, when the subject stood on a plate at the end of the tilt bed on one leg, allowing the other leg to be relaxed for microneurography. After tilting, the subject was returned to the supine position for recovery.
Heart rate (HR), blood pressure (BP), respiratory waveforms and MSNA were recorded continuously. Cardiac output (Qc) was measured when supine and after 5, 10, 20, 30 and 40 min of 60 deg upright tilt. Blood samples were taken in the supine position, after 5 and 20 min of 60 deg tilt, and immediately after returning to supine as a result of the subject either completing 45 min of tilting or developing presyncope. Presyncope was defined as a decrease in systolic BP to <80 mmHg; a decrease in systolic BP to <90 mmHg associated with symptoms of lightheadedness, nausea, sweating or diaphoresis; or progressive symptoms of presyncope accompanied by a request from the subject to discontinue the test (Levine et al. 1991). The time of presyncope was demarcated as the time from the start of 60 deg tilt to the onset of presyncope. A true hypotensive end-point was reached in all subjects who were declared presyncopal in the present study.
Measurements
MSNA
MSNA signals were obtained with the microneurographic technique (Vallbo et al. 1979). Briefly, a recording electrode was placed in the peroneal nerve at the popliteal fossa, and a reference electrode was placed s.c. 2–3 cm from the recording electrode. The nerve signals were amplified (gain 70,000–160,000), band-pass filtered (700–2000 Hz), full-wave rectified and integrated with a resistance-capacitance circuit (time constant 0.1 s). Adequate MSNA recording was assessed by (i) pulse synchrony; (ii) facilitation during the hypotensive phase of the Valsalva manoeuvre; (iii) increases in response to breath holding; and (iv) insensitivity to a gentle skin touch or a loud shout (Vallbo et al. 1979).
Haemodynamics
HR was determined via electrocardiogram and beat-to-beat arterial BP was measured non-invasively from the middle finger using photoplethysmography (Nexfin; BMEYE BV, Amsterdam, The Netherlands). Arm-cuff BP was measured by electrosphygmomanometry (model 4240; SunTech Medical Instruments Inc., Raleigh, NC, USA) with a microphone placed over the brachial artery to detect Korotkoff sounds. Qc was determined via the acetylene rebreathing technique (Triebwasser et al. 1977), in which the disappearance rate of acetylene in expired air was measured with a mass spectrometer (Marquette Electronics Inc., Milwaukee, WI, USA). Stroke volume (SV) was calculated from Qc and the HR measured during rebreathing, and total peripheral resistance (TPR) was calculated as the quotient of mean arterial pressure (MAP) and Qc, multiplied by 80 (expressed as dynes·s·cm−5). MAP was calculated as [(systolic pressure – diastolic pressure)/3] + diastolic pressure. Respiratory excursions were identified by a nasal cannula (model 1265; Respironics California Inc., Carlsbad, CA, USA).
Data analysis
MSNA
Data were sampled at 625 Hz with a commercial data acquisition system (Biopac Systems, Santa Barbara, CA, USA). Sympathetic bursts were identified via computer software (LabView Software; National Instruments, Austin, TX, USA) using a 3:1 signal-to-noise ratio threshold within a 0.5 s search window and an expected burst reflex latency of 1.3 s from the preceding R-wave (Cui et al. 2001). All bursts were confirmed by an experienced microneurographer. Within the integrated neurogram, the burst with the largest amplitude during baseline was assigned a value of 100, and all bursts in that trial were normalized to that burst (Halliwill, 2000). Burst areas of the integrated neurogram, systolic and diastolic pressures, and R–R interval were measured simultaneously on a beat-to-beat basis. Total activity of the burst was defined as the burst area of the rectified and integrated neurogram. The number of bursts per minute (burst frequency) and per 100 heartbeats (burst incidence) and total burst area per minute (total activity) were used as quantitative indices.
Baroreflex sensitivity during the Valsalva manoeuvre
Sympathetic BRS was assessed by relating all sympathetic bursts occurring during the 20 s straining period of the Valsalva manoeuvre to the maximum fall of diastolic pressure as measured from the highest level just after the beginning of straining (phase I) to its nadir (Fu et al. 2009). Cardiovagal BRS was assessed during early phase II (i.e. hypotensive stimulus) and phase IV (i.e. hypertensive stimulus) (Fu et al. 2009). The slope of the linear relationship between changes in systolic pressure and the corresponding changes in R–R interval was calculated to evaluate cardiovagal BRS. Sympathetic and cardiovagal BRS values were calculated for each of the two Valsalva manoeuvres and reported as the mean of the two.
Statistical analysis
Data are expressed as the mean ± SD. Supine MSNA, BP and HR were averaged for 6 min. MSNA data were averaged for 30 s epochs before, throughout and during recovery from CPT. During 60 deg upright tilt, data were calculated for each minute for the first 1–5 min, and then averaged from 7–10 min (‘10’), 17–20 min (‘20’), 26–29 min (‘30’), 36–39 min (‘40’) and 42–45 min (‘45’). Some subjects had presyncope before the 45 min of 60 deg upright tilt, and the tilt test was terminated at different time points; thus, we used the last stable data carry forward method for imputing with missing values (Ali & Talukder, 2005). Data were also averaged in 20 s intervals over the last 3 min prior to presyncope in presyncopal subjects, and from 42 to 45 min in non-presyncopal subjects, aiming to determine responses to tilt across subjects with different end-points.
All statistical analyses were performed using SPSS, version 20.0 (IBM, Armonk, NY, USA). Baseline characteristics, hormones and BRS between menstrual cycle phases were compared using paired t tests. Repeated measures ANOVA was used to determine differences in haemodynamic and MSNA responses between phases and time points of upright tilt and CPT, and the Holm–Sidak method was used post hoc for multiple comparisons. A chi-squared test was used to compare the incidence of presyncope in subjects between menstrual cycle phases. Effect sizes for differences were assessed using Cohen's d. For all tests, P < 0.05 was considered statistically significant.
Results
Subject characteristics
Anthropometric characteristics of the POTS patients were the same across menstrual cycle phases (age = 27 ± 9 years, height = 167 ± 6 cm, weight = 67 ± 12 kg, body mass index = 24 ± 4 kg m–2). The physical characteristics of POTS women were similar to those of healthy control women who were investigated during a previous study in our laboratory (Fu et al. 2009).
Sex hormones and resting MSNA
Plasma oestradiol (E2) and progesterone (P) concentr-ations were both elevated during the ML compared to the EF phase (Table1). Unlike healthy women (Fu et al. 2009),who display a significantly reduced E2/P ratio during the ML compared to EF phase, POTS patients had similar E2/P ratios between phases. However, four POTS patients did not display a surge in progesterone during the estimated ML phase (P during ML < 2.1 ng mL–1), and thus were likely anovulatory. When these subjects were removed, the E2/P ratio was significantly reduced during the ML compared to EF phase (8.3 ± 2.7 vs. 50.0 ± 17.7; P < 0.01).
Table 1.
Circulating sex hormone concentrations across menstrual phases in healthy women and POTS patients
| Early follicular phase | Mid-luteal phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | POTS | Healthy | POTS | |||||||||
| E2 | P | E2/P | E2 | P | E2/P | E2 | P | E2/P | E2 | P | E2/P | |
| Mean | 32.4 | 0.93 | 42.7 | 32.4 | 0.82 | 50.3 | 91.8* | 11.1* | 12.6* | 82.6* | 7.9* | 31.6 |
| SD | 8.9 | 0.50 | 23.2 | 8.7 | 0.50 | 21.9 | 46.7 | 5.7 | 13.3 | 41.5 | 6.0 | 45.8 |
Hormones measured via blood draw on testing days. E2, oestradiol (pg mL–1); P, progesterone (ng mL–1); E2/P, oestradiol to progesterone ratio (arbitary units). Data for healthy women are from a previous study (Fu et al. 2009).
Significant difference from the EF phase in the same group (P < 0.05).
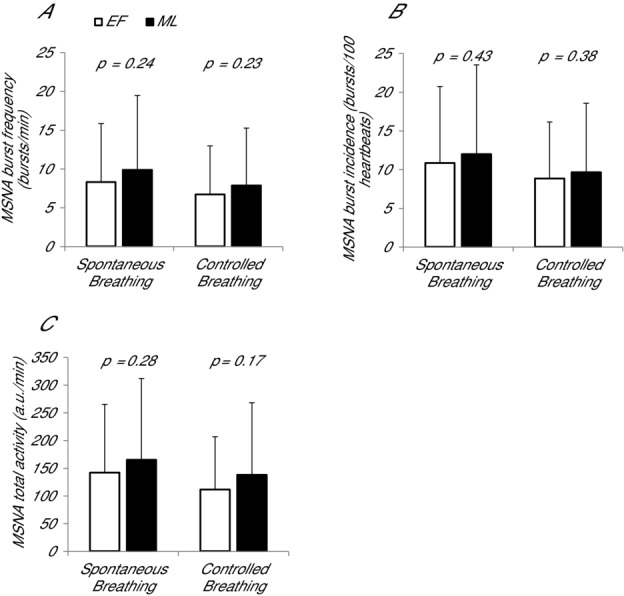
There were no differences in MSNA burst frequency or incidence between EF and ML during spontaneous or controlled breathing in the supine position (Fig. 1A and B), nor was resting MSNA different between phases when anovulatory subjects were removed from the analysis. Similarly, supine total activity did not differ between menstrual cycle phases (Fig. 1C). The delta MSNA from EF to ML was not correlated with the hormonal changes (i.e. ΔE2, ΔP or ΔE2/P; absolute r < 0.10 for each); when anovulatory subjects were removed, the relationship between delta MSNA and delta hormones became stronger but remained insignificant (ΔMSNA-ΔE2: r = –0.44; ΔMSNA-ΔP: r = 0.00; ΔMSNA-ΔE2/P: r = –0.41).
Figure 1.
Resting MSNA during EF and ML phases of the menstrual cycle
MSNA (A) burst frequency, (B) burst incidence and (C) total activity of POTS patients during spontaneous and controlled breathing when resting supine in the EF and ML phases. Error bars indicate the SD. There were no differences in resting MSNA between phases.
Baroreflex sensitivity during Valsalva manoeuvre
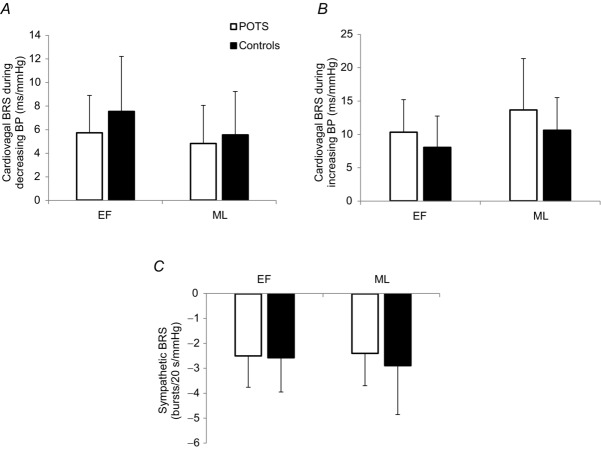
Sympathetic and cardiovagal BRS values measured during the Valsalva manoeuvre are shown in Fig. 2. There were no differences in sympathetic or cardiovagal BRS values between phases.
Figure 2.
Baroreflex sensitivity during EF and ML phases of the menstrual cycle
Cardiovagal (A and B) and sympathetic (C) BRS in POTS women and healthy controls (Fu et al. 2009) during the EF and ML phases. Error bars indicate the SD. There was no significant effect of phase on sympathetic (P = 0.652) or cardiovagal (P = 0.227 decreasing BP; P = 0.253 increasing BP) BRS in POTS women.
MSNA responses to the cold pressor test
The MSNA response to and recovery from CPT did not differ between phases in POTS women (Fig. 3). HR, systolic blood pressure (SBP) and diastolic blood pressure (DBP) all increased during the CPT, although the responses were not different between phases (P = 0.42, 0.28 and 0.33, respectively).
Figure 3.
MSNA response to CPT during EF and ML phases of the menstrual cycle
MSNA (A) burst frequency, (B) burst incidence and (C) total activity responses to the CPT. Values indicate every 30 s. BL, baseline. Error bars indicate the SD. There was no significant effect of phase on the MSNA response.
Haemodynamic and MSNA responses during upright tilt
Haemodynamic variables are shown in Fig. 4. HR rose significantly from supine to 60 deg tilt but was not different between menstrual cycle phases. Similarly, Qc and SV became lower with tilting but were not significantly different between phases. SBP, DBP, MAP and TPR increased with tilting, with SBP and MAP displaying significantly greater increases during the ML compared to EF phase. DBP and TPR also tended to increase to a greater degree during the ML compared to the EF phase (P = 0.08 for each).
Figure 4.
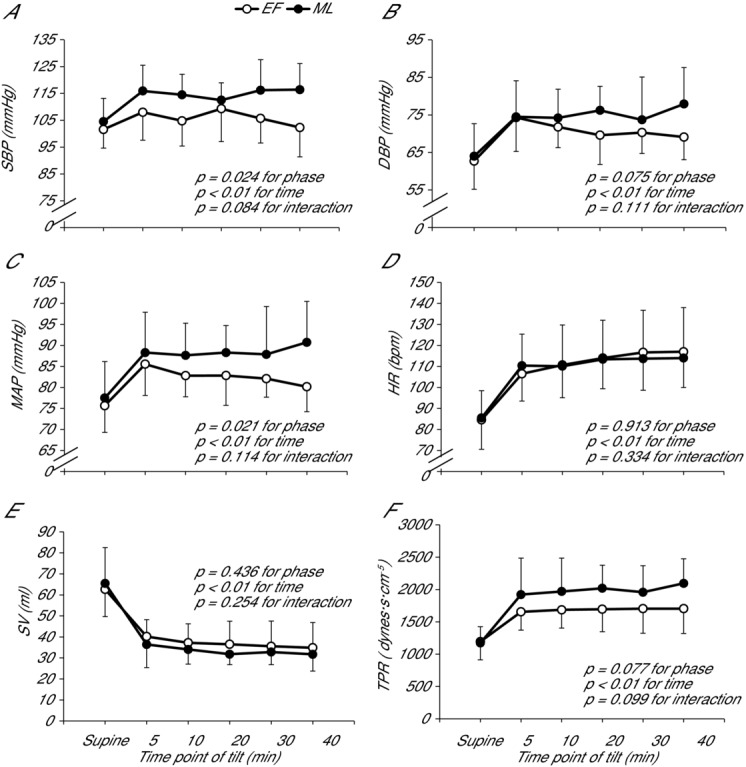
Haemodynamic responses to upright tilt during the EF and ML phases
Measures of (A) SBP, (B) DBP, (C) MAP, (D) HR, (E) SV and (F) TPR are given for supine rest and at 5, 10, 20, 30 and 40 min of tilt. Data are presented using the last stable data carry forward method for subjects who experienced presyncope. Error bars indicate the SD. All variables were significantly altered from supine to upright tilt. SBP and MAP were significantly greater during the ML compared to EF phase.
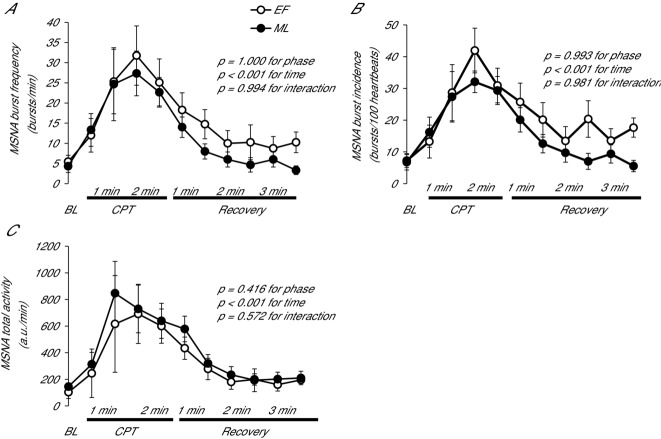
MSNA burst frequency and incidence were similar during tilting between EF and ML (P > 0.05). During both EF and ML, MSNA increased from supine to 60 deg head-up tilt, and the magnitude of increase was not different between phases (Fig. 5). When anovulatory subjects were removed, the sympathetic neural responses to orthostasis remained similar between phases.
Figure 5.
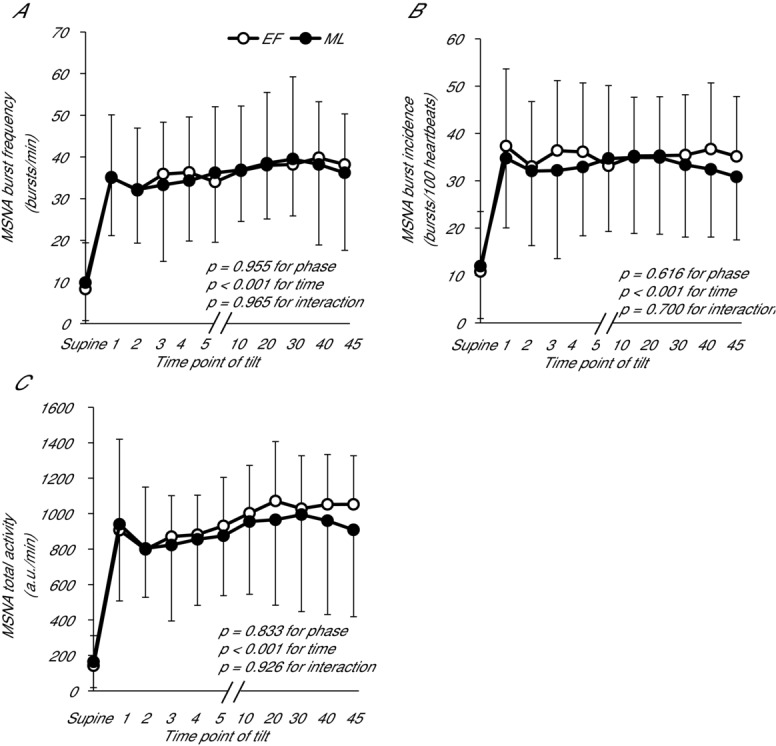
MSNA responses to upright tilt during the EF and ML phases
MSNA (A) burst frequency, (B) burst incidence and (C) total activity of POTS patients during supine and passive upright tilt at 60 deg in the EF and ML phases. Data are presented using the last stable data carry forward method for subjects who experienced presyncope. 1, 2, 3, 4, 5 indicate early minutes of tilting; 10, mean from 7–10 min; 20, mean from 17–20 min; 30, mean from 26–29 min; 40, mean from 36–39 min; 45, mean from 42–45 min. Error bars indicate the SD. There were no differences between phases in the MSNA response to upright tilt.
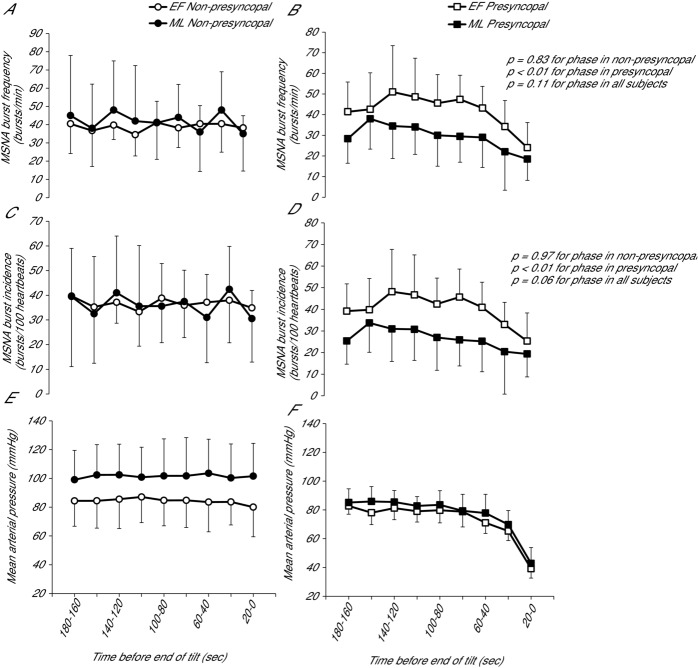
Measures of MSNA were also not significantly different during the final 3 min before the end of tilting between phases, although burst frequency and incidence tended to be greater during EF (burst frequency: P = 0.11; burst incidence: P = 0.06; total activity: P = 0.32). To further examine the effect of menstrual cycle phase on MSNA during the end of tilting, subjects were separated into those who became presyncopal and those who did not become presyncopal (Fig. 6). There were no differences between phases in MSNA burst frequency or incidence during the last 3 min of tilt in non-presyncopal women (P = 0.83 and 0.97, respectively). However, in presyncopal women, burst frequency and incidence were significantly greater during EF than ML (P < 0.01). The difference between phases observed in presyncopal subjects was primarily driven by a lower ML burst frequency/incidence compared to non-presyncopal subjects (P < 0.01).
Figure 6.
MSNA burst frequency and incidence in POTS patients who did and did not become presyncopal during the last 3 min of upright tilt in the EF and ML phases
A and B, burst frequency in non-presyncopal and presyncopal subjects, respectively. C and D, burst incidence in non-presyncopal and presyncopal subjects, respectively. E and F, MAP in non-presyncopal and presyncopal subjects, respectively. There were no differences between phases in MSNA in women who did not become presyncopal; however, MSNA was greater during EF than ML in presyncopal subjects. Error bars indicate the SD.
There was no difference in the time of tilting (i.e. to presyncope or 45 min) between phases (EF: 32 ± 15 vs. ML: 34 ± 15 min), nor in the incidence of presyncope (EF: five out of ten women vs. ML: six out of ten; χ2; P > 0.05). MSNA measures during recovery from tilt did not differ between phases (P = 0.47–0.86).
Discussion
The primary finding of the present study is that, in women with POTS, menstrual cycle phase does not affect resting sympathetic activity; sympathetic neural responses to upright tilt or cold pressor test; or baroreflex sensitivity. By contrast, the menstrual cycle does modulate blood pressure and vasoconstriction in POTS women during tilting. Given the recent retrospective analysis of several studies indicating that the degree of sympathoexcitation is dependent upon the degree of sex steroid surges in healthy women (Carter et al. 2013), the results of the present study suggest that either: (i) variable sex steroid surges among our subject population obscured meaningful differences in sympathoexcitation between phases or (ii) there is an inherent difference between healthy women and POTS patients where fluctuations in sympathoexcitation with the menstrual cycle in POTS women do not occur.
Impact of the menstrual cycle on sympathetic neural control in POTS
In healthy women, resting MSNA (Minson et al. 2000a) and MSNA total activity in response to orthostasis are blunted during the EF compared to the ML phase (Carter et al. 2009; Fu et al. 2009). In POTS women, there was no such effect of menstrual cycle phase on supine MSNA or the response to orthostasis. The degree of sex steroid surges during the ovarian cycle appears to explain, at least in part, differences in sympathoexcitation across phases in healthy women (Carter et al. 2013). In the present study, however, post hoc analysis showed that concentrations of oestradiol and progesterone, as well as the oestradiol/progesterone ratio, were not related to measures of sympathetic activity (absolute r values < 0.10), nor were the changes in hormone concentrations from EF to ML related to changes in MSNA. Thus, variable sex hormone surges probably did not obscure differences in sympathetic activity between phases in this population.
The reason why sex steroid concentrations would be related to MSNA in healthy individuals but not in POTS patients is unclear. It has been suggested that oestradiol has sympathoinhibitory effects, whereas progesterone is sympathoexcitatory, and thus the relatively larger increase in progesterone (compared to oestradiol) during the ML phase augments MSNA. In both POTS patients and healthy controls, there are significant increases in oestradiol and progesterone from the early follicular to mid-luteal testing days (P < 0.01). However, whereas the oestradiol/progesterone ratio significantly decreased in our previous study of healthy women from EF to ML (43 ± 23 to 13 ± 13; P = 0.005; Fu et al. 2009), the ratio did not change across the menstrual cycle in POTS women, presumably because a number of POTS patients were anovulatory (50 ± 22 to 32 ± 46; P = 0.19). Patients with POTS report a greater incidence of hormone-related gynecological disease compared to healthy women (Peggs et al. 2012), and anovulatory cycles may be another, previously unreported, example of gynecological disorders in POTS. However, the lack of relationship between hormones and MSNA in the present study remained when anovulatory subjects were removed. Differences in blood volume may also partially explain the stability in MSNA across the menstrual cycle in POTS patients compared to healthy euvolaemic women because it is well-established that POTS patients are characterized by reduced blood volume (Fu et al. 2010a). This low blood volume could enhance resting MSNA, effectively resulting in a ‘ceiling’ such that MSNA cannot increase further despite hormonal fluctuations in POTS women.
Responses to Valsalva and CPT
Cardiovagal and sympa-thetic BRS were comparable between POTS women and healthy women tested under similar conditions (Fu et al. 2009); menstrual cycle phase did not affect these measures of BRS (Fig. 2). This is consistent with previous studies describing a similar resting BRS between POTS patients and healthy controls (Muenter Swift et al. 2005; Masuki et al. 2007; Fu et al. 2010a). The consistency in sympathetic and cardiovagal BRS across phases is similar to that observed in healthy women (Minson et al. 2000a; Fu et al. 2009), although some studies have found greater sympathetic BRS during the ML phase (Minson et al. 2000a).
Previously, we have shown that autonomic function as assessed by the CPT is intact in POTS patients (Fu et al. 2010a). Accordingly, the response to the CPT in POTS women in the present study was similar to that of healthy controls (Fu et al. 2009) and did not differ between phases. These results suggest that the central integration of vasomotor sympathetic processes and their efferent pathways is stable, despite changes in sex hormones, and thus is appropriate in this population.
MSNA during presyncope
Interestingly, women who developed presyncope during orthostasis displayed substantially different MSNA responses prior to the end of tilting between the EF and ML phases, with lower MSNA during ML. These differences are opposite to the blunted MSNA response to tilt during EF observed in healthy women. Recent work by Wenner et al. (2011) showed that women with low orthostatic tolerance, in contrast to those with high orthostatic tolerance, are less sensitive to the cutaneous vasoconstrictor effect of progesterone, perhaps via an impairment in the cyclooxygenase pathway. Although these previous findings cannot necessarily be extended to other vascular beds (e.g. muscle), they may help explain our observation indictating that the severe decline in BP in presyncopal POTS patients over the final 3 min of tilt was similar between menstrual cycle phases, despite differences in MSNA. On the other hand, the higher MSNA response during EF in presyncopal women may be the result of a lower oestrogen concentration (i.e. less sympathoinhibition) relative to during ML, which becomes appreciable with an extreme adrenergic stimulus.
Haemodynamics during orthostasis in POTS
An unexpected finding of the present study was the greater arterial pressures and vascular resistance during orthostasis in the ML compared to EF phase. There is a lack of consistent evidence for a BP difference between menstrual cycle phases; however, decreases in systemic vascular resistance and BP during the ML phase have been reported previously in healthy women (Chapman et al. 1997). The potential for menstrual cycle differences in vascular resistance and/or BP have been attributed to ovulation-mediated increases in vasoactive substances that have both renal and systemic vasodilating properties. The results of the present study stand in contrast to previous findings in healthy women. The POTS patients in the present study displayed increases in oestradiol similar to those of healthy individuals from the EF to ML phase, which would presumably enhance nitric oxide bioavailability (Weiner et al. 1994; Chen et al. 1999) and decrease noradrenaline spillover and adrenergic vasoconstriction (Cheng & Gruetter, 1992; Sudhir et al. 1997). The differential findings between POTS patients in the present study and previous evidence suggesting vasodilatation (and thus decreased TPR and BP) during the ML phase may be a result of the renin–angiotensin–aldosterone system (RAAS). RAAS activation is greater during the ML than the EF phase and can lead to salt and water retention and vasoconstriction (Fu et al. 2010b). Chapman et al. (1997) have previously shown that hormonal changes of the menstrual cycle have a specific vasodilatating effect during the ML phase, which overrides secondary activation of other renal vasoconstricting systems, such as the RAAS. However, it is possible that POTS women may display greater sensitivity and/or density of oestrogen and progesterone receptors that mediate RAAS activation. Thus, our findings obtained during short-term orthostasis are consistent with RAAS-mediated vasoconstriction during the ML phase. Ultimately, the lower BP and TPR during the EF phase may explain, at least in part, the sensations of orthostatic intolerance prior to or during menstruation.
Interestingly, the findings of the present study also contrast haemodynamic parameters during long-term orthostasis (e.g. 2 h of standing) in POTS patients, where TPR was lower, whereas BP was not different, during the ML compared to EF phase (Fu et al. 2010b). Although haemodynamic and autonomic responses during acute (e.g. 5 min) orthostasis are similar between passive tilting and active standing (Bloomfield et al. 1997), differences between the two may become apparent during longer-duration orthostasis. It is possible that greater blood pooling during tilt compared to standing causes enhanced sympathetic-mediated vasoconstriction, overriding the oestradiol-mediated vasodilatation that characterizes the ML phase.
Because MSNA was not different during orthostasis between menstrual cycle phases, the greater TPR and BP during the ML phase was probably a result of one or more other contributors, including differences in catecholamines, myogenic factors, blood volume, sympathetic vascular transduction or vasoconstriction in other vascular beds. Increased circulating catecholamines could contribute to the differences in blood pressure and vascular resistance observed with the menstrual cycle; however, there were no differences in plasma noradrenaline between phases in the present study. Additionally, our laboratory has previously reported that the menstrual cycle phase has no effect on blood volume in POTS patients (Fu et al. 2010b). On the other hand, given the increased TPR and BP despite unchanged MSNA, it is possible that POTS patients in the present study demonstrated greater sympathetic vascular transduction during the ML compared to EF phase. The hormonal fluctuations of the menstrual cycle do not appear to affect sympathetic transduction in healthy women (Minson et al. 2000a), although this finding is not consistent. For example, in women taking hormonal contraceptives, transduction of sympathetic activity into vascular outcomes may be lower during the ML (or high-hormone) compared to EF (low-hormone) phase (Minson et al. 2000b; Usselman et al. 2013). A more comprehensive assessment of sympathetic vascular transduction and/or non-adrenergically mediated vasoconstriction throughout the menstrual cycle in women with POTS has yet to be performed.
Conclusions
Although it has been established that MSNA in healthy euvolaemic women varies with hormonal changes of the menstrual cycle, the present study is the first to examine sympathetic activity across the menstrual cycle in women with POTS. The reported fluctuations in orthostatic tolerance across the menstrual cycle in POTS patients do not appear to be related to differences in sympathetic neural control of blood pressure. The differences are more probably a consequence of renal–adrenal and haemodynamic responses to orthostasis, and thus targeted pharmacological or lifestyle interventions aiming to improve orthostatic tolerance throughout the menstrual cycle in POTS women should be developed accordingly.
Glossary
- BP
blood pressure
- BRS
baroreflex sensitivity
- CPT
cold pressor test
- DBP
diastolic blood pressure
- E2
oestradiol
- EF
early follicular
- HR
heart rate
- MAP
mean arterial pressure
- ML
mid-luteal
- MSNA
muscle sympathetic nerve activity
- P
progesterone
- POTS
postural orthostatic tachycardia syndrome
- Qc
cardiac output
- RAAS
renin–angiotensin–aldosterone system
- SBP
systolic blood pressure
- SV
stroke volume
- TPR
total peripheral resistance
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
ASLS was responsible for analysis and interpretation of data, drafting the manuscript, and revising the manuscript. TBV was responsible for the collection and analysis of data. BDL was responsible for providing the laboratory where the experiments were performed, conceiving and designing the experiments, and revising the manuscript. QF was responsible for providing the laboratory where the experiments were performed, conceiving and designing the experiments, the collection, analysis and interpretation of data, and revising the manuscript.
Funding
This study was supported by a National Institutes of Health K23 grant (HL075283).
References
- Ali MW. Talukder E. Analysis of longitudinal binary data with missing data due to dropouts. J Biopharm Stat. 2005;15:993–1007. doi: 10.1080/10543400500266692. [DOI] [PubMed] [Google Scholar]
- Bloomfield DM, Kaufman ES, Bigger JT, Jr, Fleiss J, Rolnitzky L. Steinman R. Passive head-up tilt and actively standing up produce similar overall changes in autonomic balance. Am Heart J. 1997;134:316–320. doi: 10.1016/s0002-8703(97)70140-6. [DOI] [PubMed] [Google Scholar]
- Carter JR, Fu Q, Minson CT. Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61:395–399. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Klein JC. Schwartz CE. Effects of oral contraceptives on sympathetic nerve activity during orthostatic stress in young, healthy women. Am J PhysiolRegulIntegrComp Physiol. 2010;298:R9–R14. doi: 10.1152/ajpregu.00554.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Lawrence JE. Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J PhysiolEndocrinolMetab. 2009;297:E85–E91. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT. Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J PhysiolRenal Physiol. 1997;273:F777–F782. doi: 10.1152/ajprenal.1997.273.5.F777. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME. Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DY. Gruetter CA. Chronic estrogen alters contractile responsiveness to angiotensin II and norepinephrine in female rat aorta. Eur J Pharmacol. 1992;215:171–176. doi: 10.1016/0014-2999(92)90025-y. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA. Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF. Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol. 2009;587:2019–2031. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD. Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol. 2006;577:679–687. doi: 10.1113/jphysiol.2006.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS. Levine BD. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010a;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH. Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010b;56:82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Witkowski S. Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Levine BD, Lane LD, Buckey JC, Friedman DB. Blomqvist CG. Left ventricular pressure-volume and Frank–Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- Low PA. Update on the evaluation, pathogenesis, and management of neurogenic orthostatic hypotension: introduction. Neurology. 1995;45:S4–5. [PubMed] [Google Scholar]
- Masuki S, Eisenach JH, Schrage WG, Dietz NM, Johnson CP, Wilkins BW, Dierkhising RA, Sandroni P, Low PA. Joyner MJ. Arterial baroreflex control of heart rate during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103:1136–1142. doi: 10.1152/japplphysiol.00176.2007. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM. Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000a;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM. Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000b;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- Muenter Swift N, Charkoudian N, Dotson RM, Suarez GA. Low PA. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J PhysiolHeart CircPhysiol. 2005;289:H1226–H1233. doi: 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A. Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstetr. 2012;118:242–246. doi: 10.1016/j.ijgo.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SR. What is the optimal orthostatic stress to diagnose orthostatic hypotension? Clin Auton Res. 2005;15:67–68. doi: 10.1007/s10286-005-0265-8. [DOI] [PubMed] [Google Scholar]
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- Schondorf R, Benoit J, Wein T. Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75:192–201. doi: 10.1016/s0165-1838(98)00177-5. [DOI] [PubMed] [Google Scholar]
- Seals DR. Sympathetic activation during the cold pressor test: influence of stimulus area. Clin Physiol. 1990;10:123–129. doi: 10.1111/j.1475-097x.1990.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Elser MD, Jennings GL. Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK. Low PA. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC. Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- Usselman CW, Luchyshyn TA, Gimon TI, Nielson CA, Van Uum SH. Shoemaker JK. Hormone phase dependency of neural responses to chemoreflex-driven sympathoexcitation in young women using hormonal contraceptives. J Appl Physiol. 2013;115:1415–1422. doi: 10.1152/japplphysiol.00681.2013. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE. Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG. Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG. Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner MM, Taylor HS. Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol. 2011;589:975–986. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Cooke WH, Reed KS. Carter JR. Sex differences in hemodynamic and sympathetic neural firing patterns during orthostatic challenge in humans. J Appl Physiol. 2012;112:1744–1751. doi: 10.1152/japplphysiol.01407.2011. [DOI] [PubMed] [Google Scholar]