Abstract
Abstract
By producing H2O2, the NADPH oxidase Nox4 is involved in hypoxia-induced angiogenesis, as present in vascular remodelling of the hypertrophic heart or blood flow recovery after hind limb ischaemia. In the present study, we hypothesized that Nox4 contributes to proper capillary growth in the retina and in exercised muscles and investigated this in wild-type and Nox4−/− mice. Exercise, as induced by voluntary running in a running wheel or forced running on a treadmill, stimulated capillary growth in wild-type but not Nox4−/− mice. As an underlying mechanism, we identified both vascular endothelial growth factor (VEGF) expression to be reduced and angiopoietin 1 (Ang1) expression to be increased in response to Nox4 knockout. To differentiate the two factors, oxygen-induced retinopathy was investigated. In this model, deletion of Nox4 protected from neo-angiogenesis and stabilized the network of regrown vessels, which is a typical feature of Ang1. However the angiogenesis in the developing retina was similar between Nox4−/− and wild-type mice. Thus, Nox4 contributes to exercise- and hypoxia-induced angiogenesis through a dual mechanism of maintaining VEGF and preventing Ang-1 expression, whereas the developmental angiogenesis is Nox4 independent.
Key points
We provide evidence for two distinct functions of the NADPH oxidase Nox4 in angiogenesis using Nox4 knockout mice.
First, Nox4 maintains vascular endothelial growth factor expression and prevents an increase in angiopoietin 1 expression, thereby contributing to angiogenesis in exercise.
Second, deletion of Nox4, via an enhanced angiopoietin 1 expression, contributes to stabilization of new formed vessels and prevents an exacerbated neo-angiogenesis in oxygen-induced retinopathy.
By contrast, Nox4 does not influence developmental angiogenesis.
Introduction
Angiogenesis is a major prerequisite for the proper deve-lopment and adaptation of tissue to changes in demand or nutrient supply. The start signal for angiogenesis is an inflammation-like state, with endothelial cell activation, macrophage recruitment and cytokine production at the site of needed changes in the capillary network. Activated endothelial cells start to form tip cells that migrate toward where the new vessel is needed and, subsequently, by the formation of stalk cells that follow the tip cell, proliferate and form the lumen of the new vessel (Herbert, Stainier & Didier, 2011).
In earlier work, we reported that Nox4 is the predominant isoform of NADPH oxidases in endothelial cells and that genetic deletion of Nox4 attenuates angiogenesis in response to ischaemia after femoral artery ligation (Schröder et al. 2012). Among the Nox enzymes, Nox4 is an exception. Different from other NADPH oxidases, Nox4 is constitutively active and produces H2O2 (Brandes et al. 2014). Furthermore hypoxia as the main force for the induction of angiogenesis increases Nox4 expression (Diebold et al. 2010). These features enable Nox4 to elicit the long lasting and adaptive signalling processes involved in differentiation or angiogenesis.
Developmental angiogenesis has been studied extensively in the murine retina because mice are born blind and retina vascularization develops after birth. In humans, retina angiogenesis is complete shortly before normal term birth (Stahl et al. 2009) and dysfunctional retinal angiogenesis is a frequent problem in preterm infants. Angiogenesis in the eye of adults can be a consequence of hyperglycaemia in diabetes, thrombosis in vein occlusions or developmental delays in retinopathy of prematurity. Under such conditions, the problem is not non-functional angiogenesis, but rather an excessive, unlimited angiogenesis. Therefore, reducing angiogenesis and promoting vessel stabilization in adult eye disease are the goals of many therapeutic approaches (Todorich et al. 2014).
A situation of physiological angiogenesis is exercise. In addition to adaptation to a more demanding muscle workload, angiogenesis in skeletal muscle is also an efficient therapy for peripheral artery disease. Acute exercise obviously increases the energy consumption of the muscle. Consequently, blood flow during exercise increases 15- to 20-fold, enabling an adequate supply with nutrients and oxygen. In accordance with the Hagen–Poiseulle law, a minor increase in diameter is sufficient to considerably raise the blood flow in the target organ or muscle because vessel resistance is a function of the radius to the power of four. In muscle, this is reflected by the high flow reserve. Oxygen supply to muscle is not limited by the dilator capacity of the resistance vessel but instead by perfusion and thus the number of capillaries surrounding a muscle fibre. Increased capillarization results in a longer mean transit time and improved diffusion conditions. The formation of new capillaries by angiogenesis is therefore an essential step in the adaptive response to exercise.
We hypothesize that Nox4 plays an important role in angiogenesis of the retina and in exercised skeletal muscle and investigated this using knockout mice.
Methods
Animals
All animal experiments were conducted in accordance with the German Animal Protection Act and were approved by the District Government of Darmstadt (approval numbers V54-19c20/15-F28/31 and -F28/23), Germany. All adult animals in the present study were killed by cervical dislocation after isoflurane anaesthesia (Forene®; AbbVie, Ludwigshafen am Rhein, Germany), whereas pups were de-capitated under anaesthesia. C57/BL6J Nox4−/− mice have been described previously (Schröder et al. 2012). Animals had been backcrossed for 10 generations onto a C57BL6/J background and C57BL/6J mice served as controls. Nox2y/– mice were obtained from Charles Rivers and Nox1y/– mice were kindly provided by Karl-Heinz Krause, Geneva (Gavazzi et al. 2006). All exercise experiments were initiated at a mouse age of 6–8 weeks and only male animals were used. Mice were housed in a specified pathogen-free facility under a 12:12 h light/dark cycle with free access to chow and water.
Animal models
Serum vascular endothelial growth factor (VEGF) level was measured by MyRiad RBM (Austin, TX, USA) using the RodentMAP, version 2.0, antigen panel (MyRiad RBM). Treadmill exercise training was performed on a four-chamber running belt system (TSE Systems GmbH, Bad Homburg, Germany). For repeated forced endurance exercise, mice were trained daily for 1 h with additional warm-up and cool-down phase. The 10 days of training was performed initially at 10 m min–1 and a 5% incline with a gradual increase to 15 m min–1 and 10% incline equal for all mice. Mice in the control groups remained in their cages in the treadmill room throughout the exercise bouts. For the voluntary running experiment, mice randomly assigned to the 4 weeks running group (n = 6–8) were provided with a running wheel equipped with an activity counter (running distance). It would be an oversimplification to assume that treadmill running and voluntary running in a running wheel only differ in the intensity of exercise. Numerous other factors are of relevance: wheel running is a burst exercise, which occurs throughout the whole night; it is not associated with the psychological stress of the treadmill and takes place during the maximum physiological circadian activity of the mice. At the end of the experiments, mice were sacrificed immediately after the last training and muscles were quickly excised, rinsed with ice-cold phosphate-buffered saline (PBS), blotted dry, snap-frozen and stored in liquid nitrogen or Tissue-Tek (Sakura, Heppenheim, Germany) for subsequent analyses.
The murine model of oxygen-induced retinopathy (OIR) was performed as described previously (Connor et al. 2009). Briefly, at postnatal day 7 (P7), pups with their mothers were transferred into the hyperoxia system (Biospherix, Lacona, NY, USA) and exposed to 75 ± 2% oxygen for 5 days (P7–P12) followed by a subsequent return to normoxia (room air). Pups were killed at day 12, 14 or 17 and retinas were stained with fluorescein isothiocyanate Griffonia (Bandeiraea) simplicifolia BS-I lectin (1:100) in 1% Triton X-100 (both Sigma-Aldrich, St Louis, MO, USA) in 0.1 m PBS overnight, washed and mounted with mounting media (DakoCytomation, Glostrup, Denmark). Images were taken with the aid of a digital microscope (Carl Zeiss, Oberkochen, Germany). Image J software (National Institutes of Health, Bethesda, MD, USA), together with the appropriate plug-ins and macros, was used to analyse vessel regrowth and neo-angiogenesis (Stahl et al. 2009). Very similar developmental retina angiogenesis was analysed as described previously (Pitulescu et al. 2010). The formation of the superficial vascular plexus was analysed at days 3, 5.5 and 7 using whole mount staining with a CD31 antibody.
Histochemical analysis of skeletal muscle
To determine capillary density, cryostate cross-sections of the gastrocnemius and soleus muscles embedded in Tissue-Tek were used. After fixation in phosphate-buffered formalin (4% in PBS), the tissue was blocked with 1% Rotiblock (Carl Roth GmbH, Karlsruhe, Germany) and permeabilized with 0.5% Triton X-100, followed by incubation with directly labelled anti-CD31 (BD Pharmingen, Heidelberg, Germany) and anti-laminin antibodies (Abcam, Cambridge, UK), and imaged by confocal microscopy on a LSM 510 META (Carl Zeiss).
Analysis of mRNA expression
Total RNA was extracted from the muscle tissue with TRIzol in accordance with the manufacturer's instructions (Qiagen, Hildenberg, Germany). From 1 μg of RNA, cDNA synthesis was carried out with SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and random hexamer primers; semiquantitative real-time PCR was performed with Fast Plus EvaGreen Master Mix for quantitative PCR w/Low ROX (2x, 100 rxn) (Biotium, Hayward, CA, USA) in a Mx3005 cycler (Stratagene, La Jolla, CA, USA) with the indicated primers. We attempted to use several standard housekeeping genes, such as EF, GAPDH or β-actin, and all of them were regulated upon exercise. Eventually, we found B2M to be stably expressed in all forms of exercise performed by the mice. Relative expressions of target genes were normalized using B2M as a housekeeping gene, analysed by the ΔΔCt method and given as a ratio compared to control experiments. The primers used are listed in Table1.
Table 1.
Primers used in the present study
| Target | Sequence (5’ to 3’) |
|---|---|
| mB2M fw | GTCTTTCTGGTGCTTGTCTC |
| mB2M rev | GTATGTTCGGCTTCCCATTC |
| mSema6d fw | TGACGTGGAGGTCCAGACAG |
| mSema6d rev | CTGCACATCGGGTTGAAAGC |
| mSema6a fw | CTAGACAGGCTGACGTAGAC |
| mSema6a rev | CCAAGGTATCGACCCTGTAG |
| h,m,r VEGF A fw | GTGGACATCTTCCAGGAGTA |
| h,m,r VEGF A rev | GCTGTAGGAAGCTCATCTCT |
| mAngiopoietin1 fw | GTATAAAATGGGTTTTGGGAATCC |
| mAngiopoietin1 rev | TTGCCTGCTGTCCCTGTGTGACC |
| mAngiopoetin2 fw | GGGAAGGCAACGAGGCGCATT |
| mAngiopoetin2 rev | CGCGGTCCCCGTGAGTCCTG |
| mTie2 fw | ATGGCTCAGGCATTCCAGAACAG |
| mTie2 rev | TGGCCTTCCTGTTAAGGGCCAGA |
Cell culture
C2C12 cells were obtained from ATCC and kept in non-confluent undifferentiated culture. Satellite cells were isolated from 5- to 6-week-old male mice using a protocol similar to that established by Danoviz & Yablonka-Reuveni (2012). In brief, muscle tissue was minced into small pieces, digested with 0.1% pronase, triturated with a 10 ml pipette, filtered and directly plated onto collagen coated dishes. Differentiation was monitored by the expression of myosin heavy chain isoforms (data not shown). Cells were kept in culture at 37°C and 5% CO2 in proliferation medium consisting of Dulbecco's modified Eagle's medium, 1% penicillin–streptomycin, 4 mm glutamine, 1.5 g L–1 sodium bicarbonate, 1 mm sodium pyruvate and 20% fetal calf serum. For the experiments, cells were allowed to reach confluence and were differentiated for 7 days in differentiation medium containing Dulbecco's modified Eagle's medium, 1% penicillin–streptomycin, 4 mm glutamine, 1.5 g L–1 sodium bicarbonate, 1 mm sodium pyruvate and 4% horse serum (all Invitrogen).
Cyclic stretch and hypoxia
Cyclic stretch was performed as described previously (Fisslthaler et al. 2001). Differentiated cells were seeded on flexible-bottomed six-well culture plates coated with collagen (BioFlex; Flexcell International Corp., Hillsborough, NC, USA). After 7 days of differentiation, the cells were mounted onto loading plates in a FlexerCell FX-3000 strain unit (Flexcell International Corp.) and placed in an incubator. Cells were stretched with an average strain of 6% at a rate of 1 Hz, and static control experiments were performed on cells on stretch plates not exposed to cyclic strain.
For hypoxia, differentiated cells were incubated for 24 h at 1% O2 in a hypoxic incubator (Invivo2 400; Ruskinn Technology, Leeds, UK).
Statistical analysis
Unless otherwise indicated, data are provided as the mean ± SEM. Statistical analysis for multiple groups was performed by ANOVA, followed by the Bonferroni least significant difference post hoc test and, for two group comparisons, by a two-tailed t test for normally distributed values. Not normally distributed values were analysed by the Mann–Whitney U test. P < 0.05 was considered statistically significant.
Results
Physiological retina angiogenesis is not mediated by NADPH oxidases
In C57BL/6J mice, the superficial vascular plexus forms during the first week after birth by radial outgrowth of vessels. Within 8 days of birth, the radial vessels reach the edge of the retina. Although basal VEGF levels in adult mice are slightly but significantly reduced in the absence of Nox4 (wild-type: 118 ± 4 vs. Nox4−/−: 104 ± 5 pg ml–1; P < 0.05), we did not find a reduced formation of the vascular plexus as shown, for example, for day 5.5 after birth (Fig.1) or any other vascularization of the retina (data not shown). Similarly, developmental angiogenesis was similar between wild-type and Nox1 and Nox2 knockout mice, respectively (Fig.1). Thus, at least global constitutive knockout models do not suggest that Nox1, Nox2 and Nox4 are indispensable for developmental retina angiogenesis.
Figure 1.
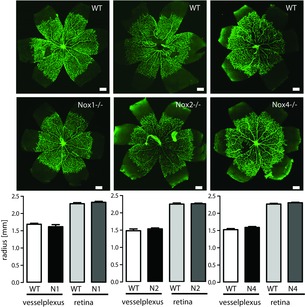
Retina angiogenesis is not mediated by NADPH oxidases
Representative images are shown of retinas from wildtype (WT), Nox1y/–, Nox2y/– or Nox4−/− mice at day 5.5 after birth, stained for endothelial cells with CD31 (green) to show capillaries in whole flat mounts. Statistics are provided below the images. Scale bar = 200 μm (n > 5).
Exercise-induced angiogenesis is mediated by Nox4
Next, exercise was studied as a model of stimulated angiogenesis. This was performed with two different protocols: 10 days of forced exercise on a tread mill and 4 weeks of voluntary running. In the voluntary group, mice had free access to running wheels and both strains ran similar distances (wild-type: 5646 ± 930 m vs. Nox4−/−: 4352 ± 955 m, n = 6, not significant).
Both 10 days of tread mill exercise or 4 weeks of voluntary running increased the endothelial to muscle fibre ratio in wild-type mice. Importantly, this effect was not observed in Nox4 knockout mice (Fig.2A, C, D and F).
Figure 2.
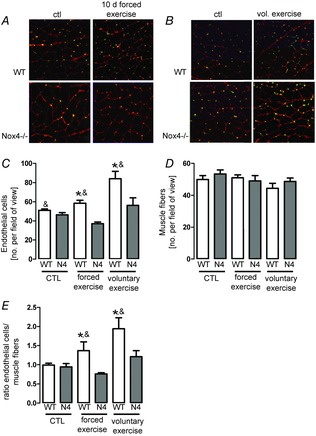
Exercise-induced capillarization is mediated by Nox4
A and B, representative images of gastrocnemius muscle from sedentary (ctl) and exercised (Ex.) wild-type (WT) or Nox4−/− mice, stained for CD31 (green) to show capillaries, as well as laminin (red) to define muscle fibres (see the online version for colours). Sections were made after 10 days (A) and after 4 weeks of voluntary (B) exercise. C–E, statistics. C, endothelial cells per field of view. D, muscle fibre per field of view. E, ratio: endothelial cells per muscle fibre. (n > 5). *P < 0.05 (ctl vs. Ex.); &P < 0.05 (WT vs. Nox4−/−).
Nox4 contributes to stretch and hypoxia mediated expression of VEGF
Exercise-induced angiogenesis is driven by VEGF, which is induced in response to hypoxia and stresses such as increases in stretch. To explore the impact of Nox4 on VEGF expression under more controlled conditions, we first utilized C2C12 satellite cells, which, prior to the experiments, were differentiated into myofibroblasts. When these cells were exposed to hypoxia with 2% O2 for 8 h, hypoxia inducible factor 1α (Hif1α) protein abundance increased, as did Nox4 mRNA expression (Fig.3A). Consequently, hypoxia induced the expre-ssion of VEGF mRNA and this was inhibited by the flavoprotein inhibitor diphenyleneiodonium (DPI), which blocks most reactive oxygen species (ROS) sources. By contrast, the addition of H2O2 even potentiated VEGF expression (Fig.3B). Similar results were obtained when the cells were exposed to cyclic stretch to simulate one aspect of exercise. VEGF mRNA expression increased and this was inhibited when ROS were reduced either by DPI or the H2O2-decomposing enzyme catalase (Fig.3C). Taken together, these experiments indicate a role for H2O2 in hypoxia- and stretch-induced VEGF expression. To study the specific involvement of Nox4, satellite cells were isolated from wild-type and Nox4−/− mice. Hypoxia, as well as stretch, induced an increase in VEGF mRNA in both wild-type and Nox4−/−. However, the effect was significantly smaller in Nox4-deficient cells compared to wild-type cells. Thus, Nox4 contributes to hypoxia- and stretch-induced VEGF-A expression in satellite cells.
Figure 3.
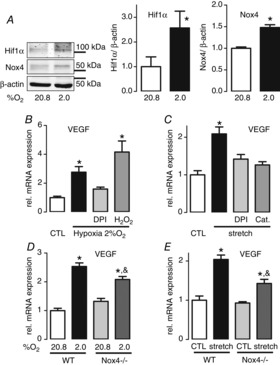
Nox4 contributes to stretch- and hypoxia-induced expression of VEGF
A, Nox4 and Hif1α protein expression analysed by western blotting. B and C, quantitative PCR analysis for VEGF mRNA expression. After 3 days of differentiation from myoblasts to myotubes, C2C12 cells were treated with or without DPI (1 μm), catalase (500 U ml–1) or H2O2 (400 μm) for 8 h in 2% O2 hypoxia (B) or subjected to cyclic stretch with the Flexcell system for 1 h (C). Mean ± SEM (n > 3) *P < 0.05. VEGF expression in satellite cells isolated from skeletal muscle of wild-type (WT) or Nox4−/− mice with or without 2% O2 hypoxia (D) or 1 h cyclic stretch with the Flexcell system (E). Mean ± SEM (n > 5 with each n = cells from one mouse) *P < 0.05 (ctl vs. Ex.); &P < 0.05 (WT vs. Nox4−/−). Ctl, control; Ex., exercised.
Exercise induced VEGF and Ang1 expression in skeletal muscle is differentially modified by Nox4
To seek in vivo confirmation of the cell culture expe-riments, VEGF mRNA expression was analysed in murine muscle tissue after exercise. VEGF mRNA expression was increased only in the initiation phase of exercise and later returned to baseline (Fig.4A). To confirm these findings, the expression of the VEGF A-dependent genes semaphorin 6A and 6D (Segarra et al. 2012) was determined, which was found to parallel that of VEGF.
Figure 4.
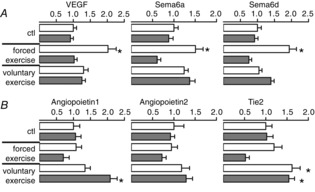
Exercise-induced VEGF and Ang1 expression are differentially modified by Nox4
Quantitative PCR for the genes indicated performed from muscle of mice subjected to 10 days or 4 weeks of voluntary exercise. Mean ± SEM (n > 5); *P < 0.05 (ctl vs. Ex.). Ctl, control; Ex., exercised.
Importantly, the effects on VEGF and semaphorins were restricted to wild-type animals, whereas no exercise-induced increase of these genes was observed in Nox4−/− mice.
As a second HIF1α- and angiogenesis-associated system, the angiopoietins (Ang1 and 2) were analysed (Fig. 4B). Ang1 is expressed by perivascular cells and promotes vessel maturation, quiescence, migration and survival of endothelial cells. By contrast, Ang2 is expressed in endothelial cells and promotes angiogenic sprouting (Eklund & Saharinen, 2013). Both angiopoietins bind to the same receptor Tie2. In our analyses, no significant changes in angiopoietin expression occur in forced exercise. However, in skeletal muscles of voluntarily running mice, Ang1 and Tie2 mRNA expression was increased and Ang1 mRNA expression in Nox4−/− mice was higher than in wild-type mice. Taken together with the finding that only wild-type animals respond with an increase in capillary density in voluntary exercise, this indicates that Ang1 signal transduction may be enhanced in the absence of Nox4.
Nox4 deficiency improves healing after oxygen-induced retinopathy
To confirm the findings reported above, we analysed a second model of angiogenesis regulated by Ang1, namely the OIR model of mice. In this model, Ang1 was described to promote healthy vascular network formation by inhibiting abnormal neo-angiogenesis (Lee et al. 2013). Indeed, we found that late healing after OIR was enhanced in Nox4-deficient mice (Fig.5). This effect was accompanied by fewer regions of neo-angiogenesis. Thus, it appears that VEGF induced neo-angiogenesis is impaired in Nox4-deficient retinas, whereas Ang1 induced stabilization of the vessels improved the rescue of the retinal vessels from oxygen-induced retinopathy.
Figure 5.
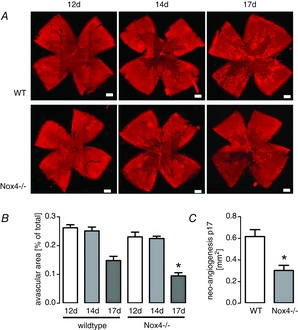
Nox4 deficiency promotes healing after oxygen-induced retinopathy
A, representative images of retinas from wild-type (WT) or Nox4−/− mice exposed to oxygen-induced retinopathy, stained for endothelial cells with lectin (red) to show capillaries. Whole flat mounts were made from pups at age 12, 14 and 17 days. B and C, statistics of the relative avascular area (B) and the area of neo-angiogenesis (C) (n > 5) *P < 0.05 (WT vs. Nox4−/−).
Discussion
NADPH oxidases have been shown to impact on angiogenesis in several disease models. Nox1 has been implicated in tumour vascularization (Garrido-Urbani et al. 2011) and, after hindlimb ischaemia, Nox1, Nox2 and Nox4 have all been reported as being relevant for vessel regrowth (Tojo et al. 2005; Schröder et al. 2012). To date, the role of Nox enzymes for developmental angiogenesis has not been studied in depth and the data on spontaneous retina angiogenesis reported in the present study suggest that Nox enzymes are dispensable for this process.
The present study provides evidence indicating that Nox4 is required for exercise-induced angiogenesis. Trained muscles exhibit an enhanced oxygen exchange capacity and repetitive training promotes angiogenesis (Richardson et al. 1999; Tesch, 1988). The training-induced formation of new vessels is probably a consequence of a greater abundance of growth factors such as VEGF and exercise endurance capacity has previously been shown to depend on VEGF expression in the muscle (Olfert et al. 2009). Recently, it was reported that especially skeletal muscle derived VEGF plays a pivotal role in exercise-induced angiogenesis and muscle-adaptation in mice (Delavar et al. 2014). Accordingly, in the early phase of forced repeated exercise, an induction of VEGF expression along with an increase in neo-angiogenesis was observed in wild-type mice but, interestingly, not in Nox4−/− mice in the present study. Up-regulation of VEGF is a consequence of cell signalling in response to muscle contraction and occurs even after a single treadmill run (Annex et al. 1998; Tang et al. 2010). Moreover, exercise by numerous mechanisms, including relative hypoxia, increased nitric oxide formation, exercise-induced cellular inflammatory activation and alterations in protein stability, can lead to Hif1α stabilization and, consequently, to increases in VEGF expression (Diebold et al. 2010). We have shown previously that Nox4 maintains a proper expression of Hif1α and other studies have reported that Nox4 is an oxygen sensor (Nisimoto et al. 2014; Zhang et al. 2010). Potentially, Nox4-deficient cells recognize the drop in O2-tension less efficiently than wild-type cells and this is also a consequence of attenuated Hif1α expression. In the present study, we observed that both stretch and hypoxia stimulate the expression of VEGF in an ROS- and Nox4-dependent manner. After long-term voluntary exercise, we found no significant increase in VEGF mRNA expression but still more neo-vascularization in wild-type than in Nox4-deficient mice, which suggests that there is adaptation to a new steady state. Indeed, growing evidence supports the view that exercise not only induces pro-angiogenic factors such as VEGF, but also regulates anti-angiogenic factors (Olenich et al. 2013). In the present study, we found that the expression of Ang1 was increased in muscle from Nox4−/− compared to wild-type mice. In the vascular system, pericytes, which stabilize vessels, produce Ang1 (Ribatti et al. 2011). Pericytes and Ang1 work together to prevent the formation of new vessels. They stabilize the vessel and thereby inhibit neo-angiogenesis. Endothelial cells produce platelet-derived growth factor that recruits pericytes, whereas TGFβ contributes to the differentiation of pericytes into myofibroblasts (Humphreys, 2012). Importantly, TGFβ is one of the most potent inducers of Nox4 and Nox4 is involved in the differentiation of other mesenchym-derived cells such as adipocytes or myofibroblasts (Hecker et al. 2009; Schröder et al. 2009). Therefore, it is likely that the pericyte to myofibroblast transition also is regulated by Nox4, which, however, still needs to be established and is beyond the scope of the present study. VEGF negatively regulates pericyte function and vessel maturation (Greenberg et al. 2008). Although highly speculative, it is possible that more pericytes are present on the vessels of Nox4-deficient mice as a result of less myocyte differentiation and a lower VEGF level in Nox4−/− mice. Indeed, we found that Nox4-deficient mice, when allowed to perform voluntary exercise, express more Ang1 in skeletal muscles than wild-type mice. Lee et al. (2013) found that Ang1 overexpression, as well as Ang1 supplementation, improved vessel regrowth and prevented neo-angiogenesis in a model of oxygen-induced retinopathy. Using the same model, we found that Nox4-deficiency reduced the number of neo-vascularization spots without preventing vessel regrowth. At least in part, this is in agreement with the recent finding showing that only Nox1, and neither Nox2 nor Nox4, is involved in vessel regrowth in the OIR model (Wilkinson-Berka et al. 2014). We conclude that pericytes and Ang1 play an important role in Nox4-regulated angiogenesis. A major shortcoming of our work is that we measure Ang1, Tie2, VEGF-A and HIF1a mainly on the mRNA and not at the protein level. Given that several of these factors adhere to matrix and that the protein and mRNA levels are often different, the results of mRNA measurements should not be over-interpreted. Although they provide an important impact of Nox4 on the mRNA of the cytokines measured, they cannot demonstrate any causal link between differences in mRNA and protein-mediated functional consequences.
Nevertheless, the present study provides evidence for a role of Nox4 as a double-edged sword in angiogenesis. As a result of its contribution to VEGF-expression, Nox4 supports exercise-induced angiogenesis. By contrast, Nox4 deficiency may contribute to vessel stabilization in rethinopathy via an enhanced expression of Ang1 and thereby prevents neo-vascularization of the retina.
Acknowledgments
We thank Flávia Figueiredo de Rezende, Susanne Schütz and Maria Walter for their excellent support of our study. We especially thank Ralf P. Brandes for critically reading the manuscript and for supporting the whole study.
Glossary
- Ang1
angiopoietin 1
- Ang2
angiopoietin 2
- DPI
diphenyleneiodonium
- Hif1α
hypoxia inducible factor 1α
- IL
interleukin
- Nox
NADPH oxidase
- OIR
oxygen-induced retinopathy
- PBS
phosphate-buffered saline factor
- ROS
reactive oxygen species
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
J.V. and C.K. were responsible for the conception and design of the experiments, as well as collection, analysis and interpretation of data. M.Z. was responsible revising the manuscript critically for important intellectual content. K.S. was responsible for the conception and design of the experiments, as well as collection, analysis and interpretation of data, and drafting the article. All authors approved the final version of the manuscript; all persons designated as authors qualify for authorship; and all those who qualify for authorship are listed.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (SFB815/TP1 and SCHR 1241/1-1 to KS), the Heinrich und Fritz-Riese-Stiftung, British Heart Foundation and the DFG excellence cluster ECCPS.
Translational perspective
Exercise is the most important non-medical treatment for several diseases such as peripheral arterial occlusive disease or even diabetes. In the present study, we provide evidence that Nox4-derived ROS are required for exercise-induced angiogenesis. Therefore, anti-oxidants should be supplemented with caution because they may impair the training effect of skeletal muscle (Strobel et al. 2011; Venditti et al. 2014). By contrast, in retinal vascular diseases such as proliferative diabetic retinopathy and retinopathy of prematurity, the inhibition of Nox4 might promote recovery because such an approach would prevent an overshoot in VEGF expression and thereby exacerbated neo-angiogenesis.
References
- Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG. Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol Heart Circ Physiol. 1998;274:H860–H8607. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N. Schröder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL. Smith, Lois EH. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoviz ME. Yablonka-Reuveni Z. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol Biol. 2012;798:21–52. doi: 10.1007/978-1-61779-343-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D. Breen EC. Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol. 2014;306:R586–R595. doi: 10.1152/ajpregu.00522.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold I, Petry A, Hess J. Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund L. Saharinen P. Angiopoietin signaling in the vasculature. Special Issue: Endothelial Biology. 2013;319:1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Michaelis UR, Kiss L, Fleming I. Busse R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension. 2001;38:1427–1432. doi: 10.1161/hy1201.096532. [DOI] [PubMed] [Google Scholar]
- Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH. Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F. Krause K. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N. Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ. Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, Stainier Didier YR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol. 2012;32:463–470. doi: 10.1016/j.semnephrol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim KE, Choi D, Jang JY, Jung J, Kiyonari H, Shioi G, Chang W, Suda T, Mochizuki N, Nakaoka Y, Komuro I, Yoo O. Koh GY. Angiopoietin-1 guides directional angiogenesis through integrin αvβ5 signaling for recovery of ischemic retinopathy. Sci Transl Med. 2013;5:203ra127. doi: 10.1126/scitranslmed.3006666. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y, Diebold BA, Constentino-Gomes D. Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenich SA, Gutierrez-Reed N, Audet GN. Olfert IM. Temporal response of positive and negative regulators in response to acute and chronic exercise training in mice. J Physiol. 2013;591:5157–5169. doi: 10.1113/jphysiol.2013.254979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD. Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–1767. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu ME, Schmidt I, Benedito R. Adams RH. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat Protoc. 2010;5:1518–1534. doi: 10.1038/nprot.2010.113. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B. Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J. Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol (1985) 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Schröder K, Wandzioch K, Helmcke I. Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM. Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- Segarra M, Ohnuki H, Maric D, Salvucci O, Hou X, Kumar A, Li X. Tosato G. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood. 2012;120:4104–4115. doi: 10.1182/blood-2012-02-410076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI. Smith LEH. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS. Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2011;43:1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- Tang K, Xia FC, Wagner PD. Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol. 2010;170:16–22. doi: 10.1016/j.resp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc. 1988;20:S132–S134. doi: 10.1249/00005768-198810001-00008. [DOI] [PubMed] [Google Scholar]
- Todorich B, Yiu G. Hahn P. Current and investig-ational pharmacotherapeutic approaches for modulating retinal angiogenesis. Expert Rev Clin Pharmacol. 2014;7:375–391. doi: 10.1586/17512433.2014.890047. [DOI] [PubMed] [Google Scholar]
- Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N. Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- Venditti P, Napolitano G, Barone D. Di Meo S. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic Res. 2014:1–32. doi: 10.3109/10715762.2014.937341. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Deliyanti D, Rana I, Miller AG, Agrotis A, Armani R, Szyndralewiez C, Wingler K, Touyz RM, Cooper ME, Jandeleit-Dahm KA. Schmidt, Harald HHW. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid Redox Signal. 2014;20:2726–2740. doi: 10.1089/ars.2013.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Brewer AC, Schröder K, Santos, Celio XC, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP. Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]


