Abstract
Abstract
Reflex cutaneous vasoconstriction is impaired in older adults; however, the relative roles of altered skin sympathetic nerve activity (SSNA) and end-organ peripheral vascular responsiveness are unclear. We hypothesized that in older adults whole-body cooling would elicit a blunted SSNA response and cutaneous adrenergic responsiveness would be reduced. Twelve young adults (Y; 24 ± 1 years) and 12 older adults (O; 57 ± 2 years) participated in two protocols. In Protocol 1, SSNA (peroneal microneurography) and red cell flux in the affected dermatome (laser Doppler flowmetry; dorsum of foot) were measured during whole-body cooling (mean skin temperature (Tsk) 30.5°C; water-perfused suit). Mental stress was performed at mean Tsk 34.0°C (thermoneutral) and at 30.5°C. In Protocol 2, an intradermal microdialysis fibre was placed in the skin of the lateral calf for graded infusions of noradrenaline (norepinephrine) (NA; 10−12 to 10−2 m). Cutaneous vascular conductance (CVC = flux/mean arterial pressure) was expressed as a change from baseline (ΔCVCbase). Vasoconstriction was attenuated in O. SSNA increased significantly during cooling in Y (+184 ± 37%; P < 0.05) but not O (+51 ± 12%; P > 0.05). Mental stress at Tsk 30.5°C further increased SSNA in both groups. There was no age-related difference in adrenergic responsiveness to exogenous NA (logEC50: −6.41 ± 0.24 in Y, −6.37 ± 0.25 in O; P > 0.05). While the SSNA response to whole-body cooling is impaired with ageing, SSNA can be further increased by a non-thermoregulatory stimulus. Cutaneous adrenergic sensitivity is not reduced in O. These findings suggest that alterations in afferent signalling or central processing likely contribute to blunted SSNA responses to cooling and subsequent impairments in reflex cutaneous vasoconstriction in ageing.
Key points
The reduction in skin blood flow during whole-body cooling is impaired in healthy older adults. However, the relative contributions of altered skin sympathetic nerve activity (SSNA), transduction of this efferent neural outflow to the cutaneous vasculature, and peripheral vascular responsiveness to adrenergic stimuli to the impaired reflex vasoconstrictor response to whole-body cooling in human ageing remain unclear.
We report that the SSNA response to whole-body cooling is blunted in healthy older adults, and this attenuated sympathetic response is related to a marked impairment in reflex cutaneous vasoconstriction. Further, the reflex SSNA response to a non-thermoregulatory stimulus was preserved in older adults during cooling.
We additionally show that cutaneous vascular responsiveness to adrenergic stimuli is not reduced in older adults.
These results further our understanding of the physiological mechanisms underlying impaired thermal-cardiovascular integration in healthy ageing.
Introduction
Advancing age is a primary risk factor for the development of cardiovascular diseases, which, despite recent declines in prevalence, remain the leading cause of morbidity and mortality in modern societies (Go et al. 2014). In older adults, cardiovascular risk increases acutely during environmental cold exposure (Sheth et al. 1999; Curriero et al. 2002; Panagiotakos et al. 2004; Morabito et al. 2005; Wolf et al. 2009). During whole-body cold stress, a reflex increase in skin sympathetic nerve activity (SSNA) mediates peripheral cutaneous vasoconstriction (Green & Kepchar, 1959; Fox & Edholm, 1963), contributing to the maintenance of body temperature and prevention of hypothermia. Reflex cutaneous vasoconstriction is markedly impaired in older adults (Frank et al. 2000; Thompson & Kenney, 2004; Degroot & Kenney, 2007; Lang et al. 2009a; Stanhewicz et al. 2013), and even during mild cold stress (ambient temperature 22°C, minimal clothing) healthy older adults exhibit a relative inability to defend body core temperature (Degroot & Kenney, 2007). Given that adults over the age of 65 account for half of all cold-exposure deaths each year (Curriero et al. 2002; Centers for Disease Control and Prevention, 2005), and coupled with potential age-related abnormalities in the central control of sympathetic outflow (Grassi et al. 2003), elucidating the mechanisms underlying aberrant sympathetic nervous system regulation of the cutaneous circulation during cold exposure in older adults is clinically relevant.
Compromised reflex vasoconstriction in healthy ageing may result from functional deficits at multiple points along the sympathetic reflex axis (Holowatz & Kenney, 2010). Reflex cutaneous vasoconstriction is mediated by increases in efferent SSNA, which stimulates the release of adrenergic neurotransmitters (i.e. noradrenaline (NA)) and non-adrenergic cotransmitters (i.e. neuropeptide Y and ATP) (Stephens et al. 2001, 2004; Thompson & Kenney, 2004). However, older adults exhibit functionally absent cotransmitter-mediated vasoconstriction (Thompson & Kenney, 2004) and instead rely entirely on an impaired adrenergic mechanism to elicit reductions in cutaneous blood flow during cold exposure (Kenney & Armstrong, 1996; Degroot & Kenney, 2007). Age-related reductions in NA biosynthesis and subsequent axonal release from peripheral sympathetic nerve terminals probably contribute to attenuated cutaneous vasoconstriction in response to cold exposure (Lang et al. 2009a, 2010; Stanhewicz et al. 2013). To date only one study has examined the effect of ageing on efferent skin sympathetic outflow during whole-body cooling, reporting blunted increases in SSNA in elderly adults during exposure to low ambient temperature (Grassi et al. 2003). However, the cooling stimulus (ambient air) was not well controlled in that study, and there was no simultaneous measurement of either the physiological stimulus (skin temperature) or effector response (cutaneous blood flow), which are significant limitations to the interpretation of these findings. To our knowledge, no study has attempted to simultaneously assess the relative contributions of alterations in efferent SSNA and cutaneous vasoconstriction, including potential age-related differences in peripheral vascular responsiveness to adrenergic stimuli, to the impaired reflex vasoconstrictor response to whole-body cooling in human ageing.
Therefore, the purpose of the present study was to more comprehensively examine sympathetic control of reflex cutaneous vasoconstriction during whole-body cooling in healthy older men and women (aged 55–70 years). We hypothesized that whole-body cold stress would elicit blunted SSNA responses in healthy older compared to young adults and that this attenuated neural outflow would be related to impaired reflex cutaneous vasoconstriction. By superimposing a non-thermoregulatory stimulus (mental stress) during cooling (Delius et al. 1972; Muller et al. 2013a), we further hypothesized that the age-related attenuation in the SSNA response to cold stress would not be due to a central inability to further increase SSNA during whole-body cooling. Finally, we hypothesized that cutaneous adrenergic sensitivity would be reduced in older adults, also implicating altered end-organ responsiveness in the relative inability of the sympathetic nervous system to evoke peripheral vasoconstriction during cold exposure in older adults.
Methods
Subjects
All experimental procedures and protocols were approved by The Pennsylvania State University Institutional Review Board. Informed verbal and written consent were obtained voluntarily from all subjects prior to participation. The study conformed to the standards outlined in the Declaration of Helsinki. All participants were screened for neurological, cardiovascular and dermatological diseases and underwent a complete medical screening including a resting 12-lead electrocardiogram, physical examination, and 12 h fasting blood chemistry (Quest Diagnostics, Pittsburgh, PA, USA). All subjects were normotensive (resting systolic blood pressure (BP) < 120 mmHg and diastolic BP < 80 mmHg), non-diabetic, normally active and not taking over-the-counter or prescription medications or supplements with primary or secondary cardiovascular effects (e.g. statins, antihypertensives, anticoagulants, antidepressants, etc.). Subjects were non-obese (body mass index < 30 kg m−2) and did not use tobacco products. Young women were tested during the early follicular phase of their menstrual cycle or during the placebo phase if using oral contraceptives. Women taking hormone replacement therapy or who had recently taken hormone replacement therapy were excluded. All subjects were familiarized with the equipment and experimental protocols before the testing visits. Prior to the experimental sessions, subjects abstained from eating for 4 h, caffeinated and alcoholic beverages for 12 h, and strenuous physical activity for 24 h. The protocols were performed in a thermoneutral laboratory (22°C).
Protocol 1: SSNA responses to whole-body cold stress
Measurements
To control skin temperature, subjects wore a water-perfused suit that covered the entire body except for the head, hands, feet and lower left leg. Copper–constantan thermocouples were affixed to the surface of the skin at six sites: calf, thigh, abdomen, chest, shoulder and back. The unweighted mean of these sites was used for continuous measurement of mean skin temperature (Tsk). To obtain an index of cutaneous blood flow, red blood cell flux was continuously measured directly on the dorsum of the foot and the lateral calf (each within the region of sympathetic innervation) with a laser Doppler flowmetry probe placed in a local heating unit (moorLab, Temperature Monitor, SHO2; Moor Instruments, Axminster, UK). To specifically isolate reflex mechanisms, the local heater was clamped at 33°C throughout baseline and whole-body cooling.
Arterial BP was obtained on a beat-to-beat basis using photoplethysmography (Finapres; Finapres Medical Systems, Amsterdam, The Netherlands) obtained from the middle finger of the left hand held at heart level. Automated brachial artery BP (Cardiocap; GE Healthcare, Milwaukee, WI, USA) was measured every 3 min throughout the experiment and used to verify absolute finger BP measurements. Heart rate was measured via a single-lead electrocardiogram (Cardiocap; GE Healthcare). Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA, USA). Multiunit recordings of postganglionic SSNA were obtained by inserting unipolar tungsten microelectrodes percutaneously through the intact, unanaesthetized skin and positioned into skin nerve fascicles of the peroneal nerve near the fibular head. A reference electrode was inserted 2–3 cm from the recording electrode site. Recordings of SSNA were considered acceptable when (1) stroking the skin elicited afferent impulses; (2) bursts of neural activity increased in response to arousal stimuli (loud noise) or deep inspiration, but not during end-expiratory breath-holds or Valsalva manoeuvres; and (3) bursts of neural activity displayed no regular pulse synchronicity (Vallbo et al. 1979). Neural signals were amplified, filtered (bandwidth; 700–2000 Hz), rectified, and integrated (time constant 0.1 s) (Nerve Traffic Analyser; University of Iowa Bioengineering, Iowa City, IA, USA). Mean voltage neurograms were visually displayed and routed to a loudspeaker for monitoring throughout the study. SSNA responsiveness to auditory stimuli was confirmed at the very end of the protocol to ensure a consistent recording site.
Protocol
All subjects were tested in the supine position. After subject instrumentation and the obtainment of a suitable nerve recording, baseline data were collected for 10 min. Throughout baseline, mean Tsk was held at thermoneutrality by perfusing ∼32°C water through the suit. Each subject then performed 3 min of mental arithmetic, as previously described (Carter et al. 2005, 2013; Muller et al. 2013a,b2013). During mental arithmetic, subjects continuously subtracted the number 7 from a three-digit number. Subjects answered verbally and were encouraged by an investigator to subtract as quickly as possible. An investigator provided a new number from which to subtract every 5–10 s. Subjects were asked to rate perceived stress using the following standard five-point scale: 0, not stressful; 1, somewhat stressful; 2, stressful; 3, very stressful; and 4, very, very stressful (Callister et al. 1992). After mental stress, subjects rested quietly for 15–20 min until SSNA and cardiovascular variables returned to resting baseline values. Thereafter, cool water (∼16°C) was perfused through the suit to gradually lower mean Tsk from 34°C to 30.5°C (∼30 min). This cooling stimulus elicits progressive gradual declines in mean Tsk but does not alter core temperature (Thompson & Kenney, 2004; Holowatz & Kenney, 2010; Wilson et al. 2010). Mean Tsk was then clamped at 30.5°C and a second bout of mental stress was performed, as described above. During the mental stress trials, acceptable SSNA recordings were obtained in nine young adults and eight older adults.
Protocol 2: cutaneous adrenergic responsiveness to exogenous NA
Measurements
Using sterile technique and after the skin was temporarily anaesthetized with ice (Hodges et al. 2009), one intradermal microdialysis fibre (10 mm, 20 kDa cutoff membrane, MD 2000; Bioanalytical Systems, West Lafayette, IN, USA) was placed in the dermal layer of the lateral calf, within the area of cutaneous innervation of the common and superficial peroneal nerves (i.e. the nerves from which SSNA was directly recorded), and perfused with lactated Ringer solution (2 μl min−1; Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems) for 60–90 min after placement to allow for the resolution of local hyperaemia. Red blood cell flux, an index of skin blood flow, was measured by a laser Doppler flowmeter probe throughout the protocol. The probe was placed in a local heater (moorLAB, Temperature Monitor SHO2; Moor Instruments), which was affixed to the skin directly above the microdialysis membrane. Temperature of the local heater was clamped at 33°C for the duration of the protocol to ensure that changes in skin blood flow were due to the exogenous infusion of vasoactive agents. Brachial artery BP (Cardiocap 5, GE Healthcare) was measured every 5 min throughout the protocol.
Protocol
All subjects were tested in the supine position. After the recovery period from probe placement, baseline measurements were made for 20 min while lactated Ringer solution was perfused through the microdialysis fibre. Thereafter, increasing doses of NA were continuously infused (10−12 m, 10−11 m, 10−10 m, 10−9 m, 10−8 m, 10−7 m, 10−6 m, 10−5 m, 10−4 m, 10−3 m, 10−2 m); each dose was for infused for 5 min, as previously described (Wilson et al. 2004; Greaney et al. 2014a). NA (Sigma, St Louis, MO, USA) was mixed just before use and dissolved in lactated Ringer solution with 1 mg ml−1 (5.7 mm) ascorbic acid as a preservative, extending the half-life from 8.5 min to over 180 min (Sigma) (Hughes & Smith, 1978; Thompson et al. 2005; Stanhewicz et al. 2013; Greaney et al. 2014a), as prolonged infusion of NA at higher concentrations causes uncoupling and desensitization of G protein receptors (Seasholtz et al. 1997; Cabrera-Wrooman et al. 2010; Akinaga et al. 2013). Although local ascorbate administration has been shown to inhibit the adrenergic vasoconstrictor response to local cooling in human skin (Yamazaki, 2010), in pilot testing in our laboratory, the perfusion of ascorbic acid alone did not induce a cutaneous vascular response. As such, it is unlikely that the addition of ascorbic acid to NA contributed to the cutaneous vasoconstrictor responses observed in the present study. Solutions were filtered using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI, USA) and wrapped in foil to prevent degradation due to light exposure.
Data analysis
All data were recorded at either 1000 Hz (Protocol 1) or 40 Hz (Protocol 2; Powerlab and LabChart; ADInstruments, Bella Vista, NSW, Australia). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial pressure (MAP) and expressed as both a change from baseline values (ΔCVCbase; Protocols 1 and 2) and also as a percentage of baseline (%CVCbase; Protocol 2). In Protocol 1, cardiovascular variables were calculated as mean values over an initial 5 min thermoneutral baseline and in 1 min averages at each 0.5°C decrease in mean Tsk during whole-body cooling. In Protocol 2, cardiovascular variables were averaged over the last minute of each NA dose.
SSNA was evaluated from the integrated neurogram using a customized computer program (LabChart, ADInstruments). The asymmetry of SSNA bursts makes calculation of burst frequency difficult (e.g. multiple peaks within one burst) (Hagbarth et al. 1972; Cui et al. 2006; Young et al. 2009a,b2009; Strom et al. 2011; Muller et al. 2013b); therefore, SSNA is presented as total integrated activity, which is the sum of the total area under all bursts detected in a given time period. Additional support for the use of total integrated activity rather than frequency is based on findings from single unit recordings which suggest that an increase in SSNA is primarily governed by the recruitment of additional neurons instead of an increase in firing frequency of individual units (Macefield & Wallin, 1999). This is especially relevant for SSNA because bursts are not limited by the cardiac cycle and thus the recruitment of additional neurons likely contributes to the characteristic wider burst morphology observed during sympathetic activation (Vallbo et al. 1979; Macefield & Wallin, 1999). Because total integrated activity is largely affected by the position of the microelectrode within the nerve fascicle, which cannot be determined, direct comparisons of basal SSNA between groups are typically not performed (Young et al. 2009b). Therefore, due to intersubject variability in SSNA, values were normalized to and expressed as a percentage of the baseline value to provide an estimate of relative changes in integrated activity in each group (Young et al. 2009a; Toma et al. 2011; Muller et al. 2013a,b2013). This analysis method for SSNA allows for the assessment of sympathetic reactivity to a given stimulus and is consistent with previous studies examining SSNA responsiveness (Wilson et al. 2001; Cui et al. 2004; Muller et al. 2013a,b2013). A 1 min segment of SSNA at thermoneutral baseline was assigned a value of 100, and 1 min segments at each 0.5°C decrease in mean Tsk during whole-body cooling were used to compare the increase in SSNA from baseline. For each mental stress trial, data were averaged during an additional 1 min of baseline before the onset of mental stress and during the first minute of mental arithmetic, during which the largest SSNA response is observed (Muller et al. 2013b). Importantly, the SSNA responses to a variety of acute sympathetic vasoconstrictor perturbations, including mental stress and intermittent static handgrip exercise (Leuenberger et al. 2003; Muller et al. 2013b), have been demonstrated to be reproducible within a subject (1) between successive trials during the same experimental visit, and (2) between trials during a different experimental visit.
Statistical analysis
Subject characteristics were compared using unpaired Student's t tests (SAS v9.1.3; Cary, NC, USA). For Protocol 1, comparisons of neural cardiovascular variables during whole-body cooling between groups were conducted using two-way mixed model repeated-measures ANOVA. A three-way mixed model repeated-measures ANOVA was conducted to detect temperature and condition (i.e. mental stress) differences in neural cardiovascular variables between groups. When appropriate, Bonferroni post hoc comparisons were performed and corrected for multiple comparisons. Pearson correlations were used to examine the relation between SSNA and CVC in each group, and linear regression analysis was used to probe group differences.
For Protocol 2, NA doses were transformed to logarithmic concentrations, and CVC was normalized such that baseline CVC = 100% (i.e. pre-NA). Sigmoidal dose–response curves with variable slope were generated using four-parameter non-linear regression modelling (Wenner et al. 2011; Greaney et al. 2014a), with constraints set for the top (100) to best fit parameters of the model (Prism v5.0, GraphPad, San Diego, CA, USA). Responsiveness to NA was determined by the effective concentration causing 50% of the maximal response (logEC50), and the extent of maximal vasoconstrictor capacity (Emax) was also determined. Differences between groups were analysed using an F test for repeated measures comparisons (Wenner et al. 2011; Greaney et al. 2014a), which takes into account all points over the entire curve as opposed to each specific dose (Cook & Bielkiewicz, 1984). Results are reported as means ± SEM, and the α level was set at P < 0.05.
Results
Subject characteristics
Subject characteristics are presented in Table1. The groups were well matched for anthropometric characteristics, resting BP and blood biochemistry. Although total cholesterol and HbA1c (glycated hemoglobin) were significantly higher in older adults, these values were within the normal recommended age-specific range.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| n: total (M/F) | 12 (7/5) | 12 (8/4) |
| Age (years) | 24 ± 1 | 57 ± 2* |
| Height (cm) | 174 ± 3 | 175 ± 3 |
| Mass (kg) | 73 ± 4 | 81 ± 4 |
| BMI (kg m−2) | 24.1 ± 1.1 | 26.5 ± 0.7 |
| Systolic BP (mmHg) | 116 ± 2 | 116 ± 2 |
| Diastolic BP (mmHg) | 69 ± 2 | 70 ± 1 |
| Heart rate (beats min−1) | 60 ± 2 | 62 ± 2 |
| Blood biochemistry | ||
| HbA1c (%) | 5.3 ± 0.1 | 5.5 ± 0.1* |
| Fasting total cholesterol (mg dl−1) | 159.3 ± 9.1 | 188.5 ± 8.5* |
| Fasting HDL (mg dl−1) | 58.2 ± 4.1 | 61.1 ± 6.1 |
| Fasting LDL (mg dl−1) | 88.1 ± 8.9 | 107.3 ± 8.6 |
| Fasting triglycerides (mg dl−1) | 94.4 ± 12.5 | 108.2 ± 10.5 |
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Values are the mean ± SEM.
P < 0.05 vs. Young.
SSNA responses to whole-body cooling are impaired in older adults
Baseline mean Tsk was not different between groups (young: 34.4 ± 0.1 vs. older: 34.3 ± 0.03°C; P = 0.21). By study design, whole-body cooling decreased mean Tsk to 30.5°C in all subjects. The rate of cooling was not different between groups (young: 0.14 ± 0.01 vs. older: 0.14 ± 0.01Δ°C min−1; P = 0.55). Absolute CVC at baseline (mean Tsk = 34.0°C) was not different between groups (young: 0.09 ± 0.02 vs. older: 0.10 ± 0.02 flux mmHg−1; P = 0.86).
Original recordings of SSNA at mean Tsk 34.0°C and Tsk 30.5°C in one young and one older adult are presented in Fig.1. Whole-body cooling elicited progressive increases in SSNA throughout the protocol in young, but not older, adults (Figs 1 and 2A). Concurrently, reflex cutaneous vasoconstriction was blunted in older adults both on the dorsum of the foot (Fig.2B) and in the lateral calf (young: Δ −0.04 ± 0.01 vs. older: Δ −0.01 ± 0.01 flux mmHg−1; P = 0.03). There was a significant relation between the change in SSNA and cutaneous vasoconstriction during cold exposure in both young (r2 = 0.97, P < 0.01) and older adults (r2 = 0.66, P = 0.01; Fig.3). Moreover, there were no age-related differences in the slope of the linear regression between ΔSSNA and ΔCVCbase (young: −2.02 × 10−4 ± 1.45 × 10−5 vs. older: −2.24 × 10−4 ± 6.53 × 10−5; P > 0.05).
Figure 1.
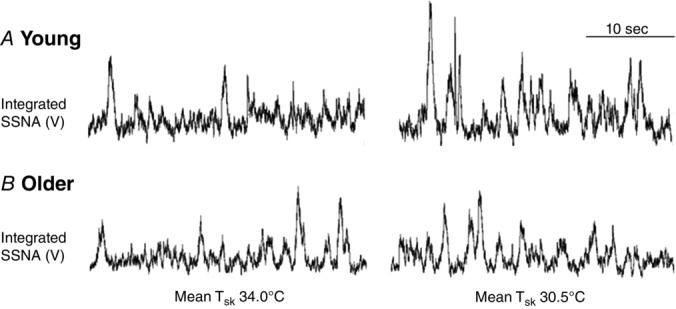
Original records from one young and one older subject illustrating integrated skin sympathetic nerve activity (SSNA) at baseline (mean Tsk 34.0°C) and during whole-body cooling (mean Tsk 30.5°C)
SSNA increased during whole-body cooling in young, but not older, adults.
Figure 2.
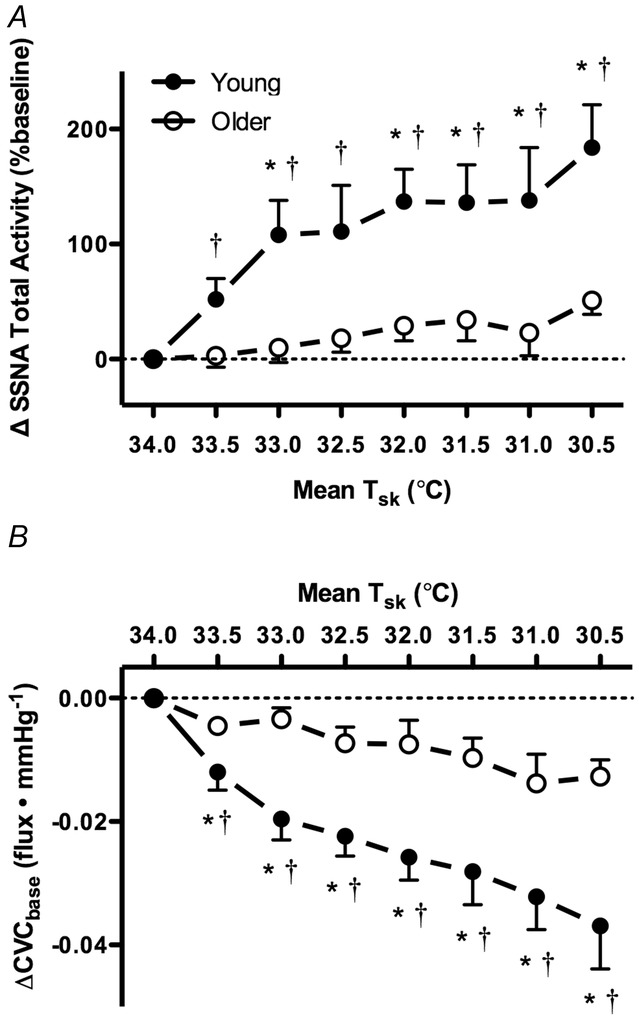
Group summary data for SSNA (ΔSSNA total activity; A) and cutaneous vascular conductance (ΔCVCbase; B) at each 0.5°C decrease in mean skin temperature (Tsk) during whole-body cooling in young (filled symbols) and older adults (open symbols)
Reflex cutaneous vasoconstriction was blunted and SSNA did not change during whole-body cooling in older adults. *P < 0.05 vs. Older; †P < 0.05 vs. mean Tsk 34°C.
Figure 3.
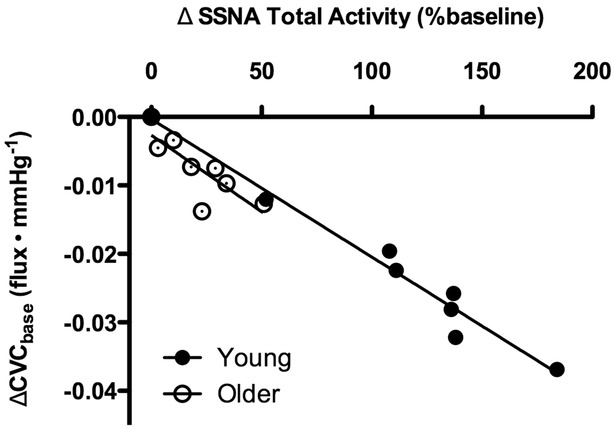
The relation between the change in SSNA (ΔSSNA total activity) and cutaneous vascular conductance (ΔCVCbase) during whole-body cooling in young (filled symbols) and older adults (open symbols)
There was a significant relation in both subject groups (P < 0.05).
BP increased during cooling in both subject groups; however, the increases in both systolic BP and MAP were exaggerated in the older adults (Table2). There were no age-related differences in the heart rate response to whole-body cooling (Table2).
Table 2.
Cardiovascular responses to whole-body cooling
| Mean Tsk 34.0°C | Mean Tsk 30.5°C | Δ | |
|---|---|---|---|
| Systolic BP (mmHg) | |||
| Young | 122 ± 4 | 126 ± 5† | 4 ± 1 |
| Older | 118 ± 3 | 129 ± 4† | 11 ± 2* |
| Diastolic BP (mmHg) | |||
| Young | 72 ± 3 | 77 ± 2† | 5 ± 1 |
| Older | 74 ± 2 | 81 ± 3† | 7 ± 2 |
| MAP (mmHg) | |||
| Young | 89 ± 3 | 93 ± 3† | 4 ± 1 |
| Older | 89 ± 2 | 97 ± 3† | 8 ± 2‡ |
| Heart rate (beats min−1) | |||
| Young | 59 ± 2 | 60 ± 2 | 1 ± 1 |
| Older | 59 ± 2 | 59 ± 1 | 0 ± 1 |
Tsk, skin temperature; BP, blood pressure; MAP, mean arterial pressure. Values are the mean ± SEM.
P < 0.05 vs. Young
P = 0.055 vs. Young
P < 0.05 vs. mean Tsk 34.0°C.
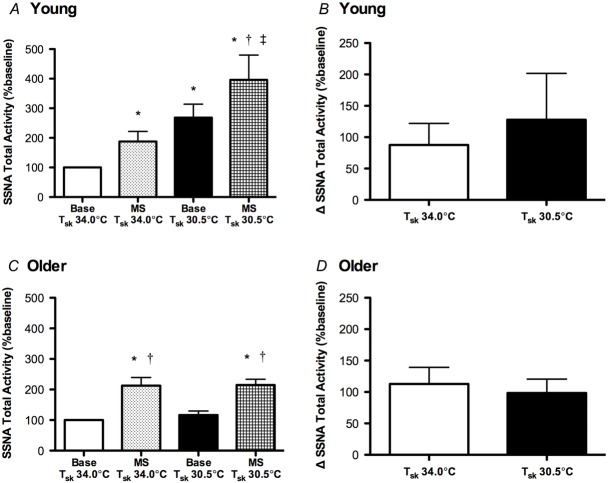
SSNA responses to mental stress are preserved during whole-body cooling in older adults
The reflex SSNA responses to mental stress are presented in Fig.4. Mental stress significantly increased SSNA in young (Fig.4A) and older adults (Fig. 4C) at both mean Tsk 34.0°C and 30.5°C. In young adults, the increase in SSNA during mental stress at mean Tsk 30.5°C occurred in addition to the significant cold-induced increase in sympathetic outflow directed to the skin. Further, whole-body cooling did not affect the SSNA response to mental stress, represented as the increase in total activity from the preceding baseline, in either group (Fig.4B for young, 4D for older). There was no age-related difference in the magnitude of the increase in SSNA during mental stress at either temperature condition (Fig.4).
Figure 4.
Group summary data for absolute SSNA (total activity) during baseline (base) and during the first minute of mental stress (MS) at mean skin temperature (Tsk) 34°C and Tsk 30.5°C in young and older adults (A and C, respectively), as well as the increase in SSNA during mental stress from the preceding baseline in each temperature condition for young and older adults (B and D, respectively)
The SSNA response to mental stress (i.e. the increase in SSNA) was not different during cold stress in either group. *P < 0.05 vs. Base Tsk 34°C; †P < 0.05 vs. Base Tsk 30.5°C; ‡P < 0.05 vs. MS Tsk 34°C.
The BP and heart rate responses to mental stress are presented in Table3. As expected, mental stress increased BP and heart rate in both young and older subjects at both mean Tsk = 34.0°C and 30.5°C. Similar to the SSNA responses to mental stress, there were no temperature-specific differences in the pressor or heart rate response to mental stress in either group (Table3). In addition, there were no group differences in the increase in BP or heart rate during mental stress at either thermoneutral or cold stress conditions (Table3). There were no temperature- or age-related differences in perceived stress level during mental stress (young: 2 ± 0.2 units at Tsk 34.0°C vs. 2 ± 0.3 units at Tsk 30.5°C; older: 2 ± 0.3 units at Tsk 34.0°C vs. 2 ± 0.2 units at Tsk 30.5°C; P > 0.05 for all comparisons).
Table 3.
Cardiovascular responses to mental stress
| Mean Tsk 34.0°C | Mean Tsk 30.5°C | |
|---|---|---|
| ΔSystolic BP (mmHg) | ||
| Young | 6 ± 2 | 6 ± 1 |
| Older | 9 ± 2 | 10 ± 3 |
| ΔDiastolic BP (mmHg) | ||
| Young | 2 ± 1 | 6 ± 1† |
| Older | 6 ± 2 | 6 ± 2 |
| ΔMAP (mmHg) | ||
| Young | 3 ± 1 | 6 ± 1 |
| Older | 7 ± 2 | 7 ± 2 |
| ΔHeart rate (beats min−1) | ||
| Young | 7 ± 1 | 7 ± 1 |
| Older | 6 ± 2 | 7 ± 2 |
Tsk, skin temperature; BP, blood pressure; MAP, mean arterial pressure. Values are the mean ± SEM.
P < 0.05 vs. mean Tsk 34.0°C.
Cutaneous vascular responsiveness to exogenous NA is not impaired in older adults
Baseline CVC was not different between groups (young: 0.23 ± 0.05 vs. older: 0.17 ± 0.02 flux mmHg−1; P = 0.20). NA dose–response curves, expressed both as a percentage of baseline and as the absolute change from baseline, are presented in Fig. 5. There were no age-related differences in cutaneous vascular responsiveness to exogenous NA, assessed by the logEC50 (young: −6.41 ± 0.24 vs. older: −6.37 ± 0.25 %CVCbase, P = 0.93; young: −6.80 ± 0.35 vs. older: −6.91 ± 0.35 ΔCVCbase, P = 0.86). In a subset of subjects (n = 17) there was acceptable test–retest reliability in the sensitivity to exogenous NA (Cronbach's α = 0.679; P < 0.05). Maximal vasoconstriction in response to exogenous NA was blunted in older adults (young: −0.18 ± 0.02 vs. older: −0.12 ± 0.01 ΔCVCbase; P < 0.05).
Figure 5.
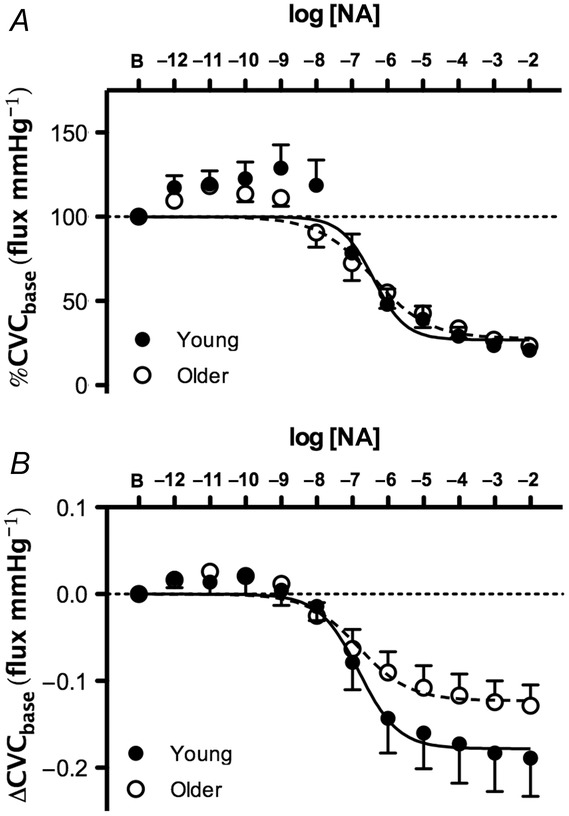
Group summary data for exogenous noradrenaline (NA)-induced cutaneous vasoconstriction in young (filled symbols) and older adults (open symbols)
Data are expressed as a percentage of baseline cutaneous vascular conductance (%CVCbase; A) and as an absolute change from baseline (ΔCVCbase; B). There were no age-related differences in cutaneous vascular sensitivity to exogenous NA.
Discussion
The primary findings of this study were that (1) the SSNA response to whole-body cooling is absent in healthy older adults, and (2) this attenuated efferent sympathetic response to cooling is related to a marked impairment in reflex cutaneous vasoconstriction. Additionally, the ability to increase SSNA during mental stress in the cold suggests that the impaired SSNA response to cooling in the older subjects was not due to a central inability to further increase skin sympathetic outflow. Lastly, contrary to our initial hypothesis, cutaneous adrenergic sensitivity to exogenous NA was not reduced in older adults, refuting the hypothesis that altered end-organ responsiveness to adrenergic stimuli contributes to impaired peripheral cutaneous vasoconstriction during cold exposure in healthy ageing. Taken together, these findings suggest that alterations in either afferent signalling from cutaneous thermoreceptors or central integration of converging afferent signals, or both, contribute to a reduced efferent SSNA response to whole-body cold stress and subsequent impairments in reflex cutaneous vasoconstriction in healthy older adults.
In the present study, whole-body cooling to Tsk = 30.5°C elicited large increases in SSNA in young adults, corroborating several previous reports (Sawasaki et al. 2001; Grassi et al. 2003; Cui et al. 2006). Moreover the increases in SSNA during cooling correlated with reductions in skin blood flow, demonstrating that SSNA (which is primarily adrenergic vasoconstrictor in origin during cold stress; Bini et al. 1980) effectively evokes reflex vasoconstriction in young skin. Healthy older adults, however, demonstrated a markedly attenuated reduction in skin blood flow during cooling, as previously reported by our laboratory (Kenney & Armstrong, 1996; Thompson & Kenney, 2004; Lang et al. 2009a,b2009, 2010; Stanhewicz et al. 2013; Greaney et al. 2014b) and others (Collins et al. 1977; Wagner & Horvath, 1985; Frank et al. 2000). The only previous study to examine the effect of age on SSNA during cold stress reported lower absolute non-normalized SSNA in elderly adults during exposure to low ambient temperature (Grassi et al. 2003); however, age-related reductions in basal skin sympathetic outflow were also reported in that study, and these baseline differences were not accounted for when examining SSNA during cooling. In addition, because no data normalization procedures were applied, it is difficult to interpret the reported magnitude of the SSNA response to cooling in elderly adults (Grassi et al. 2003). Using a standardized whole-body cooling protocol designed to elicit precise reductions in mean Tsk via a water-perfused suit (Thompson & Kenney, 2004; Lang et al. 2009a,b2009, 2010; Stanhewicz et al. 2013; Greaney et al. 2014b), coupled with accepted normalization procedures for the analysis of SSNA (Cui et al. 2006; Young et al. 2009a,b2009; Strom et al. 2011), we demonstrated that SSNA did not increase significantly above baseline during cooling in healthy older adults.
Importantly, we extend previous findings by showing that the impaired efferent sympathetic response to cooling is linearly related to impairments in reflex cutaneous vasoconstriction, evidenced by a relative inability to reduce skin blood flow during cooling in older adults. The change in SSNA during cooling was related to the corresponding change in cutaneous blood flow measured in the area of neural innervation (i.e. the dorsum of the foot) in both age groups, further validating our measurement of adrenergic vasoconstrictor SSNA during whole-body cold stress. Interestingly, the slope of the relation between cutaneous sympathetic outflow and skin blood flow was similar between age groups, suggesting that the relative inability of older adults to decrease skin blood flow during whole-body cooling is not a result of diminished sensitivity of the reflex response. Rather, older adults appear to operate within a reduced range of efferent SSNA responsiveness during cooling. Thus, because it appears that older adults are capable of eliciting similar reductions in skin blood flow for a given increase in SSNA compared to young adults within this limited response range, their attenuated reflex vasoconstrictor response is likely the result of impairments in the neural reflex axis and not peripheral vascular sensitivity to adrenergic stimuli.
Grassi et. al. reported blunted increases in SSNA during cold exposure in older compared to young adults, but similar SSNA responses to an acoustic stimulus (Grassi et al. 2003). However, the auditory stimulus was not performed during cold exposure (Grassi et al. 2003), making it difficult to draw conclusions specific to the cold stress. Therefore, to more specifically isolate the cooling stimulus itself, as well as to examine potential age-related alterations in the central ability to elicit further skin sympathetic responses, we assessed SSNA during the non-thermoregulatory stimulus of mental stress at baseline (thermoneutral) as well as when superimposed on whole-body cooling. Mental stress elicits sustained and reproducible increases in SSNA in young adults (Muller et al. 2013a,b2013). Our results demonstrate that mental stress increased SSNA in both young and older adults at mean Tsk of 34.0°C and 30.5°C. Importantly, whole-body cooling did not affect the central ability to increase SSNA during mental stress in either group. This preserved SSNA response to mental stress during whole-body cooling in older adults suggests that a central inability to elicit further increases in SSNA does not explain the lack of an increase in skin sympathetic outflow during cooling in healthy ageing.
Increased efferent SSNA during cold stress stimulates the release of NA, as well as the non-adrenergic cotransmitters neuropeptide Y and ATP, which together mediate cutaneous vasoconstriction (Stephens et al. 2001, 2004; Thompson & Kenney, 2004). Because sympathetic cotransmitter mechanisms are functionally absent in the cutaneous vasculature of healthy older adults (Thompson & Kenney, 2004), they must rely entirely on (impaired) adrenergic-mediated peripheral mechanisms for vasoconstriction. Consequently, reductions in cutaneous adrenergic sensitivity and altered end-organ responsiveness, in addition to blunted increases in sympathetic outflow, could contribute to the relative inability of the sympathetic nervous system to evoke adequate peripheral vasoconstriction during whole-body cooling in older adults. To address this potential mechanism, we assessed cutaneous adrenergic responsiveness to exogenous NA in the lower leg, which is the limb to which peroneal skin sympathetic outflow is directed. We found no evidence for age differences in the cutaneous vasoconstrictor responsiveness to infusion of exogenous NA in a wide range of concentrations from physiological to supraphysiological. Indeed, the sensitivity to NA, quantified as the logEC50 of the NA dose–response curve, was remarkably similar between young and older adults. Although maximal vasoconstriction in response to exogenous NA was blunted in older adults, consistent with earlier findings (Thompson et al. 2005), this only occurs at supraphysiological doses of NA that likely exceed those that occur during whole-body cooling (Frank et al. 2000; Thompson & Kenney, 2004). The current findings appear to be in contrast to the attenuated cutaneous vasoconstrictor responses to exogenous NA in aged adults previously reported by our laboratory (Thompson et al. 2005), and although the reason(s) for the contrasting conclusions between studies are not entirely clear, methodological differences such as study protocol, anatomical location of measurement, and mathematical modelling and statistical analyses may have contributed. However, the findings of the present investigation are similar to those reported by the only other study to date to characterize cutaneous adrenergic responsiveness in older adults. Wilson and colleagues (Wilson et al. 2004) found no age-related difference in cutaneous vasoconstrictor responsiveness to a standard NA dose–response protocol. Therefore, the current results appear to rule out the hypothesis that reduced cutaneous vascular responsiveness to adrenergic stimuli contributes to the impaired reflex vasoconstriction seen in healthy ageing. The maintained ability of the aged cutaneous vasculature to respond to a given pharmacological NA stimulus and to a given physiological increase in SSNA during cooling (albeit in a reduced operating range) collectively suggest that the deficits in reflex vasoconstriction characteristic of healthy human ageing result from functional impairments in the afferent arm or central signal processing within the neural reflex axis.
Despite our finding that end-organ responsiveness to physiological concentrations of exogenous NA was not different between age groups, there is substantial evidence demonstrating that the peripheral vascular responses to whole-body cooling are impaired in older adults (Collins et al. 1977; Wagner & Horvath, 1985; Kenney & Armstrong, 1996; Frank et al. 2000; Thompson & Kenney, 2004; Lang et al. 2009a,b2009, 2010; Stanhewicz et al. 2013; Greaney et al. 2014b). During cooling, neuropeptide Y and ATP are co-stored and co-released with NA from sympathetic adrenergic perivascular nerve terminals (Lundberg et al. 1990; Burnstock, 1999), contributing considerably to reflex cutaneous vasoconstriction (Thompson & Kenney, 2004). However, because older adults have a functionally absent non-adrenergic cotransmitter contribution (Thompson & Kenney, 2004), along with attenuated efferent skin sympathetic outflow during cooling, reflex cutaneous vasoconstriction remains impaired, even in the face of maintained cutaneous vascular responsiveness to NA itself.
In previous studies in our laboratory, older subjects exhibited attenuated vasoconstriction in response to the local perfusion of tyramine, which evokes NA release from storage vesicles in postganglionic sympathetic nerve terminals (Lang et al. 2009a, 2010). Together with the present findings, these data suggest that the synthesis of NA within the nerve terminal may be compromised in older adults. The potential for blunted NA synthesis and subsequent axonal release from sympathetic nerves to contribute to impaired reflex cutaneous vasoconstriction has been further examined in a series of studies designed to functionally restore peripheral NA bioavailability via direct intradermal microdialysis of vasoactive agents to the peripheral vasculature as well as by chronic oral pharmaceutical administration (Lang et al. 2009a, 2010; Stanhewicz et al. 2013). Exogenous local administration of either l-tyrosine, the amino acid substrate for tyrosine hydroxylase, the rate-limiting enzyme in the NA biosynthetic pathway, or tetrahydrobiopterin, an essential cofactor for tyrosine hydroxylase (Lang et al. 2009a, 2010), improves reflex vasoconstriction in aged skin. Moreover, oral administration of pharmaceutical tetrahydrobiopterin (sapropterin) likewise restores reflex cutaneous vasoconstriction in older adults (Stanhewicz et al. 2013), presumably by augmenting NA biosynthesis and storage in the perivascular nerve terminals, thereby allowing for greater NA release during sympathetic stimulation. Interestingly, emerging evidence indicates that oral sapropterin may also centrally modulate efferent sympathetic outflow (Park et al. 2015). However, future studies directly investigating the potential for strategies targeted at increasing efferent SSNA to similarly restore reflex cutaneous vasoconstriction in healthy ageing are necessary.
In conclusion, the principal finding of the present study was that the age-related impairments in reflex cutaneous vasoconstriction are mediated, at least in part, by the lack of an increase in SSNA during whole-body cold stress. The reflex SSNA response to a non-thermoregulatory stimulus during cooling was preserved in older adults, indicating that the attenuated increase in SSNA during cold exposure is likely not due to a central inability to elicit SSNA responses to cold exposure. Lastly, cutaneous adrenergic sensitivity was not reduced in older adults, suggesting that altered end-organ responsiveness does not explain the relative inability of the sympathetic nervous system to evoke adequate peripheral vasoconstriction during cold exposure in aged skin. Collectively, these findings suggest that neural deficits, potentially in afferent signalling from cutaneous thermoreceptors or the central processing of converging inputs at the level of the hypothalamus, or both, contribute to a reduced efferent sympathetic response to whole-body cold stress and the resultant reductions in reflex cutaneous vasoconstriction in healthy older adults.
Perspectives and significance
Given the significant increase in cardiovascular risk during environmental cold exposure in older adults (Sheth et al. 1999; Curriero et al. 2002; Panagiotakos et al. 2004; Morabito et al. 2005; Wolf et al. 2009), understanding the physiological mechanisms underlying impaired thermal-cardiovascular integration in this population is clinically significant. Because advancing age is the primary risk factor for the development of cardiovascular disease (Go et al. 2014), elucidating the neural mechanisms mediating impaired reflex cutaneous vasoconstriction during cold exposure in healthy older adults may potentially lead to novel treatment and prevention strategies to alleviate this cardiovascular risk. In addition, the findings of the current series of studies in healthy ageing may provide novel mechanistic insight for further understanding alterations in sympathetic neural control of the vasculature in cardiovascular pathophysiology.
Acknowledgments
The time and effort expended by all volunteer subjects are greatly appreciated. The authors are grateful for the assistance of Jessica L. Kutz, Susan Slimak and Jane Pierzga.
Glossary
- BP
blood pressure
- CVC
cutaneous vascular conductance
- MAP
mean arterial pressure
- NA
noradrenaline (norepinephrine)
- SSNA
skin sympathetic nerve activity
- Tsk
skin temperature
Additional information
Competing interests
None declared.
Author contributions
J.L.G., W.L.K. and L.M.A. contributed to the conception and design of the research; J.L.G. and A.E.S. collected data; J.L.G. analysed data; J.L.G., A.E.S., W.L.K. and L.M.A. interpreted data; J.L.G. drafted the manuscript; J.L.G., A.E.S., W.L.K. and L.M.A. edited and revised the manuscript; J.L.G., A.E.S., W.L.K. and L.M.A. approved the final version of the manuscript. This study was conducted in the Department of Kinesiology at The Pennsylvania State University.
Funding
This research was supported by HL120471-02 (J.L.G.), AG007004-23 (W.L.K.) and HL093-238-04 (L.M.A.).
References
- Akinaga J, Lima V, Kiguti LR, Hebeler-Barbosa F, Alcántara-Hernández R, García-Sáinz JA. Pupo AS. Differential phosphorylation, desensitization, and internalization of α1A-adrenoceptors activated by norepinephrine and oxymetazoline. Mol Pharmacol. 2013;83:870–881. doi: 10.1124/mol.112.082313. [DOI] [PubMed] [Google Scholar]
- Bini G, Hagbarth KE, Hynninen P. Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol. 1980;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic cotransmission. Brain Res Bull. 1999;50:355–357. doi: 10.1016/s0361-9230(99)00103-3. [DOI] [PubMed] [Google Scholar]
- Cabrera-Wrooman A, Romero-Ávila MT. García-Sáinz JA. Roles of the α1A-adrenergic receptor carboxyl tail in protein kinase C-induced phosphorylation and desensitization. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:499–510. doi: 10.1007/s00210-010-0569-7. [DOI] [PubMed] [Google Scholar]
- Callister R, Suwarno NO. Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–387. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Cooke WH. Ray CA. Forearm neurovascular responses during mental stress and vestibular activation. Am J Physiol Heart Circ Physiol. 2005;288:H904–H907. doi: 10.1152/ajpheart.00569.2004. [DOI] [PubMed] [Google Scholar]
- Carter JR, Schwartz CE, Yang H. Joyner MJ. Fish oil and neurovascular reactivity to mental stress in humans. Am J Physiol Regul Integr Comp Physiol. 2013;304:R523–R530. doi: 10.1152/ajpregu.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Hypothermia-related deaths - United States 2003–2004. MMWR Morb Mortal Wkly Rep. 2005;54:173–175. [PubMed] [Google Scholar]
- Collins KJ, Dore C, Exton-Smith AN, Fox RH, MacDonald IC. Woodward PM. Accidental hypothermia and impaired temperature homoeostasis in the elderly. Br Med J. 1977;1:353–356. doi: 10.1136/bmj.1.6057.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DA. Bielkiewicz B. A computer-assisted technique for analysis and comparison of dose-response curves. J Pharmacol Methods. 1984;11:77–89. doi: 10.1016/0160-5402(84)90017-2. [DOI] [PubMed] [Google Scholar]
- Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL. Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2006;290:H1601–H1609. doi: 10.1152/ajpheart.00025.2005. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE. Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci. 2004;116:54–61. doi: 10.1016/j.autneu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L. Patz JA. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155:80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- Degroot DW. Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:R103–R108. doi: 10.1152/ajpregu.00074.2006. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A. Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Fox RH. Edholm OG. Nervous control of the cutaneous circulation. Br Med Bull. 1963;19:110–114. doi: 10.1093/oxfordjournals.bmb.a070027. [DOI] [PubMed] [Google Scholar]
- Frank SM, Raja SN, Bulcao C. Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R349–R354. doi: 10.1152/ajpregu.2000.279.1.R349. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D. Turner MB. Executive summary: Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Turri C, Bertinieri G, Dell'Oro R. Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729–735. doi: 10.1161/01.CIR.0000081769.02847.A1. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Stanhewicz AE, Kenney WL. Alexander LM. Lack of limb or sex differences in the cutaneous vascular responses to exogenous norepinephrine. J Appl Physiol (1985) 2014a;117:1417–1423. doi: 10.1152/japplphysiol.00575.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Stanhewicz AE, Kenney WL. Alexander LM. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol (1985) 2014b;117:648–657. doi: 10.1152/japplphysiol.00516.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HD. Kepchar JH. Control of peripheral resistance in major systemic vascular beds. Physiol Rev. 1959;39:617–686. doi: 10.1152/physrev.1959.39.3.617. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjörk HE. Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hodges GJ, Chiu C, Kosiba WA, Zhao K. Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol (1985) 2009;106:1112–1118. doi: 10.1152/japplphysiol.91508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA. Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (1985) 2010;109:1538–1544. doi: 10.1152/japplphysiol.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IE. Smith JA. The stability of noradrenaline in physiological saline solutions. J Pharm Pharmacol. 1978;30:124–126. doi: 10.1111/j.2042-7158.1978.tb13179.x. [DOI] [PubMed] [Google Scholar]
- Kenney WL. Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol (1985) 1996;80:512–515. doi: 10.1152/jappl.1996.80.2.512. [DOI] [PubMed] [Google Scholar]
- Lang JA, Holowatz LA. Kenney WL. Local tetrahydrobiopterin administration augments cutaneous vasoconstriction in aged humans. J Physiol. 2009a;587:3967–3974. doi: 10.1113/jphysiol.2009.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JA, Holowatz LA. Kenney WL. Localized tyrosine or tetrahydrobiopterin supplementation corrects the age-related decline in cutaneous vasoconstriction. J Physiol. 2010;588:1361–1368. doi: 10.1113/jphysiol.2009.185694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JA, Jennings JD, Holowatz LA. Kenney WL. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol. 2009b;297:H1792–H1797. doi: 10.1152/ajpheart.00509.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger UA, Mostoufi-Moab S, Herr M, Gray K, Kunselman A. Sinoway LI. Control of skin sympathetic nerve activity during intermittent static handgrip exercise. Circulation. 2003;108:2329–2335. doi: 10.1161/01.CIR.0000093280.40118.30. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Hemsén A, Lacroix JS. Pernow J. Pharmacology of noradrenaline and neuropeptide tyrosine (NPY)-mediated sympathetic cotransmission. Fundam Clin Pharmacol. 1990;4:373–391. doi: 10.1111/j.1472-8206.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Wallin BG. Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurones to human skin. J Physiol. 1999;516:303–314. doi: 10.1111/j.1469-7793.1999.303aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G. Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol. 2005;105:288–293. doi: 10.1016/j.ijcard.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Muller MD, Sauder CL. Ray CA. Melatonin attenuates the skin sympathetic nerve response to mental stress. Am J Physiol Heart Circ Physiol. 2013a;305:H1382–H1386. doi: 10.1152/ajpheart.00470.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Sauder CL. Ray CA. Mental stress elicits sustained and reproducible increases in skin sympathetic nerve activity. Physiol Rep. 2013b;1:e00002. doi: 10.1002/phy2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos DB, Chrysohoou C, Pitsavos C, Nastos P, Anadiotis A, Tentolouris C, Stefanadis C, Toutouzas P. Paliatsos A. Climatological variations in daily hospital admissions for acute coronary syndromes. Int J Cardiol. 2004;94:229–233. doi: 10.1016/j.ijcard.2003.04.050. [DOI] [PubMed] [Google Scholar]
- Park J, Liao P, Sher S, Lyles RH, Deveaux DD. Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2015;308:R208–R218. doi: 10.1152/ajpregu.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasaki N, Iwase S. Mano T. Effect of skin sympathetic response to local or systemic cold exposure on thermoregulatory functions in humans. Auton Neurosci. 2001;87:274–281. doi: 10.1016/S1566-0702(00)00253-8. [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Gurdal H, Wang HY, Johnson MD. Friedman E. Desensitization of norepinephrine receptor function is associated with G protein uncoupling in the rat aorta. Am J Physiol. 1997;273:H279–H285. doi: 10.1152/ajpheart.1997.273.1.H279. [DOI] [PubMed] [Google Scholar]
- Sheth T, Nair C, Muller J. Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol. 1999;33:1916–1919. doi: 10.1016/s0735-1097(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Stanhewicz AE, Alexander LM. Kenney WL. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol (1985) 2013;115:1025–1031. doi: 10.1152/japplphysiol.00626.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DP, Aoki K, Kosiba WA. Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol. 2001;280:H1496–H1504. doi: 10.1152/ajpheart.2001.280.4.H1496. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad AR, Bennett LA, Kosiba WA. Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1404–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- Strom NA, Meuchel LW, Mundy DW, Sawyer JR, Roberts SK, Kingsley-Berg SM. Charkoudian N. Cutaneous sympathetic neural responses to body cooling in type 2 diabetes mellitus. Auton Neurosci. 2011;159:15–19. doi: 10.1016/j.autneu.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CS, Holowatz LA. Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1108–R1113. doi: 10.1152/ajpregu.00839.2004. [DOI] [PubMed] [Google Scholar]
- Thompson CS. Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol. 2004;558:697–704. doi: 10.1113/jphysiol.2004.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma K, Walkowski S, Metzler-Wilson K. Wilson TE. Acupuncture attenuates exercise-induced increases in skin sympathetic nerve activity. Auton Neurosci. 2011;162:84–88. doi: 10.1016/j.autneu.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjörk HE. Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wagner JA. Horvath SM. Cardiovascular reactions to cold exposures differ with age and gender. J Appl Physiol (1985) 1985;58:187–192. doi: 10.1152/jappl.1985.58.1.187. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Wilson TE, Davis SL. Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol (1985) 2011;111:1703–1709. doi: 10.1152/japplphysiol.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J. Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol. 2001;536:615–623. doi: 10.1111/j.1469-7793.2001.0615c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Gao Z, Hess KL. Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1627–R1633. doi: 10.1152/ajpregu.00099.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Monahan KD, Short DS. Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1230–R1234. doi: 10.1152/ajpregu.00467.2004. [DOI] [PubMed] [Google Scholar]
- Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE. Peters A. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation. 2009;120:735–742. doi: 10.1161/CIRCULATIONAHA.108.815860. [DOI] [PubMed] [Google Scholar]
- Yamazaki F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol (1985) 2010;108:328–333. doi: 10.1152/japplphysiol.00814.2009. [DOI] [PubMed] [Google Scholar]
- Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD. Fadel PJ. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol. 2009a;587:4977–4986. doi: 10.1113/jphysiol.2009.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CN, Keller DM, Crandall CG. Fadel PJ. Comparing resting skin sympathetic nerve activity between groups: caution needed. J Appl Physiol (1985) 2009b;106:1751–1752. doi: 10.1152/japplphysiol.91538.2008. [DOI] [PubMed] [Google Scholar]