Abstract
Abstract
Cardiovascular adjustments during heat stress are generally attenuated in healthy aged humans, which could be due to lower increases in sympathetic activity compared to the young. We compared muscle sympathetic nerve activity (MSNA) between 11 young (Y: 28 ± 4 years) and 10 aged (A: 70 ± 5 years) subjects prior to and during passive heating. Furthermore, MSNA responses were compared when a cold pressor test (CPT) and lower body negative pressure (LBNP) were superimposed upon heating. Baseline MSNA burst frequency (Y: 15 ± 4 vs. A: 31 ± 3 bursts min−1, P ≤ 0.01) and burst incidence (Y: 26 ± 8 vs. A: 50 ± 7 bursts (100 cardiac cycles (CC))−1, P ≤ 0.01) were greater in the aged. Heat stress increased core temperature to a similar extent in both groups (Y: +1.2 ± 0.1 vs. A: +1.2 ± 0.0°C, P = 0.99). Absolute levels of MSNA remained greater in the aged during heat stress (burst frequency: Y: 47 ± 6 vs. A: 63 ± 11 bursts min−1, P ≤ 0.01; burst incidence: Y: 48 ± 8 vs. A: 67 ± 9 bursts (100 CC)−1, P ≤ 0.01); however, the increase in both variables was similar between groups (both P ≥ 0.1). The CPT and LBNP further increased MSNA burst frequency and burst incidence, although the magnitude of increase was similar between groups (both P ≥ 0.07). These results suggest that increases in sympathetic activity during heat stress are not attenuated in healthy aged humans.
Key points
Cardiovascular adjustments to heat stress are attenuated in healthy aged individuals, which could contribute to their greater prevalence of heat-related illnesses and deaths during heat waves.
The attenuated cardiovascular adjustments in the aged could be due to lower increases in sympathetic nerve activity during heat stress.
We examined muscle sympathetic nerve activity (MSNA) and plasma catecholamine concentrations in healthy young and aged individuals during whole-body passive heat stress.
The main finding of this study is that increases in MSNA and plasma catecholamine concentrations did not differ between young and aged healthy individuals during passive heating. Furthermore, the increase in these variables did not differ when a cold pressor test and lower body negative pressure were superimposed upon heating.
These findings suggest that attenuated cardiovascular adjustments to heat stress in healthy aged individuals are unlikely to be related to attenuated increases in sympathetic activity.
Introduction
When humans are exposed to whole-body passive heat stress, cardiovascular adjustments contribute to the substantial increase in skin blood flow that promotes transfer of heat between the body and the surrounding environment. In young individuals, increases in cardiac output of ∼4–6 l min−1 (Rowell et al. 1969; Minson et al. 1998) and an ∼1 l min−1 redistribution of blood from the renal and splanchnic vasculatures (Rowell et al. 1970; Minson et al. 1998) combine to facilitate an estimated increase in skin blood flow upwards to ∼5–7 l min−1 (Rowell, 1974; Minson et al. 1998). In contrast, the increase in cardiac output (∼1–2 l min−1) and redistribution of blood from the renal and splanchnic vascular beds (∼0.7 l min−1) are greatly attenuated in the aged (≥64 years), ultimately contributing to lower skin blood flow (Minson et al. 1998). Although these findings clearly highlight age-related differences in the cardiovascular adjustments to heat stress, the physiological mechanism(s) underlying these differences remain relatively unknown.
In young humans, mild whole-body heat stress (∼0.5–0.7°C in core temperature) causes a 40–90% increase in muscle sympathetic nerve activity (MSNA), with continued elevations observed when core temperature is further increased (Niimi et al. 1997; Low et al. 2011). These increases in MSNA during heat stress are thought to reflect general sympathetic activation that drives the increased cardiac output and blood redistribution from the renal and splanchnic vascular beds (Low et al. 2011). It is therefore possible that attenuated sympathetic activity contributes to altered cardiovascular adjustments seen in aged humans during whole-body passive heat stress. Consistent with this hypothesis, aged rats demonstrate an attenuated increase in renal and splanchnic sympathetic nerve discharge during heat stress compared to young rats (Kenney & Fels, 2002, 2003; Margiocco et al. 2010). In humans, there is evidence that sympathetic responses to thermal stress are altered in the aged. For example, the responsiveness of skin sympathetic nerve activity to mild (±8°C) changes in ambient room temperature is attenuated in the aged (Grassi et al. 2003). Furthermore, increased MSNA is observed in aged humans during whole-body cold stress, while no increase is observed in the young (Greaney et al. 2014). Overall, these studies suggest that differences in sympathetic activity may be involved in the altered cardiovascular adjustments observed in the aged during heat stress. However, the sympathetic response to whole-body passive heat stress has not been characterized in aged humans.
The purpose of this study was to examine MSNA and plasma catecholamine concentrations in healthy young (18–35 years) and aged (62–80 years) humans during whole-body passive heat stress. A secondary objective was to compare the increase in these variables between age groups during the superimposition of sympathoexcitatory stimuli. We hypothesized that increases in MSNA and plasma catecholamine concentrations would be attenuated in the aged during heat stress alone, as well as during combined heat stress and sympathoexcitatory stimuli.
Methods
Subjects
Eleven young (Y) and ten aged (A) subjects volunteered for the study. Subject characteristics are presented in Table1. All subjects were free of any known cardiovascular, respiratory, neurological or metabolic diseases and were not taking any related medications. Aged subjects on low dose aspirin therapy were asked to hold their medication for 36 h prior to their participation in the study. Young female subjects participated during the first to seventh day of their self-reported menses (2 subjects) or during the no pill/placebo phase of oral contraceptive use (2 subjects). All aged female subjects were postmenopausal and were not on hormonal replacement therapy. The experimental procedures were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Centre and at Texas Health Presbyterian Hospital Dallas. Written informed consent was obtained from all subjects prior to their participation in the study and all procedures conformed to the standards set by the Declaration of Helsinki.
Table 1.
Subject characteristics
| Group | ||
|---|---|---|
| Young | Aged | |
| Males/females | 7/4 | 7/3 |
| Age (years) | 28 ± 4 | 70 ± 5* |
| Height (cm) | 179 ± 12 | 170 ± 8 |
| Weight (kg) | 80.7 ± 14.0 | 74.2 ± 8.6 |
| SBP (mmHg) | 117 ± 11 | 124 ± 9 |
| DBP (mmHg) | 64 ± 9 | 76 ± 7* |
| MAP (mmHg) | 82 ± 8 | 92 ± 7* |
| Heart rate (beats min−1) | 61 ± 12 | 62 ± 11 |
Values are means ± standard deviation. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Significantly different from young (P ≤ 0.05).
Measurements
Subjects were dressed in a two-piece, tube-lined suit (Med-Eng, Ottawa, ON, Canada) that covered the entire body, except for the head, hands, feet, one forearm and one lower leg. Core temperature was measured by an ingestible telemetric pill (HQ Inc., Palmetto, FL, USA) that was swallowed by the subject upon arrival at the laboratory, approximately 2 h before data collection. Mean skin temperature was measured as the weighted average of six thermocouples attached to the skin surface on the abdomen (14%), calf (11%), chest (22%), lower back (19%), thigh (14%) and upper back (19%). Heart rate was obtained from an electrocardiogram (GE Healthcare, Milwaukee, WI, USA) that was interfaced with a cardiotachometer (CWE, Ardmore, PA, USA). Blood pressure was measured by auscultation of the brachial artery (Tango+; SunTech Medical, Morrisville, NC, USA). Multifibre recordings of MSNA were obtained using a tungsten microelectrode positioned in the peroneal nerve. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The position of the recording electrode was adjusted until bursts of MSNA were identified based upon the following criteria (Delius et al. 1972a,b1972,c1972): (i) synchronicity of discharges with pulse rate; (ii) increases in activity during inspiratory apnoea; and (iii) lack of responsiveness to mental or somatosensory stimulation of the innervated region. Nerve signals were amplified, passed through a bandpass filter with a band width of 700–2000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA, USA). Plasma catecholamine concentrations were determined by drawing ∼3 ml of venous blood in tubes containing K2EDTA (Vacutainer system, Franklin Lakes, NJ, USA) which were immediately put in ice. Blood samples were then centrifuged to isolate plasma which was frozen at −80°C and sent to a biochemistry laboratory for high performance liquid chromatography analysis (Arup Laboratory, Salt Lake City, UT, USA). Urine specific gravity was measured in duplicate using a handheld refractometer (PAL-10S, Atago Inc., Bellevue, WA, USA).
Experimental protocol
Upon arrival at the laboratory the subjects swallowed the telemetric pill, provided a urine sample and weighed themselves nude. The subjects were then instrumented for the measurements of mean skin temperature, heart rate and blood pressure before being dressed in the tube-lined suit. The subjects then assumed a supine position on a patient bed with their legs sealed at the waist within a custom-made lower body negative pressure (LBNP) box. The subjects were allowed to rest quietly, with the lights dimmed, for 10 min to determine resting blood pressure and heart rate. Mean skin temperature was subsequently clamped at normothermic levels by circulating 34°C water though the tube-lined suit while the MSNA signal was obtained. Once an adequate signal was obtained, the subjects rested for a 10 min baseline period following which a cold pressor test (CPT) was performed by immersing one of the subject's hands up to the wrist in circulated ice-water slurry for 3 min. Blood pressure and MSNA were then allowed to return to baseline values (∼10 min), after which the pressure within the LBNP box was reduced to 15 mmHg for 5 min followed by another 5 min period at 30 mmHg LBNP. At the end of LBNP, blood pressure and MSNA were once again allowed to return to baseline values (∼10 min) before the temperature of the water circulating through the tube-lined suit was increased to ∼49°C. Whole-body heating continued until core temperature increased ∼1.2°C, after which the temperature of the water perfusing the suit was reduced to ∼46°C to maintain core temperature relatively constant while the CPT and LBNP periods were repeated. Following the LBNP period, the temperature of the water perfusing the suit was reduced to cool the subject and core temperature was monitored until it returned to near baseline values.
Data analysis
Data were collected with data acquisition software (Biopac MP150, Santa Barbara, CA, USA) at a minimum sampling frequency of 50 Hz. The electrocardiogram and MSNA data were collected at a sampling frequency of 200 Hz. Data during whole-body passive heat stress were analysed at increases in core temperature of 0.6°C and 1.2°C. Thermal and cardiovascular variables were analysed as 1 min averages for the baseline and heating periods; data during the CPT periods represent an average of the last 15 s; data during the LBNP periods represent an average of the last 15 s of the 3rd minute. MSNA data were analysed as a 3 min average during baseline and 1 min averages during the heating, CPT and LBNP periods. MSNA data during the CPT represent the data during the last minute, while for the LBNP periods they represent the data during the 3rd minute. Bursts of MSNA were evaluated using LabView software that identified bursts according to fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (Cui et al. 2001). Integrated MSNA was normalized by assigning a value of 100 to the mean amplitude of the five largest bursts during the normothermic baseline period. Total activity was identified from burst areas of the integrated neurogram. MSNA data are expressed as burst frequency (bursts min−1), burst incidence (bursts (100 cardiac cycles (CC))−1), and changes in total activity (units min−1). In five subjects (2 young, 3 aged), the MSNA microelectrode was repositioned during the heating period. Changes in total activity from these subjects were therefore omitted from the statistical analyses where appropriate. During the normothermic LBNP periods, MSNA was not maintained in one aged subject at 15 mmHg and four aged subjects at 30 mmHg. During the heat stress LBNP periods, MSNA was not maintained in two subjects (1 young, 1 aged) at 15 mmHg and eight subjects (3 young, 5 aged) at 30 mmHg. Data from these subjects were therefore omitted from the statistical analyses where appropriate.
Statistical analysis
Each period of interest was analysed using separate mixed-model analyses of variance with a repeated factor of time and the non-repeated factor of age (young and aged). For the heating period, there were three repeated factors (normothermic baseline; core temperature increase 0.6°C, core temperature increase 1.2°C). For the normothermic CPT period, there were two repeated factors (normothermic baseline and normothermic CPT). For the normothermic LBNP periods, there were two repeated factors for each LBNP level (normothermic baseline and LBNP 15 or 30 mmHg). For the hyperthermic CPT period, there were two repeated factors (hyperthermic baseline and hyperthermic CPT). For the hyperthermic LBNP periods, there were two repeated factors for each LBNP level (hyperthermic baseline and hyperthermic LBNP 15 or 30 mmHg). The level of significance was set at an α of P ≤ 0.05 and a Holm–Sidak correction was applied for multiple comparisons. Single time point comparisons between groups were analysed using independent samples t tests. Statistical analyses were performed using commercially available statistical software (Prism 6, Graphpad Software Inc., La Jolla, CA, USA). All variables are reported as the mean ± 95% confidence intervals.
Results
Subject characteristics
Both groups had similar height (P = 0.06), weight (P = 0.21), resting systolic blood pressure (P = 0.14) and resting heart rate (P = 0.79). However, aged individuals had greater resting diastolic (P ≤ 0.002) and mean (P ≤ 0.001) blood pressures (Table1). Urine specific gravity was similar between young (1.019 ± 0.002) and aged (1.016 ± 0.003, P = 0.28) groups prior to beginning the experimental protocol.
Normothermic baseline
Baseline core (Y: 37.1 ± 0.2 vs. A: 36.9 ± 0.2°C, P = 0.45) and mean skin (Y: 34.8 ± 0.3 vs. A: 34.6 ± 0.7°C, P = 0.33) temperatures did not differ between groups. Heart rate (Y: 59 ± 5 vs. A: 64 ± 6 beats min−1, P = 0.44) was also similar between groups while mean arterial pressure (Y: 86 ± 6 vs. A: 96 ± 5 mmHg, P ≤ 0.02) was greater in the aged. Baseline MSNA burst frequency (Y: 15 ± 4 vs. A: 31 ± 3 bursts min−1, P ≤ 0.01) and burst incidence (Y: 26 ± 8 vs. A: 50 ± 7 bursts (100 CC)−1, P ≤ 0.01), as well as plasma noradrenaline concentration (Y: 145 ± 22 vs. A: 288 ± 51 pg ml−1, P ≤ 0.01) were greater in the aged. In contrast, there were no group differences in plasma adrenaline concentration (Y: 27 ± 16 vs. A: 34 ± 24 pg ml−1, P = 0.62).
Normothermic CPT
In both groups, the CPT caused an increase (P ≤ 0.01) in mean arterial pressure, heart rate, MSNA burst frequency, burst incidence and total activity, as well as in plasma noradrenaline concentration. However, there were no age × time interactions for these variables (all P ≥ 0.34), as the increases observed during the CPT did not differ between young and aged (Table2). Plasma adrenaline concentration did not change during the CPT (P = 0.07).
Table 2.
Haemodynamic and neural responses to a cold pressor test (CPT) performed under normothermic and heat stress conditions
| Normothermia | Heat stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Young (n = 11) | Aged (n = 10) | Young (n = 11) | Aged (n = 10) | |||||
| Baseline | CPT | Baseline | CPT | Baseline | CPT | Baseline | CPT | |
| Heart rate (beats min−1) | 59 ± 5 | 66 ± 6* | 64 ± 6 | 70 ± 9* | 100 ± 12* | 99 ± 9 | 91 ± 9*,‡ | 92 ± 10 |
| MAP (mmHg) | 86 ± 6 | 104 ± 6* | 96 ± 5† | 115 ± 8*,† | 82 ± 8 | 98 ± 9# | 90 ± 5* | 95 ± 5 |
| Burst frequency (bursts min−1) | 15 ± 4 | 33 ± 7* | 31 ± 3† | 48 ± 11*,† | 47 ± 6* | 58 ± 8# | 63 ± 11*,† | 69 ± 10 |
| Burst incidence (bursts (100 CC)−1) | 26 ± 8 | 48 ± 11* | 50 ± 7† | 66 ± 9*,† | 48 ± 8* | 57 ± 9# | 67 ± 9*,† | 75 ± 9†,# |
| ΔTotal activity (units min−1) | 0 ± 0 | 486 ± 173* | 0 ± 0 | 688 ± 549* | 0 ± 0 | 356 ± 184# | 0 ± 0 | 342 ± 280# |
| Plasma [A] (pg ml−1) | 27 ± 16 | 56 ± 28 | 34 ± 24 | 46 ± 23 | 47 ± 13 | 137 ± 57# | 39 ± 20 | 90 ± 41 |
| Plasma [NA] (pg ml−1) | 145 ± 22 | 234 ± 53* | 288 ± 51† | 419 ± 53*,† | 327 ± 90* | 432 ± 94# | 419 ± 82* | 512 ± 57# |
Values are means ± 95% confidence intervals. MAP, mean arterial pressure; [A], adrenaline concentration; [NA], noradrenaline concentration.
Significantly different from normothermic baseline.
Significantly different from young for the indicated time point (P ≤ 0.05).
Significantly different from heat stress baseline.
Significantly different change from normothermia compared to the change in this variable in the young (P ≤ 0.05).
Normothermic LBNP
Heart rate (P = 0.48) and plasma adrenaline concentration (P = 0.25) did not change during 15 mmHg LBNP. In contrast, mean arterial pressure, MSNA burst frequency, burst incidence and total activity, as well as plasma noradrenaline concentration changed from baseline during 15 mmHg LBNP (all P ≤ 0.03). However, no age × time interactions were observed (all P ≥ 0.19), as the change in these variables did not differ between groups (Table3).
Table 3.
Haemodynamic and neural responses to 15 mmHg lower body negative pressure (LBNP 15) performed under normothermic and heat stress conditions
| Normothermia | Heat stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Young (n = 11) | Aged (n = 9) | Young (n = 10) | Aged (n = 9) | |||||
| Baseline | LBNP 15 | Baseline | LBNP 15 | Baseline | LBNP 15 | Baseline | LBNP 15 | |
| Heart rate (beats min−1) | 59 ± 5 | 60 ± 5 | 62 ± 5 | 63 ± 7 | 99 ± 13 | 108 ± 10# | 88 ± 8 | 93 ± 10 |
| MAP (mmHg) | 86 ± 6 | 87 ± 5 | 96 ± 5† | 101 ± 6*,† | 82 ± 9 | 85 ± 10 | 90 ± 6 | 83 ± 10 |
| Burst frequency (bursts min−1) | 15 ± 4 | 25 ± 5* | 31 ± 4† | 40 ± 7*,† | 48 ± 6 | 57 ± 7# | 59 ± 5 | 67 ± 8# |
| Burst incidence (bursts (100 CC)−1) | 26 ± 8 | 43 ± 10* | 52 ± 7† | 65 ± 12*,† | 49 ± 9 | 55 ± 7 | 65 ± 9† | 72 ± 9† |
| ΔTotal activity (units min−1) | 0 ± 0 | 247 ± 86* | 0 ± 0 | 246 ± 103* | 0 ± 0 | 236 ± 144 | 0 ± 0 | 227 ± 384 |
| Plasma [A] (pg ml−1) | 27 ± 16 | 16 ± 4 | 30 ± 26 | 27 ± 20 | 52 ± 13 | 75 ± 16# | 42 ± 21 | 67 ± 30# |
| Plasma [NA] (pg ml−1) | 145 ± 22 | 210 ± 37* | 282 ± 48† | 365 ± 53*,† | 317 ± 97 | 426 ± 88# | 401 ± 82 | 531 ± 106# |
Values are means ± 95% confidence intervals. MAP, mean arterial pressure; [A], adrenaline concentration; [NA], noradrenaline concentration.
Significantly different from normothermic baseline.
Significantly different from young for the indicated time point (P ≤ 0.05).
Significantly different from heat stress baseline for the indicated group. Note: the different baseline values in Tables4 are due to differences in the number of subjects from whom data were obtained throughout the applied perturbation.
During 30 mmHg LBNP (Table4), heart rate, MSNA burst frequency, incidence and total activity, as well as plasma noradrenaline concentration changed from baseline (P ≤ 0.01). Except for the change in heart rate (P ≤ 0.05), the change in these variables did not differ between groups (all age × time interactions, P ≥ 0.28). Mean arterial pressure (P = 0.09) and plasma adrenaline concentration (P = 0.29) were unaffected by normothermic 30 mmHg LBNP.
Table 4.
Haemodynamic and neural responses to 30 mmHg lower body negative pressure (LBNP 30) performed under normothermic and heat stress conditions
| Normothermia | Heat stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Young (n = 11) | Aged (n = 6) | Young (n = 8) | Aged (n = 5) | |||||
| Baseline | LBNP 30 | Baseline | LBNP 30 | Baseline | LBNP 30 | Baseline | LBNP 30 | |
| Heart rate (beats min−1) | 59 ± 5 | 70 ± 4* | 61 ± 8 | 67 ± 9* | 103 ± 13 | 122 ± 12# | 85 ± 14 | 95 ± 20# |
| MAP (mmHg) | 86 ± 6 | 87 ± 5 | 94 ± 7 | 98 ± 6† | 83 ± 10 | 79 ± 12 | 89 ± 12 | 73 ± 16# |
| Burst frequency (bursts min−1) | 15 ± 4 | 30 ± 6* | 29 ± 4† | 46 ± 6*,† | 49 ± 8 | 66 ± 9# | 53 ± 9 | 62 ± 12# |
| Burst incidence (bursts (100 CC)−1) | 26 ± 8 | 44 ± 8* | 49 ± 9† | 72 ± 17*,† | 51 ± 11 | 57 ± 10 | 60 ± 10 | 66 ± 12 |
| ΔTotal activity (units min−1) | 0 ± 0 | 359 ± 105* | 0 ± 0 | 501 ± 412* | 0 ± 0 | 334 ± 326 | 0 ± 0 | 287 ± 483 |
| Plasma [A] (pg ml−1) | 27 ± 16 | 19 ± 6 | 39 ± 41 | 29 ± 20 | 48 ± 12 | 235 ± 198 | 39 ± 28 | 87 ± 37 |
| Plasma [NA] (pg ml−1) | 145 ± 22 | 278 ± 47* | 268 ± 64† | 402 ± 85*,† | 307 ± 121 | 476 ± 161 | 339 ± 114 | 619 ± 271 |
Values are means ± 95% confidence intervals. MAP, mean arterial pressure; [A], adrenaline concentration; [NA], noradrenaline concentration.
Significantly different from normothermic baseline.
Significantly different from young for the indicated time point (P ≤ 0.05).
Significantly different from heat stress baseline for the indicated group. Note: the different baseline values in Tables4 are due to differences in the number of subjects from whom data were obtained throughout the applied perturbation.
Heat stress
The heating period lasted 65 ± 10 min in young and 66 ± 8 min in the aged (P = 0.89). Whole-body passive heating increased mean skin and core temperatures to a similar extent between groups at the midpoint (Y: mean skin +3.9 ± 0.3, core +0.6 ± 0.1 vs. A: mean skin +4.2 ± 0.4°C, core +0.6 ± 0.1°C, both P ≥ 0.35) and end (Y: mean skin +3.9 ± 0.4, core +1.2 ± 0.1 vs. A: mean skin +4.1 ± 0.5°C, core +1.2 ± 0.0°C, both P ≥ 0.46) of the heating period (Fig.1). Heart rate increased during heating (Fig.2), with the change in heart rate being different between groups (age × core temperature interaction, P ≤ 0.01). This was due to an attenuated increase in heart rate in the aged at an increase in core temperature of 0.6°C (Y: +32 ± 6 vs. A: +19 ± 4 beats min−1, P ≤ 0.01) and 1.2°C (Y: +41 ± 8 vs. A: +27 ± 6 beats min−1, P ≤ 0.01). In contrast, mean arterial pressure decreased during heat stress in both groups (P ≤ 0.01), and the change was similar between young and aged (age × core temperature interaction, P = 0.55). MSNA burst frequency (Fig.3), burst incidence and total activity (Fig.4), as well as plasma noradrenaline concentration (Fig.5) increased throughout the heating period in both groups (P ≤ 0.01). However, no age × core temperature interactions (all P ≥ 0.39) were observed for these variables. Heating did not cause any changes in plasma adrenaline concentration (P = 0.17).
Figure 1.
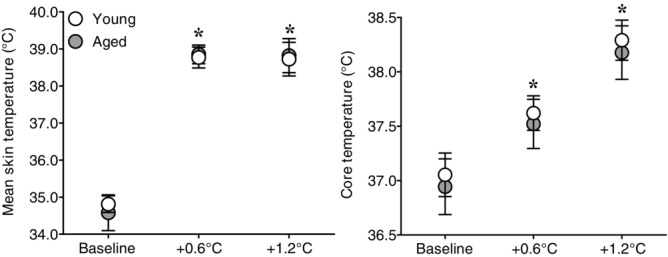
Effect of whole-body passive heat stress on skin and core temperatures of healthy young and aged subjects
Mean skin (left panel) and core (right panel) temperatures at normothermic baseline and during whole-body passive heat stress sufficient to elevate core temperature by 0.6°C (+0.6°C) and 1.2°C (+1.2°C) in healthy young (n = 11) and aged (n = 10) subjects. Values are mean ± 95% confidence intervals. *Significantly different from baseline (P ≤ 0.05).
Figure 2.
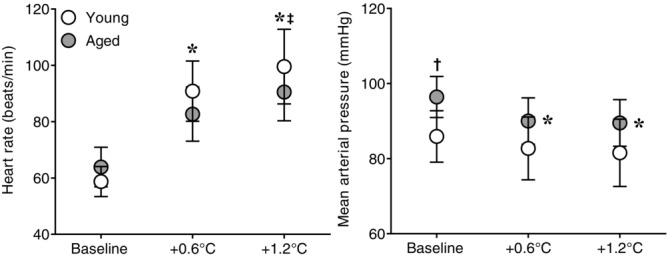
Effect of whole-body passive heat stress on heart rate and mean arterial pressure in healthy young and aged subjects
Heart rate (left panel) and mean arterial pressure (right panel) at normothermic baseline and during whole-body passive heat stress sufficient to elevate core temperature by 0.6°C (+0.6°C) and 1.2°C (+1.2°C) in healthy young (n = 11) and aged (n = 10) subjects. Values are mean ± 95% confidence intervals. *Significantly different from baseline (P ≤ 0.05). †Significantly different between young and aged (P ≤ 0.05). ‡Significantly different change from baseline between young and aged (P ≤ 0.05).
Figure 3.
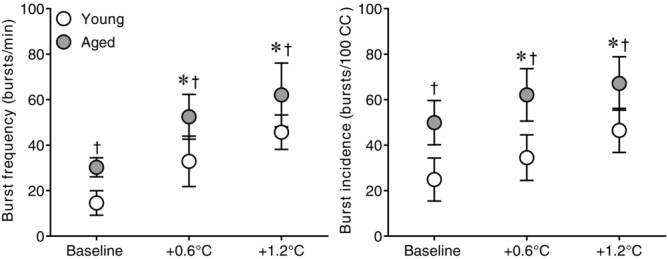
Effect of whole-body passive heat stress on MSNA burst frequency and burst incidence in healthy young and aged subjects
Muscle sympathetic nerve activity burst frequency (left panel) and burst incidence (right panel) at normothermic baseline and during whole-body passive heat stress sufficient to elevate core temperature by 0.6°C (+0.6°C) and 1.2°C (+1.2°C) in healthy young (n = 11) and aged (n = 10) subjects. Values are mean ± 95% confidence intervals. *Significantly different from baseline (P ≤ 0.05). †Significantly different between young and aged (P ≤ 0.05).
Figure 4.
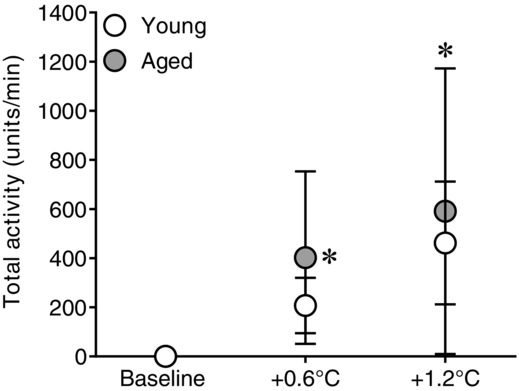
Effect of whole-body passive heat stress on total MSNA in healthy young and aged subjects
Changes from normothermic baseline in total muscle sympathetic nerve activity during whole-body passive heat stress sufficient to elevate core temperature by 0.6°C (+0.6°C) and 1.2°C (+1.2°C) in healthy young (n = 9) and aged (n = 7) subjects. Values are mean ± 95% confidence intervals. *Significantly different from baseline (P ≤ 0.05).
Figure 5.
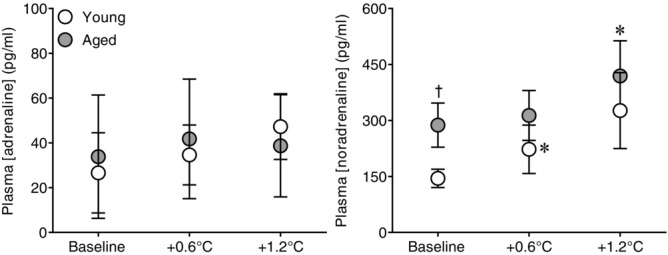
Effect of whole-body passive heat stress on plasma adrenaline and noradrenaline concentrations in healthy young and aged subjects
Plasma adrenaline (left panel) and noradrenaline (right panel) concentrations at normothermic baseline and during whole-body passive heat stress sufficient to elevate core temperature by 0.6°C (+0.6°C) and 1.2°C (+1.2°C) in healthy young (n = 11) and aged (n = 10) subjects. Values are mean ± 95% confidence intervals. *Significantly different from baseline (P ≤ 0.05). †Significantly different between young and aged (P ≤ 0.05).
Hyperthermic CPT
The superimposition of a CPT upon heat stress did not cause any change in heart rate in either group (P = 0.93), while it increased mean arterial pressure (P ≤ 0.01). However, the increase in mean arterial pressure was less in the aged (age × time interaction, P ≤ 0.02, Table2). The CPT caused further increases in MSNA burst frequency, burst incidence and total activity, as well as in plasma concentrations of noradrenaline and adrenaline (P ≤ 0.01). However, there were no age × time interactions (all P ≥ 0.17) for these variables.
Hyperthermic LBNP
The superimposition of 15 mmHg LBNP upon heat stress (Table3) increased heart rate in both groups (P ≤ 0.01), the change being similar between groups (age × time interaction, P = 0.30). Furthermore, 15 mmHg LBNP did not cause any changes in mean arterial pressure (P = 0.67). However, it did cause further increases in MSNA burst frequency, burst incidence, and total activity, as well as in plasma concentrations of noradrenaline and adrenaline (all P ≤ 0.03). There were no age × time interactions for these variables (all P ≥ 0.54), as the increases observed did not differ between groups.
During combined heat stress and 30 mmHg LBNP (Table4), changes were evident in heart rate (P ≤ 0.01), mean arterial pressure (P ≤ 0.01), MSNA burst frequency (P ≤ 0.01) and total activity (P = 0.05), as well as plasma noradrenaline concentration (P ≤ 0.03). In contrast, MSNA burst incidence (P = 0.06) and plasma adrenaline concentration (P = 0.07) were unaffected by 30 mmHg LBNP. For all measured variables, there were no age × time interactions (all P ≥ 0.07).
Discussion
The current study examined sympathetic activity in healthy young and aged humans during whole-body passive heat stress. The main results show that healthy ageing does not affect the increase in MSNA and plasma noradrenaline concentration during heat stress. Furthermore, healthy ageing does not affect the increase in these variables during sympathoexcitatory stimuli performed in normothermic and hyperthermic conditions. Contrary to our hypothesis, these results suggest that the attenuated cardiovascular adjustments observed in healthy aged humans during heat stress may not be due to attenuated increases in sympathetic activity.
Muscle sympathetic nerve activity can be modified by a number of factors (Wallin & Charkoudian, 2007), a few of which could be responsible for the sympathoexcitation observed during whole-body passive heat stress. For example, passive heat stress can be associated with decreased body water content, baroreceptor unloading, and increased ventilation, all of which have been shown to independently increase MSNA (Sundlof & Wallin, a1978; Seals et al. 1993; Rabbitts et al. 2009). However, increases in MSNA during passive heat stress occur at elevations in core temperature that are generally not associated with changes in arterial blood pressure and ventilation (Niimi et al. 1997; Low et al. 2011) and are not reversed by baroreceptor loading through rapid saline infusion (Crandall et al. 1999). Furthermore, sympathetic nerve discharge increases to a similar extent between baroreceptor-denervated and baroreceptor-intact rats (Kenney et al. 1995; Kenney & Fels, 2002). Rather than being associated with any of these factors, increased MSNA during whole-body passive heat stress is thought to reflect general activation of the central nervous system (Low et al. 2011) and therefore the sympathetic drive for cardiovascular adjustments (increased cardiac output, blood redistribution out of the splanchnic/renal beds). Given that increases in cardiac output and blood redistribution from the renal and splanchnic vasculatures are attenuated in aged humans during heat stress (Minson et al. 1998, 1999), we hypothesized they could be due, in part, to attenuated increases in sympathetic activity. In contrast to this hypothesis, the aged group had similar increases in MSNA (regardless of how it was expressed) and plasma concentrations of catecholamines at both mild (∼0.6°C) and relatively greater (∼1.2°C) increases in core temperature compared to the young. These results suggest that increases in sympathetic activity during passive heat stress are not attenuated by healthy human ageing.
Since the current results suggest that differences in sympathetic activity may not contribute to observed age-related differences in cardiovascular adjustments to heat stress (Minson et al. 1998, 1999), it can be hypothesized that these differences are associated with other factors, such as altered adrenergic responsiveness and/or cardiac function in the aged. Healthy ageing is generally accompanied by reduced adrenergic responsiveness (Seals & Dinenno, 2004), such that appropriate increases in sympathetic activity during heat stress may nonetheless result in attenuated cardiovascular adjustments. For example, we observed a significantly lower increase in heart rate in the aged during heat stress, despite similar increases in core temperature and, presumably, sympathetic drive compared to the young, although altered cardiac vagal responses may have contributed to these differing heart rate responses. Furthermore, we observed that similar increases in MSNA between groups during the hyperthermic CPT period resulted in less of an increase in mean arterial pressure in the aged. In addition to any potential reductions in adrenergic responsiveness, age-related differences in cardiovascular adjustments during heat stress could be related to differences in cardiac function. Although healthy ageing generally does not affect changes in echocardiographic indices of diastolic and systolic function during passive heating (Lucas et al. 2015), sedentary and healthy ageing in the absence of heat stress is associated with diminished cardiac ventricular compliance that shifts the Frank–Starling curve downwards and to the right (Arbab-Zadeh et al. 2004). An upward and leftward shift in the Frank–Starling curve is an important mechanism by which stroke volume is preserved during heat stress in young humans (Wilson et al. 2009; Bundgaard-Nielsen et al. 2010). It is therefore possible that a lack of, or altered, shift in the Frank–Starling curve in the aged contributes to an attenuated increase in cardiac output with heat stress, particularly since the lower cardiac output in the aged is associated with an inability to maintain stroke volume (Minson et al. 1998). Future studies are required to examine these possibilities.
The results of the current study are in contrast to data from animal studies in which ageing is associated with attenuated increases in sympathetic activity during passive heat stress (Kenney & Fels, 2002, 2003; Margiocco et al. 2010). In particular, Kenney & Fels (2002) clearly demonstrate that renal and splanchnic sympathetic nerve discharge during passive increases in core temperature are greatly attenuated in aged compared to young rats. A number of factors should be considered when comparing the current results from these animal studies, which could explain the divergent findings. First, the current study examined muscle sympathetic nerve activity as a measure of sympathetic activity, while the aforementioned animal studies have shown that renal and splanchnic sympathetic nerve discharge is reduced in aged rats. Sympathetic activity to various organs is not consistently homogeneous in humans (Esler et al. 1984). For example, differential sympathetic responses are observed between skeletal muscle (Delius et al. 1972a) and skin (Hagbarth et al. 1972) for a number of perturbations. It is therefore conceivable that increases in renal and/or splanchnic nerve activity were lower in the aged group of the current study, despite similar increases in MSNA. That said, MSNA is a relatively good index of sympathetic activity to the heart and kidneys in humans, as it correlates positively with noradrenaline spillover at rest in the coronary (Wallin et al. 1992) and renal circulations (Wallin et al. 1996). However, these studies were performed under normothermic conditions and it remains unknown whether the same relationships are observed with heat stress. Second, the animals in the aforementioned studies were exposed to appreciably greater levels of heat stress (changes in core temperature of ≥3°C). It is therefore possible that differences in sympathetic activity between healthy young and aged humans may be evident at greater increases in core temperature than those elicited by the current experimental protocol. Finally, the divergent results could be related to the fact that the animals in the aforementioned studies were anaesthetized as well as to differences between species. Future studies examining sympathetic activity to various organs during heat stress in humans are needed to address these possibilities.
Healthy ageing generally does not affect sympathetic activation during various sympathoexcitatory stimuli performed under normothermic conditions (Seals & Esler, 2000). Similarly, changes in MSNA and plasma catecholamine concentrations during the normothermic CPT and LBNP periods did not differ as a function of age in the current study. The current study extends previous findings by showing that healthy ageing does not affect increases in sympathetic activity during heat stress alone, as well as during combined heat stress and sympathoexcitatory stimulation. Since healthy ageing is associated with increased MSNA at rest (Sundlof & Wallin, b1978; Ng et al. 1993), the aged group in the current study had the potential to reach a ‘ceiling’ in sympathetic activity during heat stress as they maintained greater absolute levels of MSNA throughout the heating period. Such a ceiling would have implied that aged individuals have little reserve for further increases in sympathetic activity to defend against acute decreases in blood pressure or further reductions in central blood volume while exposed to passive heat stress. The similar increases in MSNA between groups during the hyperthermic CPT and LBNP periods, however, suggest that healthy aged individuals maintain a sufficient reserve for further sympathetic activation, at least at the increase in core temperature elicited by the current experimental protocol.
Considerations
Only MSNA was measured in the current study, therefore conclusions about sympathetic activity to other organs (e.g. renal and splanchnic vascular beds) remain limited. We also did not measure skin sympathetic nerve activity (SSNA), which differs between healthy young and aged humans during passive exposure to mild changes in ambient room temperature (Grassi et al. 2003). Future studies are needed to determine whether similar age-related differences in SSNA are observed during more stressful heat exposure that elicits greater changes in mean skin and/or core temperatures. Furthermore, the results of the current study pertain primarily to healthy aged individuals. Sympathetic activity is modulated by various disease states in the absence of heat stress (Malpas, 2010). It is therefore possible that disease may affect sympathetic responses during heat stress in the aged. Interestingly, however, sympathetic activity to the skin during heat stress is not altered in heart failure patients, despite substantially lower increases in skin blood flow and slightly lower sweat rates compared to healthy matched controls (Cui et al. 2013). Nonetheless, future studies examining MSNA and/or SSNA during heat stress in various clinical populations are required.
Perspectives
The frequency and intensity of climatic heat waves are expected to increase in the coming years, placing the general population at greater risk of heat-related morbidity and/or death. The aged represent the population at greatest risk of suffering from the negative effects of climatic heat waves. Impaired cardiovascular adjustments to heat exposure could explain the greater prevalence of heat-related deaths in the aged (Kenney & Munce, 2003; Kenney et al. 2014). However, few studies have examined age-related differences in cardiovascular adjustments to heat stress, which is an important avenue of future research as cardiovascular causes contribute significantly to heat-related deaths during climatic heat waves (Semenza et al. 1996, 1999; Wainwright et al. 1999; Kaiser et al. 2007). Understanding the mechanisms underlying the altered cardiovascular adjustments observed in healthy aged humans during heat stress (Minson et al. 1998) could lead to a better understanding of the factors responsible for the greater risk of death during climatic heat waves in this population and in individuals with cardiovascular disease. Furthermore, it may lead to intervention strategies that reduce cardiovascular strain in at-risk populations and therefore improve their overall health and well-being in hot environments. The results of the current study do not support the hypothesis that attenuated increases in sympathetic activity contribute to attenuated cardiovascular adjustments seen in the aged during heat stress. As such, future studies should focus on potential age-related differences in adrenergic responsiveness and/or the shift in the Frank–Starling curve during heat stress.
Conclusion
The current study examined potential differences in MSNA and plasma catecholamine concentrations between healthy young and aged humans during whole-body passive heat stress. The results show that core temperature-induced increases in MSNA and plasma catecholamine concentrations did not differ as a function of age. Furthermore, the increase in these variables did not differ between age groups when sympathoexcitatory stimuli were superimposed upon heat stress. Overall, these results suggest that attenuated increases in sympathetic activity do not contribute to the attenuated cardiovascular adjustments observed in healthy aged humans during passive heat stress.
Acknowledgments
We would like to thank Amy Adams, Matthew Cramer, Naomi Kennedy, Jena Kern, Paula Poh and Eric Rivas for their contributions to the study.
Glossary
- A
aged
- CC
cardiac cycles
- CPT
cold pressor test
- LBNP
lower body negative pressure
- MSNA
muscle sympathetic nerve activity
- Y
young
Additional information
Competing interests
The authors have no competing interests to report.
Author contributions
All experiments were performed at the Institute for Exercise and Environmental Medicine. All authors contributed to the conception and design of the experiment, as well as to data collection. D.G. analysed the data and drafted the manuscript. All authors contributed to the interpretation of the data and to revising the manuscript for important intellectual content. All authors have approved the final version of the manuscript.
Funding
This project was supported by NIH grants HL61388 and HL84072 (C.G.C.). D.G. is supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada.
References
- Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D. Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- Bundgaard-Nielsen M, Wilson TE, Seifert T, Secher NH. Crandall CG. Effect of volume loading on the Frank-Starling relation during reductions in central blood volume in heat-stressed humans. J Physiol. 2010;588:3333–3339. doi: 10.1113/jphysiol.2010.191981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA. Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol. 1999;277:H2348–H2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- Cui J, Boehmer JP, Blaha C, Lucking R, Kunselman AR. Sinoway LI. Chronic heart failure does not attenuate the total activity of sympathetic outflow to skin during whole-body heating. Circ Heart Fail. 2013;6:271–278. doi: 10.1161/CIRCHEARTFAILURE.112.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA. Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol (1985) 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A. Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972a;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A. Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972b;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A. Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972c;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Blombery P, Sacharias N. Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol. 1984;247:E21–E28. doi: 10.1152/ajpendo.1984.247.1.E21. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Turri C, Bertinieri G, Dell'Oro R. Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729–735. doi: 10.1161/01.CIR.0000081769.02847.A1. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Stanhewicz AE, Kenney WL. Alexander LM. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol (1985) 2014;117:648–657. doi: 10.1152/japplphysiol.00516.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjörk HE. Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Le Tertre A, Schwartz J, Gotway CA, Daley WR. Rubin CH. The effect of the 1995 heat wave in Chicago on all-cause and cause-specific mortality. Am J Public Health. 2007;97:S158–162. doi: 10.2105/AJPH.2006.100081. (Suppl. 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Barney CC, Hirai T. Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. J Appl Physiol (1985) 1995;78:881–889. doi: 10.1152/jappl.1995.78.3.881. [DOI] [PubMed] [Google Scholar]
- Kenney MJ. Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R513–520. doi: 10.1152/ajpregu.00683.2001. [DOI] [PubMed] [Google Scholar]
- Kenney MJ. Fels RJ. Forebrain and brain stem neural circuits contribute to altered sympathetic responses to heating in senescent rats. J Appl Physiol (1985) 2003;95:1986–1993. doi: 10.1152/japplphysiol.00438.2003. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Craighead DH. Alexander LM. Heat waves, aging, and human cardiovascular health. Med Sci Sports Exerc. 2014;46:1891–1899. doi: 10.1249/MSS.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney WL. Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol (1985) 2003;95:2598–2603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- Low DA, Keller DM, Wingo JE, Brothers RM. Crandall CG. Sympathetic nerve activity and whole body heat stress in humans. J Appl Physiol (1985) 2011;111:1329–1334. doi: 10.1152/japplphysiol.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RA, Sarma S, Schlader ZJ, Pearson J. Crandall CG. Age-related changes to cardiac systolic and diastolic function during whole-body passive hyperthermia. Exp Physiol. 2015 doi: 10.1113/expphysiol.2014.083014. (in press; DOI: 10.1113/expphysiol.2014.083014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- Margiocco ML, Borgarelli M, Musch TI, Hirai DM, Hageman KS, Fels RJ, Garcia AA. Kenney MJ. Effects of combined aging and heart failure on visceral sympathetic nerve and cardiovascular responses to progressive hyperthermia in F344 rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1555–R1563. doi: 10.1152/ajpregu.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA. Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985) 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA. Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol. 1999;276:R203–R212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG. Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G. Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Rabbitts JA, Strom NA, Sawyer JR, Curry TB, Dietz NM, Roberts SK, Kingsley-Berg SM. Charkoudian N. Influence of endogenous angiotensin II on control of sympathetic nerve activity in human dehydration. J Physiol. 2009;587:5441–5449. doi: 10.1113/jphysiol.2009.176693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Blackmon JR. Murray JA. Redistribution of blood flow during sutained high skin temperature in resting man. J Appl Physiol. 1970;28:415–420. doi: 10.1152/jappl.1970.28.4.415. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL. Murray JA. Cardiovascular responses to sutained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Seals DR. Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- Seals DR. Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG. Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- Semenza JC, McCullough JE, Flanders WD, McGeehin MA. Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med. 1999;16:269–277. doi: 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL. Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335:84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- Sundlof G. Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol. 1978a;278:525–532. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G. Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978b;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright SH, Buchanan SD, Mainzer HM, Parrish RG. Sinks TH. Cardiovascular mortality – the hidden peril of heat waves. Prehosp Disaster Med. 1999;14:222–231. [PubMed] [Google Scholar]
- Wallin BG. Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R. Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL. Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491:881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH. Crandall CG. Effect of thermal stress on Frank–Starling relations in humans. J Physiol. 2009;587:3383–3392. doi: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]


