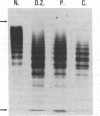
Abstract
Three preparations of purified von Willebrand factor (vWF), obtained from unrelated patients affected by type IIB von Willebrand disease, were found to have normal sialic acid content (between 129 and 170 nmol/mg of vWF, as compared with 158 +/- 17 nmol/mg in four normal preparations) and to induce platelet aggregation in the presence of physiologic levels of divalent cations and without addition of ristocetin. A monoclonal antibody that blocks the vWF binding domain of the platelet glycoprotein (GP)Ib caused complete inhibition of IIB vWF-induced aggregation. In contrast, a monoclonal antibody that blocks the receptor for adhesive proteins on the platelet GPIIb/IIIa complex failed to inhibit the initial response of platelets to high concentrations of IIB vWF. Moreover, IIB vWF caused agglutination of formalin-fixed platelets that was blocked only by the anti-GPIb antibody, suggesting that the binding of vWF to GPIb, even in the absence of ristocetin, results in platelet-platelet interaction that is followed by exposure of the GPIIb/IIIa receptors for adhesive proteins. Endogenous ADP, normally active platelet metabolism and fibrinogen binding to GPIIb/IIIa were necessary for maximal and irreversible platelet aggregation. In the absence of fibrinogen, however, aggregation was mediated by vWF binding to GPIIb/IIIa. A 52/48-kD tryptic fragment containing the GPIb binding domain of normal vWF completely blocked the aggregation induced by all three IIB vWF preparations. The present study defines in detail the mechanisms involved in IIB vWF-induced platelet aggregation. Moreover, it establishes that the GPIb binding domain of normal and IIB vWF are closely related and that desialylation is not required for the direct interaction of IIB vWF with GPIb.
Full text
PDF

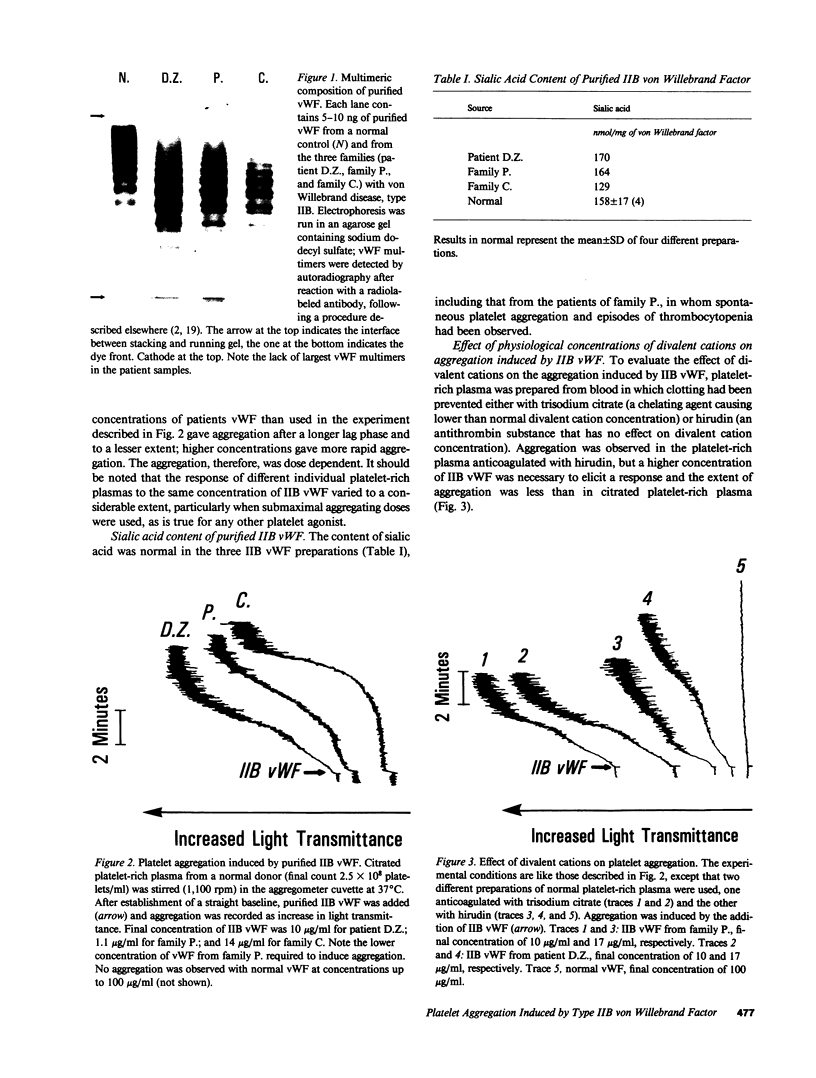
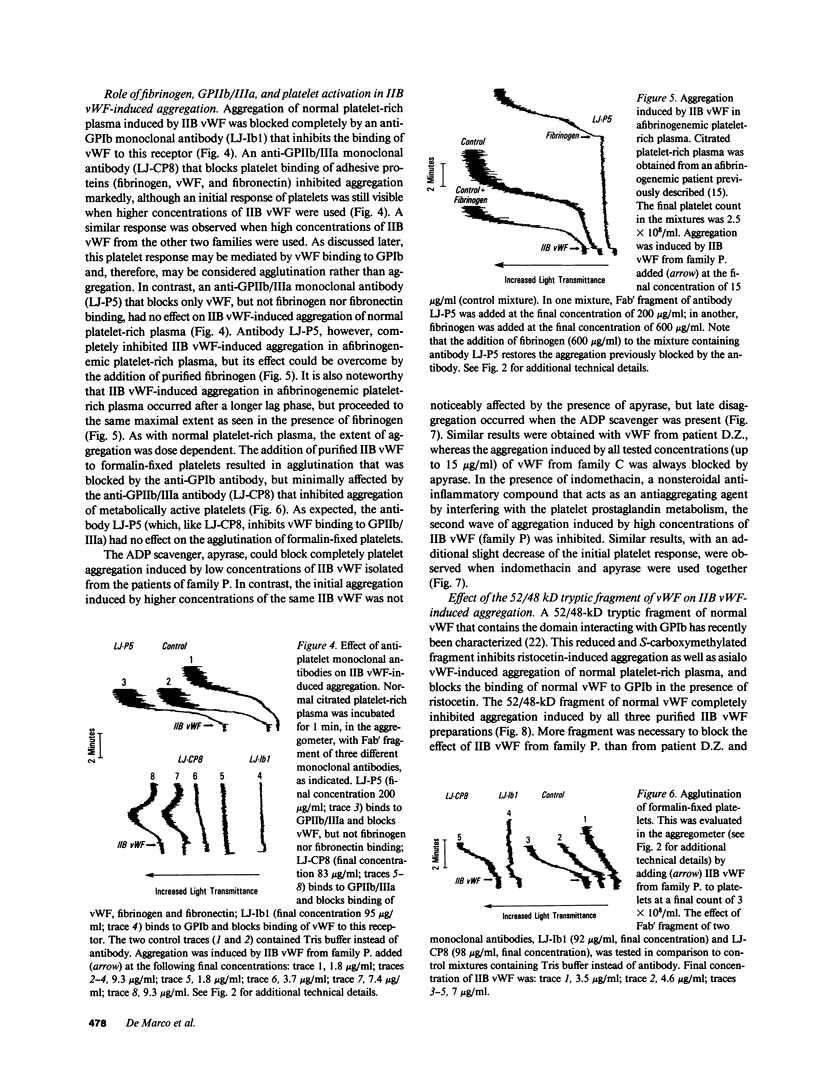
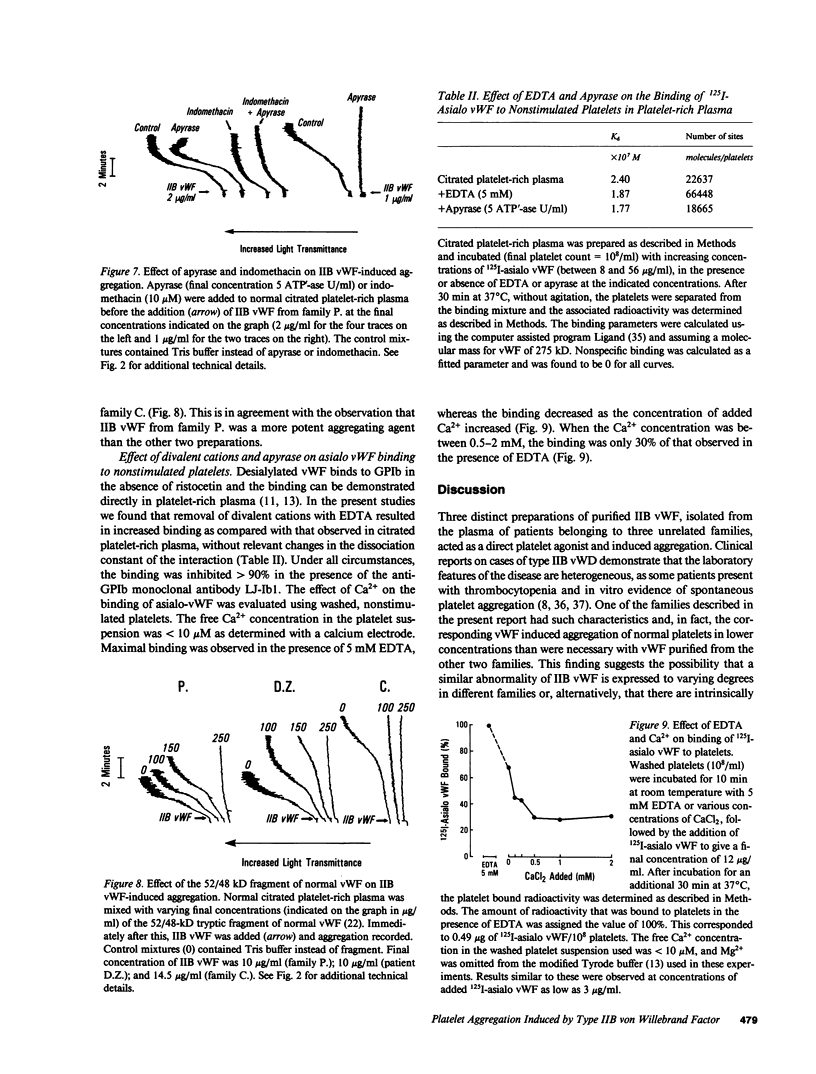



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz S. D., Dent J., Roberts J., Fujimura Y., Plow E. F., Titani K., Ruggeri Z. M., Zimmerman T. S. Epitope mapping of the von Willebrand factor subunit distinguishes fragments present in normal and type IIA von Willebrand disease from those generated by plasmin. J Clin Invest. 1987 Feb;79(2):524–531. doi: 10.1172/JCI112843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Chediak J., Telfer M. C., Vander Laan B., Maxey B., Cohen I. Cycles of agglutination-disagglutination induced by ristocetin in thrombasthenic platelets. Br J Haematol. 1979 Sep;43(1):113–126. doi: 10.1111/j.1365-2141.1979.tb03726.x. [DOI] [PubMed] [Google Scholar]
- De Marco L., Girolami A., Russell S., Ruggeri Z. M. Interaction of asialo von Willebrand factor with glycoprotein Ib induces fibrinogen binding to the glycoprotein IIb/IIIa complex and mediates platelet aggregation. J Clin Invest. 1985 Apr;75(4):1198–1203. doi: 10.1172/JCI111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Girolami A., Zimmerman T. S., Ruggeri Z. M. Interaction of purified type IIB von Willebrand factor with the platelet membrane glycoprotein Ib induces fibrinogen binding to the glycoprotein IIb/IIIa complex and initiates aggregation. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7424–7428. doi: 10.1073/pnas.82.21.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Girolami A., Zimmerman T. S., Ruggeri Z. M. von Willebrand factor interaction with the glycoprotein IIb/IIa complex. Its role in platelet function as demonstrated in patients with congenital afibrinogenemia. J Clin Invest. 1986 Apr;77(4):1272–1277. doi: 10.1172/JCI112430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Shapiro S. S. Properties of human asialo-factor VIII. A ristocetin-independent platelet-aggregating agent. J Clin Invest. 1981 Aug;68(2):321–328. doi: 10.1172/JCI110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Holland L. Z., Russell S. R., Roberts J. R., Elder J. H., Ruggeri Z. M., Zimmerman T. S. von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986 Jan 5;261(1):381–385. [PubMed] [Google Scholar]
- Fulcher C. A., Ruggeri Z. M., Zimmerman T. S. Isoelectric focusing of human von Willebrand factor in urea-agarose gels. Blood. 1983 Feb;61(2):304–310. [PubMed] [Google Scholar]
- Girolami A., De Marco L., Virgolini L., Peruffo R., Fabris F. Platelet adhesiveness and aggregation in congenital afibrinogenemia. An investigation of three patients with post-transfusion, cross-correction studies between two of them. Blut. 1975 Feb;30(2):87–100. doi: 10.1007/BF01633963. [DOI] [PubMed] [Google Scholar]
- Grainick H. R., Williams S. B., Coller B. S. Asialo von Willebrand factor interactions with platelets. Interdependence of glycoproteins Ib and IIb/IIIa for binding and aggregation. J Clin Invest. 1985 Jan;75(1):19–25. doi: 10.1172/JCI111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainick H. R., Williams S. B., McKeown L. P., Rick M. E., Maisonneuve P., Jenneau C., Sultan Y. Von Willebrand's disease with spontaneous platelet aggregation induced by an abnormal plasma von Willebrand factor. J Clin Invest. 1985 Oct;76(4):1522–1529. doi: 10.1172/JCI112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa M., Titani K., Holland L. Z., Roberts J. R., Ruggeri Z. M. The von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Characterization by monoclonal antibodies and partial amino acid sequence analysis of proteolytic fragments. J Biol Chem. 1986 Sep 25;261(27):12579–12585. [PubMed] [Google Scholar]
- Holmberg L., Kristoffersson A. C., Lamme S., Nilsson I. M., Awidi A., Solum N. O. Platelet--von Willebrand factor interactions in type IIB von Willebrand's disease. Scand J Haematol. 1985 Sep;35(3):305–314. doi: 10.1111/j.1600-0609.1985.tb01710.x. [DOI] [PubMed] [Google Scholar]
- Holmberg L., Nilsson I. M., Borge L., Gunnarsson M., Sjörin E. Platelet aggregation induced by 1-desamino-8-D-arginine vasopressin (DDAVP) in Type IIB von Willebrand's disease. N Engl J Med. 1983 Oct 6;309(14):816–821. doi: 10.1056/NEJM198310063091402. [DOI] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Packham M. A., Reimers H. J., Cazenave J. P., Mustard J. F. Mechanisms of platelet shape change, aggregation, and release induced by collagen, thrombin, or A23,187. J Lab Clin Med. 1977 Oct;90(4):707–719. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lombardo V. T., Hodson E., Roberts J. R., Kunicki T. J., Zimmerman T. S., Ruggeri Z. M. Independent modulation of von Willebrand factor and fibrinogen binding to the platelet membrane glycoprotein IIb/IIIa complex as demonstrated by monoclonal antibody. J Clin Invest. 1985 Nov;76(5):1950–1958. doi: 10.1172/JCI112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane D. E., Stibbe J., Kirby E. P., Zucker M. B., Grant R. A., McPherson J. Letter: A method for assaying von Willebrand factor (ristocetin cofactor). Thromb Diath Haemorrh. 1975 Sep 30;34(1):306–308. [PubMed] [Google Scholar]
- Miller J. L., Castella A. Platelet-type von Willebrand's disease: characterization of a new bleeding disorder. Blood. 1982 Sep;60(3):790–794. [PubMed] [Google Scholar]
- Miller J. L., Kupinski J. M., Castella A., Ruggeri Z. M. von Willebrand factor binds to platelets and induces aggregation in platelet-type but not type IIB von Willebrand disease. J Clin Invest. 1983 Nov;72(5):1532–1542. doi: 10.1172/JCI111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., DIXON D. J. EVIDENCE FOR LINKAGE OF UNIVALENT FRAGMENTS OR HALF-MOLECULES OF RABBIT GAMMA-GLOBULIN BY THE SAME DISULFIDE BOND. Biochemistry. 1964 Sep;3:1338–1342. doi: 10.1021/bi00897a025. [DOI] [PubMed] [Google Scholar]
- Palascak J. E., Martinez J. Dysfibrinogenemia associated with liver disease. J Clin Invest. 1977 Jul;60(1):89–95. doi: 10.1172/JCI108773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Lombardi R., Gatti L., Bader R., Valsecchi C., Zimmerman T. S. Type IIB von Willebrand's disease: differential clearance of endogenous versus transfused large multimer von willebrand factor. Blood. 1982 Dec;60(6):1453–1456. [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Bader R., Barbui T. Factor VIII-related properties in platelets from patients with von Willebrand's disease. J Lab Clin Med. 1978 Jan;91(1):132–140. [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Lombardi R., Federici A. B., Zimmerman T. S. Multimeric composition of factor VIII/von Willebrand factor following administration of DDAVP: implications for pathophysiology and therapy of von Willebrand's disease subtypes. Blood. 1982 Jun;59(6):1272–1278. [PubMed] [Google Scholar]
- Ruggeri Z. M., Pareti F. I., Mannucci P. M., Ciavarella N., Zimmerman T. S. Heightened interaction between platelets and factor VIII/von Willebrand factor in a new subtype of von Willebrand's disease. N Engl J Med. 1980 May 8;302(19):1047–1051. doi: 10.1056/NEJM198005083021902. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba H. I., Saba S. R., Dent J., Ruggeri Z. M., Zimmerman T. S. Type IIB Tampa: a variant of von Willebrand disease with chronic thrombocytopenia, circulating platelet aggregates, and spontaneous platelet aggregation. Blood. 1985 Aug;66(2):282–286. [PubMed] [Google Scholar]
- Takahashi H., Nagayama R., Hattori A., Ihzumi T., Tsukada T., Shibata A. Von Willebrand disease associated with familial thrombocytopenia and increased ristocetin-induced platelet aggregation. Am J Hematol. 1981;10(1):89–99. doi: 10.1002/ajh.2830100113. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Zirinis P. Platelet aggregation induced by arachidonic acid is accompanied by release of potential inflammatory mediators distinct from PGE2 and PGF2. Nat New Biol. 1973 Jul 25;244(134):114–116. doi: 10.1038/newbio244114a0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Walsh P. N., Mills D. C., White J. G. Metabolism and function of human platelets washed by albumin density gradient separation. Br J Haematol. 1977 Jun;36(2):287–296. doi: 10.1111/j.1365-2141.1977.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Meyer D., Rabinowitz R., Pietu G., Girma J. P., Vicic W. J., Rogers J. Pseudo-von Willebrand's disease. An intrinsic platelet defect with aggregation by unmodified human factor VIII/von Willebrand factor and enhanced adsorption of its high-molecular-weight multimers. N Engl J Med. 1982 Feb 11;306(6):326–333. doi: 10.1056/NEJM198202113060603. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Dent J. A., Ruggeri Z. M., Nannini L. H. Subunit composition of plasma von Willebrand factor. Cleavage is present in normal individuals, increased in IIA and IIB von Willebrand disease, but minimal in variants with aberrant structure of individual oligomers (types IIC, IID, and IIE). J Clin Invest. 1986 Mar;77(3):947–951. doi: 10.1172/JCI112394. [DOI] [PMC free article] [PubMed] [Google Scholar]