Abstract
Objective
To investigate whether water influx into cerebrospinal fluid (CSF) space is reduced in Alzheimer’s patients as previously shown in the transgenic mouse model for Alzheimer’s disease.
Methods
Ten normal young volunteers (young control, 21-30 years old), ten normal senior volunteers (senior control, 60-78 years old, MMSE ≥ 29), and ten Alzheimer’s disease (AD) patients (study group, 59-84 years old, MMSE: 13-19) participated in this study. All AD patients were diagnosed by neurologists specializing in dementia based on DSM-IV criteria. CSF dynamics were analyzed using positron emission tomography (PET) following an intravenous injection of 1,000 MBq [15O]H2O synthesized on-line.
Results
Water influx into CSF space in AD patients, expressed as influx ratio, (0.755 ± 0.089) was significantly reduced compared to young controls (1.357 ± 0.185; p < 0.001) and also compared to normal senior controls (0.981 ± 0.253, p < 0.05). Influx ratio in normal senior controls was significantly reduced compared to young controls (p < 0.01).
Conclusion
Water influx into the CSF is significantly reduced in AD patients. β-amyloid clearance has been shown to be dependent on interstitial flow and CSF production. The current study indicates that reduction in water influx into the CSF may disturb the clearance rate of β-amyloid, and therefore be linked to the pathogenesis of AD.
Trial Registration
UMIN Clinical Trials Registry UMIN000011939
Introduction
The brain lacks conventional lymphatics for clearing interstitial fluid of solutes not absorbed across capillaries. The cerebrospinal flow (CSF) system has long been suggested to play a role equivalent to the systemic lymphatic system. Nevertheless, the role of CSF as the brain’s lymphatic system has not received much attention. Recently, however, the classic CSF circulation theory where CSF is almost exclusively produced by choroid plexus is found to be incomplete. It is now understood that influx from the peri-capillary (Virchow-Robin) space into the CSF system, classically referred to as interstitial flow, plays a major role in CSF production [1–3]. Furthermore, a number of studies have now shown that this interstitial flow plays a critical role in the clearance of β-amyloid [4–8]. Prealbumin (transthyretin) in the CSF has been identified to play the role of chaperon to β-amyloid, and prevents β-amyloid's natural tendency to aggregate and form plaques [9]. It is highly conceivable, therefore, that disturbance of interstitial flow may play a significant role in the pathogenesis of senile plaque (SP) formation.
Water homeostasis of the Virchow-Robin space and, therefore, interstitial flow is regulated by aquaporin-4 (AQP-4) [2,3,10], an isoform of water channels abundant in the brain. Using JJ vicinal coupling proton exchange (JJVCPE) imaging [11,12], a novel non-invasive magnetic resonance imaging (MRI) method capable of tracing exogenously applied substrates in a manner similar to positron emission tomography (PET), we had previously demonstrated that water influx into the CSF system is significantly reduced in senile plaque bearing transgenic AD model mice to the extent similar to AQP-4 knockout mice [12,13]. Using PET, we confirmed in this study that water influx into CSF is also reduced in AD patients.
Materials and Methods
Subjects
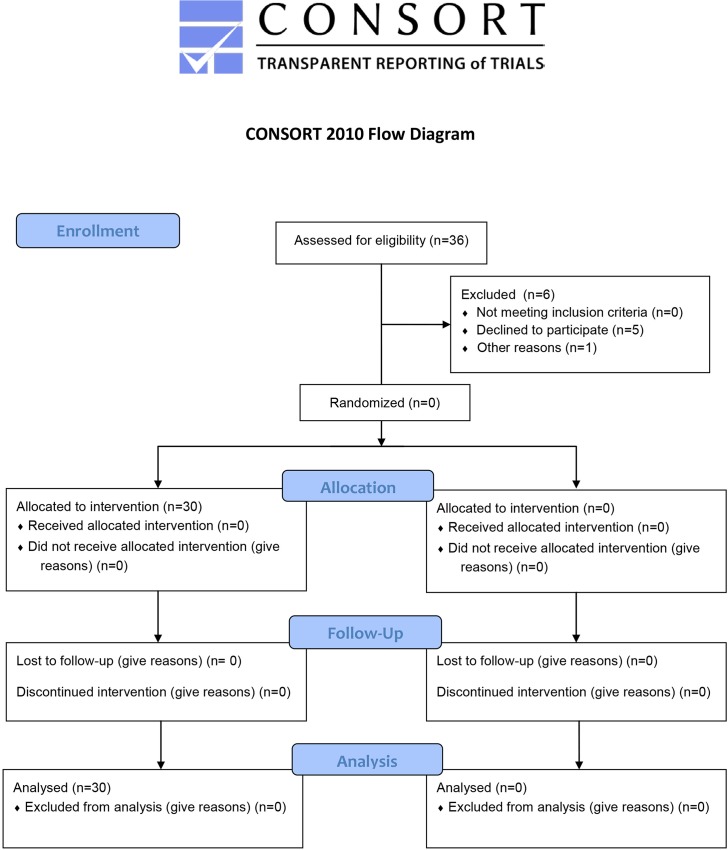
Ten normal young volunteers (10 males, ages 21–30 years), ten normal senior volunteers (7 males and 3 females, ages 60–78 years), and ten Alzheimer’s disease (AD) patients (6 males and 4 females, ages 59–84 years) participated in this study. Participant recruitment was completed between October 1, 2013, and April 4, 2014 (Fig 1). All volunteers were free of any significant medical conditions such as hypertension, diabetes, chronic pulmonary diseases, and were not taking any prescribed, over the counter, or herbal medications. AD patients were recruited from the Memory Clinic, Niigata University Hospital. The diagnosis of AD was determined based on DSM-IV criteria by neurologists specializing in dementia. The neurologists further determined whether the potential subject had decision making capacity to consent for the study. Age-matched senior volunteers were assessed to have no functional and no cognitive impairment (Mini-Mental State Examination (MMSE) score ≥29), and had no neurological disease. In compliance with the Institutional Review Board of University of Niigata, the study protocol was explained in detail to all potential subjects, or their proxy where appropriate. Written informed consent was obtained from all subjects or, where the Alzheimer’s subject was deemed to have no decision making capacity to consent for the study, by his/her proxy. All imaging studies were performed between October 2013 and April 2014. The study was approved by the Ethics Review Board of the Internal Review Board of University of Niigata. The project was registered at the UMIN Clinical Trials Registry as UMIN000011939 (http://www.umin.ac.jp/ctr/index.htm). Participants were provided with contact names and telephone numbers in the event of any adverse event related to the study. All participants were followed up within one month of the study to further confirm the absence or occurrence of adverse events related to the study, not otherwise self-reported by the study participant or proxy to the study coordinator. Alzheimer’s patients are additionally followed up annually at the Memory Clinic.
Fig 1. Consort Flow Diagram providing details of participant enrolment.
PET imaging
PET imaging was performed using a combined PET/CT scanner (Discovery ST Elite, GE Healthcare, Schenectady NY, USA) with a 15 cm field of view (FOV) positioned in the region of the cerebrum. To correct for photon attenuation, low-dose CT imaging was acquired in helical mode. The subject’s head rested on a foam cushioned headrest. A head strap was applied to minimize head movement.
1000 MBq [15O]H2O, synthesized on-line ([15O]CO2 + 4H2 → 2[15O]H2O + CH4) was injected intravenously into an antecubital vein via an automatic water injection system (AM WR01, JFE Technos, Yokohama, Japan). The system delivered a 10 ml bolus over 10 seconds at 1 ml/sec with both pre- and post- flush of an inert saline solution. Immediately after starting the administration, emission data were acquired over 20 minutes in three-dimensional list mode with a 25.6 cm axial FOV and sorted into 40 time frames (40 × 30 seconds).
All emission scans were normalized for detector inhomogeneity and corrected for random coincidences, dead time, scattered radiation, and photon attenuation. For optimization of image quality, the 40 frames of the dynamic emission scans were reconstructed using 3D-OSEM (Ordered-Subset Expectation Maximization) with 2 iterations and 28 subsets. The resultant image quality allowed manual identification of regions of interest (ROIs). For the reconstruction algorithms, the data were collected in a 128 × 128 × 47 matrix with a voxel size of 2.0 × 2.0 × 3.27mm.
Data Analysis
The CT and PET image data were transferred to a Xeleris 1.1 workstation (GE Healthcare) for PET data analysis. Manually defined ROIs on the attenuation corrected axial images and CT images (lateral and third ventricle, cortex of occipital lobe) were used to obtain the time-activity data of the scans of each subject. The tissue activity concentration in each ROI was expressed as the standardized uptake value (SUV, g/ml), corrected for the subject’s body weight and administrated dose of radioactivity. Each tissue time activity concentration was determined by fitting the data using the following equation:
where y0 is the baseline SUV. Subsequently, the ratio between SUVs of ventricle and cortex was defined as the influx ratio. The numerical data were subjected to the Mann-Whitney-Wilcoxon rank sum test for group analysis using IBM SPSS version 19.5 (IBM Corporation, Armonk, NY, USA).
Results
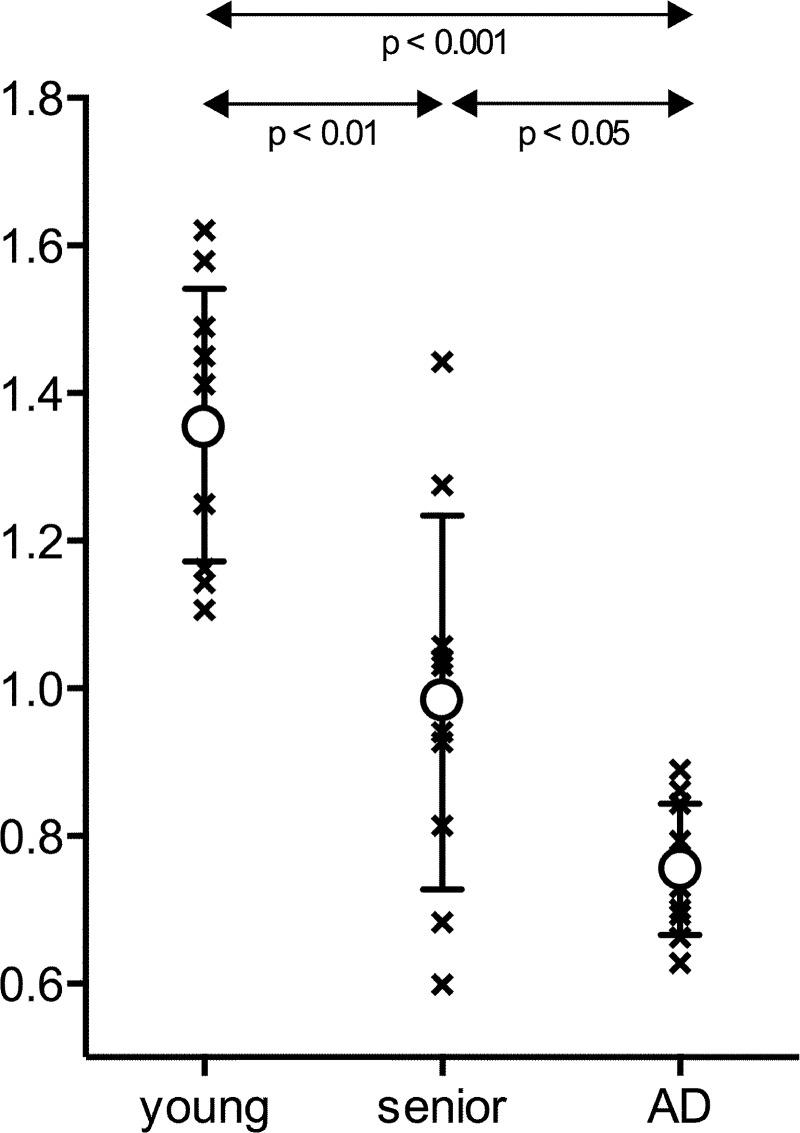
Results are shown in Table 1 and summarized in Fig 2. Water influx into CSF space in AD patients, expressed as influx ratio (0.755 ± 0.089), was significantly reduced compared to young controls (1.357 ± 0.185, p < 0.001) and senior controls (0.981 ± 0.253, p < 0.05). Furthermore, the influx ratio of senior controls compared to that of young controls was significantly reduced (p < 0.01) as well. The observed large range of influx ratio (0.599–1.442) in the senior controls suggested that the reduction in water influx into CSF represented an aging process.
Table 1.
| Age | Influx Ratio | |
|---|---|---|
| Young | 21 | 1.10620 |
| 21 | 1.35608 | |
| 21 | 1.45009 | |
| 21 | 1.14260 | |
| 22 | 1.16180 | |
| 22 | 1.62079 | |
| 22 | 1.24990 | |
| 24 | 1.41213 | |
| 24 | 1.48959 | |
| 30 | 1.57873 | |
| Senior | 60 | 0.68299 |
| 63 | 0.92728 | |
| 63 | 1.03987 | |
| 63 | 1.05720 | |
| 65 | 1.44195 | |
| 68 | 0.94050 | |
| 68 | 1.27526 | |
| 69 | 0.81396 | |
| 74 | 1.03050 | |
| 78 | 0.59892 | |
| AD | 59 | 0.69261 |
| 61 | 0.79321 | |
| 63 | 0.86105 | |
| 63 | 0.62796 | |
| 71 | 0.70088 | |
| 71 | 0.73065 | |
| 73 | 0.74510 | |
| 79 | 0.66232 | |
| 80 | 0.84389 | |
| 84 | 0.88935 |
Fig 2. Schematic presentation of the results with mean (circle) and standard deviation (bar).
Water influx into CSF space is expressed as influx ratio (IR): the ratio between the standardized uptake value (SUV, g/ml) of the ventricle to that of cortex. IR in Alzheimer’s disease patients (AD) is significantly reduced compared to both young controls (p < 0.001) and senior controls (p < 0.05), Mann-Whitney-Wilcoxon rank sum test. Note that there is no overlap in data points between AD and young controls. Reduction of influx ratio in senior controls compared to that in young control is found to be significant (p < 0.01) as well. A large range of influx ratio in senior controls suggests that the observed reduction likely represents one of the aging processes.
Discussion
Fluid-filled canals surrounding perforating arteries and veins in the brain parenchyma were recognized in the early era of modern medicine. They became known as the Virchow Robin space, so named after the first two scientists who described the structure in detail, namely, Rudolph Virchow and Charles Philippe Robin [14,15]. It was soon recognized that fluid in the Virchow Robin space may play a role similar to systemic lymphatics [4–8,16]. Studies based on modern technologies disclosed some of the physiological roles of the Virchow Robin space and interstitial fluid flow, ranging from the regulation of regional blood flow to β-amyloid clearance during sleep [17–19].
The unique anatomical features of the brain’s vascular system ensure a properly functioning blood brain barrier (BBB) and are a result of modification of the systemic circulation system. A critical property of the BBB is prevention of free water permeability across capillary walls (tight endothelium). This is in strong contrast to systemic capillaries which possess a leaky endothelium. The molecular basis of the tight endothelium is the formation of tight junctions. Various tight junction proteins such as claudin and occludin play the role of “gating control” of paracellular transport. Passive water movement across brain capillaries is strongly restricted by these tight junctions. In systemic capillaries, active water transport across endothelial cells is accomplished by the water channel aquaporin 1 (AQP-1). In brain capillaries, such water movement is totally abolished through the active suppression of AQP-1 [20]. Accordingly, water motion between capillary lumen into the peri-capillary Virchow Robin space is highly limited to restricted passive movement.
In contrast to the above situations, the peri-capillary Virchow Robin space receives constant water influx through AQP-4, another isoform of the aquaporin family, abundantly expressed in the perivascular endfeet of astrocytes [21]. Virchow Robin space water homeostasis and, hence, interstitial flow is primarily dependent on water influx through AQP-4 [2,3,10]. Accordingly, brain interstitial flow, which plays a role equivalent to the systemic lymphatic system, is now considered to be an AQP-4 dependent system [13]. The basic function of lymphatics is drainage of cellular debris that has been subjected to molecular scrutiny before being returned to venous circulation. β-amyloid has been shown to be essential for synaptic formation [22]. Disturbance in the proper clearance of β-amyloid, and negative balance between its clearance and production has been implicated to play a significant role in senile plaque formation and ultimately the pathogenesis of Alzheimer’s disease. The various components of amyloid homeostasis have become a target of therapeutic strategies [23–25]. Since under physiological conditions interstitial flow plays a critical role in the clearance of β-amyloid [4–8], disturbance in AQP-4 functionality and resultant reduction in interstitial flow could cause significant β-amyloid accumulation. Transgenic mice lacking endothelial cell expression of Agrin are shown to have reduced AQP4 while BBB remained intact. These mice have significant accumulation of β-amyloid[26].
Although it is difficult to determine whether the observed reduction in water influx into the CSF system in AD patients is the primary abnormality or merely related to β-amyloid deposits in the brain, the clear cut differences between young normal volunteers and AD patients unequivocally indicated that AD patients have significant reduction in interstitial flow. Furthermore, the theoretical possibility of contribution by choroid plexus or the leptomeningeal vasculature to the water detected in the CSF compartment cannot be totally excluded. Nevertheless, the observed large range of influx ratios in senior controls without cognitive dysfunction, strongly suggests that reduction of water influx into the CSF itself is not a sufficient factor for the pathogenesis of AD, a situation similar to senile plaque formation. There must be additional factors that eventually lead to neural death and cortical dysfunction.
Drainage of β-amyloid by interstitial flow through the Virchow Robin space into CSF is essential for maintaining proper homeostasis between β-amyloid production and clearance. The balance between β-amyloid production and its clearance appears to be vital for maintaining proper neural function. Disruption in β-amyloid homeostasis may play a critical role in the pathogenesis of senile plaque formation, and, hence, the development of Alzheimer’s disease. Assessment of the dynamic indices of production and clearance of β-amyloid in various cohort groups, including mild cognitive impairment (MCI), is warranted.
Supporting Information
(PDF)
Acknowledgments
The study in part was presented at the 66th AAN Annual Meeting of the American Academy of Neurology, April 2014, Philadelphia, USA and Alzheimer’s Imaging Consortium, July 2014, Copenhagen, Denmark.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology (Japan) (TN).
References
- 1. Orešković D, Klarica M (2010) The formation of cerebrospinal fluid: Nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev 64: 241–262. 10.1016/j.brainresrev.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 2. Igarashi H, Tsujita M, Kwee IL, Nakada T (2014) Water Influx into Cerebrospinal Fluid (CSF) is Primarily Controlled by Aquaporin-4, not by Aquaporin-1: O-17 JJVCPE MRI Study in Knockout Mice. Neuroreport 25: 39–43. 10.1097/WNR.0000000000000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haj-Yasein NN, Jensen V, Østby I, Omholt SW, Voipio J, Kaila K, et al. (2012) Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: A gene deletion study in mouse hippocampus. Glia 60: 867–874. 10.1002/glia.22319 [DOI] [PubMed] [Google Scholar]
- 4. Johnston M, Papaiconomou C (2002) Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci 17: 227–230 [DOI] [PubMed] [Google Scholar]
- 5. Abott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45: 545–552 [DOI] [PubMed] [Google Scholar]
- 6. Weller RO, Djuanda E, Yow HY, Carare RO (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117: 1–14 10.1007/s00401-008-0457-0 [DOI] [PubMed] [Google Scholar]
- 7. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weller RO (1998) Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J Neuropathol Exp Neurol 57: 885–894 [DOI] [PubMed] [Google Scholar]
- 9. Xinyi L, Buxbaum JN (2011) Transthyretin and the brain re-visited: Is neuronal synthesis of transthyretin protective in Alzheimer’s disease? Mol Neurodegener 6: 79 10.1186/1750-1326-6-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitaura H, Tsujita M, Huber VJ, Kakita A, Shibuki K, Sakimura K, et al. (2009) Activity-dependent glial swelling is impaired in aquaporin-4 knockout mice. Neurosci Res 64: 208–212. 10.1016/j.neures.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki K, Igarashi H, Huber VJ, Kitaura H, Kwee IL, et al. (In Press) Ligand based molecular MRI: O-17 JJVCPE amyloid imaging in transgenic mice. J. Neuroimag 2014;24:595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Igarashi H, Suzuki Y, Kwee IL, Nakada T (2014) Water Influx into cerebrospinal fluid is significantly reduced in senile plaque bearing transgenic mice, supporting β-Amyloid clearance hypothesis of Alzheimer’s disease. Neurol Res 2014;36:1094–1098 10.1179/1743132814Y.0000000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakada T (2014) Review: Virchow-Robin space and aquaporin-4: New insights on an old friend. Croatian Med J 55: 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virchow R (1851) Ueber die Erweiterung kleinerer Gefaesse. Arch Pathol Anat Physiol Klin Med 3: 427–462 [Google Scholar]
- 15. Robin C (1859) Recherches sur quelques particularites de la structure des capillaires de l’encephale. J Physiol Homme Animaux 2: 537–548 [Google Scholar]
- 16. Esiri MM, Gay D (1990) Immunological and neuropathological significance of the Virchow-Robin space. J Neurol Sci 100: 3–8 [DOI] [PubMed] [Google Scholar]
- 17.Nakada T (2015) The molecular mechanisms of neural flow coupling: A new concept. J Neuroimg 2015 Feb 20. 10.1111/jon.12219 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 18. Lulu X, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. (2013) Sleep drives metabolite clearance from the adult brain. Science 342: 373–377 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Igarashi H, Tsujita M, Huber VJ, Kwee IL, Nakada T (2013) Inhibition of Aquaporin-4 significantly increases regional cerebral blood flow. NeuroReport 24: 324–328 10.1097/WNR.0b013e32835fc827 [DOI] [PubMed] [Google Scholar]
- 20. Dolman D, Drndarski S, Abbott NJ, Rattray M (2005) Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J Neurochem 93: 825–833 [DOI] [PubMed] [Google Scholar]
- 21. Huber VJ, Tsujita M, Nakada T (2012) Aquaporins in drug discovery and pharmacotherapy. Mol Aspects Med 33: 691–703 10.1016/j.mam.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 22. Parihar MS, Brewer GJ (2010) Amyloid-β as a modulator of synaptic plasticity. J Alzheimer’s Dis 22: 741–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y-J, Zhou H-D, Zhou X-F (206) Clearance of amyloid-beta in Alzheimer's disease: progress, problems and perspectives. Drug Discovery Today 11:931–938 [DOI] [PubMed] [Google Scholar]
- 24. Muthuraj B, Hussaina S, Iyer PK (2013) A rapid and sensitive detection of ferritin at a nanomolar level and disruption of amyloid β fibrils using fluorescent conjugated polymer. Polym Chem 4:5096–5107 [Google Scholar]
- 25. Deane R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of Amyloid-β peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8:16–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rauch SM, Huen K, Miller MC, Chaudry H, Lau M, Sanes JR, et al. (2011) Changes in brain β-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J Neuropathol Exp Neurol 70: 1124–1137 10.1097/NEN.0b013e31823b0b12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.