A study shows customized order-entry sets for antiretroviral therapy to manage human immunodeficiency virus reduced the potential for prescribing incorrect regimens and may be useful when HIV-specific medication reconciliation is unavailable.
Keywords: ARV, HIV, medication errors, antimicrobial stewardship, computerized order entry
Abstract
Background:
Patients with human immunodeficiency virus (HIV) infection on antiretroviral (ARV) therapy are at increased risk for medication errors during transitions of care between the outpatient and inpatient settings. This can lead to treatment failure or toxicity. Previous studies have emphasized the prevalence of medication errors in such patients, but few have reported initiatives to prevent errors from occurring.
Methods:
The study was conducted in a 1,400-bed health care center with a state-designated Acquired Immunodeficiency Syndrome (AIDS) Center in the Bronx, New York. The antimicrobial stewardship team and HIV specialists developed customized order-entry sets (COES) to guide ARV prescribing and retrospectively reviewed their effect on error rates of initial ARV orders for inpatients before reconciliation. Patient records were reviewed in six-month periods before and after intervention. The student’s t-test or Mann–Whitney U test was used to compare continuous variables; chi-square or Fisher’s exact test was used for categorical variables.
Results:
A total of 723 and 661 admissions were included in the pre-intervention and post-intervention periods, respectively. Overall, error rates decreased by 35% (38.0% to 24.8%, P < 0.01) with COES. Wrong doses and drug interactions decreased by more than 40% (P < 0.005). Error reductions were observed in protease inhibitor (PI)-based (43.6% versus 28.7%, P < 0.01) and non–PI-based (38.0% versus 24.4%, P = 0.02) regimens with COES. A shift in predominant drug-class errors was observed as there was a trend toward increased usage of non-PI regimens post-intervention. Admission in the pre-intervention period (adjusted odds ratio [AOR], 1.79; 95% confidence interval [CI], 1.39–2.31) and use of PI-based regimens (AOR, 2.03; 95% CI, 1.53–2.70) remained significantly associated with ARV prescribing errors after controlling for confounding factors.
Conclusion:
Detailed COES improved ARV prescribing habits, reduced the potential for prescribing incorrect regimens, and can prove useful and cost-effective where HIV-specific medication reconciliation is unavailable.
INTRODUCTION
At least 1.5 million preventable adverse drug events occur each year in the U.S. because of medication errors.1 Several studies have found that many of these occur during the transition of care between the outpatient and inpatient settings.2–5 Individuals infected with human immunodeficiency virus (HIV) are particularly vulnerable during periods of transition.6 Prescribing errors involving patients receiving antiretroviral (ARV) therapy are significantly higher than rates reported in individuals without HIV infection.3,4,7 It is estimated that more than 84% of hospitalized patients with HIV infection may experience an ARV-related error.7 The etiology of these errors is often multifactorial and includes lack of provider knowledge or familiarity with the disease state,1 complexity of regimens, and frequent regimen revisions. The widespread use of potent combination ARV therapy has reduced hospitalizations for opportunistic infections and other HIV-related conditions,8 has increased life expectancy for those with HIV infections, and has resulted in an increase in hospitalizations for other comorbid medical problems. Increasingly, medical staff members without formal training in HIV management or infectious diseases have been providing inpatient care to HIV patients. In addition, comorbidities may increase the potential for clinically significant drug interactions and polypharmacy.9 ARV prescribing errors may lead to treatment failure, development of resistance, and suboptimal dosing or drug toxicity. It is therefore crucial that these patients’ therapies are appropriately continued during their hospitalizations.
Many studies have emphasized the prevalence of medication errors in hospitalized patients with HIV infection.1–4,7,10–26 Frequently cited errors include medication omission, dosing errors, incorrect scheduling, and drug interactions; errors are associated with all major drug classes, including nucleos(t)ide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).
In our institution, monthly in-service training sessions for house staff, physician assistants, and pharmacists were conducted along with the provision of reference materials. An ARV medication chart was developed, which included routine doses; interactions of commonly used medications as well as other ARV medications; weight and renal dosing recommendations; and contraindications for use of other medications. Pop-up alerts in the computerized prescriber order-entry system (CPOE; CareCast version 3.04) were added to remind ordering prescribers of the need for complete regimens, deferring ordering until correct regimens could be confirmed. ARV orders are routinely reviewed by the AIDS Center nurse practitioner and hospital pharmacy staff members, who alert prescribing staff to the need for order corrections. However, errors for ARV orders can still occur.
The antimicrobial stewardship team and HIV specialists determined that an enhanced system would be necessary to prevent ARV errors before they actually occur. In 2011, customized order-entry sets (COES) for ARV medications were developed and implemented in our institution to further prevent ARV-related errors prospectively in addition to CPOE and periodic educational in-service sessions (Figure 1). CPOE with COES prevents prescribers from ordering incomplete regimens, provides dosing guidance based on renal function and concomitant drug therapy, and flags contraindicated drug interactions prior to the commission of potential ARV errors in real time. The objective of this study was to evaluate the effect of CPOE with COES on error rates of initial ARV orders for inpatients with HIV infection prior to reconciliation of orders by HIV clinical specialists and pharmacists.
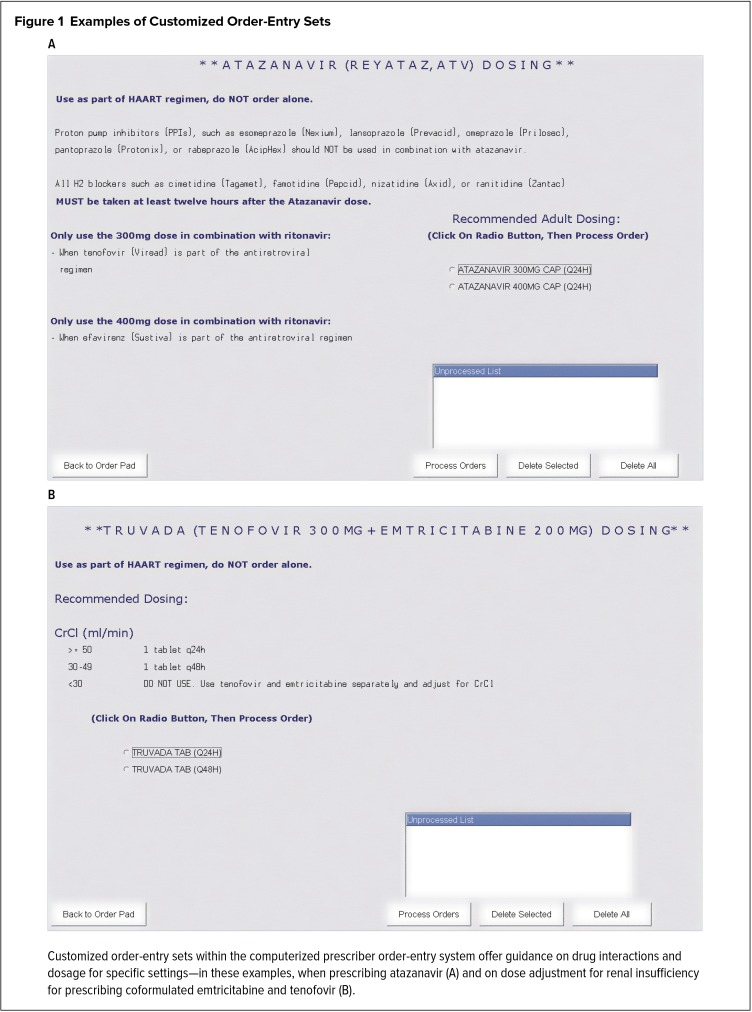
Figure 1.
Examples of Customized Order-Entry Sets
Customized order-entry sets within the computerized prescriber order-entry system offer guidance on drug interactions and dosage for specific settings—in these examples, when prescribing atazanavir (A) and on dose adjustment for renal insufficiency for prescribing coformulated emtricitabine and tenofovir (B).
METHODS
Study Population and Setting
Our institution is a 1,400-bed academic health care system in the Bronx, New York, with more than 90,000 inpatient admissions annually, of which more than 1,700 are for HIV-infected individuals. In a Bronx population of nearly 1.4 million people, more than 23,000 are living with HIV.27 The system is one of the few regional hospitals with a dedicated team consisting of a medical director, a nurse practitioner, and social workers who monitor the care of any patient admitted with the diagnosis of HIV. Our institution does not maintain a dedicated HIV patient-care unit; rather, patients are cared for throughout the hospital on units appropriate to their medical or surgical needs.
The study was approved by the Montefiore Medical Center/Albert Einstein College of Medicine institutional review board.
Data Collection
The time frames reviewed were September 2010 to February 2011 (a six-month period before implementation of the COES in August 2011) and September 2011 to February 2012. The charts of all hospitalized adult patients (older than 18 years of age) for whom ARVs were ordered were retrospectively reviewed. Patients without HIV infection who were prescribed lamivudine or tenofovir for hepatitis B infection were excluded. Patient characteristics obtained included age, sex, race, medical service, HIV viral load, CD4 cell count, creatinine clearance (estimated based on the Cockcroft–Gault equation), ARV regimens initially prescribed, and medications with the potential for drug–drug interactions. The CD4 cell counts and HIV viral load results used in this study were those values within six months of and closest to the dates of admission.
ARV regimens were classified as consisting of a single-formulation preparation (e.g., emtricitabine/tenofovir/efavirenz [Atripla, Gilead Sciences]), protease inhibitor (PI)-based, or non–PI-based. Prescribing errors were categorized as incomplete regimens, incorrect ARV drug, incorrect dosing of at least one ARV drug, incorrect scheduling frequency, incorrect dosing of at least one drug for renal insufficiency, and regimens that included an agent with interaction potential with another prescribed drug.
Prescribing errors were enumerated differently based on analysis parameters. For determination of admissions with any ARV errors, one or more prescribing error(s) within an ARV regimen was tallied as one admission with ARV prescribing error(s). For determination of a specific type of error within an ARV regimen, one or more error(s) of a specific type (e.g., wrong dose) was counted as one type-specific error against a regimen. Finally, for determination of a specific type of error based on drug classes, each error was tallied individually.
Analysis
Chi-square and Fisher’s exact tests as appropriate were used to assess associations between categorical variables; the Mann–Whitney test was used to compare continuous variables among groups. Multivariate logistic regression analysis was performed to determine variables independently associated with ARV prescribing errors. Variables with a P value of less than 0.20 on univariate analysis, intervention period, and internal medicine as admitting service were included in the logistic regression model. A two-tailed P value of less than 0.05 represented statistical significance for all statistical comparisons. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
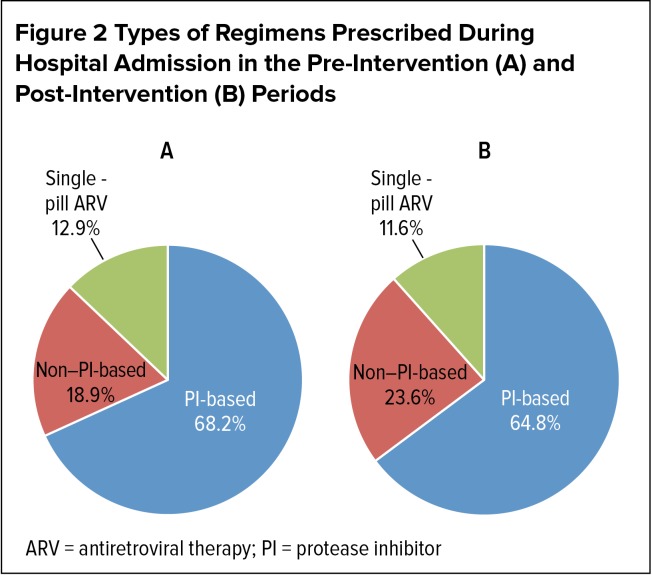
A total of 1,486 and 1,316 admissions involved patients with a diagnosis of HIV during the pre-intervention and post-intervention periods, respectively. Among these hospitalizations, ARVs were prescribed in 723 admissions involving 519 patients in the pre-intervention period and 661 admissions involving 489 patients in the post-intervention period. Patient demographics for the two study periods were comparable (Table 1). The median age of the study population was approximately 50 years; approximately 46% of the patients were female. Nearly three-quarters of the study population identified themselves as either African-American or multiracial. The majority of study patients (more than 70%) were admitted to an internal medicine service, while relatively fewer patients were admitted to a surgical service or critical-care unit. Forty-nine percent and 54% of patients had undetectable viral loads (40 copies/mL or less) during the pre-intervention and post-intervention periods, respectively. CD4 counts were more than 200 cells/mcL in approximately 60% of patients and more than 500 cells/mcL in more than 25% of patients. Types of regimens prescribed during hospital admissions in the pre-intervention and post-intervention periods were similar; PI-based regimens were the most commonly prescribed during both study periods (Table 1, Figure 2).
Table 1.
Demographics of Hospitalized Patients With Human Immunodeficiency Virus Infection on Antiretroviral Therapy During the Pre-Intervention and Post-Intervention Periods
| Characteristics | Pre-Intervention Period (n = 723) | Post-Intervention Period (n = 661) | P Values |
|---|---|---|---|
| Median age, years (range) | 51 (18–88) | 52 (22–93) | 0.78 |
| Female, n (%) | 333 (46.1) | 315 (47.7) | 0.55 |
| Race, n (%) | 0.17 | ||
| African-American | 318 (44.0) | 333 (50.4) | |
| Multiracial | 218 (30.2) | 178 (26.9) | |
| Hispanic | 63 (8.7) | 50 (7.6) | |
| Caucasian | 62 (8.6) | 56 (8.5) | |
| Other | 62 (8.6) | 44 (6.7) | |
| Admitting service, n (%) | 0.32 | ||
| Internal medicine | 528 (73.0) | 486 (73.5) | |
| Surgery | 50 (6.9) | 43 (6.5) | |
| Critical care | 2 (0.3) | 7 (1.1) | |
| Other* | 143 (19.8) | 125 (18.9) | |
| Median length of stay, days (range) | 5 (1–135) | 5 (1–126) | 0.41 |
| Viral load > 40 copies/mL, n (%) | 312/616 (50.7) | 272/592 (46.0) | 0.10 |
| CD4 count < 200 cells/mcL, n (%) | 257/671 (38.3) | 230/623 (36.9) | 0.61 |
| PI-based ARV regimen | 493 (68.2) | 428 (64.8) | 0.10 |
“Other” includes services such as family medicine, oncology, etc.
ARV = antiretroviral; PI = protease inhibitor
Figure 2.
Types of Regimens Prescribed During Hospital Admission in the Pre-Intervention (A) and Post-Intervention (B) Periods
ARV = antiretroviral therapy; PI = protease inhibitor
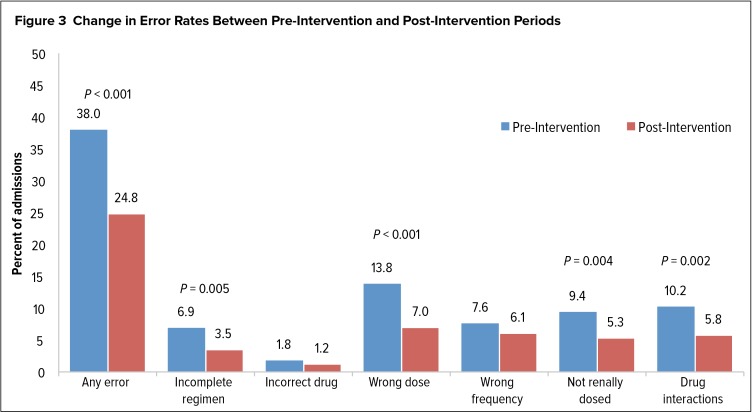
Prior to the initiation of COES, ARV prescribing errors of any type were observed in 275 admissions (38.0%). Use of COES, along with prescriber education, reduced ARV prescribing errors in 164 admissions (24.8%), representing an error reduction rate of 34.7% (P < 0.01). The most commonly observed errors were use of an incorrect ARV dose (13.8%) and drug interaction with an ARV (10.2%) during the pre-intervention period. With the use of COES, reductions of 49% in wrong dose and 43% in drug-interaction errors were observed. COES, however, did not significantly reduce errors of prescribing the incorrect ARV (1.8% pre-intervention versus 1.2% post-intervention, P = 0.39) or wrong frequency (7.6% versus 6.1%, P = 0.29). Figure 3 further details changes in error rates between the pre-intervention and post-intervention periods.
Figure 3.
Change in Error Rates Between Pre-Intervention and Post-Intervention Periods
When stratified by regimen type, significant error reductions in PI-based regimens (43.6% versus 28.7%, P < 0.01) and non–PI-based regimens (38.0% versus 24.4%, P = 0.02) were observed with use of COES. A greater than 50% reduction in single-pill combination ARV errors was also observed, albeit not statistically significant due to the small number of patients on this type of therapy (8.6% versus 3.9%, P = 0.35).
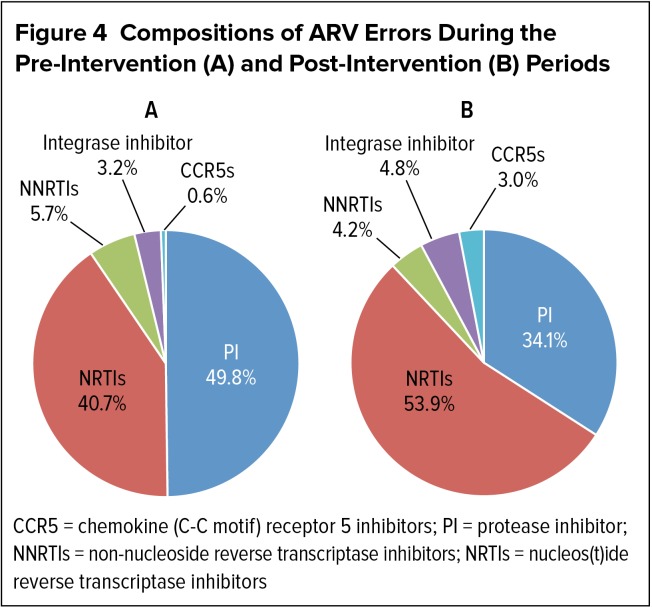
When stratified by drug class, a change in the composition of prescribing errors was observed between the two study periods (Figure 4). Prior to the introduction of COES, PI errors were the most common among the five ARV classes, accounting for 49.8% of 319 errors. Errors involving NRTIs were the next most common at 40.7%. With the use of COES, NRTI prescribing errors became the most common (53.9% of 167 errors), followed by PI errors (34.1%). The percentage of errors involving the remaining three classes of ARVs was not significantly different with or without implementation of COES.
Figure 4.
Compositions of ARV Errors During the Pre-Intervention (A) and Post-Intervention (B) Periods
CCR5 = chemokine (C-C motif) receptor 5 inhibitors; PI = protease inhibitor; NNRTIs = non-nucleoside reverse transcriptase inhibitors; NRTIs = nucleos(t)ide reverse transcriptase inhibitors
Closer examination of different types of errors within each drug class revealed wrong dose/frequency and drug-interaction errors with protease inhibitors were significantly reduced after the introduction of COES (Table 2). Errors related to NRTI dose adjustment due to renal dysfunction also significantly declined. Prescribing errors associated with NNRTIs, chemokine (C-C motif) receptor 5 (CCR5) inhibitors, and integrase inhibitors did not change significantly between the two study periods, most likely because of the small number of patients using these drug classes.
Table 2.
Prescribing Errors During Pre-Intervention and Post-Intervention Periods Stratified by Pharmacological Class
| Characteristics | Pre-Intervention Period (n = 723)a | Post-Intervention Period (n = 661)a | % Change | P Values |
|---|---|---|---|---|
| NRTI errors | ||||
| Wrong dose | 34 (4.7) | 26 (3.9) | −17.0 | 0.51 |
| Wrong frequency | 21 (2.9) | 27 (4.1) | +41.4 | 0.31 |
| Not renally dosed | 68 (9.4) | 35 (5.3) | −43.6 | < 0.01b |
| Drug interaction | 6 (0.8) | 2 (0.3) | −62.5 | 0.29 |
| NNRTI errors | ||||
| Wrong dose | 9 (1.2) | 2 (0.3) | −75.0 | 0.07 |
| Wrong frequency | 4 (0.6) | 4 (0.6) | 0.0 | 1.00 |
| Drug interaction | 5 (0.7) | 1 (0.2) | −71.4 | 0.22 |
| PI errors | ||||
| Wrong dose | 65 (9.0) | 16 (2.4) | −73.3 | < 0.01b |
| Wrong frequency | 30 (4.2) | 9 (1.4) | −66.7 | < 0.01b |
| Drug interaction | 63 (8.7) | 32 (4.8) | −44.8 | < 0.01b |
| CCR5 errors | ||||
| Wrong dose | 1 (0.1) | 2 (0.3) | +200.0 | 0.61 |
| Wrong frequency | 0 (0.0) | 0 (0.0) | 0.0 | 1.00 |
| Drug interaction | 1 (0.1) | 3 (0.5) | +400.0 | 0.35 |
| Integrase inhibitor errors | ||||
| Wrong dose | 1 (0.1) | 3 (0.5) | +400.0 | 0.35 |
| Wrong frequency | 8 (1.1) | 5 (0.8) | −27.3 | 0.58 |
| Drug interaction | 1 (0.1) | 0 (0.0) | −100.0 | 1.00 |
Data presented as n (%)
Statistically significant
CCR5 = chemokine (C-C motif) receptor 5 inhibitor; NNRTI = nonnucleoside reverse-transcriptase inhibitor; NRTI = nucleos(t)ide reverse-transcriptase inhibitor; PI = protease inhibitor
In addition to intervention period and internal medicine as admitting service, the variables of race, viral load greater than 40 copies/mL, and ARV regimen (all with P values of less than 0.20 on univariate analysis, Table 1) were included in the logistic regression analysis model. After controlling for potential confounders, pre-intervention period (adjusted odds ratio [AOR], 1.79; 95% confidence interval [CI], 1.39–2.31; P < 0.01) and PI-based ARV regimen (AOR, 2.03; 95% CI, 1.53–2.70, P < 0.01) remained significantly associated with ARV prescribing errors (Table 3).
Table 3.
Multivariate Logistic Regression Analysis of Factors Associated With Antiretroviral Prescribing Errors
| Variables | Adjusted OR (95% CI) | P Values |
|---|---|---|
| Pre-intervention period | 1.79 (1.39–2.31) | < 0.01* |
| African-American | 0.96 (0.74–1.23) | 0.73 |
| Viral load > 40 copies/mL | 0.79 (0.61–1.02) | 0.07 |
| PI-based ARV regimen | 2.03 (1.53–2.70) | < 0.01* |
| Admitted to Internal Medicine | 1.02 (0.77–1.36) | 0.88 |
Statistically significant
ARV = antiretroviral; OR = odds ratio; PI, protease inhibitor
DISCUSSION
It is becoming increasingly difficult for physicians to stay abreast of changes in HIV drug management. The expanding number of ARVs approved by the Food and Drug Administration and the potential number of drug combinations, as well as frequent changes in clinical practice guidelines, can be overwhelming to medical providers. Improved measures to assist prescribers with error prevention are much needed.
This study demonstrated that ARV prescribing errors in an inpatient setting can be substantially reduced by CPOE with COES. More importantly, COES prevented a large number of these errors prior to patients receiving their first ARV doses during hospitalization. To our knowledge, this is the largest and only study to date in a region of high HIV prevalence that reviews the effects of such an ordering system on the reduction of potential medication errors before they occur, rather than rectifying mistakes after they have transpired. The multidisciplinary collaboration between the departments of infectious diseases, pharmacy, and information technology made the CPOE with COES possible for our institution. We constructed a COES for each ARV that is simple in design (Figure 1) and can be easily reproduced and implemented by most small or large institutions with CPOE. These ordering screens can be especially important for reducing ARV errors if the institution does not have designated pharmacists or medical staff trained in infectious diseases to review ART on a daily basis.
Overall, COES reduced errors of any type by 34.7%, primarily due to reductions in PI and renal dosing errors. Error rates were significantly reduced for incomplete regimens, incorrect dose, and drug interactions. There was no significant impact on incorrect ARV choice and on dosing frequency, but a trend toward reduction was observed. The lack of impact on incorrect ARV choice is likely multifactorial in etiology. COES cannot distinguish between the choices of protease inhibitors (e.g., atazanavir [Reyataz, Bristol-Myers Squibb] versus darunavir [Prezista, Janssen]), NRTIs (e.g., emtricitabine/tenofovir [Truvada, Gilead Sciences] versus lamivudine/abacavir [Epzicom, ViiV Healthcare]), or NNRTIs (e.g., nevirapine [Viramune, Boehringer Ingelheim] versus efavirenz [Sustiva, Bristol-Myers Squibb]).
In almost all cases, ARV regimens of hospitalized patients are initiated by outside HIV care providers. The occasional lack of access to the correct ARV regimen, especially for those patients whose records are not part of the hospital’s medical record system, poses challenges for inpatient house staff. Prompt communication with the primary HIV care provider will help to reduce the number of errors for ARV choice and address the inability of COES to make intra-class distinctions. Incorrect creatinine clearance calculations may have contributed to incorrect dosing frequency errors. Further education is needed on using the Cockcroft–Gault equation for creatinine clearance calculations in order to reduce error in dosing frequency.
Error rates for PI-based and non–PI-based regimens similarly improved after the introduction of COES (pre-intervention: PI-based, 43.6%, non–PI-based, 38%; post-intervention: PI-based, 28.7%, non–PI-based, 24.4%). A number of studies4,21–23 have found that the ordering of PIs is more likely to be associated with errors as a result of multiple available formulations, the use of ritonavir for so-called boosted regimens, and interactions with proton pump inhibitors, histamine receptor 2 blockers, and statins. This is consistent with findings from this study that suggest PI-based regimens were independently associated with ARV prescribing errors (Table 3). Possibly, the decrease in concomitant use of potentially interacting drugs and prescribers’ increased familiarity with ritonavir’s use as a booster may have contributed to a reduction in PI-associated errors after the introduction of COES. The reduction in the percentage of PI-associated errors likely accounts for the increase in the proportion of NRTI errors.
The prescribing of single-pill formulations such as efavirenz/emtricitabine/tenofovir and emtricitabine/rilpivirine/tenofovir (Complera, Gilead Sciences) among hospitalized patients remained just over 10% between the two study periods. With a decreased pill burden and potential for increased medication adherence, these formulations can be expected to be prescribed more frequently. However, their use in persons with renal insufficiency will be limited. Errors associated with prescribing of these formulations occurred in our population precisely for this reason. In a prospective French study involving HIV sero-positive hemodialysis patients, underdosing and overdosing errors occurred among 59% of ARV medications prescribed.18
A variety of interventions to reduce ARV prescribing errors have been investigated. Several studies are based primarily on the intervention by the pharmacy, in which inpatient pharmacy staff members reconcile outpatient regimens with inpatient orders and often consult the patients’ medical care providers or families.3,9,11,21,23 Other studies have used a multidisciplinary approach that typically included a combination of computerized alerts, physician and pharmacy review, and educational measures. Yehia et al. used such an approach with a CPOE with drug–drug interaction alerts and calculated creatinine clearance but did not flag incomplete regimens or errors in medication dosing or scheduling.20 In another study, a primarily pharmacy-driven resolution was achieved through daily medication review, superimposed on education, CPOE alerts, and formulary conversions.22
With the goal of achieving optimal clinical outcomes related to antimicrobial use and minimizing adverse drug events and the potential for drug resistance, antimicrobial stewardship is well positioned to intervene in the problem of ARV prescribing errors. This approach was recently reported by Sander et al., in which an intervention that included education, modification of electronic records, collaboration with infectious disease (ID) specialists, and prospective auditing by ID clinical pharmacists of HIV-related medication orders resulted in a significantly higher (74% versus 35%) post-intervention error resolution rate.25 In our study, the stewardship effort was further enhanced by the use of COES superimposed on established educational tools and multidisciplinary communication between prescribers, ID specialists, and ID pharmacists.
CPOE systems have been observed to both reduce and facilitate medication errors.21,28 The potential for ordering errors is likely to be increased for unusual ARV regimens, especially in the highly treatment-experienced patient with multiple genotypic resistance mutations, and with the ordering of nonformulary ARVs. Therefore, to reduce such ordering errors, COES must be constantly updated and HIV clinical specialists and pharmacists must be readily available.
There are a number of potential limitations to this study. The retrospective nature of the review may have allowed unrecognized bias. The study is based on data from a single large tertiary-care teaching hospital in an urban setting; the results may not be applicable to all hospital settings. Multiple hospitalizations per patient may have occurred during each study period as well as during both study periods, but each admission presented as an opportunity for ARV ordering. Patient characteristics (such as degree of renal insufficiency, comorbidities, or reasons for hospitalization) before and after the introduction of COES may have differed in unrecognized ways. The pattern of ARV prescribing or prescriber characteristics may have changed over time. Familiarity with ARVs and management may have differed between the two periods studied. Possibly, newer ARVs and formulations and increased ARV resistance between the two populations studied may have confounded our analysis of the impact of COES. We attempted to control for possible seasonal effects on hospital admissions by reviewing the same period of the year; we also intended that this would control for the experience of medical residents, who comprise a significant proportion of prescribers at our institution.
Although ARV medication errors cannot be prevented completely, health care providers must exercise caution when prescribing ARVs. It is sobering to consider that ARV regimens incorrectly prescribed for even brief periods could have both short- and long-term consequences with adverse effects, viral resistance, and clinical outcomes. A multifaceted approach to error prevention under the auspices of an antimicrobial stewardship program should include education with updated, user-friendly tools to increase awareness, active real-time auditing of ARV ordering, consultation with HIV ID specialists or dedicated clinical pharmacists, and effective reconciliation of inpatient orders with outpatient records. Upon patient discharge, it is also crucial to ensure that the patient will be provided with prescriptions for the correct ARV regimens during the transition in care.
In conclusion, COES can further prevent ARV prescribing errors before they occur among hospitalized patients with HIV infection in large urban areas with high HIV prevalence and may prove to be a useful, stand-alone, and cost-efficient means of reducing ARV prescribing errors in hospitals that do not have HIV clinical specialists and pharmacists. Vigilance must be maintained to keep the ordering system updated to ensure correctness in prescribing, especially as newer medications and formulations become available and drug interactions are better described. Future studies might focus additionally on the use of COES in other health care settings, such as long-term care facilities, clinics, ambulatory surgical centers, and dialysis centers, and on the use of medications for primary and secondary prophylaxis of opportunistic infections.
Acknowledgments
We thank Charles Abam for his assistance with data sorting and for his design and management of the customized order-entry set screens.
Footnotes
Disclosure: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Arshad S, Rothberg M, Rastegar DA, et al. Survey of physician knowledge regarding antiretroviral medications in hospitalized HIV-infected patients. J Int AIDS Soc. 2009;12:1. doi: 10.1186/1758-2652-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok S, Minson Q. Drug-related problems in hospitalized patients with HIV infection. Am J Health-Syst Pharm. 2008;65(1):55–59. doi: 10.2146/ajhp070011. [DOI] [PubMed] [Google Scholar]
- 3.Eginger KH, Yarborough LL, Inge LD, et al. Medication errors in HIV-infected hospitalized patients: a pharmacist’s impact. Ann Pharmacother. 2013;47(7):953–960. doi: 10.1345/aph.1R773. [DOI] [PubMed] [Google Scholar]
- 4.Commers T, Swindells S, Sayles H, et al. Antiretroviral medication prescribing errors are common with hospitalization of HIV-infected patients. J Antimicrob Chemother. 2014;69(1):262–267. doi: 10.1093/jac/dkt323. [DOI] [PubMed] [Google Scholar]
- 5.Osorio SN, Abramson E, Pfoh E, et al. Risk factors for unexplained medication discrepancies during transitions in care. Fam Med. 2014;46(8):587–596. [PubMed] [Google Scholar]
- 6.Yehia B, Kangovi S, Frank I. Patients in transition: avoiding detours on the road to HIV treatment success. AIDS. 2013;27(10):1529–1533. doi: 10.1097/QAD.0b013e328360104e. [DOI] [PubMed] [Google Scholar]
- 7.Pastakia SD, Corbett AH, Raasch RH, et al. Frequency of HIV-related medication errors and associated risk factors in hospitalized patients. Ann Pharmacother. 2008;42(4):491–497. doi: 10.1345/aph.1K547. [DOI] [PubMed] [Google Scholar]
- 8. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed January 28, 2015.
- 9.Edelman EJ, Gordon KS, Glover J, et al. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30(8):613–628. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heelon M, Skiest D, Tereso G, et al. Effect of a clinical pharmacist’s interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health-Syst Pharm. 2007;64(19):2064–2068. doi: 10.2146/ajhp070072. [DOI] [PubMed] [Google Scholar]
- 11.Carcelero E, Tuset M, Martin M, et al. Evaluation of antiretroviral-related errors and interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med. 2011;12(8):494–499. doi: 10.1111/j.1468-1293.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 12.Saberi P, Dong BJ, Johnson MO, et al. The impact of HIV clinical pharmacists on HIV treatment outcomes: a systematic review. Patient Pref Adherence. 2012;6:297–322. doi: 10.2147/PPA.S30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rastegar DA, Knight AM, Monolakis JS. Antiretroviral medication errors among hospitalized patients with HIV infection. Clin Infect Dis. 2006;43(7):933–938. doi: 10.1086/507538. [DOI] [PubMed] [Google Scholar]
- 14.Horace A, Philips M. Identification and prevention of antiretroviral medication errors at an academic medical center. Hosp Pharm. 2010;45(12):927–933. [Google Scholar]
- 15.Snyder AM, Klinker K, Orrick JJ, et al. An in-depth analysis of medication errors in hospitalized patients with HIV. Ann Pharmacother. 2011;45(4):459–468. doi: 10.1345/aph.1P599. [DOI] [PubMed] [Google Scholar]
- 16.Willig JH, Westfall AO, Allison J, et al. Nucleoside reverse transcriptase inhibitor dosing errors in an outpatient clinic in the electronic medical record era. Clin Infect Dis. 2007;45(5):658–661. doi: 10.1086/520653. [DOI] [PubMed] [Google Scholar]
- 17.Rao N, Patel V, Grigoriu A, et al. Antiretroviral therapy prescribing in hospitalized HIV clinic patients. HIV Med. 2012;13(6):367–371. doi: 10.1111/j.1468-1293.2011.00977.x. [DOI] [PubMed] [Google Scholar]
- 18.Tourret J, Tostivint I, Tezenas Du Montcel S, et al. Antiretroviral drug dosing errors in HIV-infected patients undergoing hemodialysis. Clin Infect Dis. 2007;45(6):779–784. doi: 10.1086/521168. [DOI] [PubMed] [Google Scholar]
- 19.Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34(7–8):833–838. doi: 10.1345/aph.19399. [DOI] [PubMed] [Google Scholar]
- 20.Yehia BR, Mehta JM, Ciuffetelli D, et al. Antiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adults. Clin Infect Dis. 2012;55(4):593–599. doi: 10.1093/cid/cis491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siemianowski LA, Sen S, George JM. Impact of pharmacy technician-centered medication reconciliation on optimization of antiretroviral therapy and opportunistic infection prophylaxis in hospitalized patients with HIV/AIDS. J Pharm Pract. 2013;26(4):428–433. doi: 10.1177/0897190012468451. [DOI] [PubMed] [Google Scholar]
- 22.Daniels LM, Raasch RH, Corbett AH. Implementation of targeted interventions to decrease antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm. 2012;69(5):422–430. doi: 10.2146/ajhp110172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauzevis S, Chaix F, Lazzerini C. Evaluation of a strategy aimed at reducing errors in antiretroviral prescriptions for hospitalized HIV-infected patients. Med Mal Infect. 2013;43(9):391–397. doi: 10.1016/j.medmal.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Flanigan TP, Wools-Kaloustain K, Harwell J, et al. Highly active anti-retroviral therapy (HAART)—plus: next steps to enhance HAART in resource-limited areas? Clin Infect Dis. 2007;45(11):1499–1501. doi: 10.1086/522992. [DOI] [PubMed] [Google Scholar]
- 25.Sanders J, Pallotta A, Bauer S, et al. Antimicrobial stewardship program to reduce antiretroviral medication errors in hospitalized patients with human immunodeficiency virus infection. Infect Control Hosp Epidemiol. 2014;35(3):272–277. doi: 10.1086/675287. [DOI] [PubMed] [Google Scholar]
- 26.Gray J, Hicks RW, Hutchings C. Antiretroviral medication errors in a national medication error database. AIDS Patient Care STDS. 2005;19(12):803–812. doi: 10.1089/apc.2005.19.803. [DOI] [PubMed] [Google Scholar]
- 27.Bureau of HIV/AIDS Epidemiology, AIDS Institute, New York State Department of Health New York State HIV/AIDS Surveillance Annual Report for Cases Diagnosed Through December 2011. Aug, 2013. pp. 1–90. Available at: http://www.health.ny.gov/diseases/aids/general/statistics/annual/2011/2011-12_annual_surveillance_report.pdf. Accessed April 13, 2015.
- 28.Reckmann MH, Westbrook JI, Koh Y, et al. Does computerized provider order entry reduce prescribing errors for hospital inpatients? A systematic review. J Am Med Inform Assoc. 2009;16(5):613–623. doi: 10.1197/jamia.M3050. [DOI] [PMC free article] [PubMed] [Google Scholar]