Abstract
Importance
Sleep disturbances have been linked to increased morbidity and mortality, yet it is unknown whether improving sleep quality in older adult patients with insomnia alters biomarkers of diabetes and cardiovascular disease risk.
Objective
Determine the comparative efficacy of cognitive behavioral therapy (CBT), tai chi chih (TCC), and a sleep seminar control (SS) to reduce multisystem biomarkers of disease risk in older adults with insomnia.
Design
Randomized controlled comparative efficacy trial.
Setting
Los Angeles community
Participants
A population-based sample of 109 older adults with chronic and primary insomnia
Intervention
Random assignment to CBT, TCC, or SS for 2-hour group sessions weekly over 4 months with a 16-month evaluation (1 year after follow-up).
Main Outcome(s) and Measure(s)
Multisystem biological risk comprised of 8 biomarkers: high-density lipoprotein, low-density lipoprotein, triglycerides, hemoglobinA1c, glucose, insulin, C-reactive protein, and fibrinogen. Using clinical laboratory cutoffs defined as abnormal, a multisystem risk score was computed representing a sum of the deviation around the cutoffs across the 8 biomarkers. In addition, high risk grouping was classified if subjects exhibited 4 or more biomarkers in the abnormal laboratory range.
Results
An interaction of time-by-treatment-by-high risk group was found (F(4,197.2)=3.14, p=.02) in which both TCC (p=.04) and CBT (p=.001) showed significantly lower risk scores as compared to SS at 16-months. CBT reduced risk of being in the high risk group at 4-months (odds ratio [OR]=.21 [95%CI, .03–1.47], p<.10) and at 16-months (OR=0.06 [95%CI, .005–.669]; p<.01). TCC reduced the risk at 16-months (OR=.10 [95%CI, .008–1.29]; p<.05) but not at 4 months. Of participants who were classified in the high risk category at baseline, improvements in sleep quality, as defined by a clinical severity threshold, reduced the likelihood of being in the high risk group at 16-months, OR=.08 (95% CI, .008–.78); p = .01.
Conclusions and Relevance
Participants classified as having high multisystem biological risk at entry and assigned to CBT or TCC show improvements in risk scores after one year follow-up. Given that these clinical biomarkers are associated with cardiovascular, metabolic, and inflammatory disease risk, improving sleep quality has the potential to reduce the risk of chronic disease in older adults with insomnia.
Clinical Trial Registration # and name
ClinicalTrials.gov: NCT00280020, Behavioral Treatment of Insomnia in Aging
Keywords: Sleep, Insomnia, Allostatic Load, Multisystem, Disease Risk, Intervention
1.0 Introduction
Insomnia symptoms are characterized as impairments in sleep quantity and/or quality that can result from difficulties in falling or staying asleep, frequent arousals, and/or obtaining restorative sleep.1–3 Insufficient sleep and poor sleep quality have been linked to disease risk and mortality.1,4–8 Not getting enough quality sleep causes repeated disruptions to multiple regulatory systems including metabolic, cardiovascular, and immune.9–11 This is particularly salient in older adults, where the risk for disease and death is elevated, with as many as half of adults over 65 years old reporting insomnia symptoms.12 Sleep is a modifiable behavior with established behavioral treatments that improve sleep quality.13,14 Although disturbed sleep has been associated with increased risk for disease and death, it is not clear whether treating sleep problems would reduce laboratory markers of disease risk, particularly among those with elevated levels of such risk markers. Further research is needed to determine whether the treatment of sleep disturbances, such as insomnia, has the potential to aid in the prevention of disease.
There are a number of pathways through which sleep problems likely contribute to poorer health. Among them, inadequate sleep has been associated with adverse effects on levels of glucose, lipids, inflammation, and blood pressure.3,9,11,15–21 Inadequate and fragmented sleep disrupts the normal diurnal rhythm across regulatory systems and interferes with the restorative nature of sleep.9,22 Although each system is important in understanding disease risk, recent work has found that higher multisystem biological risk (a combination of biomarkers representing cardiovascular, metabolic, immune, nervous, and endocrine systems) is a stronger predictor of morbidity and mortality outcomes as compared to the predictive role of each biologic system alone.23–28 Hence, this work is grounded in the hypothesis that the pathway to disease is a dynamic interface between multiple biologic systems that influence the whole and may not be fully captured when measuring individual components. Importantly, multisystem biological risk has been linked to sleep, with shorter sleep duration and poor sleep quality associated with an elevated multisystem risk.11,29
An unanswered question is whether treating sleep disturbances such as insomnia in individuals with elevated markers of disease risk can then reduce such levels, with implications for disease. Cognitive Behavioral Therapy (CBT) is an effective treatment for insomnia in young and older adults.13,30–34 Tai Chi Chih (TCC) has also been proposed as an alternative treatment, with evidence that it improves sleep quality in older adults.14,35–38 With the exception of our initial findings showing decreases in C-reactive protein following the successful treatment of insomnia,38 we know of no published trials examining the efficacy of an RCT for insomnia in older adults on biomarkers of diabetes and cardiovascular disease risk. An observed reduction in risk via changes in clinical laboratory biomarkers that traditionally signal risk (e.g., LDL cholesterol, hemoglobin A1c, CRP) following the successful improvement of sleep quality would suggest that the treatment of sleep problems among individuals showing initially elevated risk might be included as part of a disease prevention strategy. Following our initial analyses showing that CBT and TCC are efficacious at improving sleep quality in older adults,38 we report on the efficacy of this RCT to reduce multisystem biological risk among individuals classified as higher risk at the beginning of the trial based on several key clinical laboratory biomarkers indicative of disease risk.
2.0 Methods
2.1 Study Design
Methods of the study design and intervention modalities have been reported previously.38 Briefly, after obtaining UCLA institutional review board approval, subjects were recruited from the surrounding Los Angeles community through advertisements. Following eligibility screening, older adults were invited to participate in the RCT, which was conducted from the period of April 2006 through August 2011. Each participated in 120 minutes of group class time weekly for 4-months, with assessments at baseline (pre-intervention), 4-months (post-intervention), and 16-months (one year follow-up, after completion of the intervention). There were no changes to the methods after trial commencement as previously described.38
2.2 Study Participants
To be eligible for the study, participants had to be 55 years of age or older, free of major medical illness and sleep apnea (determined through an overnight stay using polysomnography; apnea/hypopnea index (AHI) >15), and to fulfill Diagnostic and Statistical Manual (4th Ed.)39 criteria for primary insomnia as well as the International Classification of Sleep Disorders (2nd Ed.)40 for insomnia (determined through a structured interview). To meet criterion, patients had to report difficulty in initiating or maintaining sleep or have non-restorative sleep for at least one month, and also report significant distress and daytime impairment. Eligible participants were randomly assigned in a 2:2:1 ratio to CBT, TCC, or Sleep Seminar (SS) by permuted block design of 7–10 subjects.
2.3 Interventions
Research personnel who were involved in enrollment and assessment of subjects were blind to random allocation. Assignment was known only after eligibility was determined, and the individual managing randomization did not interact with participants prior to assignment. Participants assigned to CBT, TCC, and SS received 120-minutes of class instruction time weekly for 4-months. As reported previously,30,38 the CBT intervention was modified to include behavioral strategies to improve mood and manage daytime cognitive activity. The TCC intervention taught participants slow-paced movements designed to learn control over physical function and arousal. SS informed participants about sleep hygiene and educated them on various factors contributing to sleep problems. [See Irwin et al.38 and related online supplement for details]
2.4 Measures
The primary outcome of the RCT, insomnia remission, has been previously reported along with improvement in insomnia symptom severity and sleep quality using the Pittsburgh Sleep Quality Index (PSQI)41, and a daily diary of sleep activity. Additional secondary outcomes have also been reported previously including fatigue, depressive symptoms, and an inflammatory marker (C-reactive protein; CRP).38
Improvements in Sleep Quality
The PSQI was used to compute a global score of sleep quality across seven domains at each visit. Scores greater than 5 indicate poor sleep quality and elevated sleep disturbances, whereas scores of 5 or less indicate good sleep quality.41
This analysis focused on an a priori secondary outcome, multisystem biological risk, that was derived from fasting blood samples taken at baseline, 4-months, and 16-months. Laboratory personnel processing biomarker outcomes were blind to intervention assignment throughout the trial. Multisystem biological risk is comprised of eight different biomarkers, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, fibrinogen, C-reactive protein (CRP), glucose, insulin, and glycosylated hemoglobin (HgA1C). All biomarker values were determined by a Clinical Laboratory Improvement Amendments (CLIA) certified UCLA Medical Clinical Laboratory using standardized methodologies and reporting guidelines. For CRP, samples were diluted 1:20 then run using a high-sensitivity immunoassay using a BN-II System (Dade-Behring, Newark, DE). Limit of detection is .175 mg/L and coefficients of variation of < 4% for both intra- and inter-assay reliability.
Cut points for risk are reported in Table 1, and were selected based on clinical target guidelines for each biomarker when available.142–47 The clinical cutoffs were defined by existing guidelines for LDL, HDL, glucose, HgA1C, and CRP. The National Cholesterol Education Program guidelines classify LDL 130–159 mg/dL as borderline high in the ATP III guidelines, and recommend target below 130 mg/dL to reduce risk.44 Recommendation for HDL cholesterol is >40 in men and >50 in women,44 and adopted by AHA and NHLBI to be used in the diagnosis of metabolic syndrome.45 Based on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, the American Diabetes Association’s (ADA) defines impaired fasting glucose at ≥ 100, which indicates risk for diabetes.45,46 Individuals with HgA1C ≥ 6.0 are at high risk for developing diabetes.46,47,48 The AHA/Center for Disease Control (CDC) scientific statement reported CRP > 3 mg/L corresponds with a high relative risk.43 For three of our biomarkers (triglycerides, fibrinogen, and insulin), we used the upper quartile for risk due to either a restricted distribution in our sample (triglycerides), or a lack of clearly defined risk cutoffs (fibrinogen, insulin). Current guidelines consider elevated triglycerides,42 fibrinogen,43 and insulin to be indicators of risk.48–51
Table 1.
Descriptive Statistics By Treatment Category. Data represent means (standard deviations), percentage (%), or cut point for risk where indicated.
| Cut point, % High Risk | Cognitive Behavioral Therapy N=47 |
Tai Chi N=39 |
Sleep Seminar N=23 |
|
|---|---|---|---|---|
| Age | 64.8(6) | 67.3 (7.5) | 66(7.7) | |
| Gender, % Female | 78.7% | 64.1% | 69.6% | |
| Race, % White | 87% | 84.2% | 86.4% | |
| BMI | 25.4(3.3) | 26.3(4) | 26.1(4.1) | |
| PSQI Global Score | 10.4(2.9) | 10.4(3.2) | 11.2(3) | |
| Glucose, mg/dL | >100, 30.2% | 95.4(25.5) | 99.7(22.2) | 106.8(28.8) |
| Insulin, mlU/mL | ≥10, 26.9% | 7.4(5.7) | 7.4(4.7) | 8.6(7.4) |
| HA1C | ≥6.0, 68.3% | 6.0(.9) | 5.8(.9)a | 6.7(2.2) |
| LDL, mg/dL | ≥130, 27.4% | 115.4(34.3) | 109(31.3) | 106.4(29.1 |
| HDL, mg/dL | ≤ 40 men, 35.5% | 45.4(8.5) | 42.5(8.3) | 41.9(15.6) |
| ≤ 50 women, 34.6% | 60.7(15.2) | 56(19.9) | 56.8(12.1) | |
| Triglycerides, mg/dL | ≥128, 28.4% | 114.3(57.4) | 106.7(39.4) | 101.4(31.2) |
| CRP, mg/L | ≥3, 21.9% | 2.0(1.9) | 2.1(2) | 2.1(2.2) |
| Fibrinogen, mg/dL | ≥335, 26% | 307.6(37) | 303.1(52) | 316.5(49.8) |
| Multisystem biological riskb | −3.2(3.9) | −3.2(3.3) | −2.1(4.3) | |
| Risk status at baseline, % High Risk | 27.7% | 23.1% | 34.8% |
TCC vs. SS difference, p < .05
Multisystem biological risk score is the sum of each z-score from the individual biomarkers (See methods for more detail).
2.5 High Risk/Low Risk Groups
To examine whether the treatments would reduce clinically meaningful laboratory biomarkers, we designated individuals as being in the high risk group if they had 4 or more biomarkers in the abnormal laboratory range (cutoffs specified in Table 1) at baseline (CBT 27.7%; TCC 23%; SS 34.8%). This designation was selected based on two factors. First, previous research has shown the highest risk for declines in health and mortality occur when 3 to 7+ biomarkers are in the abnormal ranges,23,27,28 hence, our selection of 4 or more avoided the lower boundary. Secondly, within our sample, this risk group cut point allowed for stable estimates to be obtained while maintaining sample size to ensure adequate power.
2.6 Multisystem Biological Risk Score
Given that the relationship of the biomarker with disease risk is often linear and doesn’t necessarily function as a threshold effect (e.g., CRP: 1–3 mg/L is associated with a moderate risk52), we also computed a multisystem risk score by estimating the distance a person’s value is from the cutoff for each biomarker. To do this, multisystem biological risk scores were also computed to estimate deviation from the designated cut point, as described above and displayed in Table 1. Here we used the cut point for risk as the mean, and generated a z-score around that cut point by taking the sample value and subtracting the cut point value, then dividing by the standard deviation of the group for that biomarker. Individual z-scores for each biomarker were then averaged and multiplied by eight (the number of biomarkers) to create a total sum multisystem biological risk score which represents the summed standardized distance from the cut points. Negative values suggest that their average is below the risk cut point, while positive values imply the average is above the risk cut point. All z-scores, prior to creating the sum, were restricted to 4 SD away from the cut point. Table 1 displays means and standard deviations of biomarkers within treatment group prior to z-transformation, denotes the defined cutoffs for risk, and reports percentage within high risk for each biomarker.
2.6 Statistical Analyses
No existing data are available to generate effect estimates for a treatment trial to impact multisystem biological risk scores. However in our sample individuals with high CRP are at greater likelihood to fall in the high risk group, with 53% classified as HR compared to 18% of those with low CRP (χ2=9.99, p=.002). Given this, we used effect estimates (0.76) from our prior work showing reduction of CRP with insomnia by treatment,38 which suggests a sample size of 23 per group is needed to detect improvements. All statistical analyses were performed using IBM SPSS for Windows v.22. The present analyses include 109 (CBT, n=47; TCC, n=39; SS, n=23) participants for whom multisystem biological risk measures were obtained; the demographic and clinical characteristics of this sample (n=109) did not differ from the total number of participants who were enrolled in the insomnia treatment trial previously reported (n=123).38 In addition, 12 of the 109 subjects had missing data at baseline and these missing values were imputed using a regression equation derived from available biomarker data and an estimated error using random number generation within the standard error of the distribution of that biomarker. Testing of the models with and without the imputed data did not alter the results.
Independent sample t-tests were performed to examine differences between treatment groups in age, BMI, PSQI scores, and individual biomarkers. To test for the effect of the treatment on multisystem biological risk scores among those who met high risk criterion compared to those with lower risk, we used mixed linear model analyses testing for a 2(high risk, low risk) × 3(TCC, CBT, SS) × 3(baseline, 4-month, 16-month) interaction. The mixed model allows for values to be missing as long as they are missing at random (i.e., missingness is not dependent on the value of unobserved variables); model estimates are unbiased under such conditions. This approach retains completers with missing data, which is ideal for RCT analyses. The total missingness was <8%. Pairwise comparisons using the least significant difference (LSD) method were used to test differences between groups at each time point. For high risk subjects (n=30), additional analyses examined the intervention effects on the proportion lowering risk at 4- and 16-months, which includes evaluation of the odds ratio of CBT and TCC vs. SS. Chi-square analysis and odds ratios were used to test whether those with improved sleep disturbances (PSQI scores < 5 at 4- and 16-months) also were at lower risk of remaining in the high risk group. Data was available on >90% of the subjects at all time points among those who completed follow-up assessments.
3.0 Results
Of the 294 who underwent the baseline assessment, a total of 207 participants met further eligibility criteria and 123 agreed to participate. These subjects completed the entire baseline, agreed to participate in the clinical trial, and were randomly assigned to treatment (Figure 1 CONSORT). Of these 123 participants, 109 subjects had biomarker data and are included in this secondary analysis. Treatment groups were similar in demographics (Table 1). Data on completers and non-completers has been reported previously.38 As a primary outcome, the CBT group at 4-months showed a greater rate of insomnia remission (using the DSM-IVTR criteria) compared to TCC and SS (p′s<.01). Sleep disturbance severity using the PSQI also revealed significant treatment effects for both CBT and TCC compared to SS at 4-months (p’s<.05), which remained lower for CBT but not TCC by 16-months (See Irwin et al., 2014 for primary outcome reporting).38 No harms or unintended side effects were reported during the trial.
Figure 1.
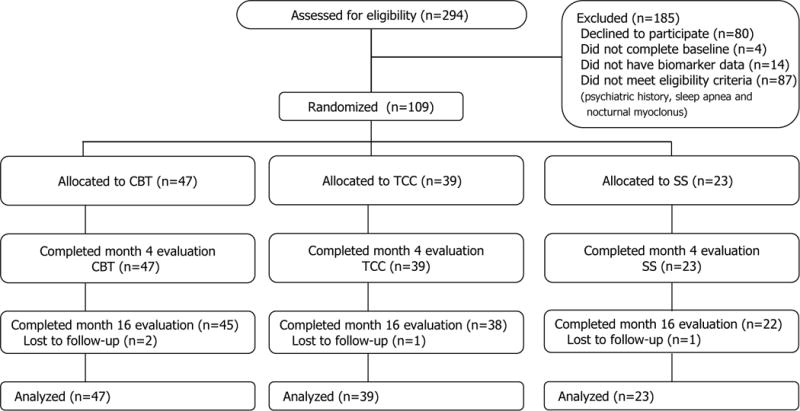
CONSORT Flow Diagram of the Treatment Trial For Sleep Disturbances Assessing Changes in Multisystem Biological Risk.
Descriptive statistics of baseline levels of biomarkers within groups are shown in Table 1. Independent sample t-tests for differences between mean multisystem biological risk in CBT vs. SS and TCC vs. SS at baseline were not significant (p’s>.30). Initial mixed linear model analyses found no significant treatment by time interaction (p=.30) in the prediction of multisystem biological risk. Among those in the high risk group, CBT and TCC were associated with decreases in multisystem biological risk from baseline to 16-months. Mixed linear model analyses, adjusting for baseline multisystem biological risk, revealed a 3-way interaction of time by treatment by risk group in the prediction of multisystem biological risk, F(4,197.2)=3.14, p=.02. As compared to SS, TCC and CBT were associated with improvements in multisystem biological risk by 16-months among those participants entering the trial in the high risk category. Further adjustment by BMI and changes from baseline to 16-months in physical activity did not alter this effect. At 4 months, post hoc analyses of group differences in mean multisystem biological risk revealed no significant differences between treatment groups in participants who were categorized as high risk at entry (all p′s>.40). At 16-months, both TCC (M=−.98; p=.04) and CBT (M=−2.3; p=.001) showed significantly lower multisystem biological risk scores compared to SS (M=1.3) in participants who were categorized as high risk at entry.
To examine whether improvements in sleep quality altered the likelihood of remaining in the high risk group from baseline to 4- or 16-months, the odds of remaining in the high risk group was tested for those who had persistent poor sleep quality (PSQI scores >5) as compared to those who had improvements in sleep quality (PSQI scores ≤ 5). As compared to those with persistent poor sleep quality, those who showed improvement in sleep quality had a decreased likelihood of being in the high risk at 4-months, odds ratio [OR]=.21 (95% CI, .3–1.39), p=.09, and at 16-months, OR=.08 (95% CI, .008–.78); p=.01. Improvement in sleep quality (≤5 PSQI) at 16-months was associated with a significant shift into the lower risk group; 88.8% of those participants entering the trial in the high risk group were re-categorized into the lower risk group following improvement in sleep quality (χ2=6.08, p=.01; Figure 2).
Figure 2. Percentage of Subjects in High Risk Group at 4-months and 16-months Stratified by Good- and Poor- Sleep Quality.
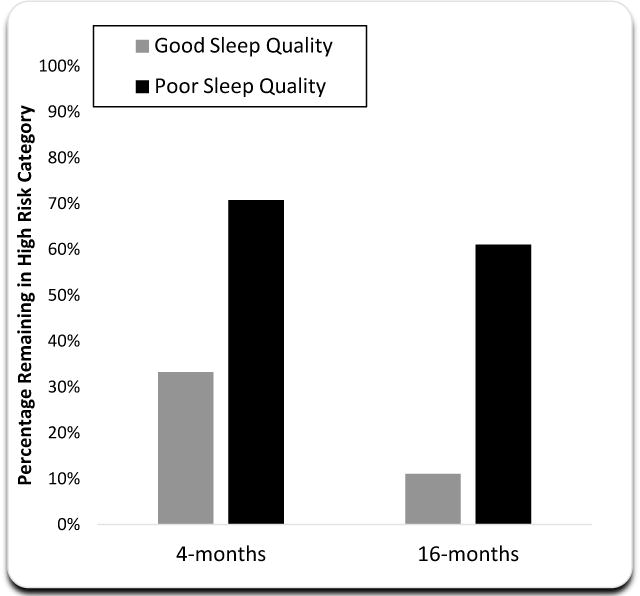
All subjects began the treatment trial at high risk (n = 30). High risk was defined by having 4+ biomarkers above the cutoff for risk. As compared to those with poor sleep quality, those with improved sleep quality had a reduced odds of being in the high risk post intervention (odds ratio [OR] = .21 (95% CI, .3–1.39), p = .09), which reached significance by 16-months, (OR = .08 (95% CI, .008–.78); p = .01.). Good sleep quality at 4-months (n = 6) and 16-months (n = 9).
To examine whether the treatment type altered the likelihood of remaining in the high risk group from baseline to 4- or 16-months, the odds of remaining in the high risk group was tested for those who received CBT or TCC as compared to SS. As compared to the SS, CBT was associated with a decreased likelihood of being in the high risk group at 4-months, OR=.21 (95% CI, .03–1.47, p<.10), and at 16-months, OR=0.06 (95% CI, .005–.67; p<.01). As compared to SS, TCC did not show an effect at 4-months (p>.4) but did show a reduced likelihood of being in the high risk group at 16-months, OR=.10 (95% CI, .008–1.29; p<.05). As compared to TCC, CBT was associated with a significant decreased likelihood of being in the high risk at 4-months, OR=.08 (95% CI, .007–.83; p<.05), but by 16-months this difference was not present, OR=.56 (95% CI, .08–3.9; p=.92). Figure 3 displays the percentage of subjects by treatment within the high risk group at baseline who continue to be in the high risk category at 4-months and 16- months. For individuals who were classified as low risk prior to treatment, no changes in risk across treatments was observed (x2=1.35, p=.51).
Figure 3. Percentage of Subjects in High Risk Group at 4-months and 16-months Stratified by Treatment.
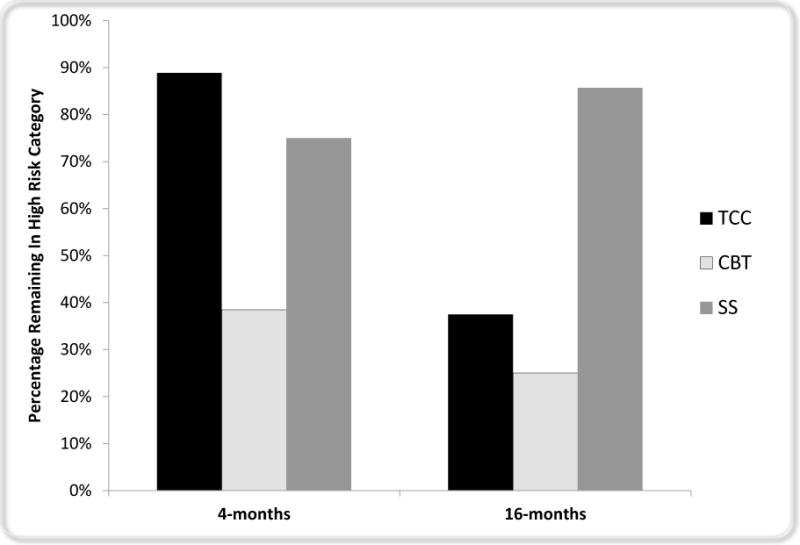
All subjects began the treatment trial at high risk (CBT n = 13; TCC n = 9; SS n = 8). High risk was defined by having 4+ biomarkers above the cutoff for risk. As compared to SS, CBT was associated with a reduced risk of being classified as high risk post intervention (odds ratio [OR] = .21 [95% CI, .03–1.47], p < .10), which reached significance by 16-months, OR = 0.06 (95% CI, .005–.669; p < .01). TCC, as compared to SS, was not associated with reduced risk at post intervention (p > .4) but did show reduced risk by 16-months (OR=.10 [CI, .008–1.29]; p < .05).
4.0 Discussion
The present paper presents the first randomized controlled trial in older adults with insomnia that examines the efficacy of the treatment on reducing multisystem biological markers of disease risk among subjects who begin the trial with elevated risk. We report that high risk insomnia subjects who experienced improvements in sleep quality showed reduced biological risk. In addition, among these individuals who exhibit elevated risk at the beginning of the treatment trial, both CBT and TCC treatments for insomnia were associated with reduced biological risk at 16-months, 1 year after treatment completion. Based on our classification of high risk, CBT and TCC treatment of insomnia combined reduced risk in over half of participants at 16-months, which indicates treating insomnia may also benefit lipid metabolic, glucose metabolic, and inflammatory profiles. Interestingly, reduction in risk was more rapid in the CBT compared to the TCC treatment, with greater reduction at 4-months; however, by 16-months risk reduction was similar in both treatment conditions. These findings advocate for the treatment of insomnia in older patients at higher risk for disease based on biomarkers indicating risk, and demonstrate that improving sleep quality with CBT or TCC may be important in reducing long term disease risk.
Our CBT was unique from other interventions in that it lasted considerably longer (so as to be comparable to the TCC intervention) and included a mood enhancement module (further detail regarding this additional module can be found in the online supplement of Irwin et al., 2014 SLEEP38). These features of the intervention may have contributed to our successful reduction in biomarkers of risk in the CBT group, and future work should consider whether this addition to the CBT intervention is necessary for treatment to reduce disease risk.
Although the present RCT is novel in demonstrating reductions in risk in older adults with insomnia, a prior intervention study has demonstrated that sleep extension improved blood pressure in short sleepers with pre-hypertension or hypertension.53 Similarly, there is growing interest in developing interventions to treat insomnia as a means to improve metabolic regulation and reduce type 2 diabetes,54 including an ongoing trial for sleep extension in an obese sample.55 In light of this work, and the present results, additional treatment trials are warranted.
4.1 Mechanisms
Inadequate and fragmented sleep disrupts the normal diurnal rhythm across regulatory systems and interfere with the restorative qualities of sleep.9,22 Such disruptions put demands on regulatory systems to maintain homeostasis under adverse conditions (i.e., allostasis). This is thought to cause gradual deterioration across systems, as there is an accumulation of wear and tear and the absence of adequate restoration, termed allostatic load.27,56 Evidence of allostatic load is seen in changes to physiological set points for biological indicators within each system, including elevated LDL and triglycerides, poor glucose regulation, and increased inflammatory activity. Epidemiological evidence links poor quality and inadequate quantity of sleep to increased allostatic load9–11 and other diseases related to allostatic load, such as diabetes, hypertension, metabolic syndrome, and cardiovascular disease.1,4–8,16–20 In the current study, we assessed HDL, LDL, triglycerides, fibrinogen, CRP, glucose, HA1C, and insulin, using cutoffs that are commonly used to identify risk for diabetes and cardiovascular disease. These biomarkers also have considerable overlap with metabolic syndrome. Here, we report that intervening to improve sleep appears to help re-establish healthy patterns for these biomarkers. This improvement represents healthier functioning of the respective regulatory systems that may otherwise, with unremitting insomnia, drive more dysfunction and lead to disease.9
One plausible mechanism through which the observed changes in biomarkers of risk occur may be through the re-establishment of healthy circadian rhythm dynamics. The circadian clock regulates neuronal and hormonal secretory patterns and peripheral organs rhythms altering numerous biological processes including metabolism and blood glucose regulation (See Reitrakul & Van Cauter, 2014 for a review).54 Future research should consider whether the circadian patterns are restored across systems after a clinical trial for the treatment of insomnia to more fully elucidate the hypothesized mechanism.
4.2 Limitations
The present study included adults 55 years and older, and while these are the individuals at highest risk for disease, the generalizability of these findings to younger adults is limited. Future work should determine whether treating sleep disturbances in adults under the age of 55 years with elevated biological risk would have the same beneficial effect on multisystem risk. Secondly, we cannot rule out the possibility of biases in self report. However, biological data are not thought to be influenced by such bias, lending strength to the findings. The findings may not be generalizable to the population as a whole due to over sampling of women and careful exclusion of co-morbid medical and psychiatric illness. In addition, our selection of a high risk group was performed after trial completion, and future research should specifically target high risk individuals to evaluate the efficacy of the intervention to improve biomarkers of risk. The present results are also constrained by the small sample size in our high risk groups within treatment conditions limiting our power to detect effects. Further research should consider the selection of a larger high risk sample for a treatment trial. Although we captured 8 different biomarkers, our multisystem risk measure does not include other frequently used biomarkers representing the cardiovascular (e.g., blood pressure, heart rate), autonomic (e.g., heart rate variability), and neuroendocrine systems (e.g., cortisol, catecholamines), which have been linked to mortality risk.23,26 Likewise, changes in adiposity may also be related to reductions in risk, however we were unable to capture this in the present sample. Future research examining treatment efficacy should include these additional indices of risk. Given the sample of older adults recruited for the present study required them to be free of major medical illnesses, the results may not be generalizable to older adults with comorbid chronic disease. Of note, major strengths of the current design include the randomization of participants to treatment conditions, a careful assessment of clinical biomarkers of risk prior to treatment, post-treatment, and 1 year after treatment, and a high completion rate.
4.3 Conclusions
The present research documents clinically meaningful declines in biomarkers of risk among older adults with elevated risk who receive treatment to improve sleep disturbances. This is the first study to report the efficacy of a clinical treatment trial for insomnia on clinically meaningful biomarkers of risk for diabetes and cardiovascular disease. These results highlight the importance of treating sleep disturbances in later life, particularly among individuals with laboratory values for HDL, LDL, triglycerides, CRP, fibrinogen, glucose, insulin, and/or HA1c that indicate risk. Clinicians should consider treatment of sleep disturbances within these groups who have elevated clinical biomarkers of risk as a crucial part of a comprehensive disease prevention strategy. As the identification and treatment of modifiable risk factors to prevent disease are of vital importance, physician queries of behavioral risk factors such as physical activity and smoking should also now include an assessment of patient’s sleep health for the identification of sleep disturbances.57 This modification to current practice should also include a referral to treat the sleep disturbances using CBT or TCC.
Highlights.
Individuals starting the trial with high multisystem biological risk and assigned to CBT or TCC, but not to the sleep seminar control condition, showed improvements in risk scores after one year follow-up.
These findings suggest that improving sleep quality has the potential to reduce the risk of chronic disease in older adults with sleep disturbances.
This is the first study to report the efficacy of a treatment trial for insomnia in older adults on clinical markers of risk for diabetes and cardiovascular disease.
Acknowledgments
Supported by grant R01-AG034588 from the National Institute of Aging; NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124. Other grant support to JEC from the National Institute of Aging K01-AG044462 and American Sleep Medicine Foundation, and from the National Institutes of Health to MRI including R01-CA119159; R01-HL079955; R01 HL095799; P30-AG028748; UL RR 033176. This work was also supported by the Cousins Center for Psychoneuroimmunology and UCLA Claude D. Pepper Older Americans Independence Center. We thank all the study participants for their involvement in this research project. Institutional Review Board approval was obtained from the University of California, Los Angeles.
Role of Funding Source: The following supported the conduct of the research and/or preparation of the article, but had no involvement in the study design, collection, analysis, or interpretation of the data, or in the writing of the report or decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No known conflicts of interest for JEC, RO, RS, GM, PN, RB, & MRI. TES served on an advisory board for a NIH funded National Health and Aging Trends study and received an honoraria. She also received honoraria for giving a keynote speech at the University of Alabama, Birmingham Health Disparities conference.
In the case of triglycerides, we used the upper quartile for risk do to a restricted distribution in our sample.
Financial Disclosure: None
All authors have contributed to the design, analyses, and writing of the manuscript and have consented to publication.
References
- 1.Center for Disease Control. CDC Data & Statistics | Feature: Insufficient Sleep Is a Public Health Epidemic. 2011 Available at: http://www.cdc.gov/Features/dsSleep/. Accessed August 4, 2011.
- 2.Colten HR, Altevogt BM, Research I of MUS. C on SM . Sleep disorders and sleep deprivation: an unmet public health problem. Vol. 2006. National Academies Press; p. 404. [PubMed] [Google Scholar]
- 3.Motivala SJ. Sleep and Inflammation: Psychoneuroimmunology in the Context of Cardiovascular Disease. Ann Behav Med. 2011;42(2):141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med (Baltim) 2009;49(2–3):135–41. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rod NH, Kumari M, Lange T, Kivimäki M, Shipley M, Ferrie J. The joint effect of sleep duration and disturbed sleep on cause-specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9(4):e91965. doi: 10.1371/journal.pone.0091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55(10 Suppl 2):S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124(19):2049–51. doi: 10.1161/CIRCULATIONAHA.111.062190. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Redline S, Shields AE, Williams DR, Williams MA. Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann Epidemiol. 2014;24(8):612–19. doi: 10.1016/j.annepidem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep. 2008;31(7):1001–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Redwine L. Effects of Sleep and Sleep Deprivation on Interleukin-6, Growth Hormone, Cortisol, and Melatonin Levels in Humans. J Clin Endocrinol Metab. 2000;85(10):3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99(5):2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 18.Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19(13):2409–19. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- 19.Van Cauter E, Holmback U, Knutson K, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 20.Ju S-Y, Choi W-S. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes. 2013;3:e65. doi: 10.1038/nutd.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robles TF, Carroll JE. Restorative biological processes and health. Soc Personal Psychol Compass. 2011;5(8):518–837. doi: 10.1111/j.1751-9004.2011.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci U S A. 2006;103(38):14158–63. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeman T, Gruenewald T, Karlamangla A, et al. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. Am J Hum Biol. 2010;22(4):463–72. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc. 2009;57(9):1525–31. doi: 10.1111/j.1532-5415.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom Med. 2006;68(3):500–7. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- 27.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation–allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–68. [PubMed] [Google Scholar]
- 28.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98(8):4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci U S A. 2013;110(42):17149–53. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie Ca, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein DR, Sidani S, Bootzin RR, Belyea MJ. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. Sleep. 2012;35(6):797–805. doi: 10.5665/sleep.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 35.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55(4):511–7. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen MH, Kruse A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin Interv Aging. 2012;7:185–90. doi: 10.2147/CIA.S32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of tai chi chih. Am J Geriatr Psychiatry. 2012;20(9):764–72. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive Behavioral Therapy vs. Tai Chi for Late Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial. Sleep. 2014;37(9):1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diagnostic and Statistical Manual of Mental Disorders DSM-IV. 4. American Psychiatric Association; 2000. [Google Scholar]
- 40.International Classification of Sleep Disorders (ICSD-2) American Academy of Sleep Medicine (AASM); 2005. [Google Scholar]
- 41.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 43.Pearson TA. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 44.Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 45.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation. 2005;112(17):e285–e290. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 46.Genuth S, Alberti KGMM, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 47.Qaseem A. Glycemic Control and Type 2 Diabetes Mellitus: The Optimal Hemoglobin A_1c Targets. A Guidance Statement from the American College of Physicians. Ann Intern Med. 2007;147(6):417. doi: 10.7326/0003-4819-147-6-200709180-00012. [DOI] [PubMed] [Google Scholar]
- 48.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1(Supplement_1)):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 49.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 50.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61–e99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jordan J. Obesity, insulin, and hypertension: why outliers count. J Hypertens. 2014;32(4):740–741. doi: 10.1097/HJH.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108(12):e81–e85. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- 53.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 55.Cizza G, Piaggi P, Rother KI, Csako G. Hawthorne effect with transient behavioral and biochemical changes in a randomized controlled sleep extension trial of chronically short-sleeping obese adults: implications for the design and interpretation of clinical studies. PLoS One. 2014;9(8):e104176. doi: 10.1371/journal.pone.0104176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 57.Buysse D. Sleep health: Can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]


