Abstract
Members of the urea transporter (UT) family mediate rapid, selective transport of urea down its concentration gradient. To date, crystal structures of two evolutionarily distant UTs have been solved. These structures reveal a common UT fold involving two structurally homologous domains that encircle a continuous membrane-spanning pore, and indicate that UTs transport urea via a channel-like mechanism. Examination of the conserved architecture of the pore, combined with crystal structures of ligand-bound proteins, molecular dynamics simulations, and functional data on permeation and inhibition by a broad range of urea analogs and other small molecules, provides insight into the structural basis of urea permeation and selectivity.
Keywords: urea transporter, urea channel, membrane proteins, crystallography, permeation
5.1. Structural studies of urea transporters
Proteins that mediate the movement of ions and small molecules across the lipid bilayer are typically classified into one of two categories based on their mechanism of transport: channels or transporters. Channels function by transporting the substrate along a continuous, semi-rigid pore that spans from one side of the bilayer to the other. In contrast, transporters have a central binding site for their substrates that is exposed alternately to one side of the bilayer or the other by a series of conformational changes. These different mechanisms have consequences for the functional properties of the transport protein. Channels are able to mediate extremely rapid flux, and can discriminate between different substrates based on their size, shape and other physical and chemical properties, but can only transport substrates along the direction of their electrochemical gradient; transporters typically have slower rates but are able to mediate “uphill” transport by coupling their conformational changes to an energetic driving force, such as a chemical reaction or the downhill transport of a secondary substrate. An indispensible tool for understanding how the functional properties of membrane transport proteins arise is an atomistic understanding of their three-dimensional structures. In this chapter, the structures of two members of the UT family are discussed and interpreted in terms of their mechanisms of permeation and selectivity.
Primary structure and function of UT
The amino acid sequences of members of the UT family are characterized by a number of shared features. UTs generally contain 10 predicted transmembrane helices, or in the case of the UT-A1 isoform, 20 predicted transmembrane helices from two UT domains in tandem. The regions corresponding to the first and last five transmembrane helices in each UT domain have significant homology to each other, indicating that the protein may have arisen from the duplication of an ancestral five-transmembrane helix protein (Minocha et al. 2003). Another notable feature determined from comparison of UT sequences is that each five-transmembrane repeat contains a conserved signature motif with the consensus sequence LPXXTXPF, which was proposed to play an important role in urea permeation (Rousselet et al. 1996). Functional studies of UTs revealed that the proteins mediate transport of urea passively down its concentration gradient at estimated single-protein rates in the range of 104–106 molecules per second, are selective for urea and a small number of urea analogs, and that their rates of transport saturate at high concentrations of urea (Mayrand and Levitt 1983; Mannuzzu et al. 1993; Zhao et al. 2007; Maciver et al. 2008). These properties are consistent with the behavior of a membrane channel.
Determination of UT structures by X-ray crystallography
A high-resolution structure is generally a prerequisite to understanding the mechanistic basis of a protein’s function. Since bacterial proteins are typically more stable and easier to crystallize, initial efforts to obtain a UT structure by X-ray crystallography focused on bacterial homologs. Bacteria can utilize urea for two purposes: as a source of nitrogen for building amino acids and nucleotides; and, in the case of enteric bacteria, to produce ammonia as a buffer against the highly acidic environment of the gastrointestinal system (Young et al. 1996; Marcus et al. 2005). In 2002, Sebbane and colleagues cloned and sequenced a urea transporter from the enteric pathogen Yersinia pseudotuberculosis, and demonstrated that it could complement UreI, a proton-gated urea channel from the ulcer-causing pathogen Helicobacter pylori (Sebbane et al. 2002). Surprisingly, yUT possessed no detectable homology to UreI, but instead shared 21–25% sequence identity to the mammalian urea transporters. As more bacterial genomes became available, other bacterial UTs were identified and characterized, including ApUT from Actinobacillus pleuropneumoniae (Godara et al. 2009). ApUT was the first UT to be purified in detergent and reconstituted into proteoliposomes, which were used in stopped-flow fluorimetry assays of urea permeation (Raunser et al. 2009). ApUT was shown to mediate rapid, downhill flux of urea, and was sensitive to inhibition by phloretin, a characteristic property of mammalian UTs (Hediger et al. 1996). These results demonstrated that bacterial UTs were suitable model systems for structural studies of the UT family. The first crystal structure of a UT family member was the homolog from the bacterium Desulfovibrio vulgaris, dvUT (Levin et al. 2009). This structure was later followed by the structure of UT-B from bovines, obtained by overexpressing the mammalian protein in insect cells (Levin et al. 2012).
5.2. Characteristics of the urea transporter fold
Topology and fold of dvUT and UT-B
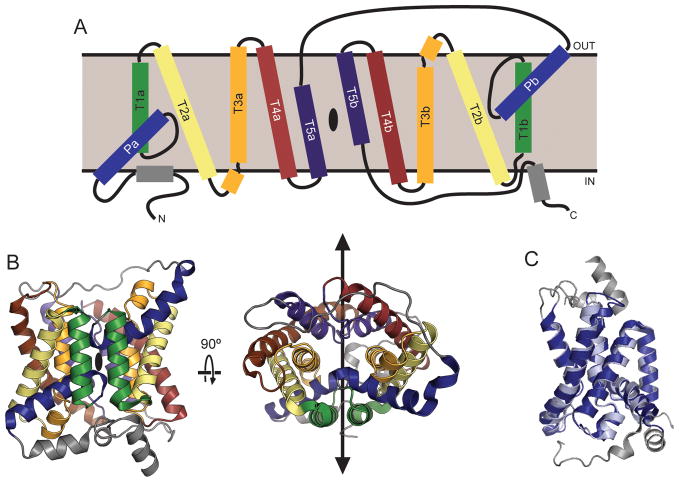
The dvUT and bovine UT-B structures are highly similar, and both possess the same overall fold. Both proteins form trimers with parallel orientations in the membrane (Figure 5.1A), with a large cavity at the trimer interface (Figure 5.1B). The fold of the individual protomers contains a total of fourteen α-helices, including ten that are transmembrane helices, and two short helices in the soluble regions of the N- and C-termini (Figure 5.2A). The remaining two helices are “re-entrant” helices; that is, they form part of structures that enter the membrane on one side, penetrate only partway into the bilayer, and then re-exit on the same side. The two re-entrant helices in the UT structures extend roughly halfway into the membrane, and are tilted at approximately 45° relative to the bilayer plane. Assuming that the UT proteins obey the “positive-inside” rule (von Heijne and Gavel 1988), the N- and C-termini are oriented towards the cytoplasm. This topology is consistent with the location of known sites of post-translational modifications, including N-glycosylation sites on the extracellular loop between the fifth and sixth transmembrane helix of both UT-A and UT-B (You et al. 1993; Tsukaguchi et al. 1997), and phosphorylation sites on the N-terminus of UT-A1 and UT-A3 (Bansal et al. 2010).
Figure 5.1. Oligomeric structure of urea transporters.
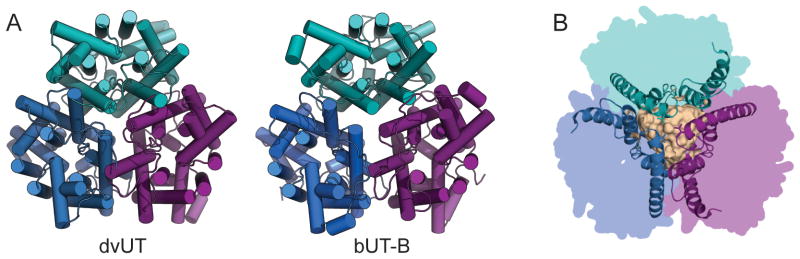
A. Crystal structures of the dvUT (left) and bovine UT-B (right) trimers, viewed from the extracellular side. B. Helices surrounding the cavity at the center of the trimer interface. The surface of the cavity is shown in tan.
Figure 5.2. Topology and fold of urea transporters.
A. Topology diagram of a UT protomer. Helices that are equivalent in the inverted repeats are colored in pairs; helices not part of the pseudosymmetry are gray. The location of the pseudosymmetry axis is marked with a black oval. B. Cartoon representation of the bUT-B protomer from two orientations, colored according to the same scheme as in panel A. The pseudosymmetry axis is marked with a black oval (left) or with a black line (right). C. The N- (light blue) and C-terminal (dark blue) halves of bUT-B, comprising the first and last five transmembrane helices, respectively, are shown superposed on each other.
The first five and last five transmembrane helices in the UT protomer each form a separate domain with an approximate semicircular shape; the two domains also each contain one re-entrant helix (Figure 5.2B). Consistent with their sequence homology (Minocha et al. 2003), the two domains are similar and can be aligned with a root mean square deviation (RMSD, the standard deviation of distances between equivalent atoms in two structures) of less than 1 Å (Figure 5.2C). Because their topology is inverted with respect to each other, the two repeats give the UT fold twofold pseudosymmetry along an axis parallel to the plane of the membrane. Inverted internal structural repeats are found frequently in membrane proteins, and have been observed previously in a number of channel and transporter folds (Abramson et al. 2003; Huang et al. 2003; Khademi et al. 2004; Yernool et al. 2004; Yamashita et al. 2005; Hu et al. 2011; Liao et al. 2012). At the center of the interface between the two domains, intersecting with the pseudosymmetry axis, lies a solvent-accessible pore that spans from one side of the bilayer to the other (Figure 5.3A).
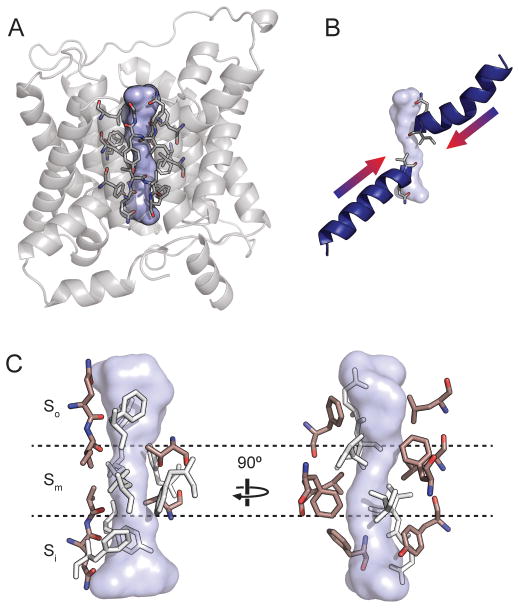
Figure 5.3. The urea transporter permeation pathway.
A. The central pore in a bUT-B protomer is shown as a blue surface. Residues lining the pore are shown as sticks. B. The locations of the pore helices are shown relative to the UT pore. C. The UT selectivity filter shown from two orientations, divided into the So, Sm, and Si regions. Conserved residues capable of forming hydrophilic (left) or hydrophobic (right) interactions with ligands within the filter are highlighted in pink.
Conservation with other UTs
Mammals have two UT genes that, via alternative splicing, are used to construct different proteins with at least five unique transmembrane domains. The bovine UT-B and dvUT have only approximately 33% sequence identity and yet the RMSD between their transmembrane helices is less than 1 Å. It is therefore likely that the basic features of the core hydrophobic regions of UT protomers, including the ten transmembrane-helix topology and the location of the permeation pathway, are conserved across the UT family. The soluble regions of the different mammalian UT proteins show more variation: for example, the UT-A isoforms UT-A1 and UT-A3 have an ~90 residue N-terminal domain, predicted to be unstructured, while the N-terminus of UT-B is relatively short, and UT-A1 has a more than 100-residue long intracellular linker connecting the two UT domains. Post-translational modifications in these regions likely account for differences in how the mammalian UT isoforms are regulated and trafficked (Klein et al. 2011).
While the individual subunits of different UT proteins likely have highly similar folds, it is not as clear whether all homologs possess the same oligomeric state. Native gel electrophoresis and a low-resolution projection map from 2-D crystals suggested that the bacterial homolog ApUT forms a dimer (Raunser et al. 2009). Because the UT-A1 isoform contains two tandem urea transporter domains, it cannot form a three-domain homotrimer similar to UT-B. It is also unlikely that UT-A1 forms a heterotrimer with UT-B or one of the single-domain UT-A isoforms because they are spatially segregated in renal tissues. UT-A3 and UT-A1 are both expressed in the epithelia of the inner medullary collecting ducts, but UT-A1 is localized to the apical membrane (Nielsen et al. 1996), while UT-A3 is localized to the basolateral membrane (Stewart et al. 2004). One possibility is that UT-A forms a higher order complex than UT-B, such as a hexamer. Because the permeation pathway is enclosed within a single protomer, differences in the number of subunits may not have a significant effect on the function of the protein. Alternatively, urea binding to dvUT was shown to exhibit a Hill coefficient of ~3 (Levin et al. 2009), which could be indicative of strong positive cooperativity between the different subunits. Additional structural studies on the UT-A homologs will likely be necessary to resolve this issue.
5.3. Features of the permeation pathway
The UT trimer contains a large cavity at the center of the subunit interface (Figure 5.1B), but it is blocked from the solvent on both sides of the membrane by hydrophobic residues, and the cavity interior is packed with lipid molecules. It is therefore more likely that the permeation pathway for urea and other solutes is the pore in the middle of each subunit, lined by highly conserved residues (Figure 5.3A). The structure of this pore is discussed in detail below.
Architecture of the UT selectivity filter
The UT pore can be divided into three regions: two hydrophilic vestibules forming the entrances to the pore on either side, which are likely of sufficient width for an entering urea molecule to retain its hydration waters, and a narrower region approximately 15 Å long at the center of the pore, lined with highly conserved residues. This constricted region, referred to as the selectivity filter, ranges in diameter from ~1 to 3 Å across and is roughly rectangular in cross section. The psuedosymmetry axis of the protein runs directly through the center of the pore. The structural elements forming the four walls of the pore are the two pore helices (Figure 5.3B), the third transmembrane helices from each psuedosymmetry repeat (T3a and T3b), and the fifth transmembrane helices from each repeat (T5a and T5b). These last two helices are actually shorter than the full length of the membrane, so that the regions forming the selectivity filter are unwound and expose backbone elements to the lumen of the pore. This region also corresponds to the location of the conserved signature motifs (Rousselet et al. 1996).
The selectivity filter can be further subdivided into outer, middle and inner regions, referred to as So, Sm, and Si (Figure 5.3C). So and Si are related by the pseudosymmetry axis and have a similar architecture. One wall of the pore at these sites is formed by a row of oxygen atoms contributed by backbone carbonyls and side chains located on the C-terminal ends of the pore helices, which point directly into the selectivity filter. The ability of these atoms to act as hydrogen bond acceptors would therefore likely be strengthened by the helix dipole moments. These oxygen atoms are flanked by hydrophobic residues from T3a and T3b, which form the two perpendicular walls of the selectivity filter. The Sm site is the narrowest region of the pore, and is hydrophobic except for a pair of threonine residues from the conserved LPXXTXPF motifs on the N-terminal ends of T5a and T5b. These residues form a hydrogen bond that crosses the center of the selectivity filter.
Comparison to other solute channels
Urea transporters are one of three unrelated families of proteins of known structure that transport urea by a channel-like mechanism. The others are the proton-gated UreI channels (Strugatsky et al. 2013), most notable for conferring acid resistance to Helicobacter pylori (Weeks et al. 2000), and some members of the aquaporin family, often referred to as the aquaglyceroporins, which are capable of transporting urea in addition to water and other small polar molecules such as glycerol (Newby et al. 2008). Surprisingly, there is no apparent similarity between the permeation pathways of UTs and UreI, whose selectivity filter is characterized by two constrictions ringed by aromatic residues (McNulty et al. 2013; Strugatsky et al. 2013). In contrast, the permeation pathways of aquaporins show obvious parallels to the UT pore, particularly in the presence of reentrant helices and exposed backbone carbonyls that stabilize the permeant water molecules through hydrogen bonds. Interestingly, the aquaporins also have a pseudosymmetry axis that intersects the center of the permeation pathway. The center of the pore harbors the NPA motifs, containing two pseudosymmetry-related asparagines that are reminiscent of the central threonines in UT, and that contribute to selectivity in aquaporins (Murata et al. 2000; Wang et al. 2005; Wree et al. 2011).
There are also elements of similarity between the UT pore and the permeation pathways of some ion channels. In tetrameric K+ channels, the central permeation pathway is encircled by four re-entrant pore helices (Doyle et al. 1998), whose dipole moments are thought to help stabilize K+ within the hydrophobic core of the membrane (Roux and MacKinnon 1999). Exposed backbone carbonyls are also key features of the K+ channel selectivity filter and provide octahedral coordination to replace the hydration sphere on K+, although these oxygens are located on non-helical segments following the pore helices, rather than being located directly on the helix C-termini. Tilted re-entrant helices also play a role in permeation of chloride ions in the CLC channels (Dutzler et al. 2002).
Interestingly, the spatial organization of the ten transmembrane helices in the UT fold is similar to that of the first ten helices in the ammonia transporters of the Amt/Rh family (Khademi et al. 2004; Lupo et al. 2007; Gruswitz et al. 2010). Because the ammonia transporters lack equivalents to the UT pore helices, their largely hydrophobic pores bear little resemblance to the pores of the UT proteins. However, the similarities in their folds suggest a possible shared evolutionary origin.
5.4. Interactions with ligands, and the structural basis of selectivity and inhibition
The structures of dvUT and bovine UT-B provide a framework for understanding the mechanism of binding and permeation of urea and urea analogs, which, although not naturally occurring compounds, can be useful tools for understanding the structural determinants of selectivity in UTs. The structures may also aid in optimizing the binding of clinically useful UT inhibitors. The structural basis of interactions between UTs and various substrates and inhibitors is discussed below, based on information from crystal structures bound to urea analogs, molecular dynamics simulations, functional studies and docking models.
Interactions with urea and urea analogs
There are currently no crystal structures available for any UT protein bound to urea, but structures have been solved for dvUT bound to the urea analog 1,3-dimethylurea (DMU) (Levin et al. 2009) and bovine UT-B bound to selenourea (Figure 5.4A) (Levin et al. 2012). Both structures contain two molecules of the substrate bound to the selectivity filter, one in So and the second in Si (Figure 5.4B–C). Both DMU and selenourea are oriented with their amide nitrogens positioned to form hydrogen bonds with the oxygen atoms at the ends of the pore helices. In the DMU-bound structure, ordered water molecules are also visible forming hydrogen bonds with the oxygen atoms on both DMU molecules, suggesting that the substrate is not fully desolvated in these regions. Comparison of the binding sites of the two substrates shows that their locations are not exactly equivalent: DMU bound to So is positioned to potentially form hydrogen bonds with the first and second oxygen atoms, while selenourea is positioned to interact with the second and third. Similarly, the binding sites are slightly offset in Si as well. This suggests a mechanism for how the selectivity filter could continuously provide at least two hydrogen bonds to a urea molecule migrating stepwise through the So and Si sites. No electron density corresponding to substrates was observed in the Sm region; however, it remained ambiguous whether this was due to the absence of a stable binding site in this region, or because the larger, impermeable DMU and selenourea analogs could not enter the narrowest region of the pore.
Figure 5.4. Ligand binding sites in the urea transporter selectivity filter.
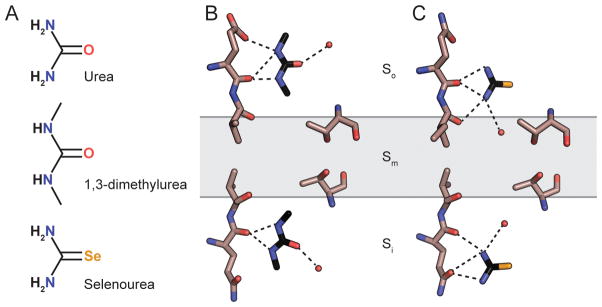
A. The chemical structures of urea, dimethylurea (DMU), and selenourea. B–C. The locations of DMU molecules in the dvUT structure (A) and selenourea molecules in the bUT-B structure (B) are shown relative to the oxygens lining one side of the selectivity filter. The central threonines in the Sm site and ordered water molecules within hydrogen bonding distance to the bound substrates are shown as well.
To gain additional information on the interaction between urea and the pore, particularly in the Sm region, molecular dynamics simulations were carried out on bovine UT-B and used to calculate a potential of mean force (PMF) for urea permeation, representing the change in the total energy of the system as a function of the position of urea in the pore (Levin et al. 2012). Multiple local energy minima were observed in the So and Si sites, indicating that these regions contain a series of low affinity urea binding sites. These minima agree well with the observed positions of urea analogs in the crystal structures. Also in agreement with the ligand-bound crystal structures, urea in the So and Si sites was still partly solvated and oriented in alignment to the pore helix dipoles. Upon entering the Sm site, urea was completely stripped of hydration waters and the PMF exhibits a large energy barrier, with a maximal ΔG of ~5 kcal/mol. No conformational changes other than thermal fluctuations were required for urea to pass completely through the pore, confirming that UTs are channels, not transporters.
Of the different UTs, the selectivity of UT-B has been the most thoroughly characterized due in part to the early development of assays for measuring substrate flux through UT-B in erythrocytes (Levitt and Mlekoday 1983; Chasan and Solomon 1985; Yang and Verkman 1998). This approach has been used to test the selectivity of UT-B with a large number of urea analogues (Zhao et al. 2007). The channel can permeate formamide and acetamide at rates close to those for urea, indicating that loss or substitution of one amide nitrogen is tolerated. Transport is significantly decreased by changes to the substrate that increase the size and decrease the strength of potential hydrogen bonds, such as substitution of the oxygen atom with sulfur in thiourea, or N-methylation of the amides in DMU; however both of these compounds are effective inhibitors of UT-B. The structural data discussed above provide a rationale for this behavior: DMU and thiourea are able to compete with urea for binding to the So and Si sites, but have difficulty permeating through the constricted Sm site.
In comparison to UT-B, functional data on UT-A is relatively scarce; however, transport through mouse UT-A2 and UT-A3 has been measured in vesicles derived from oocyte plasma membranes (Maciver et al. 2008). Both homologs exhibited higher selectivity than UT-B and did not permeate any of the tested urea analogs, including formamide and acetamide. The residues forming the selectivity filter in both UT-A domains and UT-B are similar, and the structural basis for this difference in selectivity is not yet understood.
Interactions with other natural substrates
The permeation of water through UT-B, although initially controversial (Martial et al. 1996; Sidoux-Walter et al. 1999), has since been well established (Yang and Verkman 1998; Yang and Verkman 2002; Meng et al. 2005). Recent estimates of the rate of water permeation through UT-B are similar to those measured for aquaporins (Azouzi et al. 2013). Given the similarities between the architecture of the aquaporin and UT pores discussed above, this result is not entirely surprising. Molecular dynamics simulations of water permeation through a homology model of human UT-B, based on the bovine UT-B structure, suggest that the magnitude of the energy barrier for water permeation is similar to that observed for aquaporins (Azouzi et al. 2013). The observation that UT-B is an efficient water channel raises the question of how protons are excluded from leaking through the pore. In aquaporins, proton exclusion has been attributed primarily to two electrostatic barriers: one at a constriction formed by positively-charged residues, and another at the NPA motifs, where the positive ends of two helix dipoles point into the pore (Beitz et al. 2006; Wu et al. 2009). In contrast, the UT selectivity filter lacks any positively charged side chains, and the orientations of the two pore helices are reversed relative to aquaporins, leading to a negative potential at the Sm site rather than a positive one. Interestingly, molecular dynamics simulations predict that water permeating through UT-B undergoes a reversal of the orientation of its dipole moment correlated to passage through the Sm site (Azouzi et al. 2013). A similar change in orientation was predicted by molecular dynamics simulations in aquaporins (de Groot and Grubmuller 2001; Tajkhorshid et al. 2002), and recently gained experimental support from an ultra-high crystal structure of a yeast aquaporin (Kosinska Eriksson et al. 2013). This re-orientation was proposed to play a role in proton exclusion in aquaporins.
Permeability of human UT-B to ammonia, but not ammonium, has also been reported recently (Geyer et al. 2013). Molecular dynamics simulations suggest that the ammonia is transported via the same central pore that serves as the urea and water permeation pathway, and that the energetics of permeation are similar to those for water, including the location and size of the central energy barrier at the Sm site.
The ability to transport water and ammonia has also been assayed for UT-A. UT-A2 and UT-A3 were found to be impermeable to both compounds (Maciver et al. 2008). This observation fits with the overall pattern of higher selectivity for the UT-A isoforms. Given that the rate of flux through UT-A is 1–2 orders of magnitude slower than UT-B (Mannuzzu et al. 1993; Maciver et al. 2008), the diameter of the Sm site could be smaller, resulting in a higher energy barrier. However, it is counterintuitive to imagine that a narrower permeation pathway could result in an improved ability of the channel to reject substrates smaller than its native ligand. The residues that line the selectivity filter are well conserved between UT-A2, UT-A3, and UT-B, including at the Sm site, making it difficult to ascertain the basis for these differences without a UT-A crystal structure.
Inhibitors of UT permeation
In addition to urea analogs, there are a number of organic compounds that are structurally unrelated to urea known to inhibit UTs. The plant flavonoid phloretin was the first compound of this class to be discovered, and inhibits UTs with micromolar affinity (Chou and Knepper 1989). However, phloretin is a relatively non-selective inhibitor of a large number of structurally unrelated transport proteins (Wheeler and Hinkle 1981; Tsukaguchi et al. 1998; Wang et al. 2000; Fan et al. 2001; Xiang et al. 2007), and may modulate transport by directly affecting the physical properties of the lipid bilayer (Andersen et al. 1976; Cseh and Benz 1999). More recently, the search for novel diuretics has lead to the discovery of multiple classes of high affinity inhibitors of the mammalian UTs (Levin et al. 2007; Anderson et al. 2012; Yao et al. 2012; Esteva-Font et al. 2013; Li et al. 2013; Liu et al. 2013), including compounds that are selective for UT-B over UT-A (Yao et al. 2012) and vice versa (Esteva-Font et al. 2013). Currently there is no crystal structure available of any these compounds bound to the protein. Given the large size of the inhibitors relative to the dimensions of the pore, they seem unlikely to bind at the predicted urea binding sites within the selectivity filter itself, but curiously, the IC50s of the triazolothienopyrimidine UT-B inhibitors were sensitive to the magnitude of the urea gradient, suggesting a competitive mechanism of inhibition involving shared binding sites. In contrast, the UT-A selective compounds exhibited noncompetitive inhibition (Esteva-Font et al. 2013). By measuring the kinetics of inhibition, Yao et al. were able to demonstrate that the triazolothienopyrimidine-derived UT-B selective inhibitors must diffuse through the bilayer and bind to the intracellular side of protein (Yao et al. 2012); similar experiments demonstrated that non-selective thienoquinolin inhibitors block from the intracellular side (Li et al. 2013), and selective UT-A inhibitors bound to either the intracellular or extracellular side (Esteva-Font et al. 2013). Computational docking models predict that these compounds block the pores by binding to the vestibules immediately outside of the selectivity filter.
Acknowledgments
This work was supported by the National Institutes of Health (5R01DK088057 and 5R01GM098878), the American Heart Association (12EIA8850017) and the Cancer Prevention and Research Institute of Texas (R12MZ).
References
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- Andersen OS, Finkelstein A, Katz I, Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976;67:749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MO, Zhang J, Liu Y, Yao C, Phuan PW, Verkman AS. Nanomolar potency and metabolically stable inhibitors of kidney urea transporter UT-B. J Med Chem. 2012;55:5942–5950. doi: 10.1021/jm300491y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouzi S, Gueroult M, Ripoche P, Genetet S, Aronovicz YC, Le Van Kim C, Etchebest C, Mouro-Chanteloup I. Energetic and Molecular Water Permeation Mechanisms of the Human Red Blood Cell Urea Transporter B. PLOS ONE. 2013;8:e82338. doi: 10.1371/journal.pone.0082338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou CL, Boja ES, Wang G, Knepper MA. Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol. 2010;21:303–315. doi: 10.1681/ASN.2009070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci U S A. 2006;103:269–274. doi: 10.1073/pnas.0507225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan B, Solomon AK. Urea reflection coefficient for the human red cell membrane. Biochim Biophys Acta. 1985;821:56–62. doi: 10.1016/0005-2736(85)90152-x. [DOI] [PubMed] [Google Scholar]
- Chou CL, Knepper MA. Inhibition of urea transport in inner medullary collecting duct by phloretin and urea analogues. Am J Physiol. 1989;257:F359–365. doi: 10.1152/ajprenal.1989.257.3.F359. [DOI] [PubMed] [Google Scholar]
- Cseh R, Benz R. Interaction of phloretin with lipid monolayers: relationship between structural changes and dipole potential change. Biophys J. 1999;77:1477–1488. doi: 10.1016/S0006-3495(99)76995-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot BL, Grubmuller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Esteva-Font C, Phuan PW, Anderson MO, Verkman AS. A small molecule screen identifies selective inhibitors of urea transporter UT-A. Chem Biol. 2013;20:1235–1244. doi: 10.1016/j.chembiol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HT, Morishima S, Kida H, Okada Y. Phloretin differentially inhibits volume-sensitive and cyclic AMP-activated, but not Ca-activated, Cl(−) channels. Br J Pharmacol. 2001;133:1096–1106. doi: 10.1038/sj.bjp.0704159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer RR, Musa-Aziz R, Enkavi G, Mahinthichaichan P, Tajkhorshid E, Boron WF. Movement of NH(3) through the human urea transporter B: a new gas channel. Am J Physiol Renal Physiol. 2013;304:F1447–1457. doi: 10.1152/ajprenal.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godara G, Smith C, Bosse J, Zeidel M, Mathai J. Functional characterization of Actinobacillus pleuropneumoniae urea transport protein, ApUT. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1268–1273. doi: 10.1152/ajpregu.90726.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, Sali A, Westhoff CM, Stroud RM. Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci U S A. 2010;107:9638–9643. doi: 10.1073/pnas.1003587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Smith CP, You G, Lee WS, Kanai Y, Shayakul C. Structure, regulation and physiological roles of urea transporters. Kidney Int. 1996;49:1615–1623. doi: 10.1038/ki.1996.235. [DOI] [PubMed] [Google Scholar]
- Hu NJ, Iwata S, Cameron AD, Drew D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature. 2011;478:408–411. doi: 10.1038/nature10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- Khademi S, O’Connell J, 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- Klein JD, Blount MA, Sands JM. Urea transport in the kidney. Compr Physiol. 2011;1:699–729. doi: 10.1002/cphy.c100030. [DOI] [PubMed] [Google Scholar]
- Kosinska Eriksson U, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R. Subangstrom resolution X-ray structure details aquaporin-water interactions. Science. 2013;340:1346–1349. doi: 10.1126/science.1234306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EJ, Cao Y, Enkavi G, Quick M, Pan Y, Tajkhorshid E, Zhou M. Structure and permeation mechanism of a mammalian urea transporter. Proc Natl Acad Sci U S A. 2012;109:11194–11199. doi: 10.1073/pnas.1207362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EJ, Quick M, Zhou M. Crystal structure of a bacterial homologue of the kidney urea transporter. Nature. 2009;462:757–761. doi: 10.1038/nature08558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MH, de la Fuente R, Verkman AS. Urearetics: a small molecule screen yields nanomolar potency inhibitors of urea transporter UT-B. FASEB J. 2007;21:551–563. doi: 10.1096/fj.06-6979com. [DOI] [PubMed] [Google Scholar]
- Levitt DG, Mlekoday HJ. Reflection coefficient and permeability of urea and ethylene glycol in the human red cell membrane. J Gen Physiol. 1983;81:239–253. doi: 10.1085/jgp.81.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lei T, Zhu J, Wang W, Sun Y, Chen J, Dong Z, Zhou H, Yang B. A novel small-molecule thienoquinolin urea transporter inhibitor acts as a potential diuretic. Kidney Int. 2013;83:1076–1086. doi: 10.1038/ki.2013.62. [DOI] [PubMed] [Google Scholar]
- Liao J, Li H, Zeng W, Sauer DB, Belmares R, Jiang Y. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science. 2012;335:686–690. doi: 10.1126/science.1215759. [DOI] [PubMed] [Google Scholar]
- Liu Y, Esteva-Font C, Yao C, Phuan PW, Verkman AS, Anderson MO. 1,1-Difluoroethyl-substituted triazolothienopyrimidines as inhibitors of a human urea transport protein (UT-B): new analogs and binding model. Bioorg Med Chem Lett. 2013;23:3338–3341. doi: 10.1016/j.bmcl.2013.03.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo D, Li XD, Durand A, Tomizaki T, Cherif-Zahar B, Matassi G, Merrick M, Winkler FK. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci U S A. 2007;104:19303–19308. doi: 10.1073/pnas.0706563104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver B, Smith CP, Hill WG, Zeidel ML. Functional characterization of mouse urea transporters UT-A2 and UT-A3 expressed in purified Xenopus laevis oocyte plasma membranes. Am J Physiol Renal Physiol. 2008;294:F956–964. doi: 10.1152/ajprenal.00229.2007. [DOI] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Macey RI. Estimate of the number of urea transport sites in erythrocyte ghosts using a hydrophobic mercurial. J Membr Biol. 1993;133:85–97. doi: 10.1007/BF00231880. [DOI] [PubMed] [Google Scholar]
- Marcus EA, Moshfegh AP, Sachs G, Scott DR. The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol. 2005;187:729–738. doi: 10.1128/JB.187.2.729-738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial S, Olives B, Abrami L, Couriaud C, Bailly P, You G, Hediger MA, Cartron JP, Ripoche P, Rousselet G. Functional differentiation of the human red blood cell and kidney urea transporters. Am J Physiol. 1996;271:F1264–1268. doi: 10.1152/ajprenal.1996.271.6.F1264. [DOI] [PubMed] [Google Scholar]
- Mayrand RR, Levitt DG. Urea and ethylene glycol-facilitated transport systems in the human red cell membrane. Saturation, competition, and asymmetry. J Gen Physiol. 1983;81:221–237. doi: 10.1085/jgp.81.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty R, Ulmschneider JP, Luecke H, Ulmschneider MB. Mechanisms of molecular transport through the urea channel of Helicobacter pylori. Nat Commun. 2013;4:2900. doi: 10.1038/ncomms3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhou X, Li Y, Zhao D, Liang S, Zhao X, Yang B. A novel mutation at the JK locus causing Jk null phenotype in a Chinese family. Sci China C Life Sci. 2005;48:636–640. doi: 10.1360/062005-127. [DOI] [PubMed] [Google Scholar]
- Minocha R, Studley K, Saier MH., Jr The urea transporter (UT) family: bioinformatic analyses leading to structural, functional, and evolutionary predictions. Receptors Channels. 2003;9:345–352. doi: 10.3109/714041015. [DOI] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Newby ZE, O’Connell J, 3rd, Robles-Colmenares Y, Khademi S, Miercke LJ, Stroud RM. Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol. 2008;15:619–625. doi: 10.1038/nsmb.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin- regulated urea transporter in rat kidney. Proc Natl Acad Sci U S A. 1996;93:5495–5500. doi: 10.1073/pnas.93.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunser S, Mathai JC, Abeyrathne PD, Rice AJ, Zeidel ML, Walz T. Oligomeric structure and functional characterization of the urea transporter from Actinobacillus pleuropneumoniae. J Mol Biol. 2009;387:619–627. doi: 10.1016/j.jmb.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet G, Ripoche P, Bailly P. Tandem sequence repeats in urea transporters: identification of an urea transporter signature sequence. Am J Physiol. 1996;270:F554–555. doi: 10.1152/ajprenal.1996.270.3.F554. [DOI] [PubMed] [Google Scholar]
- Roux B, MacKinnon R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science. 1999;285:100–102. doi: 10.1126/science.285.5424.100. [DOI] [PubMed] [Google Scholar]
- Sebbane F, Bury-Mone S, Cailliau K, Browaeys-Poly E, De Reuse H, Simonet M. The Yersinia pseudotuberculosis Yut protein, a new type of urea transporter homologous to eukaryotic channels and functionally interchangeable in vitro with the Helicobacter pylori UreI protein. Mol Microbiol. 2002;45:1165–1174. doi: 10.1046/j.1365-2958.2002.03096.x. [DOI] [PubMed] [Google Scholar]
- Sidoux-Walter F, Lucien N, Olives B, Gobin R, Rousselet G, Kamsteeg EJ, Ripoche P, Deen PM, Cartron JP, Bailly P. At physiological expression levels the Kidd blood group/urea transporter protein is not a water channel. J Biol Chem. 1999;274:30228–30235. doi: 10.1074/jbc.274.42.30228. [DOI] [PubMed] [Google Scholar]
- Stewart GS, Fenton RA, Wang W, Kwon TH, White SJ, Collins VM, Cooper G, Nielsen S, Smith CP. The basolateral expression of mUT-A3 in the mouse kidney. Am J Physiol Renal Physiol. 2004;286:F979–987. doi: 10.1152/ajprenal.00334.2003. [DOI] [PubMed] [Google Scholar]
- Strugatsky D, McNulty R, Munson K, Chen CK, Soltis SM, Sachs G, Luecke H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2013;493:255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O’Connell J, Stroud RM, Schulten K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, Hediger MA. Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Tokui T, Brown D, Hediger MA. Cloning and characterization of the urea transporter UT3: localization in rat kidney and testis. J Clin Invest. 1997;99:1506–1515. doi: 10.1172/JCI119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988;174:671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun. 2000;267:488–494. doi: 10.1006/bbrc.1999.1929. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schulten K, Tajkhorshid E. What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure. 2005;13:1107–1118. doi: 10.1016/j.str.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482–485. doi: 10.1126/science.287.5452.482. [DOI] [PubMed] [Google Scholar]
- Wheeler TJ, Hinkle PC. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J Biol Chem. 1981;256:8907–8914. [PubMed] [Google Scholar]
- Wree D, Wu B, Zeuthen T, Beitz E. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J. 2011;278:740–748. doi: 10.1111/j.1742-4658.2010.07993.x. [DOI] [PubMed] [Google Scholar]
- Wu B, Steinbronn C, Alsterfjord M, Zeuthen T, Beitz E. Concerted action of two cation filters in the aquaporin water channel. EMBO J. 2009;28:2188–2194. doi: 10.1038/emboj.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Feng M, Muend S, Rao R. A human Na+/H+ antiporter sharing evolutionary origins with bacterial NhaA may be a candidate gene for essential hypertension. Proc Natl Acad Sci U S A. 2007;104:18677–18681. doi: 10.1073/pnas.0707120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Urea transporter UT3 functions as an efficient water channel. Direct evidence for a common water/urea pathway. J Biol Chem. 1998;273:9369–9372. doi: 10.1074/jbc.273.16.9369. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Analysis of double knockout mice lacking aquaporin-1 and urea transporter UT-B. Evidence for UT-B-facilitated water transport in erythrocytes. J Biol Chem. 2002;277:36782–36786. doi: 10.1074/jbc.M206948200. [DOI] [PubMed] [Google Scholar]
- Yao C, Anderson MO, Zhang J, Yang B, Phuan PW, Verkman AS. Triazolothienopyrimidine inhibitors of urea transporter UT-B reduce urine concentration. J Am Soc Nephrol. 2012;23:1210–1220. doi: 10.1681/ASN.2011070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. Cloning and characterization of the vasopressin-regulated urea transporter. Nature. 1993;365:844–847. doi: 10.1038/365844a0. [DOI] [PubMed] [Google Scholar]
- Young GM, Amid D, Miller VL. A bifunctional urease enhances survival of pathogenic Yersinia enterocolitica and Morganella morganii at low pH. J Bacteriol. 1996;178:6487–6495. doi: 10.1128/jb.178.22.6487-6495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Sonawane ND, Levin MH, Yang B. Comparative transport efficiencies of urea analogues through urea transporter UT-B. Biochim Biophys Acta. 2007;1768:1815–1821. doi: 10.1016/j.bbamem.2007.04.010. [DOI] [PubMed] [Google Scholar]