SUMMARY
The discovery that enhancers are regulated transcription units, encoding eRNAs, has raised new questions about the mechanisms of their activation. Here, we report an unexpected molecular mechanism that underlies ligand-dependent enhancer activation, based on DNA nicking to relieve torsional stress from eRNA synthesis. Using dihydrotestosterone (DHT)-induced binding of androgen receptor (AR) to prostate cancer cell enhancers as a model, we show rapid recruitment, within minutes, of DNA topoisomerase I (TOP1) to a large cohort of AR-regulated enhancers. Furthermore, we show that the DNA nicking activity of TOP1 is a prerequisite for robust eRNA synthesis and enhancer activation, and is kinetically accompanied by the recruitment of ATR and the MRN complex, followed by additional components of DNA damage repair machinery to the AR-regulated enhancers. Together, our studies reveal a linkage between eRNA synthesis and ligand-dependent TOP1-mediated nicking a strategy exerting quantitative effects on eRNA expression in regulating AR-bound enhancer-dependent transcriptional programs.
INTRODUCTION
Research over the past few years, supported by data from GRO-seq analysis and the ENCODE project, has revealed that most developmental and regulatory transcriptional regulation programs are controlled by an extensive enhancer network (Kim et al., 2010; Shlyueva et al., 2014), with each cell type estimated to harbor 70,000–100,000 enhancers, located upstream and downstream of coding target gene promoters (Pennacchio et al., 2013). Enhancer signatures include mono-methylated H3K4 (H3K4me1) and H3K27-acetylated histones (Kim et al., 2010; Li et al., 2013a; Wang et al., 2011). These enhancers are usually characterized by a nucleosome-depleted core region where many of the cooperating transcription factors bind (Andersson et al., 2014; Hah et al., 2013; Kaikkonen et al., 2013; Lai et al., 2013; Lam et al., 2013; Li et al., 2013a; Melgar et al., 2011; Melo et al., 2013; Mousavi et al., 2013). Most surprisingly, enhancers are also transcription units, wherein their effect on target coding genes correlates with the transcription of the lncRNAs, referred to as eRNAs (Andersson et al., 2014; De Santa et al., 2010; Hah et al., 2013; Kaikkonen et al., 2013; Kim et al., 2010; Lai et al., 2013; Lam et al., 2013; Li et al., 2013a; Melgar et al., 2011; Melo et al., 2013; Mousavi et al., 2013) adding a new layer of regulation to the fundamental mechanisms underlying enhancer action (Lam et al., 2014; Natoli and Andrau, 2012).
The current prevailing belief, based on chromosome capture assays, where looping constraints are inferred from interaction frequencies between a point of interest and distal loci of the genome is that the main mechanism by which enhancers affect their target gene expression is through chromatin looping. eRNAs transcripts seem to be functionally important by contributing to the stabilization of juxtaposed enhancer-target gene promoter loops to allow for optimal gene expression (Lai et al., 2013; Li et al., 2013a). However, both eRNA synthesis and nucleosome depletion are potential sources of topological strain on enhancers that can potentially hinder transcription. The movement and rotation of RNA polymerase complex (RNAP) along DNA template during the process of RNA synthesis (Liu and Wang, 1987) can generate positive supercoils in front of the advancing RNAP, and negative supercoils behind it (Darzacq et al., 2007; Kouzine et al., 2013; Kouzine and Levens, 2007; Liu and Wang, 1987). Because RNA polymerase is a powerful torsional motor, it can alter DNA topology by creating DNA supercoils, which can propagate and affect transcription elongation (Ma and Wang, 2014). While negative supercoiling can initially facilitate transcription initiation, either by helping RNAP to form an open complex or by helping to recruit transcription factors (Ma and Wang, 2014), it can subsequently lead to the generation of R-loops resulting from hybridization of nascent RNA to the DNA strand that is being transcribed, which in turn can impede transcriptional elongation (El Hage et al., 2010). Positive or over-wound supercoiling can prevent transcription initiation and greatly diminish mRNA synthesis (Ma and Wang, 2014). Moreover, the very depletion of histones from the core region of enhancers releases unconstrained negative supercoils, which can impede transcription factor binding. One mechanism that resolves the undesirable effects of excessive supercoiling employs DNA topoisomerases, including topoisomerase I (TOP1). TOP1 can relax both negative and positive supercoils by transient single-strand breaks for the passage of individual DNA strands through one another, followed by the rejoining of the phosphodiester backbone of DNA (Pedersen et al., 2012; Pommier et al., 2006).
While TOP1 activity is well established in DNA replication, its potential functionality in enhancer activation and transcriptional initiation remains unclear. Most of the experiments hitherto examining the role of TOP1 in transcription have been limited to artificial promoter model systems which, if anything, have argued that TOP1 DNA nicking activity is not involved in transcriptional activation in such in vitro systems (Kretzschmar et al., 1993; Merino et al., 1993; Shykind et al., 1997).
However, the utilization of a nicking strategy for transcriptional initiation and enhancer regulated events would be in concert with the elegant explication of the molecular mechanisms underlying the expression of bacteriophage T4 late genes, with the participation of DNA-mounted activator of transcription, gp45 and RNAP-bound gp33. Here, a nick in the strands of the DNA and the actions of an exonuclease are required, with the DNA template single-strand nicks being essential for transcriptional activation and the nicked-DNA gp45-loading site located upstream or downstream of its target site (Herendeen et al., 1992). Also, in human cells, artificially-generated nicks (but not double-strand DNA breaks) have recently been found to be associated with transcription (Davis and Maizels, 2014). Together, these and other experiments in prokaryotes and eukaryotes suggest an intriguing link between DNA nicking and transcription but the mechanism and the factors involved remain largely unknown.
Here, we describe a molecular mechanism that operates at functional androgen-regulated enhancers and identify DNA topoisomerase I as a critical DNA-nicking enzyme involved in the process of cell-specific, ligand-driven enhancer activation. Recruitment of TOP1 to these AR-bound enhancers is of functional consequence as knockdown of the enzyme in the prostate cancer cells results in inhibition of DHT-regulated eRNA and many coding gene transcriptional targets. Additionally, we provide evidence that recruitment of a significant repertoire of DNA damage response machinery occurs on these functional enhancers, potentially to prevent undesirable effects of persistent DNA damage.
RESULTS
TOP1 Recruitment to AR-Regulated Enhancers Affects eRNA and Coding Gene Expression
To further investigate the mechanism of enhancer activation in ligand-regulated transcription, we employed an early prostate adenocarcinoma cell line, LNCaP, the growth of which is androgen-dependent (Horoszewicz et al., 1980). The cell line is exquisitely sensitive to androgen stimulation and arrests in the G1 phase of the cell cycle upon steroid depletion, despite the presence of peptide growth factors (Fig. S1A). Regulation of cyclin D expression and concomitant CDK4 activity represents one mechanism by which androgen impinges on the cell cycle to govern proliferation (Knudsen et al., 1998).
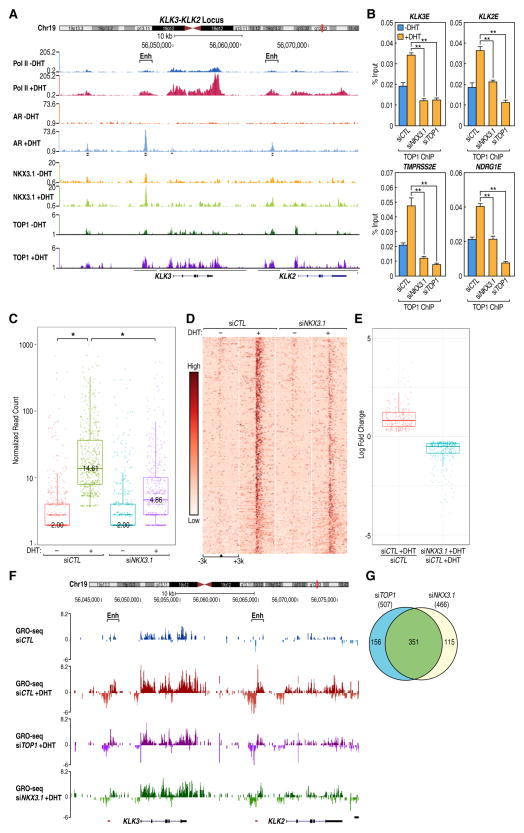
To investigate whether TOP1 played a role in ligand-regulated transcription, we undertook to examine the possible recruitment of TOP1 to enhancers; finding that it was recruited to several AR-enhancers early in response to androgen (5α-dihydrotestosterone, DHT) treatment in the ligand-dependent LNCaP prostate cancer cell line (Figure 1A, Figure S1B). These data prompted us to study genome-wide localization of this protein by performing chromatin immunoprecipitation (ChIP) coupled with next-generation sequencing (ChIP-seq). Because exhaustive efforts to identify a TOP1 antibody suitable for ChIP-seq proved unsuccessful, we generated a stable LNCaP cell line with inducible biotinylated TOP1 expression. We observed that TOP1 recruitment in response to DHT, generated enriched regions of a range of sizes (Fig. 1B), as opposed to point sources, as found for factors such as androgen receptor, or broad sources, such as observed for the H3K36me3 histone mark (Sims et al., 2014). Consistent with the observation that enhancers represent regulated transcription units, we noticed a hormone-dependent increase in RNA PolII (phospho-Ser5) occupancy predominately at these enhancers (Figure 1B, Figure S1C). As expected, we also observed increased TOP1 occupancy over promoters and gene bodies of the representative DHT-induced genes (e.g., KLK3 and KLK2), consistent with the possibility that TOP1 might be involved in both enhancer activation and transcriptional elongation events.
Figure 1. TOP1 Occupies AR-enhancers and Affects the Transcriptional Program of the Prostate Cancer Cell Line LNCaP.
(A) Recruitment of AR and TOP1 to the KLK3 and KLK2 enhancers. The highest TOP1 binding is detected at 15 min DHT treatment. Data points show mean ± s.d.; (n=3), *P<0.05, **P<0.01. (B) The UCSC genome browser screenshot of the KLK3-KLK2 locus showing the occupancy of p-S5-RNA PolII (Pol II), AR and TOP1 (all tested with and without DHT treatment). (C) GRO-seq analysis of the effect of TOP1 knockdown on nascent RNA levels shown as a heatmap for 579 (out of 644, which were upregulated by DHT treatment) with the most affected AR-enhancers at the top. (D) Heatmap showing DHT-induced TOP1 sequencing tags density increase around 644 AR-enhancer binding sites (centered on AR). (E) Boxplot: siTOP1 reduced transcription at ~ 80% of DHT-up-regulated AR-enhancers. *P 2.2e-16 (Wilcoxon test). (F) Boxplot: The response to DHT of 368 DHT-up-regulated genes was reduced after TOP1 knockdown by siRNA. (G) Knockdown of TOP1 affects the induction of both eRNA and mRNA. LNCaP cells, hormone-starved for 1 day and transfected with the indicated siRNA, were stimulated with 100 nM DHT for 1h (eRNA) or 5 h (mRNA) 48 h post transfection. Quantitative RT-PCR was performed with SYBR Green using reverse-transcribed RNA. Data represent mean ± s.d.; (n=3), **P<0.01. (H) Recruitment of ATR to the KLK3 and KLK2 enhancers following DHT stimulation of starved cells. Data represent mean ± s.d.; (n=3). **P<0.01. See also Figure S1 and Table S1.
Preliminary analysis demonstrated that TOP1 binding overlapped in particular with that of liganded androgen receptor at enhancers (Figure 1B, Fig. S1C). Genome-wide analysis revealed 6545 putative “AR-bound enhancer” sites based on the criterion of an AR-bound locus marked with H3K4me1, H3K27Ac, and more than 1kb away (in either direction), from the promoter of annotated genes, of which 96% bound TOP1, with 3921 (60%) exhibiting a DHT-stimulated increase in TOP1 binding (Fig. S1D).
To assess eRNAs induced by DHT, we took advantage of technological advances that permit mapping of the position, amount, and orientation of transcriptionally engaged RNA polymerase II on a genome-wide scale (Core et al., 2008). GRO-seq analysis (Core et al., 2008) of serum-starved LNCaP cells treated for 1h with DHT, identified 644 putative AR enhancers with significantly up-regulated eRNAs (Fig. 1C), which is the best mark of activated enhancers (Hsieh et al., 2014; Li et al., 2013a), amongst which 477 (~74%) were noted to have increased TOP1 occupancy in response to ligand at 30′ (Fig. 1D); and virtually all appear to exhibit DHT-increased TOP1 binding at 15′ (Fig. S5C). Because TOP1 has been shown to affect the transcriptional activity of RNA PolII (Kretzschmar et al., 1993), we decided to investigate whether knockdown of TOP1 would alter eRNA synthesis from the androgen-regulated enhancers. Knockdown of endogenous TOP1 by small interfering RNA (siRNA) revealed that eRNA induction was reduced in at least 79% (507 of 644) of AR-regulated enhancers (Figure 1C, E), accompanied by a decrease in the induction of 368 coding target genes in the experiment shown (Figure 1F), with similar results in repeat experiments. 92% of DHT-induced eRNAs were up-regulated more than 2-fold, with FC average of 7.6x. Analysis of 100 randomly selected housekeeping genes not regulated by DHT in our GRO-seq experiments, confirmed that the specific siRNAs used for this study had no effect on their expression (TableS 1).
To validate all major mechanistic points in this study, we chose four enhancers-gene pairs. Three of these enhancers (KLK3E, KLK2E and TMPRSS2E) are validated by previous studies (Andreu-Vieyra et al., 2011; Clinckemalie et al., 2013; Hsieh et al., 2014). The fourth one, NDRG1E, meets the criteria of others. It is an AR-bound element located not too far away from the NDRG1 gene TSS (-29kb), it is H3K4me1+, H3K27Ac+ and following hormone stimulation the transcription unit produces DHT-dependent, bidirectional eRNA, making it a strong candidate. Using these enhancer sites, we found that recruitment of the nuclear receptor co-activators (p300 and SRC-1) at AR enhancers was diminished (Figure S1E). Thus, TOP1 knockdown attenuated the induction of eRNA (1h DHT treatment) and the production of mRNA of the corresponding target genes 5h after ligand addition (Figure 1G, Figure S1F). Importantly, the fold-induction (-/+ DHT) was similar between independent experiments in which eRNA levels were measured. Surprisingly, we noted that ATR (Ataxia telangiectasia and Rad3-related), a protein involved in DNA damage repair, was recruited to AR-regulated enhancers at ~15 min following addition of ligand (Figure 1H, Figure S1G). Together, these data identify TOP1-bound genomic regions that bear enhancer marks and produce eRNA in a DHT-dependent manner. Knockdown of TOP1 reduces production of eRNA and coding gene RNA for most of these AR-regulated target genes.
NKX3.1 and TOP1 Co-occupy Enhancer Binding Sites and Regulate the AR Transcription Program
NKX3.1 is an androgen-regulated transcription factor (Bhatia-Gaur et al., 1999), which is a highly selective and specific marker of metastatic prostatic adenocarcinoma (Gurel et al., 2010). NKX3.1 has been found to interact with TOP1 to enhance formation of the TOP1-DNA complex and increase TOP1 nicking of DNA (Bowen et al., 2007). In fact, TOP1 activity in prostates of Nkx3.1 +/− and Nkx3.1−/− mice is reduced compared with wild-type mice, but not in other organs that do not express Nkx3.1 (Bowen et al., 2007). Overlap of the reported NKX3.1 ChIP-seq dataset (Tan et al., 2012) with that of AR and TOP1 revealed that NKX3.1 occupancy was highest at AR enhancers, with NKX3.1 binding sites located over regions with increased TOP1 binding (Figure 2A, Figure S2A). We observed that AR and TOP1 started to be recruited to AR-enhancers within a few minutes after DHT stimulation. Interestingly, siRNA-mediated knockdown of cellular NKX3.1 inhibited recruitment of TOP1 at enhancers of DHT-regulated genes at 5 min following DHT stimulation (Figure 2B), in line with the previous data suggesting that NKX3.1 is needed for the formation of the TOP1-DNA cleavage complex (Bowen et al., 2007). We observed that following NKX3.1 knockdown in LNCaP cells, the DHT-dependent up-regulation of ~70% enhancer eRNAs were significantly reduced (Figure 2C, D, Figure S2C). We also noted significant reduction in the expression levels of 273 DHT up-regulated genes (Figure 2E), exemplified for two representative genes (Figure 2F). Additionally, knockdown of TOP1 and NKX3.1 reduced DHT-up-regulation of eRNA at the same 351 AR-enhancers in these experiments (Figure 2G), apparently without affecting AR recruitment to the enhancer-binding sites (Fig. S2E). Together, these experiments demonstrate that NKX3.1 and TOP1 binding occurs at a subset of DHT-regulated enhancers and the knockdown of either diminishes transcription in response to ligand.
Figure 2. NKX3.1 and TOP1 Co-occupy a Subset of AR Enhancers and Co-regulate the Enhancer Program.
(A) The UCSC genome browser screenshot displaying a direct overlap between AR, NKX3.1 and TOP1 binding at enhancers of KLK3 and KLK2 genes. Regions with increased (after DHT) TOP1-binding (except regions present in the background control) are underlined. (B) Knockdown of NKX3.1 prevents TOP1 from binding at AR-regulated enhancers; siCTL-, siNKX3.1- and siTOP1-treated cells were stimulated with DHT for 5 min. Chromatin immunoprecipitation was performed with an antibody against TOP1. Data represent mean ± s.d. (n=3). **P<0.01. (C) Knockdown of NKX3.1 by siRNA affects the induced transcription of ~69% of the regulated eRNAs. *P 2.2e-16 (Wilcoxon test). (D). Heatmap of AR-enhancers sorted from most-to-least affected by siNKX3.1. (E) siNKX3.1 reduces induced transcription of 273 genes in this experiment determined by GRO-seq. (F) The UCSC genome browser screenshot showing the KLK3-KLK2 locus. Knockdown of TOP1 or NKX3.1 by siRNA reduces eRNA and genic RNA induction. (G) Knockdown of either TOP1 or NKX3.1 affects induction of the same 351 eRNAs in the same experiment, as measured by GRO-seq. See also Figure S2 and Table S1.
Catalytic Activity of TOP1 is Required for DNA Nicking and Enhancer Activation
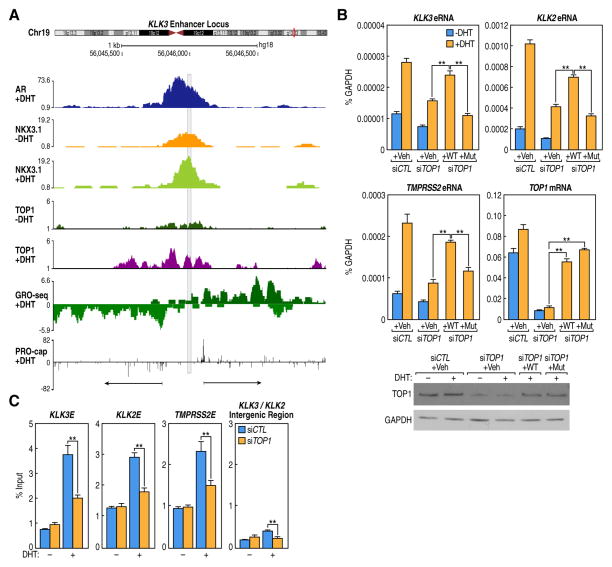
Based on its mechanism of action as a DNA nickase, by which TOP1 forms a covalent intermediate with DNA, and possesses intrinsic DNA ligase activity (Pommier et al., 1998; Champoux, 2001), it would be difficult to detect any such transient nick by available methods. Indeed, despite extensive attempts to detect such a nick in enhancers by primer extension approaches, only a few examples could be clearly visualized. Thus, using this approach to investigate whether AR-regulated enhancers might be the sites of DNA scission by the activated TOP1, we chose the KLK3 enhancer as a model and examined a region overlapped by the AR and NKX3.1 peaks and flanked by two PRO-caps, which mark the transcription initiation sites at high resolution (Kwak et al., 2013), noting that PRO-cap sites could be located on AR-regulated enhancers following hormone stimulation from the AR binding sites. (Figure 3A, Table S2). Primer extension analysis of both DNA strands with [γ32P]-ATP labeled oligonucleotides yielded several termination products consistent with a series of closely-spaced DNA nicks; the strongest band that became accentuated in response to DHT was seen on the lower strand, in support of the notion that it may be one of the major TOP1 binding/scission sites (Figure S3B). Moreover, detailed PRO-cap analysis to locate the precise start sites revealed that the RNA cap sites located, on average ~134 bp away from the center of AR peak binding site (Fig. S3C) are occupied by TOP1 (Fig. S3D), and as shown in GRO-seq experiments, continue to the end of eRNA-encoding sequence, however for the majority (75th percentile) of these transcripts the GRO-seq signal starts to fade away after 1000 bp from the TSS/cap site.
Figure 3. TOP1 Recruits toAR -Regulated Enhancers and Nicks the DNA.
(A) UCSC browser screenshot displaying the KLK3 enhancer. Arrows indicate the PRO-caps representing (putative) eRNA TSS flank the NKX3.1 peak. (B) eRNA readout assay showing that Tyr723 of TOP1 is required for eRNA induction. LNCaP cells were hormone-starved for 24 h, transfected with siTOP1 to deplete the endogenous protein and then electroporated with either empty expression vector (Veh), wild-type TOP1 (WT), or the Y723F-TOP1 mutant (Mut) before treatment with either ethanol or DHT for 1h. eRNA for KLK2, KLK3 and TMPRSS2 gene enhancers was quantified by RT-PCR. TOP1 mRNA and protein levels are also shown. qPCR data show mean ± s.d.; (n=3). **P<0.01. (C) Knockdown of endogenous TOP1 affects nick/break formation as measured by incorporation of Biotin 11-dUTP at selected AR-enhancers after 10 min DHT treatment. Data represent mean ± s.d.; (n=3). **P<0.01.
As another approach to infer the possibility of TOP1 DNA nickase actions in activation of AR-dependent enhancer we sought to mutate TOP1. TOP1 enzymatic activity depends on Tyr723 to relax superhelical DNA (Madden and Champoux, 1992). Specifically, Tyr723 of TOP1 initiates the nucleophilic attack on the backbone scissile phosphate resulting in nicked DNA and a phosphodiester link between the tyrosine and 3′ phosphate (Champoux, 2001; Pommier et al., 2010). Subsequently, the covalent intermediate is re-ligated with concomitant release of Tyr723 from the DNA (Champoux, 2001; Stewart et al., 1998). We therefore tested whether the Y723F TOP1 mutant could rescue the defect caused by TOP1 knockdown. For this purpose, endogenous TOP1 was knocked-down with specific siRNA and either the wild-type or the Y723F TOP1 mutant was then expressed in LNCaP cells. Analysis of the enhancer RNA after 1h DHT treatment revealed that the wild-type TOP1 largely reinstated eRNA induction, whereas the catalytically-inactive mutant failed to do so (Figure 3B). The incomplete rescue with the wild-type construct most likely reflected the fact that not all cells could be efficiently electroporated with the DNA expression vectors, as LNCaPs are notoriously difficult to transfect with conventional cationic liposome reagents. Interestingly, wild-type TOP1 relaxes supercoiled DNA only in the presence of NKX3.1, whereas the active site mutant does not at all (Bowen et al., 2007), consistent with the presence of TOP1 on AR-bound enhancers. These findings are of particular interest based on previous in vitro transcription system analyses. TOP1 has been shown to be essential for transcriptional activation in a system containing RNA polymerase II and other cofactors (Kretzschmar et al., 1993; Merino et al., 1993; Shykind et al., 1997) but in these artificial in vitro transcription system, the Y723F mutant did not block the transcriptional activity of the complex at promoters. Therefore, in this context, TOP1 was proposed to modulate transcription by changing the conformation of DNA at the promoter or via interactions with TBP/TFIID (Kretzschmar et al., 1993; Merino et al., 1993; Shykind et al., 1997). In contrast, on AR-regulated enhancers, the nicking activity of TOP1 appears to be required for its effects on eRNA transcription.
Incorporation of labeled nucleotide by terminal deoxynucleotidyl transferase (TdT) has been considered to label both DNA nicks and dsDNA breaks (Gavrieli et al., 1992); hence, we also employed this assay on specific enhancer sites to assess incorporation of Biotin 11-dUTP in response to DHT. Therefore, we fixed the cells with Streck Cell Preservative (Ju et al., 2006), a formulation shown not to cause DNA breaks during the fixation process. Biotin 11-dUTP incorporation with (TdT) was observed at 10 min following addition of DHT hormone treatment at the several enhancers tested, and this was strikingly reduced after TOP1 knockdown (Figure 3C) Together, these data suggested that TOP1 recruitment to enhancers co-occupied by AR and NKX3.1 occurred at regions proximal to transcription initiation sites and caused ssDNA nicks, although the possibility of a dsDNA break cannot be ruled out, especially as an unligated nick can be converted to a DSB for subsequent processing by the DSB repair pathway (Davis and Maizels, 2014).
Involvement of MRE11 in the Regulation of the AR-Program
The MRN complex, composed of the meiotic recombination 11 (MRE11), RAD50 and Nijmegan breakage syndrome 1 (NBS1) is central to the DNA damage response (DDR) pathway which is initiated upon recognition of the DNA breaks by sensor proteins (Stracker and Petrini, 2011). MRE11 regulates DNA repair by recruitment of DNA-repair proteins that load onto the chromatin at the site of the break (Price and D’Andrea, 2013). Recent evidence shows that cleavage of the covalent 3′ phosphotyrosyl bond that joins TOP1 to the DNA backbone by MRE11 generates a product carrying a 3′ phosphate end, which MRE11-RAD50 can resect in an ATP-regulated reaction, producing a 3′-hydroxyl that can prime repair synthesis (Hamilton and Maizels, 2010; Sacho and Maizels, 2011). Interestingly, the p300 transcriptional co-activator physically interacts with all three members of the MRN complex (Jung et al., 2005).
Based on these considerations and the results in Figure 3, we investigated whether MRE11 was present at AR-regulated enhancers. Kinetic ChIP experiments using a specific antibody (Figure 4A, Figure S4A) revealed that MRE11 recruitment at enhancer-binding sites peaked at 15 min of DHT treatment. On performing ChIP-seq, we identified 19,886 loci in the (−) hormone control and 30,636 loci in the cells treated with DHT for 15 min, observing that MRE11 sequencing tag density at enhancers increased with DHT treatment (Figure 4B, C, Figure S4B). We also observed similar recruitment of the RAD50 component of the MRN complex (Figure S4C). Genome-wide analysis showed indistinguishable alterations in the number of tags over promoters of these genes in response to DHT treatment, although a small increase in MRE11 occupancy at promoters of select DHT-regulated genes (e.g., KLK3, KLK2, NDRG1, and TMPRSS2) could be detected by ChIP-qPCR after DHT treatment (data not shown). GRO-seq analysis of nascent transcription revealed that induction of ~89% of detectible enhancer eRNAs induced by DHT were inhibited by MRE11 knockdown (Figure 4D, E). In addition, expression of 510 induced coding genes was reduced (Figure 4F). Knockdown of RAD50 caused a similar effect on eRNA and mRNA expression levels (Figure S4D). Given the role of ATR in sensing single-strand DNA breaks, we also investigated the potential functional role of ATR following DHT. We found that ATR is rapidly recruited, by 15 min, to AR-bound enhancers after DHT (Figure 1H). This is of functional significance, as knockdown of either MRE11 or TOP1 caused dramatic decrease in ATR recruitment to enhancers (Figure 4G) and a reduction of DHT-induced enhancer and gene transcription (Figure 4H).
Figure 4. MRE11 Regulates the AR Transcription Program.
(A) Recruitment of MRE11 to the selected DHT-regulated AR-enhancers. Data points show mean ± s.d. (n=3). *P<0.05, **P<0.01. (B) MRE11 binding (sequencing tags density) increases over AR-enhancers in a DHT-dependent manner (KLK3 and KLK2 genes shown). (C) Distribution of MRE11 and AR binding (sequencing tag density) centered over AR-enhancer binding sites with DHT-induced eRNA. (D) MRE11 knockdown reduces eRNA expression levels of 89% of DHT up-regulated eRNAs. *P 2.2e-16 (Wilcoxon test). (E) Heatmap for AR-enhancers sorted from the most downregulated by siMRE11 at the top to the least, at the bottom. (F) Boxplot showing 510 genes, where DHT-induced up-regulation of transcription (determined by GRO-seq) was reduced by MRE11. (G) Knockdown of either MRE11 or TOP1 affects recruitment of ATR at enhancers following hormone stimulation of the starved cells, measured after 15 min DHT stimulation. Data show mean ± s.d. (n=3). *P<0.05, **P<0.01. (H) siATR affects induction of eRNA (1h DHT) and mRNA (5h DHT treatment) of the corresponding gene. Data are the mean ± s.d. (n=3). *P<0.05, **P<0.01. See also Figure S4 and Table S1.
Recruitment of Components of DDR to AR-regulated Enhancers
Indeed, a mechanism that could be involved in the repair of single-strand nick would be the base excision repair pathway (BER), to process nicks that evaded TOP1 ligase activity. Therefore, we investigated whether factors involved in this or other DNA damage repair pathways might also be recruited to AR-regulated enhancers. We performed kinetic ChIP experiments using antibodies against phospho-ATM (Ataxia telangiectasia mutated), Ku80 (part of the Ku heterodimer that binds to double-strand DNA break ends), Exonuclease 1 (EXO1), the Bloom syndrome DNA helicase (BLM), and DNA ligase IV (LIGIV). Additionally, we used antibodies to proteins involved in the base excision repair pathway, including XRCC1 (X-ray repair cross-complementing protein 1), DNA polymerases β and ε, and Ligase I, observing an orderly and reproducible kinetics of recruitment after hormone treatment at enhancers including KLK3, KLK2, TMPRSS2, and NDRG1 (Figure 5, Figure S5A), as well on other DHT up-regulated enhancers identified by the GRO-seq (Figure S5B). While TOP1 and ATR were essentially recruited simultaneously at enhancers at 15′, XRCC1 was recruited between 15′-30′, consistent with the recruitment of base excision repair pathway machinery that could process any unligated nicks. Interestingly, DNA ligase IV showed maximum occupancy after 30 min, while pATM (p-S1983), Ku80, EXO1, BLM and DNA ligase I were maximally recruited to enhancers ~60 min post DHT treatment (Figure 5, Figure S5A), indicating recruitment of multiple DNA repair factors that have been conventionally considered to function in DNA damage repair (Nimonkar et al., 2011). The sequence of events would be consistent with resolving any unligated DHT/TOP1-induced ssDNA nicks; the DDR machinery primarily recruited as a ”safety net” against any DNA breaks that are not sealed by TOP1. From our data, the machineries of transcription and DNA damage repair seem to be intrinsically linked.
Figure 5. Canonical DNA Damage/Repair Machinery Components Recruit to AR-Regulated Enhancers.
Kinetic recruitment of factors implicated in the DNA damage response (DDR) to AR- enhancers. All kinetic ChIP experiments were performed at least twice with cells of similar passage number to ensure data reproducibility. Data shown as mean ± s.d. (n=3). *P<0.05, **P<0.01. See also Figure S5.
DISCUSSION
Regulated gene expression has been a subject of intense investigation over the past few decades, yet the precise mechanisms by which enhancers orchestrate tissue-specific programs with such an astonishing precision remain unclear. In particular, the finding that enhancers are also regulated transcription units, encoding eRNAs, has added to the mystery and raised new questions about how the subsequent topological strain on enhancers is handled. Both eRNA synthesis and nucleosome depletion at enhancers are potential sources of topological strain. Advancing RNA polymerase can generate both positive and negative supercoils. The amount of supercoiling is potentially enormous given that a positive and a negative supercoil is generated for every 10 bp transcribed and that the length of an eRNA transcript is typically 1-2 kbp in length. Indeed, it has been estimated that approximately seven supercoils may be generated by the transcribing polymerase per second, and that these supercoils can propagate >1 kbp from the transcription start site (Kouzine et al., 2013). At the same time, the depletion of histones from enhancers releases unconstrained negative supercoils, which, in principle, can parse to a change in DNA twist or unwinding to facilitate transcription and/or to a change in writhe that impedes transcription factor binding. To relieve torsional stress, it is tempting to predict that cells might employ actions of DNA topoisomerases, including topoisomerase I as an integral component of regulated enhancer transcription.
Here, we have elucidated the operation of just such a mechanism in prostate cell-specific enhancer activation by androgen receptor, using the LNCaP cancer cell line as a model. In a sense analogous to the role of TOP1 at origins of replication (Simmons et al., 1998; Tsao et al., 1993), we show here that this DNA nickase is rapidly recruited to a large cohort of AR/NKX3.1-occupied enhancers to putatively activate the enhancers and relieve torsional stress due to ongoing transcription (Fig. 6). Our results are consistent with observations that in yeast cells, Top1/Top2 play a role in the activation of genes characterized by high transcriptional plasticity (Pedersen et al., 2012). However, the beneficial effects of TOP1 have to be weighed against the negative effects of retention of TOP1 as an obstacle to further transcription and the deleterious effects of a single-strand nick if it is not quickly sealed by TOP1 itself, or repaired by the base excision pathway. Unrepaired nicks could lead to the formation of DNA double-strand breaks (DSB) as, for example, when a replication fork runs into and collapses at a nick (Kuzminov, 2001; Wimberly et al., 2013). It has also been suggested that a co-directional collision between the replisome and backtracked RNA polymerase transcription elongation complexes leads to DNA double-strand breaks (Dutta et al., 2011). Thus one important role for the MRN complex and other components of the DDR machinery that we observe recruited to the TOP1-bound enhancers might be for the removal of any “stalled” TOP1 from the DNA substrate, as well as repair of any possible DNA breaks that might occur despite TOP1 or the BER actions (Hamilton and Maizels, 2010; Sacho and Maizels, 2011; Davis and Maizels, 2014).
Figure 6. A Model for TOP1-mediated Activation of the AR-Enhancer.
Following androgen stimulation, AR and DNA topoisomerase I recruit to the enhancer region, premarked by the NKX3.1 pioneer transcription factor. NKX3.1 to TOP1 stimulates enzymatic activity of the topoisomerase, resulting in nicking of DNA on a single strand, followed by recruitment of ATR, XRCC1, and the MRN complex components (MRE11/RAD50). After dismissal of TOP1, ATR and the MRN, additional components of DNA repair machinery recruit to the activated enhancer. The “thin blue line” indicates the presence of low levels of residual eRNA, not totally eliminated by hormone starvation, whereas the “thick blue line” represents induced bidirectional eRNA produced by the transcription unit.
TOP1 activity is likely to be modulated by factors other than NKX3.1, suggesting that the mechanism we describe here may not be restricted to prostate cells. In this regard, it has been shown that the catalytic activity of TOP1 is stimulated by large T antigen during unwinding of the SV40 origin (Simmons et al., 1998) and overexpression of the antigen rendered LNCaP cells androgen-independent for cell cycle progression (Knudsen et al., 1998). This raises the possibility that activation of TOP1 catalytic activity, may in part, trigger a switch to androgen independence. The Werner syndrome helicase, WRN, has also been found to enhance the ability of TOP1 to relax negatively supercoiled DNA and specifically stimulate the religation step of the relaxation reaction (Laine et al., 2003). It is therefore not unlikely that there exist other, yet undiscovered, activators of TOP1 catalytic activity to regulate eRNA synthesis and gene expression programs. Alternatively, there may be other DNA nickases that initiate enhancer activation in tissues other than prostate, in signal-dependent manner, and that the activities of those nickases are modulated by enhancer-bound factors.
While the finding that ligand-dependent enhancer activation strategy would involve a DNA nick may seem counter-intuitive in terms of cellular integrity, it is noteworthy that cellular integrity is threatened daily by endogenous and extracellular agents that lead to the formation of single- and double-strand DNA breaks. For instance, the estimated number of single-strand breaks and spontaneous base losses in nuclear DNA together with other types of spontaneous damage may reach 105 lesions per cell per day (Hoeijmakers, 2009), yet the cells are programmed to survive. To maintain genomic integrity, cells constantly engage the DNA repair machinery. As such, the usage of a programmed DNA nicking/repair strategy in regulated transcription to relieve torsional stress and activate transcription in this case, while apparently surprising, is in keeping with growing evidence that components of DNA damage machinery do participate in transcriptional regulation. For instance, Reinberg and colleagues demonstrated that human RNA polymerase II complex contains components with roles in DNA repair, including Ku70, Ku80 and DNA Pol ε (Maldonado et al., 1996) and Kung and colleagues (Mayeur et al., 2005) have identified heterotrimeric DNA-dependent protein kinase subunits: Ku70, Ku80 and DNA-PKcs, as well as poly(ADP-ribose) as proteins associated with the C-terminal domain of AR and demonstrated that in LNCaP cells, Ku70 and Ku80, recruited to the KLK3 promoter and enhancer in a hormone-dependent manner. Interestingly, Ku70 and Ku80 can function outside of the Ku heterodimer that loads on double-stand DNA. Hasty and colleagues have shown that Ku80 deletion impairs the base excision pathway (BER) at the initial lesion recognition/strand scission step, arguing that free Ku70 and free Ku80, but not Ku heterodimers, associate with apurinic/apyrimidinic (AP) sites that BER corrects (Li, et al., 2013b; Choi, et al., 2014). Moreover, Mo and Dynan showed that in normally growing human cells, Ku80 associated with RNA polymerase II elongation sites. This association occurred independently of the DNA-dependent protein kinase catalytic subunit and was highly selective. In addition, there was no detectable association with the initiating isoform of RNAPII or with the general transcription initiation factors. The authors concluded that association of Ku80 with transcription sites is important for maintenance of global transcription levels, as functional disruption of a discrete C-terminal domain in the Ku80 subunit inhibited transcription in vitro and in vivo (Mo and Dynan, 2002). Importantly, LigIV, like Ku80, is commonly associated with the NHEJ pathway, but its active site has been found to be highly permissive and capable of ligating atypical DNA substrates, including nicks with gaps (Gu et al., 2007). Interestingly, in the absence of RNase H2, the suppression of mutations arising from mis-insertion of ribonucleoside monophosphates (rNMP) during DNA replication, involves Top1-mediated cleavage at an rNMP, followed by unwiding of DNA by Srs2 and digestion by Exo1 (Potenski et al., 2014). Also, earlier studies showed that TOP1 enhanced TFIID-TFIIA complex assembly during activation of transcription; however, in these biochemical studies, the catalytic activity of TOP1 was not essential to activate transcription from promoters. It is also interesting to note that the AR itself has been shown to transcriptionally regulate a network of DNA repair genes, including those implicated in DNA damage sensing (MRE11, NBN and ATR), non-homologous end joining (XRCC4 and XRCC5), homologous recombination (RAD54B and RAD51C), mismatch repair (MSH2 and MSH6), base excision repair (PARP1 and LIG3) and the Fanconi pathway (FANC1, FANCC and USP1) (Polkinghorn et al., 2013). Moreover, p53 itself binds enhancers and regulates eRNA synthesis for transcription enhancement of neighboring genes (Melo et al., 2013).
Together, the recruitment of DNA damage response machinery in specific transcriptional regulatory events is an emerging theme, from the regulation of pluripotency in embryonic stem cells by the trimeric XPC-nucleotide excision repair complex (Fong et al., 2011) to the regulation of human RARβ2 gene via XPG induced DNA breaks at the promoter region (Le May et al., 2012). Moreover, experiments with yeast have revealed that the Rad1XPF/Rad10ERCC1, Mms4Emi1 orthologs can catalyze the endonucleolytic cleavage of DNA immediately upstream from the Top1-DNA adduct (Pommier et al., 2010). Indeed, permissive chromatin architecture seems to be a crucial requirement for transcription initiation events (Fong et al., 2013). While these events are quite distinct from the TOP1-dependent regulatory events described in the present manuscript, they do suggest a common usage of the DNA damage repair machinery to regulate gene transcription.
EXPERIMENTAL PROCEDURES
Cell Culture
LNCaP cells were purchased from ATCC and maintained in RPMI-1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (Omega Scientific), 2mM L-Glutamine and Penicillin/Streptomycin. For kinetic ChIP experiments, cells were starved in phenol-free DMEM (Lonza) supplemented with 5% charcoal:dextran stripped fetal bovine serum (Omega Scientific) for 72 hours. Cells were synchronized with 2.5 μM α-amanitin (Sigma) for 2 hours, washed twice with PBS and released. 100 nM 5α-dihydrotestosterone (DHT, Sigma) was added to the starvation media to stimulate the cells.
Small Interfering RNA
siRNA-mediated knockdown was achieved by transfecting cells with Lipofectamine 2000 and specific siRNAs. The following siRNAs were used for this study: AllStars Neg. Control siRNA (1027281) was from Qiagen. Human ON-TARGETplus SMARTpool siRNAs against TOP1 (L-005278-00-0020), MRE11 (L-009271-00-0020) and RAD50 (L-005232-00-0005) were purchased from Dharmacon. Single interfering RNAs targeting AR (SASI_Hs01_00224483, SASI_Hs01_00224484), TOP1 (SASI_Hs02_00335354, SASI_Hs01_00047440), ATR (SASI_Hs01_00176270, SASI_Hs01_00176271) and NKX3.1 (SASI_Hs02_00341026, SASI_Hs01_00018365) were obtained from Sigma. Multiple siRNAs were used during the course of the study to confirm data reproducibility. For transfection, LNCaP cells were seeded on dishes in RPMI-1640 supplemented with 10% FBS and allowed to attached overnight. The following day, the cells were washed twice with PBS and fed with phenol-free DMEM supplemented with 5% charcoal:dextran FBS. One day later, the cells were transfected using Lipofectamine 2000 and 20 pmol mL−1 siRNA diluted in Opti MEM reduced serum media without phenol red (Life Technologies). The transfection media was removed after 16 h incubation and the cells were washed twice with PBS. Fresh, phenol-free DMEM supplemented with 5% charcoal:dextran FBS and Penicillin/Streptomycin was added to the dishes. Cells were harvested 48-72 h post transfection. All siRNAs used in this study were validated by vendors or by us and used only if providing >70% knockdown efficiency. Relative quantities of gene expression level were normalized to the GAPDH gene. The relative quantities of ChIP samples were normalized by individual inputs, respectively.
ChIP-qPCR
Chromatin immunoprecipitation experiments were done as previously described (Garcia-Bassets et al., 2007). All ChIPs and qPCRs were repeated at least thrice and representative results were shown. P-values were calculated by using a two-tailed Student’s t-test.
GRO-seq and PRO-cap
Global run-on sequencing (GRO-seq) was performed as detailed (Wang et al., 2011) and precision nuclear run–on sequencing of transcription initiation sites (PRO-cap) was performed as described (Kwak et al., 2013).
Antibodies
AR (N-20), TOP1 (H-300), ATR (N-19), RAD50 (H-300), XRCC1 (H-300), BLM (H-300), DNA Ligase I (C-21), DNA Ligase IV (H-300), DNA POL β (C-21), DNA POL ε 3/CHRAC17 (N-15), p300 (C-20), SRC-1 (M-341) were from Santa Cruz Biotechnology. MRE11 (Ab397) and p-S1983-ATM (Ab2888) were obtained from Abcam. Ku80 (A302-627A) and EXO1 (A302-639A) were purchased from Bethyl Laboratories.
Supplementary Material
Acknowledgments
We thank Dr. Bogdan Tanasa and Daria Merkurjev for help with statistical analyses, Charles Nelson for cell culture assistance, Tara Rambaldo for help with cell cycle analysis and Janet Hightower for assistance with figure preparation. We are particularly grateful to Dr. E. Peter Geiduschek for a critical reading of the manuscript, Drs. Kalotina Machini and Patricia Cortes for discussions and helpful suggestions and thank the members of the Rosenfeld laboratory for their comments on the work. This work was supported by grants from NIH and NCI to M.G.R. (DK018477, HL065445, NS034934, DK039949, DK074868 and CA173903). J.P. was supported, in part, by a NIDDK Mentored Research Scientist Development Award (K01DK080180). M.G.R. is an Investigator with the HHMI.
Footnotes
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.P. and M.G.R. conceived the project. J.P., A.K.A and M.G.R. designed experiments. J.P. performed most of the experiments reported. P.K. performed bioinformatics analyses. W.L. performed GRO-seq experiments and Y.T. performed the modified PRO-cap experiments. T.S. carried out screening experiments, while Z.L. generated biotin-TOP1 stable cell line and carried out TOP1 ChIP-seq. J.Z. and K.A.O. assisted in deep-sequencing library preparations and sequencing. J.P., A.K.A and M.G.R. wrote the manuscript with input from P.K.
ACCESSION NUMBERS
The Gene Expression Omnibus accession number for all sequencing data reported in this paper is GSE63202.
Supplemental Information includes Extended Experimental Procedures, five figures and two tables and can be found with this article online at http://
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–461. doi: 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Vieyra C, Lai J, Berman BP, Frenkel B, Jia L, Jones PA, Coetzee GA. Dynamic nucleosome-depleted regions at androgen receptor enhancers in the absence of ligand in prostate cancer cells. Molecular and cellular biology. 2011;31:4648–4662. doi: 10.1128/MCB.05934-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, et al. Roles for Nkx3.1 in prostate development and cancer. Genes & development. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen C, Stuart A, Ju JH, Tuan J, Blonder J, Conrads TP, Veenstra TD, Gelmann EP. NKX3.1 homeodomain protein binds to topoisomerase I and enhances its activity. Cancer research. 2007;67:455–464. doi: 10.1158/0008-5472.CAN-06-1591. [DOI] [PubMed] [Google Scholar]
- Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Li H, Son MY, Wang XH, Fornsaglio JL, Sobol RW, Lee M, Vijg J, Imholz S, Dolle ME, et al. Deletion of individual Ku subunits in mice causes an NHEJ-independent phenotype potentially by altering apurinic/apyrimidinic site repair. PloS one. 2014;9:e86358. doi: 10.1371/journal.pone.0086358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinckemalie L, Spans L, Dubois V, Laurent M, Helsen C, Joniau S, Claessens F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Molecular endocrinology. 2013;27:2028–2040. doi: 10.1210/me.2013-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH. In vivo dynamics of RNA polymerase II transcription. Nature structural & molecular biology. 2007;14:796–806. doi: 10.1038/nsmb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Maizels N. Homology-directed repair of DNA nicks via pathways distinct from canonical double-strand break repair. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E924–932. doi: 10.1073/pnas.1400236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi B, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS biology. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146:533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A, French SL, Beyer AL, Tollervey D. Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes & development. 2010;24:1546–1558. doi: 10.1101/gad.573310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong YW, Cattoglio C, Tjian R. The intertwined roles of transcription and repair proteins. Molecular cell. 2013;52:291–302. doi: 10.1016/j.molcel.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong YW, Inouye C, Yamaguchi T, Cattoglio C, Grubisic I, Tjian R. A DNA repair complex functions as an Oct4/Sox2 coactivator in embryonic stem cells. Cell. 2011;147:120–131. doi: 10.1016/j.cell.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Lu H, Tippin B, Shimazaki N, Goodman MF, Lieber MR. XRCC4:DNA ligase IV can ligate incompatible DNA ends and can ligate across gaps. The EMBO journal. 2007;26:1010–1023. doi: 10.1038/sj.emboj.7601559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M, Eberhart CG, Clark DP, Bieberich CJ, Epstein JI, et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol. 2010;34:1097–1105. doi: 10.1097/PAS.0b013e3181e6cbf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah N, Murakami S, Nagari A, Danko C, Kraus W. Enhancer transcripts mark active estrogen receptor binding sites. Genome research. 2013;23:1210–1223. doi: 10.1101/gr.152306.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NK, Maizels N. MRE11 function in response to topoisomerase poisons is independent of its function in double-strand break repair in Saccharomyces cerevisiae. PloS one. 2010;5:e15387. doi: 10.1371/journal.pone.0015387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen DR, Kassavetis GA, Geiduschek EP. A transcriptional enhancer whose function imposes a requirement that proteins track along DNA. Science. 1992;256:1298–1303. doi: 10.1126/science.1598572. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. The New England journal of medicine. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, et al. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Progress in clinical and biological research. 1980;37:115–132. [PubMed] [Google Scholar]
- Hsieh CL, Fei T, Chen Y, Li T, Gao Y, Wang X, Sun T, Sweeney CJ, Lee GS, Chen S, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7319–24. doi: 10.1073/pnas.1324151111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- Jung SY, Malovannaya A, Wei J, O’Malley BW, Qin J. Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Molecular endocrinology. 2005;19:2451–2465. doi: 10.1210/me.2004-0476. [DOI] [PubMed] [Google Scholar]
- Kaikkonen M, Spann N, Heinz S, Romanoski C, Allison K, Stender J, Chun H, Tough D, Prinjha R, Benner C, et al. Remodeling of the Enhancer Landscape during Macrophage Activation Is Coupled to Enhancer Transcription. Molecular cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray J, Costa A, Bear D, Wu J, Harmin D, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. The Journal of biological chemistry. 1998;273:20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- Kouzine F, Gupta A, Baranello L, Wojtowicz D, Ben-Aissa K, Liu J, Przytycka TM, Levens D. Transcription-dependent dynamic supercoiling is a short-range genomic force. Nature structural & molecular biology. 2013;20:396–403. doi: 10.1038/nsmb.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzine F, Levens D. Supercoil-driven DNA structures regulate genetic transactions. Frontiers in bioscience : a journal and virtual library. 2007;12:4409–4423. doi: 10.2741/2398. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Meisterernst M, Roeder RG. Identification of human DNA topoisomerase I as a cofactor for activator-dependent transcription by RNA polymerase II. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11508–11512. doi: 10.1073/pnas.90.24.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8241–8246. doi: 10.1073/pnas.131009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H, Fuda NJ, Core LJ, Lis JT. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science. 2013;339:950–953. doi: 10.1126/science.1229386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Orom U, Cesaroni M, Beringer M, Taatjes D, Blobel G, Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JP, Opresko PL, Indig FE, Harrigan JA, von Kobbe C, Bohr VA. Werner protein stimulates topoisomerase I DNA relaxation activity. Cancer research. 2003;63:7136–7146. [PubMed] [Google Scholar]
- Lam M, Cho H, Lesch H, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen M, Kim A, Kosaka M, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Li W, Rosenfeld M, Glass C. Enhancer RNAs and regulated transcriptional programs. Trends in biochemical sciences. 2014;39:170–182. doi: 10.1016/j.tibs.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May N, Fradin D, Iltis I, Bougneres P, Egly JM. XPG and XPF endonucleases trigger chromatin looping and DNA demethylation for accurate expression of activated genes. Molecular cell. 2012;47:622–632. doi: 10.1016/j.molcel.2012.05.050. [DOI] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen A, Merkurjev D, Zhang J, Ohgi K, Song X, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013a;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Marple T, Hasty P. Ku80-deleted cells are defective at base excision repair. Mutation research. 2013b;745–746:16–25. doi: 10.1016/j.mrfmmm.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang M. Interplay between DNA supercoiling and transcription elongation. Transcription. 2014;5:e28636–1. doi: 10.4161/trns.28636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KR, Champoux JJ. Overexpression of human topoisomerase I in baby hamster kidney cells: hypersensitivity of clonal isolates to camptothecin. Cancer research. 1992;52:525–532. [PubMed] [Google Scholar]
- Mayeur GL, Kung WJ, Martinez A, Izumiya C, Chen DJ, Kung HJ. Ku is a novel transcriptional recycling coactivator of the androgen receptor in prostate cancer cells. The Journal of biological chemistry. 2005;280:10827–10833. doi: 10.1074/jbc.M413336200. [DOI] [PubMed] [Google Scholar]
- Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson CW, Linn S, Reinberg D. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature. 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- Melgar M, Collins F, Sethupathy P. Discovery of active enhancers through bidirectional expression of short transcripts. Genome biology. 2011;12:R113. doi: 10.1186/gb-2011-12-11-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C, Drost J, Wijchers P, van de Werken H, de Wit E, Oude Vrielink J, Elkon R, Melo S, Léveillé N, Kalluri R, et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Molecular cell. 2013;49:524–535. doi: 10.1016/j.molcel.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Merino A, Madden KR, Lane WS, Champoux JJ, Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993;365:227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- Mo X, Dynan WS. Subnuclear localization of Ku protein: functional association with RNA polymerase II elongation sites. Molecular and cellular biology. 2002;22:8088–8099. doi: 10.1128/MCB.22.22.8088-8099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, Hager G, Sartorelli V. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Molecular cell. 2013;51:606–617. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G, Andrau JC. Noncoding transcription at enhancers: general principles and functional models. Annual review of genetics. 2012;46:1–19. doi: 10.1146/annurev-genet-110711-155459. [DOI] [PubMed] [Google Scholar]
- Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes & development. 2011;25:350–362. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JM, Fredsoe J, Roedgaard M, Andreasen L, Mundbjerg K, Kruhoffer M, Brinch M, Schierup MH, Bjergbaek L, Andersen AH. DNA Topoisomerases maintain promoters in a state competent for transcriptional activation in Saccharomyces cerevisiae. PLoS Genet. 2012;8:e1003128. doi: 10.1371/journal.pgen.1003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio LA, Bickmore W, Dean A, Nobrega MA, Bejerano G. Enhancers: five essential questions. Nature reviews Genetics. 2013;14:288–295. doi: 10.1038/nrg3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, Arora VK, Yen WF, Cai L, Zheng D, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer discovery. 2013;3:1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Barcelo JM, Rao VA, Sordet O, Jobson AG, Thibaut L, Miao ZH, Seiler JA, Zhang H, Marchand C, et al. Repair of topoisomerase I-mediated DNA damage. Progress in nucleic acid research and molecular biology. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Pourquier P, Fan Y, Strumberg D. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochimica et biophysica acta. 1998;1400:83–105. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Potenski CJ, Niu H, Sung P, Klein HL. Avoidance of ribonucleotide-induced mutations by RNase H2 and Srs2-Exo1 mechanisms. Nature. 2014;511:251–254. doi: 10.1038/nature13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price BD, D’Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152:1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacho EJ, Maizels N. DNA repair factor MRE11/RAD50 cleaves 3′-phosphotyrosyl bonds and resects DNA to repair damage caused by topoisomerase 1 poisons. The Journal of biological chemistry. 2011;286:44945–44951. doi: 10.1074/jbc.M111.299347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nature reviews Genetics. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- Shykind BM, Kim J, Stewart L, Champoux JJ, Sharp PA. Topoisomerase I enhances TFIID-TFIIA complex assembly during activation of transcription. Genes & development. 1997;11:397–407. doi: 10.1101/gad.11.3.397. [DOI] [PubMed] [Google Scholar]
- Simmons DT, Roy R, Chen L, Gai D, Trowbridge PW. The activity of topoisomerase I is modulated by large T antigen during unwinding of the SV40 origin. The Journal of biological chemistry. 1998;273:20390–20396. doi: 10.1074/jbc.273.32.20390. [DOI] [PubMed] [Google Scholar]
- Sims D, Sudbery I, Ilott NE, Heger A, Ponting CP. Sequencing depth and coverage: key considerations in genomic analyses. Nature reviews Genetics. 2014;15:121–132. doi: 10.1038/nrg3642. [DOI] [PubMed] [Google Scholar]
- Stewart L, Redinbo MR, Qiu X, Hol WG, Champoux JJ. A model for the mechanism of human topoisomerase I. Science. 1998;279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nature reviews Molecular cell biology. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PY, Chang CW, Chng KR, Wansa KD, Sung WK, Cheung E. Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival. Molecular and cellular biology. 2012;32:399–414. doi: 10.1128/MCB.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao YP, Russo A, Nyamuswa G, Silber R, Liu LF. Interaction between replication forks and topoisomerase I-DNA cleavable complexes: studies in a cell-free SV40 DNA replication system. Cancer research. 1993;53:5908–5914. [PubMed] [Google Scholar]
- Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly H, Shee C, Thornton PC, Sivaramakrishnan P, Rosenberg SM, Hastings PJ. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nat Commun. 2013;4:2115. doi: 10.1038/ncomms3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.