Abstract
Purpose
Antiangiogenic therapy is effective in blocking vascular permeability, inhibiting vascular proliferation, and slowing tumor growth, but studies in multiple cancer types have shown that tumors eventually acquire resistance to blockade of blood vessel growth. Currently, the mechanisms by which this resistance occurs are not well understood.
Experimental Design
In this study, we evaluated the effects of neutrophils on glioma biology both in vitro and in vivo and determined target genes by which neutrophils promote the malignant glioma phenotype during anti-VEGF therapy.
Results
We found that an increase in neutrophil infiltration into tumors is significantly correlated with glioma grade and in glioblastoma with acquired resistance to anti-VEGF therapy. Our data demonstrate that neutrophils and their condition media increased the proliferation rate of Glioblastoma-initating cells (GICs). In addition, neutrophils significantly increased GICs transwell migration compared to controls. Consistent with this behavior, co-culture with neutrophils promoted GICs to adopt morphologic and gene expression changes consistent with a mesenchymal signature. Neutrophil-promoting tumor progression could be blocked by S100A4 down-regulation in vitro and in vivo. Furthermore, S100A4 depletion increased the effectiveness of anti-VEGF therapy in glioma.
Conclusions
Collectively, these data suggest that increased recruitment of neutrophils during anti-VEGF therapy promotes glioma progression and may promote treatment resistance. Tumor progression with mesenchymal characteristics is partly mediated by S100A4, the expression of which is increased by neutrophil infiltration. Targeting granulocytes and S100A4 may be effective approaches to inhibit the glioma malignant phenotype and diminish antiangiogenic therapy resistance.
Keywords: bevacizumab, Neutrophil, mesenchymal phenotype, S100A4, EMT, angiogenesis
Introduction
Gliomas are the most common type of primary brain tumors in adults and classified into four grades by the world Health Organization (WHO) according to their degree of malignancy and histologic features (1). For patients with high grade gliomas (HGGs), such as glioblastoma, the prognosis remains poor with a short survival time (1, 2). Patients with low grade tumors have a comparative longer-term survival, but nearly all low-grade tumors progress to high-grade malignancy (3). Identifying the key contributors and molecular pathways that result in tumor progression is the focus of intense investigation because inhibition of malignant progression of glioma has the potential to significantly extend patient survival.
Over the past two decades, advances made in molecular technologies such as microarray technologies and genome sequencing have made it possible to evaluate the molecular and genetic changes in malignant brain tumors. Recent gene expression profiling studies have revealed 3 to 4 molecular subclasses of HGGs based on differential gene expression (4, 5). One tumor subtype is characterized by expression of neural progenitor markers (proneural, PN), which is enriched in low grade gliomas (LGGs) (6), is associated with longer survival, whereas malignant glioma with a mesenchymal (MES) signature are aggressive, commonly coincide with disease recurrence, resistant to chemothrepy, and is associated with a shorter survival time. In addition, resistance to chemotherapy is also seen in gliomas that were originally defined as proneural and later have increased mesenchymal genes expression, suggesting a shift to the mesenchymal subtype over time (4, 5). This shift from proneural to mesenchymal transition (PMT) in glioblastoma may drive its aggressive behavior and eventually cause chemotherapy failure (7). Currently, it is unknown what drives this PMT in glioblastoma.
Recent evidence provided by our group and others point to tumor microenvironment components as potential participants in the generation of tumor malignancy and chemoresistance (7-11). The tumor microenvironment contains large populations of cancer-related inflammatory cells including tumor associated neutrophils (TAN). In gliomas, increased infiltration of neutrophils has been observed in HGGs comparing to LGGs (12). Resistance to chemotherapy is also associated with increased infiltration of neutrophils. Our previous work shows that glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration and mesenchymal transition, indicating a positive correlation between them (7). Similar findings have been reported for other tumors (13, 14), suggesting that targeting TAN-promoting tumor progression can be a universal method to overcome drug resistance and tumor recurrence. F Shojaei et al. reported inherent anti-VEGF refractoriness is associated with infiltration of the tumor tissue by CD11b+Gr1+ myeloid cells. Combining anti-VEGF treatment with anti-myeloid cell therapy inhibits growth of refractory tumors more effectively than anti-VEGF alone (15, 16). Recent works by Acharyya et al. showed that inhibition of neutrophil recruitment augments the efficacy of chemotherapy against breast tumors and particularly against lung metastasis (8). It has been reported that blocking TAN infiltration into tumor tissues can influent several key aspects of tumor progression, including tumor growth (17-19), angiogenesis (16, 20, 21) and metastasis (8, 11). Identifying the mechanisms by which TAN promote tumor progression and the genetic characteristics associated with these phenotypic changes is essential to identify the key regulators of glioblastoma progression and thus guide the development of new therapies to overcome resistance.
In this study, we make use of an in vitro co-culture model to investigate the interaction between glioma cells and neutrophil progenitor cells, especially how neutrophil influence glioma phenotypes and signaling pathways. We found that co-culture of neutrophil and glioma stem cells increases the expression of S100A4 in glioma cells, which was also up-regulated in anti- VEGF resistant tumors. Down-regulating neutrophil-promoting expression of S100A4 can mitigate the neutrophil-mediated malignant phenotype in vitro and in vivo. Furthermore, S100A4 depletion prolongs the survival of anti-VEGF treated animals and partially reduces glioblastoma resistance to anti-VEGF therapy.
Materials and Methods
Brain Tumor Tissue Microarray
A human glioma tissue microarray was constructed using formalin-fixed, paraffin-embedded archival tissue blocks as described previously (22). The tissue microarray included samples from 232 primary brain tumors of varying grades taken from sites of the most phenotypically representative tumor regions. The array contained 96 glioblastoma tumors, 13 gliosarcoma tumors, 12 anaplastic mixed oligoastrocytoma tumors,32 anaplastic astrocytoma tumors, 24 anaplastic oligodendroglioma tumors, 29 oligodendroglioma tumors, 11 mixed oligoastrocytoma tumors, 4 low-grade astrocytomas. Normal brain tissue samples were included in the array as a negative control. Expression levels of MPO were evaluated by a standard indirect immunoperoxidase procedure as previously described (7). Mayer's hematoxylin nuclear staining was used as a counterstain. An intensity score was assigned to each sample that represented the average numbers of the positive staining on an arbitrary scale of 0 to 5. Statistical analysis was done using a Kruskal-Wallis analysis of ranks.
Cell culture
Glioma stem cell line GIC23, GIC11, GIC2 and GIC20 were obtained from Dr. Howard Colman (Department of Neuro-Oncology, M.D. Anderson Cancer Center, Houston, TX). GIC cells were maintained in suspension in DMEM containing epidermal growth factor, basic fibroblast growth factor (bFGF), and B27 (Invitrogen) at 37°C in 5% CO2 atmosphere. Neutrophil progenitor cell line CRL-11422 (AACR) was cultured in Iscove's modified Dulbecco's medium with 4 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate containing 10 ng/ml murine granulocyte macrophage colony stimulating factor (GM-CSF) 80%; heat-inactivated horse serum, 20%. Prior to collecting conditioned media (CM), change cells to serum-free and GM-CSF-free media. CM was collected after 24 hours incubation.
Cell cycle analysis
For cell cycle analysis, asynchronous GIC cells were fixed with ethanol and stained with 50 μg/ml PI containing 0.1 mg/ml RNaseA (both from Sigma-Aldrich). Cells were analyzed by flow cytometry to determine subG1 (apoptosis), G1, S, and G2/M cell cycle distribution (FACStar Plus Flow Cytometer, Becton-Dickinson).
Immunoblot analysis
Cells were lysed in an ice-cold lysis buffer containing 50 50mM Tris-Cl, pH 7.5, 100mM NaCl, 1mM EDTA, 1% TritonX-100, 1mM PMSF, 1μg/ml leupeptin, and 1μ/ml pepstain A. The protein concentration in the supernatant was determined using a BCA pepstain protein assay (Pierce, Rockford, IL, USA). Samples were subjected to 8–12% SDS-polyacrylamide gel electrophoresis, and the separated proteins were electrophoretically transferred to nitrocellulose membranes. Blots were incubated with the primary antibody against cyclin D1 (1:1000, CST), cyclin D2 (1:1000, CST), c-myc (1:1000, CST), Tubulin (1:3000, Sigma), Ykl-40 (1:1000, Santa Cruz Biotechnology), MMP2 (1:1000, Chemicon-Millipore), nestin (1:1000, abcam) or S100a4 (1:1000, abcam). The membranes were then incubated with horseradish peroxidase-linked secondary anti-rabbit or anti-mouse antibodies (bio-rad).
Invasion assay
Matrigel Basement Membrane Matrix (BD Labware) was used to perform the in vitro cell invasion assays. Cells were pretreated with bevacizumab for 72 h. Transwell inserts for 24-well plates were coated with diluted Matrigel, and cells were added in triplicate to the transwells. Serum-free medium was added to the bottom of the plate. Cells were allowed to invade for 24 h at 37°C. The filters were then fixed and stained with 0.1% crystal violet in 20% methanol. The invasive cells were visualized using bright-field microscopy. Transwell membranes were incubated with 2% deoxycholic acid for 20 min, and the absorbance at 595 nm was recorded.
Microarray and Ingenuity Pathway Analysis
Affymetrix GeneChip Human Genome HG-U133 Plus 2.0 arrays (Affymetrix) were used for expression profiling. The list of genes was overlaid onto a global molecular network developed from information contained in the IPA (Ingenuity Pathways Analysis) knowledge base (IPKB). For network analysis, IPA computed a score (p-score=-log (p-value)) according to the fit of the set of supplied genes and a list of biological functions stored in the IPKB. The score takes into account the number of genes in the network and the size of the network to approximate how relevant this network is to the original list of genes. A score >1.3 (p<0.05) indicates a significant change in the gene network. The network identified is presented as a graph indicating the molecular relationships between genes/gene products.
Immunofluorescence
Immunofluorescence analysis was done as previously described with minor modifications (23). Briefly, formaldehyde-fixed cells were permeabilized with Triton X-100 0.1% in PBS, and blocked with 5% serum diluted in PBS-gel (0.2% gelatin in PBS) for 30 min. The primary antibodies were incubated in blocking solution overnight at 4°C. Immuno-staining was performed using the primary antibody against Ykl-40 (1:50, Santa cruz), CD31 (1:50, abcam) and ly6B.2 (1:50, AbD Serotec). Coverslips were mounted using ProLong antifade reagent (Invitrogen). The images were acquired with an inverted deconvolution microscope. Images were taken with a Zeiss Axioskop 40 microscope equipped with AxioVision Rel.4.2 software.
Animal xenografts
For in vivo experiments, GIC cells (3 × 105) with or without CRL11422 (9 × 105) were implanted intracranially into nude mice (12 mice per group). The mice were euthanized at 3, 6, 9, 11 week, and their brains were removed and processed for analysis. All experiments were approved by the Institutional Animal Care and Use Committee of The University of Texas M. D. Anderson Cancer Center. Tumor volume analysis was done using an unpaired two-tailed Student’s test and groups were compared using the log-rank test. P < 0.05 was determined to be significant.
Immunohistochemistry
Paraffin sections from xenografts were used for immunohistochemical analysis. The slides were deparaffinized and subjected to graded rehydration. After blocking in 5% serum and an antigen retrieval step (citrate buffer, pH 6.0), the slides were incubated with the primary antibodies overnight at 4°C. After washing in PBS with Tween 20, primary antibody reactions were detected using the Vectastain ABC kit (Vector Laboratories) with the respective secondary antibody.
Transfection
Cells were plated at a density of 3×105/6 well plate 3 h prior to transfection. Transfection was carried out using HyFect reagents according to the vendor’s instructions. Transfected cultures were selected with puromycin (5 μg/ml) for 10–14 days. At that time, antibiotic-resistant colonies were picked, pooled and expanded for further analysis under selective conditions. The pGIPZ control was generated with control oligonucleotide GCTTCTAACACCGGAGGTCTT. pGIPZ S100a4 shRNA was generated with TGCTCAGCATCAAGCACGT and TGAGCTTGAACTTGTCACC.
The Cancer Genome Atlas (TCGA) analysis
S100A4 gene expression values were determined among glioblastoma datasets in The Cancer Genome Atlas, accessed through the cBio Cancer Genomics Portal (http://cbioportal.org) supplied by Memorial Sloan Kettering Cancer Center (website accessed on 25th, April, 2013) . The levels of expression of S100A4 were evaluated in the different TCGA glioblastoma subtypes (Glioblastoma, Nature 2008). For each glioma subtype, patients were divided into two groups based on gene expression (Z-score < or ≥ 2). Kaplan Meier curves were generated using these data. The difference in survival between patients with high and low expression of S100A4 was calculated for each subtype.
Results
Neutrophil infiltration is correlated with glioma grade and tumor progression
A human glioma tissue microarray (TMA) was used to evaluate for the presence of neutrophil infiltration using immunohistochemical staining for myeloperoxidase-positive (MPO). The numbers of MPO-positive cells for each tumor sample were calculating from grade II, III and IV glioma. Eighty-six percent of all glioma samples in our TMA (n=232) had some evidence of neutrophil infiltration (Fig. 1A). Grade IV glioblastoma specimens had the highest numbers of infiltration neutrophil (number of neutrophil>10/200X HPF (high powered field)) (Fig. 1A and Supplementary data Table 1). The level of neutrophil infiltration was significantly positively correlated with glioma grade. To confirm the level of neutrophil infiltration in murine models of glioma, we evaluated the numbers of neutrophils infiltrated into tumor xenografts injected into the brain of nude mice. The number of murine neutrophils characterized by the marker Ly-6B.2 (24, 25) increased during tumor growth and progression (Fig. 1B).
Figure 1.
Levels of neutrophil infiltration into gliomas correlates with tumor grades and tumor progression. A, Neutrophil infiltration into different grade glioma was detected by immunohistochemical staining using an anti-MPO antibody. (200X). Grade IV: Glioblastoma (GB), Gliosarcoma (GS); Grade III: Anaplastic mixed oligoastrocytoma (AMOA), Anaplastic oligodendroglioma (AO), Anaplastic astrocytoma (AA); Grade II: Oligodendroglioma (OL), Mixed oligoastrocytoma (MOA); Grade I: Pilocytic astrocytomas (PA); Normal brain (NB). B, Neutrophil infiltration was detected by immunostaining of Ly6B.2 in tumors collected from GICs xenograft nude mice at 2, 3, 4, 5 weeks. (200X)
Neutrophils promote the proliferation of glioma stem cells (GICs)
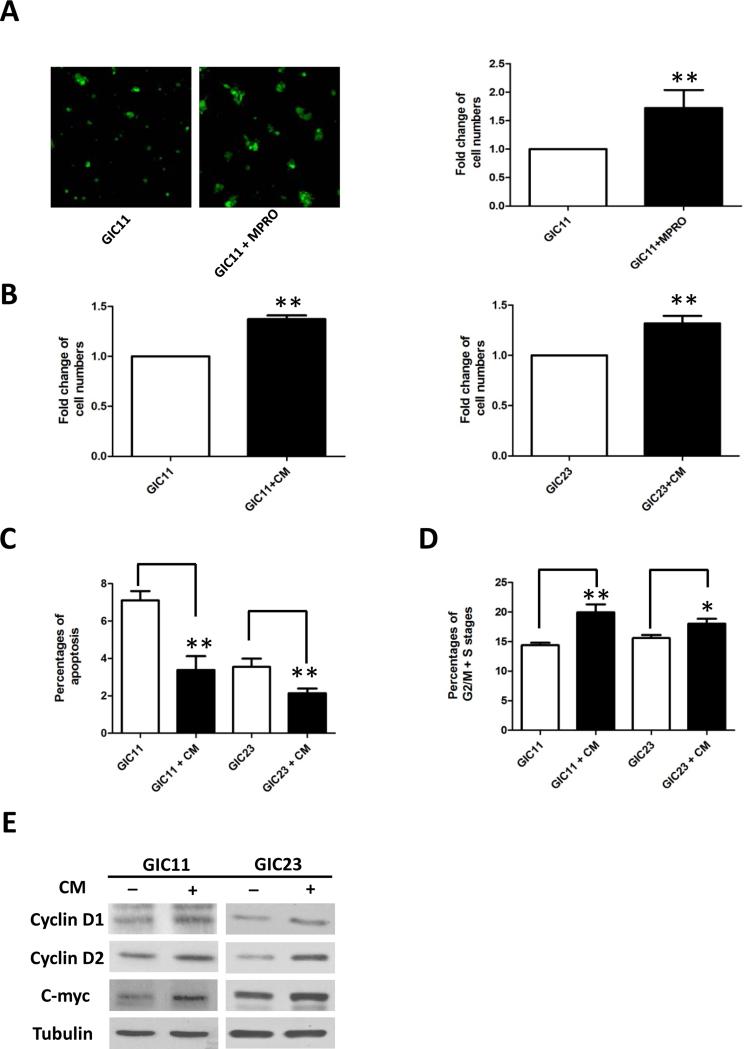
To identify the effects of neutrophils on tumor cells, we performed in vitro co-culture of glioma stem cells (GICs) with neutrophil progenitor cells (MPRO cells, CRL11422, ATCC). After 2 days of co-culture, the numbers of GFP-positive GIC11 cells were significantly increased comparing to controls (Fig. 2A). Condition media (CM) collected from MPRO cells (24 hour serum-free culture medium) also increase the numbers of GICs (Fig. 2B), suggesting that secreted factors from neutrophils may be partially responsible for the tumor proliferating effects.
Figure 2.
Neutrophils promote the proliferation of GICs. A, Morphology of GFP-positive GICs with and without co-culture with MPRO cells (at 1:1 ratio) for 3 days. (100X) Bar graph demonstrating the fold change of GFP-expressing cell numbers analyzed by Flow Cytometry. B, Fold changes of cell numbers after 3 days incubation of GIC11 or GIC23 with serum-free condition media (CM) collected from MPRO cells. Cell cycle analysis using propidium iodide (PI) staining showed CM decreased the percentages of cells in sub-G1 stages (apoptotic cells, C) and increased that in G2/M+S stages (growing cells, D). E, Expression of cyclin D1, cyclin D2, C-myc and Tubulin were detected by western blots in GICs incubated with or without CM. *: P<0.05; **: P<0.01, Student’s t test.
Further evaluation by Propidium Iodide (PI) staining identified that the increase in cell number resulted from both a decrease in GIC apoptosis (Fig. 2C) and an increase in cell proliferation (Fig. 2D). Western blot data demonstrated that CM up-regulated the expression of proliferation related proteins cyclin D1, cyclin D2 and c-myc (Fig. 2E). In addition, CM increased the phosphorylation of Akt and Erk (Supplementary data Fig. 1 A). Blocking Akt and Erk pathway activation by their inhibitors decreased the expression of cyclin D1 and c-myc induced by CM (Supplementary data Fig. 1 B). These data indicate that, at least in our in vitro model, factors secreted by neutrophils promote GIC proliferation.
Neutrophils promote a mesenchymal phenotype
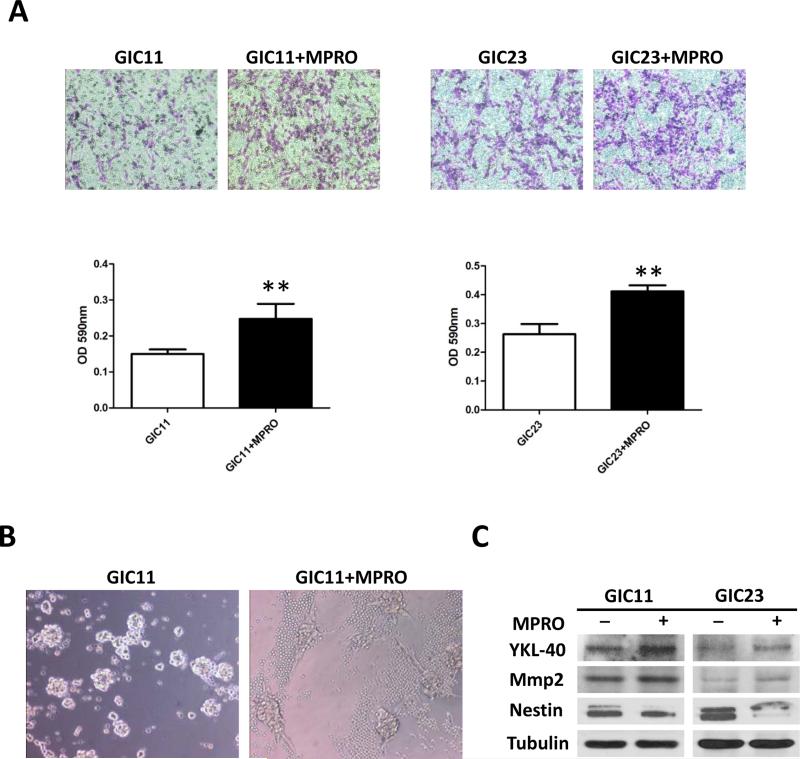
We next investigated the effect of neutrophils on the ability of GIC invasion. A Matrigel transwell assays showed an increase in transwell migration/invasion for GICs when co-cultured with MPRO cells compared to controls (Fig. 3A). GIC cells in culture demonstrated a spindle-shaped morphology, which is a typical morphology for mesenchymal cells (26). when co-culture with MPRO cells (Fig. 3B). Consistent with a more mesenchymal phenotype, Western blots showed increased expression of mesenchymal markers YKL-40 and MMP2, and less expression of Nestin, a proneural marker following co-culture (Fig. 3C). To confirm the effects of neutrophils on GICs and to investigate the underlying mechanisms by which neutrophils promote a mesenchymal glioma phenotype, we performed gene expression analysis of GIC23 treated with or without MPRO cells. IPA analysis (Supplementary data Fig. 2) showed a significant shift towards a mesenchymal gene signature and increased cell mobility in the co-culture group compared to the control group. These data indicate that neutrophils promote a mesenchymal shift in GICs. In addition, gene expression analysis demonstrated that neutrophils also promote an increase in genes related to cell division processes, consistent with the results presented in Fig. 2.
Figure 3.
Neutrophils promote a mesenchymal phenotype of GICs in vitro. A, Matrgel invasion assay for GIC11 (left panel) and GIC23 (right panel) cells. GICs (3X105) were allowed to invade for 24 h in serum-free medium with or without MPRO cells (3X105). Graphs represent absorbance at 590nm after incubation of the memberanes with deocycholic acid. Pictures shown are the most representative form the three independent experiments. *: P<0.05; **: P<0.01, Student’s t test.) B, Morphology of GICs colonies with and without MPRO cells (small round cells) co-culture (cell numbers at 1:1 ratio). 200X C, Expression of Ykl-40, Mmp2, Nestin and Tubulin were detected by western blots in GICs when co-culture with or without MPRO cells. GFP positive GICs were sorted for western blots analysis after 1:1 co-culture with MPRO cell for 3 days.
Neutrophils promote a malignant glioma phenotype through S100A4
In our previous work, we found glioblastoma resistance to anti-VEGF therapy was associated with neutrophil infiltration and a mesenchymal phenotype (7). Gene expression profiling data from these experiments also identified (Fig. 4A) multiple genes that were up-regulated during the development of resistance. Several of these genes may be involved in the regulation of neutrophil-mediated mesenchymal transformation. One of the candidate genes S100A4 was found to be highly expressed in GIC with a mesenchymal phenotype (Fig. 4B, GIC2 and GIC20), whereas no expression was observed in GICs of the proneural subclass (Fig. 4B, GIC11 and GIC23). To identify the role of S100A4, we developed a stable GIC23 cell line with a specific shRNA to S100A4 (Fig. 4C).
Figure 4.
Neutrophils promote a S100A4-mediate malignant glioma phenotype. A, Increased expression of mesenchymal related markers were observed in microarray analysis for bevacizumab-resistant mice tumors. B, S100A4 expression in GICs of proneural (PN) and mesenchymal (MES) subtypes. C, Transient transfection of S100A4 overexpression and shRNA plasmids in GIC23. D, S100A4 expression in GIC23 cells stable transfected with either Control shRNA or S100A4 shRNA when co-culture of MPRO cells. E, Flow Cytometry analysis of GFP-Control shRNA or GFP-S100A4 shRNA GIC23 cells when co-culture with or without MPRO cells (cell numbers at 1:1 ratio). F, Matrgel invasion assay for GIC23 Control shRNA and S100A4 shRNA cells. GICs (3X105) were allowed to invade for 24 h in serum-free medium with or without MPRO cells (3X105). Graphs represent absorbance at 590nm after incubation of the memberanes with deocycholic acid. Pictures shown are the most representative form the three independent experiments. *: P<0.05, Student’s t test. G, Expression of Ykl-40 and Tubulin were detected by western blots in GIC23 when co-culture with or without MPRO cells. GFP positive GICs were sorted for western blots analysis after 1:1 co-culture with MPRO cell for 3 days.
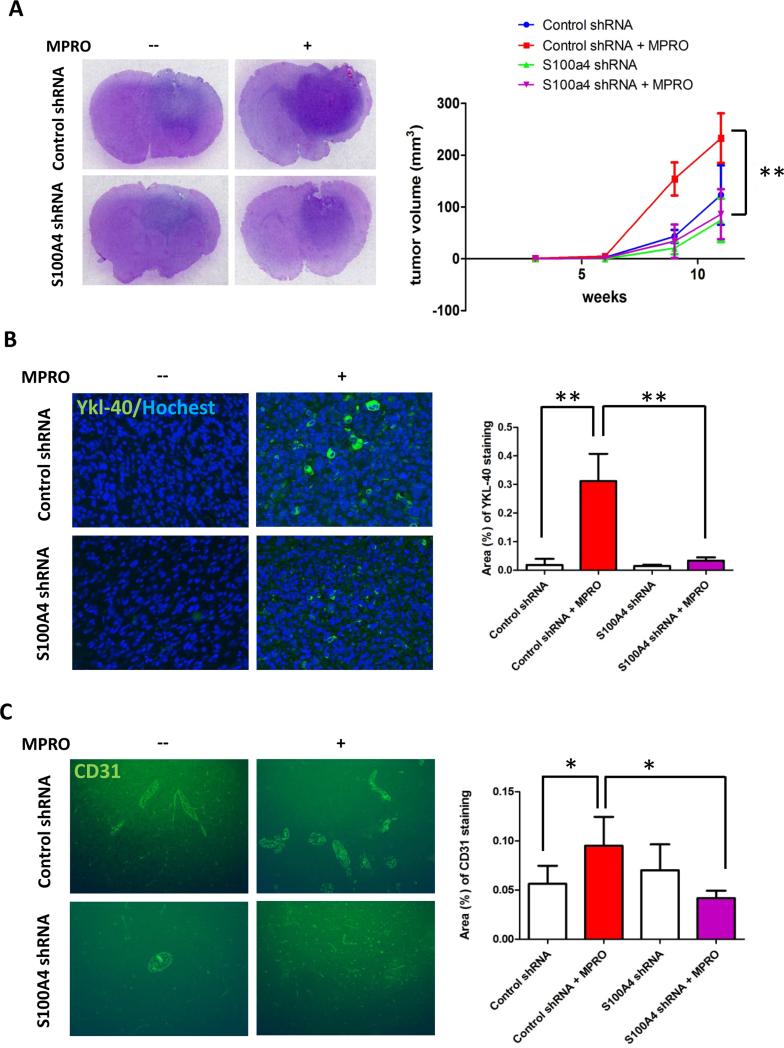
Consistent with the gene expression analysis, GICs co-culture with neutrophils increased S100A4 expression, which was not observed in the S100A4 knock down cell line. Although down regulation of S100A4 did not affect neutrophil-mediated proliferation (Fig. 4E), neutrophil promotion of tumor cell migration (Fig. 4F) and YKL-40 expression (Fig. 4G) were inhibited by S100A4 knock down. To evaluate the impact of S100A4 on tumor growth in vivo, we then co-injected neutrophils with control shRNA or S100A4 shRNA GIC23 into nude mice. Consistent with the in vitro data, neutrophils co-injection increased human S100A4 expression in tumor tissue, while almost no human S100A4 staining was observed in the S100A4 knock down group (Supplementary data Fig. 3A). Our data showed that co-injection of neutrophils with control GIC23 cells enhanced tumor growth, an effect that was significantly blunted by using cells with S100A4 knock down (Fig. 5A). Increased YKL-40 expression was also observed in control cells injected concurrently with neutropihls, but not in S100A4 knock down cells with neutrophils (Fig. 5B). Finally, neutrophil co-injection increased vascularization in control GICs group but not in the S100A4 knock down group (Fig. 5C). Taken together, these data indicate that S100A4 was partially responsible for neutrophil-mediated glioma progression.
Figure 5.
Neutrophils promote tumor progression through S100A4 in mice glioma xenograft model. A, Representative whole mounts of H&E stained brains containing glioma tumors from GIC23 Control shRNA cells alone, GIC23 Control shRNA cells co-injected with MPRO cells, GIC23 S100A4 shRNA cells alone, GIC23 S100A4 shRNA cells co-injected with MPRO cells in nude mice (left panel). Tumor growth from the above four groups (right panel). Data are shown as averages ± SD, n=9 mice per group. **: P<0.01, P values were determined by t test. B, Representative light microscopy images (left panel) showing immunostaining of Ykl-40 (green) in GICs gliomas. Images were taken at 200X. Bar gragh (right panel) demonstrating the mean staining of mesenchymal marker Ykl-40. **: P<0.01, Student’s t test. C, Representative light microscopy images (left panel) showing immunostaining of angiogenesis marker CD31 (green) in GICs gliomas. 100X. Bar gragh (right panel) demonstrating the mean vascular density in GICs gliomas. Data are shown as averages ± SD, n=9 fields taken from at least 3 mice per group at each time point. *: P<0.05, Student’s t test. Cell nuclei were counterstained with Hochest (Blue).
S100A4 depletion increases the efficacy of anti-VEGF therapy in gliomas
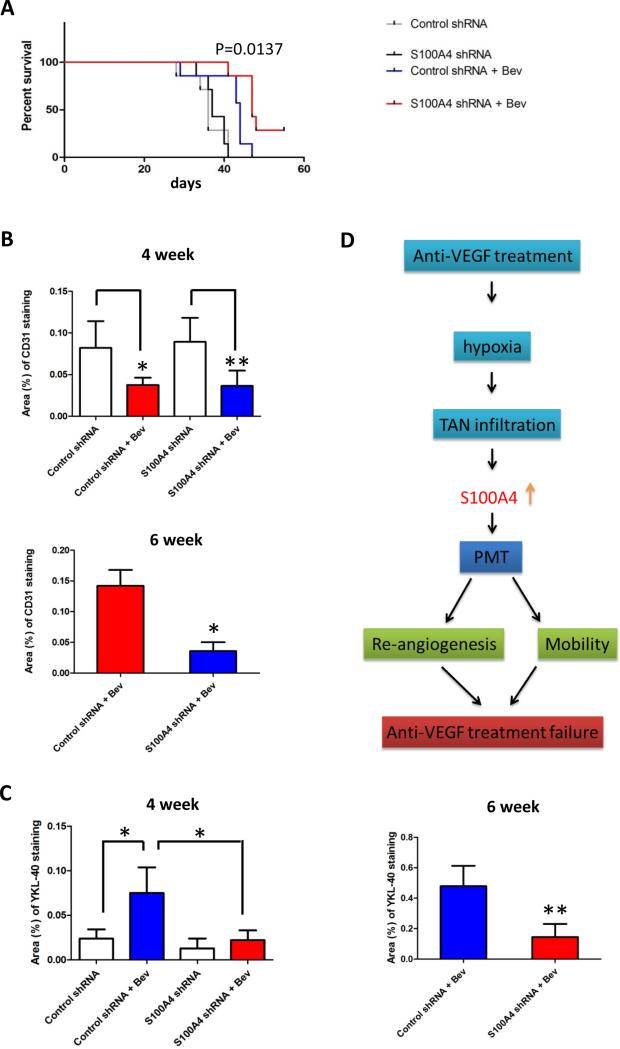
Since S100A4 depletion may block neutrophil-mediated mesenchymal transformation, which is a prominent feature of anti-VEGF resistant tumors, we next investigated whether S100A4 depletion would improve the efficacy of anti-VEGF therapy. Our survival study in animals demonstrated that mice treated with bevacizumab had a longer survival time than the no treatment group [P=0.0044, log-rank (Mantel-Cox) test; Fig. 6A], and S100A4 depletion further prolonged survival [P=0.0137, log-rank (Mantel-Cox) test; Fig.6A]. Immuno-staining data showed that the specific shRNA dramatically inhibited S100A4 expression in tumor cells (Supplementary data Fig. 3B), but this inhibition did not affect the number of infiltrated neutrophils during bevacizumab treatment (Supplementary data Fig. 3C). As reported previously (7, 27), we found the angiogenesis marker CD31 was markedly inhibited in both control cells and S100A4 depleted tumors following 4 weeks of bevacizumab treatment (Fig. 6B, 4 week), indicating the bevacizumab-treated tumor had not escaped from therapy at that time point. However, at 6 weeks tumors were bevacizumab resistant and demonstrated an increase in CD31 staining, which was not observed in the S100A4 depletion group (Fig. 6B, 6 week). Interestingly, YKL-40 staining was increased in bevacizumab treated tumors at as early as 4 weeks which was also inhibited by S100A4 down-regulation at 4 weeks and 6 weeks (Fig. 6C). These data suggest that the mesenchymal transformation of gliomas was regulated by S100A4 and may be responsible for the increase in angiogenesis during tumor progression on anti-VEGF therapy.
Figure 6.
Inhibition of S100A4 increases the efficacy of anti-VEGF therapy in gliomas. A, Kaplan-Meier survival curves for GIC11 Control shRNA and S100A4 shRNA cells injected mice treated with or without bevacizumab. GIC11 S100A4 shRNA cells injected mice treated with bevacizumab have a longer survival duration compared with treated GIC11 Control shRNA cells injected mice. [P=0.0137, long-rank (Mantel Cox) test]. Bar gragh demonstrating the mean staining of CD31 (B) and Ykl-40 (C) at 4 weeks and 6 weeks. Data are shown as averages ± SD, n=9 fields taken from at least 3 mice per group at each time point. *: P<0.05, **: P<0.01, P values were determined by t test. D, Model showing how anti-VEGF treatment failure is promoted by neutrophil through S100A4.
Discussion
Glioblastoma is recognized as the most common and lethal form of brain cancer. Current treatments for glioblastoma include temozolomide (TMZ) paired with radiotherapy, and to more recently the use of anti-angiogenic agents (28). However, anti-VEGF agents have not significantly extended the life expectancies of patients diagnosed with glioblastoma (29, 30), which has partially been attributed to drug resistance. The major aim of this study is to determine the mechanisms underlying glioblastoma resistance to antiangiogenic therapy. Although there is strong preclinical evidence supporting the efficacy of antiangiogenic therapy, tumors eventually adopt a highly resistant phenotype. In this study, we demonstrate that neutrophils may play a critical role in tumor escape from antiangiogenic therapy. Consistent with previous studies (7), we observed an increase in neutrophil infiltration into gliomas, which may be partially driven by tumor hypoxia possibly exacerbated by prolonged anti-VEGF therapy (7, 31). The extent of neutrophil infiltration was positively correlated with glioma grade. Our studies provide evidence that neutrophils promote the glioma malignant phenotype and increase the expression of mesenchymal markers in vitro and in vivo.
Neutrophils have been reported to play an important role in stimulating tumor angiogenesis (32, 33). In patients with myxofibrosarcomas, increased numbers of neutrophils were associated with increased intratumoural microvessel density (34). In vivo studies using various tumor models of angiogenesis demonstrated that neutrophils promote neovascularization (20, 21, 35). In our proneural glioma stem cell xenograft model, we found increased infiltration of neutrophils and expression of mesenchymal marker following 4 weeks of anti-VEGF treatment, with enhancement of angiogenesis at later stages of tumor escape. Mesenchymal tumors are highly vascularized consistent with the findings that the mesenchymal gene expression signature contains multiple pro-angiogenic genes (4). Our data suggests that neutrophils may promote angiogenesis by inducing a shift of gliomas from proneural to mesenchymal subtype (Fig. 6D). Although activated myeloid cells such as M2-skewed macrophages can directly secrete proangiogenic factors (36-39), tumor associated neutrophils may also regulate tumor angiogenesis in an indirect way, such as through shifting stem cells towards a mesenchymal phenotype.
In the present study, we found that a mesenchymal transformation in glioma stem cells was promoted by neutrophils through up-regulation of S100A4. S100A4 belongs to the S100 family of proteins that contain two Ca2+-binding sites including a canonical EF-hand motif (40). S100A4 is involved in the regulation of a wide range of biological effects including cell motility, survival, differentiation and contractility (41). Several of the S100A4 effects resemble processes that occur during epithelial-mesenchymal transition (EMT), and a direct link between S100A4 expression and EMT has been suggested in a number of organs (42-44). In human cancer patients, increased expression of S100A4 is positively associated with an increased incidence of metastasis, invasiveness, aggressiveness, and a worse prognosis (45-50). Our mesenchymal glioblastoma stem cells expressed S100A4 whereas GICs with a proneural signature did not. Likewise, a query of the Cancer Genome Atlas (TCGA) identified 5.8% of the glioblastoma samples (206 cases) over expressed S100A4 and most of these samples (58.6%) were of the mesenchymal subtype and associated with a shorter overall survival (P=0.003964). Conversely, 14.3% proneural glioblastoma samples have down-regulation of S100A4 and longer survival (P=0.009221). Consistent with our results that neutrophils can increased the expression of S100A4 in vitro, S100A4 and CD64, a human monocyte marker, are significantly co-expressed in glioblastoma patient samples from TCGA. Cumulatively, these data suggest that S100A4 may be a key contributor of mesenchymal transition in glioblastoma progression, and the increased expression of S100A4 can be at least partially promoted by an increase in neutrophil infiltration.
Currently, it is difficult to discern the exact mechanisms underlying S100A4-mediated mesenchymal transformation because S100A4 can function both intra- and extra-cellularly. It is likely that the intracellular and the extracellular effects involve distinct mechanisms. S100A4 has been reported to interact with multiple proteins which are known to play a role in tumor progression and metastasis, such as MMP-9 (51). Iwamoto et al showed that patients with GBM with longitudinal increases in MMP-9 had a shorter survival (52). Studies in endothelial cells and tumors demonstrate that S100A4 expression can be promoted by TGF-β1 (43, 53), which is well-known to induce EMT. Consistently, we have observed an increased the TGF- β pathway in anti-VEGF resistant tumors (7) Further studies are needed to identify the molecular mechanism by which S100A4 regulate the mesenchymal phenotype.
Although tumor associated neutrophils play a critical role in tumor progression, therapeutic targeting of this cell type in cancer will be challenging. Neutrophils are critical mediators of host defense against infection, and depletion of these cells could result in dangerous levels of immunosuppression. However, we found that down-regulation of S100A4 inhibited neutrophil-promoting tumor progression independent of the infiltration of neutrophils. Our findings provide a possible alternative strategy to targeting the specific neutrophil-activated regulator on tumor cells, S100A4. Therefore, the combination of S100A4 inhibitor with standard anti-angiogenesis therapy may inhibit glioma progression and anti-VEGF therapy resistance.
Supplementary Material
Statement of Translational Relevance.
Tumor progression is affected by a wide variety of components within the tumor microenvironment, and the importance of neutrophils in glioma has yet to be fully characterized. Although recent work, including ours, has overwhelmingly shown that neutrophils promote tumor progression, some studies suggest that neutrophils can be “polarized” to both pro-tumor and anti-tumor phenotypes by the tumor microenvironment. Therefore, therapeutic targeting these cells may be challenging. A more straightforward approach is to target the specific neutrophil-promoting factors on tumor cells. Here, for the first time, we show that S100A4 is up-regulated on tumor cells by neutrophils. Down-regulation of S100A4 can mitigate the neutrophil-mediated mesenchymal phenotype in vitro and in vivo. Furthermore, S100A4 depletion prolongs the survival of anti-VEGF treated animals and partially reduces glioblastoma resistance to anti-VEGF therapy. Therefore, the combination of an S100A4 inhibitor with antiangiogenic therapy may delay glioma progression and anti-VEGF therapy resistance.
Acknowledgments
Funding
This work was supported in part by an American Society of Clinical Oncology (ASCO) Career Development Award and a grant from the Martha G. Williams Brain Tumor Research Fund (both to J. F. D.). The MD Anderson Cancer Center is supported in part by a Core grant (CA16672).
Footnotes
Conflict of interest statement: Genentech consultant/advisory board, J. F. D.
Reference
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 4.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper LA, Gutman DA, Long Q, Johnson BA, Cholleti SR, Kurc T, Saltz JH, Brat DJ, Moreno CS. The proneural molecular signature is enriched in oligodendrogliomas and predicts improved survival among diffuse gliomas. PLoS One. 2010;5:e12548. doi: 10.1371/journal.pone.0012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piao Y, Liang J, Holmes L, Zurita AJ, Henry V, Heymach JV, de Groot JF. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro Oncol. 2012;14:1379–1392. doi: 10.1093/neuonc/nos158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Hoyer-Hansen G, Eefsen RL, Reynolds AR, Brodt P. The Multifaceted Role of the Microenvironment in Liver Metastasis: Biology and Clinical Implications. Cancer Res. 2013;73:2031–2043. doi: 10.1158/0008-5472.CAN-12-3931. [DOI] [PubMed] [Google Scholar]
- 12.Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98:349–354. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer. 1996;65:57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 15.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–269. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- 22.Liu TJ, Wang M, Breau RL, Henderson Y, El-Naggar AK, Steck KD, Sicard MW, Clayman GL. Apoptosis induction by E2F-1 via adenoviral-mediated gene transfer results in growth suppression of head and neck squamous cell carcinoma cell lines. Cancer Gene Ther. 1999;6:163–171. doi: 10.1038/sj.cgt.7700007. [DOI] [PubMed] [Google Scholar]
- 23.de Groot J, Liang J, Kong LY, Wei J, Piao Y, Fuller G, Qiao W, Heimberger AB. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget. 2012;3:1036–1048. doi: 10.18632/oncotarget.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JU, Cortini A, Rosas M, Fossati-Jimack L, Ling GS, Lewis KJ, Dewitt S, Liddiard K, Brown GD, Jones SA, et al. In vivo functional analysis and genetic modification of in vitro-derived mouse neutrophils. FASEB J. 2011;25:1972–1982. doi: 10.1096/fj.10-178517. [DOI] [PubMed] [Google Scholar]
- 26.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 27.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 28.Weller M. Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly. 2011;141:w13210. doi: 10.4414/smw.2011.13210. [DOI] [PubMed] [Google Scholar]
- 29.J Clin Oncol. 2013;31(suppl) abstr 1. [Google Scholar]
- 30.J Clin Oncol. 2013;31(suppl) abstr 2003. [Google Scholar]
- 31.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90:222–231. doi: 10.1111/j.1365-2613.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 34.Mentzel T, Brown LF, Dvorak HF, Kuhnen C, Stiller KJ, Katenkamp D, Fletcher CD. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch. 2001;438:13–22. doi: 10.1007/s004280000327. [DOI] [PubMed] [Google Scholar]
- 35.Van Coillie E, Van Aelst I, Wuyts A, Vercauteren R, Devos R, De Wolf-Peeters C, Van Damme J, Opdenakker G. Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. Am J Pathol. 2001;159:1405–1414. doi: 10.1016/S0002-9440(10)62527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–1331. doi: 10.1001/archsurg.134.12.1325. discussion 1331-1322. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow- derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heizmann CW, Cox JA. New perspectives on S100 proteins: a multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998;11:383–397. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]
- 41.Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med (Berl) 2008;86:507–522. doi: 10.1007/s00109-007-0301-3. [DOI] [PubMed] [Google Scholar]
- 42.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 44.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 45.Wang YY, Ye ZY, Zhao ZS, Tao HQ, Chu YQ. High-level expression of S100A4 correlates with lymph node metastasis and poor prognosis in patients with gastric cancer. Ann Surg Oncol. 2010;17:89–97. doi: 10.1245/s10434-009-0722-z. [DOI] [PubMed] [Google Scholar]
- 46.Yonemura Y, Endou Y, Kimura K, Fushida S, Bandou E, Taniguchi K, Kinoshita K, Ninomiya I, Sugiyama K, Heizmann CW, et al. Inverse expression of S100A4 and E-cadherin is associated with metastatic potential in gastric cancer. Clin Cancer Res. 2000;6:4234–4242. [PubMed] [Google Scholar]
- 47.Kimura K, Endo Y, Yonemura Y, Heizmann CW, Schafer BW, Watanabe Y, Sasaki T. Clinical significance of S100A4 and E-cadherin-related adhesion molecules in non-small cell lung cancer. Int J Oncol. 2000;16:1125–1131. doi: 10.3892/ijo.16.6.1125. [DOI] [PubMed] [Google Scholar]
- 48.Wang HY, Zhang JY, Cui JT, Tan XH, Li WM, Gu J, Lu YY. Expression status of S100A14 and S100A4 correlates with metastatic potential and clinical outcome in colorectal cancer after surgery. Oncol Rep. 2010;23:45–52. [PubMed] [Google Scholar]
- 49.Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005;93:1277–1284. doi: 10.1038/sj.bjc.6602856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ismail NI, Kaur G, Hashim H, Hassan MS. S100A4 overexpression proves to be independent marker for breast cancer progression. Cancer Cell Int. 2008;8:12. doi: 10.1186/1475-2867-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci U S A. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwamoto FM, Hottinger AF, Karimi S, Riedel E, Dantis J, Jahdi M, Panageas KS, Lassman AB, Abrey LE, Fleisher M, et al. Longitudinal prospective study of matrix metalloproteinase-9 as a serum marker in gliomas. J Neurooncol. 2011;105:607–612. doi: 10.1007/s11060-011-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.