Abstract
Background
To investigate the association of basic demographic data, socioeconomic status, medical services, and hospital characteristics with end-of-life expenditure in patients with oral cancer in Taiwan who died between 2009 to 2011.
Methods
This nationwide population-based, retrospective cohort study identified 5,386 patients who died from oral cancer. We evaluated medical cost in the last month of life by universal health insurance. The impact of each variable on the end-of-life expenditure was examined by hierarchical generalized linear model (HGLM) using a hospital-level random-intercept model.
Results
The mean medical cost in the last six months of life was $2,611±3,329 (U.S. dollars). In HGLM using a random-intercept model, we found that patients younger than 65 years had an additional cost of $819 over those aged ≥65 years. Patients who had a high Charlson Comorbidity Index Score (CCIS) had an additional $616 cost over those with a low CCIS. Those who survived post-diagnosis less than 6 months had an additional $659 in expenses over those who survived more than 24 months. Medical cost was $249 more for patients who had medium to high individual SES, and $319 more for those who were treated by non-oncologists.
Conclusion
This study provides useful information for decision makers in understanding end-of-life expenditure in oral cancer. We found significantly increased end-of-life expenditure in patients if they were younger than 65 years or treated by non-oncologists, or had high CCIS, medium to high individual SES, and survival of less than 6 months after diagnosis.
Introduction
Oral cancer is common worldwide, with an estimated 263,900 new cases in 2008 [1]. The current treatment is either surgery alone or in combination with radiotherapy and chemotherapy. However, the economic cost of advanced head and neck cancer is substantial and higher than that of other solid tumors [2–4]. Oral cancer has been ranked fourth in incidence and mortality in Taiwanese males since 1995. The estimated cost of treatment was approximately $1.195 billion U.S. dollars in Taiwan in 2004.
Previous studies have reported increasing aggressiveness in treatment (e.g., chemotherapy, ICU utilization, emergency department visits) for cancer near the end of life, especially in patients who received primary care from oncologists, those with higher individual socioeconomic status (SES), males and those with shorter post-diagnosis survival [5, 6]. However, the association between end-of-life expenditure and these parameters in oral cancer require further investigation. At present, no publication has shown which factors influence end-of-life expenditute in oral cancer. We hope to provide information on medical cost to help decision makers characterize the end-of-life expenditure associated with oral cancer.
This study uses the nationwide claims data from Taiwan’s National Health Insurance Research Database (NHIRD) for patients who died from oral cancer between 2009 and 2011 to investigate the determinants of end-of-life expenditure for those with oral cancer in the last month of life. This database provides basic demographic data as well as socioeconomic status, medical services, and hospital characteristics.
Patients and Methods
Ethical consideration
This study was approved by the Institutional Review Board of Buddhist Dalin Tzu Chi General Hospital, Taiwan. Review board requirements for written informed consent were waived because all personal identifying information had been removed from the dataset prior to analysis.
Study design and data sources
We identified patients who died from oral cancer from Taiwan’s NHIRD and Taiwan’s National Health Insurance (NHI) Program between 2009 and 2011 [7]. Taiwan’s NHIRD captures basic demographic data, SES, medical services and hospital characteristics. The NHI Program contains information on billing and provides universal health insurance coverage for a comprehensive array of medical services for all of Taiwan’s residents. We excluded patients younger than 18 years of age in order to provide a more precise age distribution. There were only 2 patients younger than 18 years of age each year in this study. The definition of end-of-life expenditures in our study was the medical cost in the last month of life. It was observed that the cost of every medical measurement has experienced little changes throughout these years in Taiwan. The exchange rate is 30 Taiwan dollars to 1 U.S. dollar in this study, which is similar to current exchange rate.
Measurement
Patient demographics
Patient characteristics included age, gender, post-diagnosis survival time, geographic location, urbanization level of residence, individual SES, status of advanced cancer and severity of comorbidity. The recoding and definition of SES and urbanization of residence in the insurance premium is based on income in Taiwan and several urbanization variables [8]. Severity of comorbidity was based on the modified Charlson Comorbidity Index Score (CCIS) recorded on the claims database for the last six months of each patient’s life. The CCIS is a widely accepted scale used for risk adjustment in administrative claims data sets [9]. Oral cancer metastatic status was identified by International Classification of Diseases, 9th Revision (ICD-9) codes 196.xx to 199.xx.
Characteristics of physicians and hospitals
The primary physician’s specialty was identified from NHI claims and was dichotomized into oncologist vs. other. Hospitals were categorized by hospital level (medical center, regional or district hospital), caseload volume (high, medium or low) and hospital spending intensity (high or low). The hospital caseload volume was sorted by total patient volume using unique hospital identifiers [10]. To simplify the presentation, we defined the hospital volume into three categories: low, medium, and high. The volume category cutoff points were determined by sorting samples into three approximately equal groups.
Statistical analysis
The key dependent variable of interest was medical expenditure in the last month of life. This amount included charges paid by both patients and the NHI Bureau in Taiwan. All statistical operations were performed using SPSS (version 15, SPSS Inc., Chicago, IL). The goodness of fit between the association of the end-of-life expenditures and explanatory variables by multiple linear regression was assessed with residual analysis, and we found that it was not suitable for implementing multiple linear regression due to unequal variance assumption (S1 Fig). Considering the clustering effect of physician compensation, procedures and policy in each hospital (S2 Fig), the impact of each variable on medical cost was examined by hierarchical generalized linear model using a hospital-level random-intercept model [11–13]. A two-tailed value of p<0.05 was considered significant.
Results
A total of 5,386 patients who died from oral cancer from 2009 to 2011 were identified in the Taiwan NHIRD. Table 1 summarizes basic demographic data, socioeconomic status, medical services, hospital characteristics, and the end-of-life expenditures for patients with oral cancer. The mean age at death was 56±13 years. Most patients (72.5%) died between 35 and 64 years. In all, 3,445 patients (64%) presented with advanced disease (nodal metastasis or distant metastasis). Only 554 patients (10.3%) survived more than two years. Most patients (71.1%) were treated at a medical center.
Table 1. Baseline characteristics of the patient population.
| Parameter | Year | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | 2009 | 2010 | 2011 | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Total | 5,386 | 100 | 1,369 | 100 | 1,808 | 100 | 2,209 | 100 |
| Socioeconomic status | ||||||||
| High | 1,418 | 26.3 | 355 | 25.9 | 448 | 24.8 | 615 | 27.8 |
| Medium | 2,244 | 41.7 | 551 | 40.2 | 761 | 42.1 | 932 | 42.2 |
| Low | 1,724 | 32.0 | 463 | 33.8 | 599 | 33.1 | 662 | 30.0 |
| Gender | ||||||||
| Male | 5,033 | 93.4 | 1,281 | 93.6 | 1,670 | 92.4 | 2,082 | 94.3 |
| Female | 353 | 6.6 | 88 | 6.4 | 138 | 7.6 | 127 | 5.7 |
| Mean age, years (±SD*) | 56±13 | 56±13 | 56±13 | 57±12 | ||||
| Age group, years | ||||||||
| 18–34.99 | 99 | 1.8 | 36 | 2.6 | 32 | 1.8 | 31 | 1.4 |
| 35–64.99 | 3,906 | 72.5 | 987 | 72.1 | 1,313 | 72.6 | 1,606 | 72.7 |
| more than 65 | 1,381 | 25.6 | 346 | 25.3 | 463 | 25.6 | 572 | 25.9 |
| CCIS ** | ||||||||
| 0 or 1 | 1,907 | 35.4 | 479 | 35.0 | 665 | 36.8 | 763 | 34.5 |
| 2 | 1,038 | 19.3 | 283 | 20.7 | 333 | 18.4 | 422 | 19.1 |
| 3 | 398 | 7.4 | 102 | 7.5 | 135 | 7.5 | 161 | 7.3 |
| 4 | 2,043 | 37.9 | 505 | 36.9 | 675 | 37.3 | 863 | 39.1 |
| Cancer group | ||||||||
| Local disease | 1,941 | 36.0 | 499 | 36.4 | 662 | 36.6 | 780 | 35.3 |
| Advanced disease | 3,445 | 64.0 | 870 | 63.6 | 1,146 | 63.4 | 1,429 | 64.7 |
| Post-diagnosis survival, months | ||||||||
| 0–6 | 1,499 | 27.8 | 447 | 32.7 | 504 | 27.9 | 548 | 24.8 |
| 6.01–12 | 1,683 | 31.2 | 567 | 41.4 | 544 | 30.1 | 572 | 25.9 |
| 12.01–24 | 1,650 | 30.6 | 355 | 25.9 | 602 | 33.3 | 693 | 31.4 |
| >24 | 554 | 10.3 | 0 | 0.0 | 158 | 8.7 | 396 | 17.9 |
| Primary physician’s specialty | ||||||||
| Oncologist | 831 | 15.4 | 213 | 15.6 | 284 | 15.7 | 334 | 15.1 |
| Other | 4,555 | 84.6 | 1,156 | 84.4 | 1,524 | 84.3 | 1,875 | 84.9 |
| Hospital characteristics | ||||||||
| Medical center | 3,238 | 71.1 | 975 | 71.2 | 1,291 | 71.4 | 1,562 | 70.7 |
| Regional | 1,461 | 27.1 | 364 | 26.6 | 486 | 26.9 | 611 | 27.7 |
| District | 97 | 1.8 | 30 | 2.2 | 31 | 1.7 | 36 | 1.6 |
| Caseload group | ||||||||
| High | 1,999 | 37.1 | 525 | 38.3 | 666 | 36.8 | 808 | 36.6 |
| Medium | 2,132 | 39.6 | 566 | 41.3 | 719 | 39.8 | 847 | 38.3 |
| Low | 1,255 | 23.3 | 278 | 20.3 | 423 | 23.4 | 554 | 25.1 |
| Urbanization | ||||||||
| Urban | 1,244 | 23.1 | 312 | 22.8 | 406 | 22.5 | 526 | 23.8 |
| Suburban | 2,361 | 43.8 | 614 | 44.9 | 787 | 43.5 | 960 | 43.5 |
| Rural | 1,781 | 33.1 | 443 | 32.4 | 615 | 34.0 | 723 | 32.7 |
| Geographic Region | ||||||||
| Northern | 2,351 | 43.7 | 609 | 44.5 | 767 | 42.5 | 975 | 44.2 |
| Central | 1,042 | 19.4 | 274 | 20.0 | 356 | 19.7 | 412 | 18.7 |
| Southern | 1,698 | 31.5 | 419 | 30.6 | 571 | 31.6 | 708 | 32.1 |
| Eastern | 291 | 5.4 | 67 | 4.9 | 111 | 6.1 | 113 | 5.1 |
*SD: standard deviation.
**CCIS: Charlson Comorbidity Index Score.
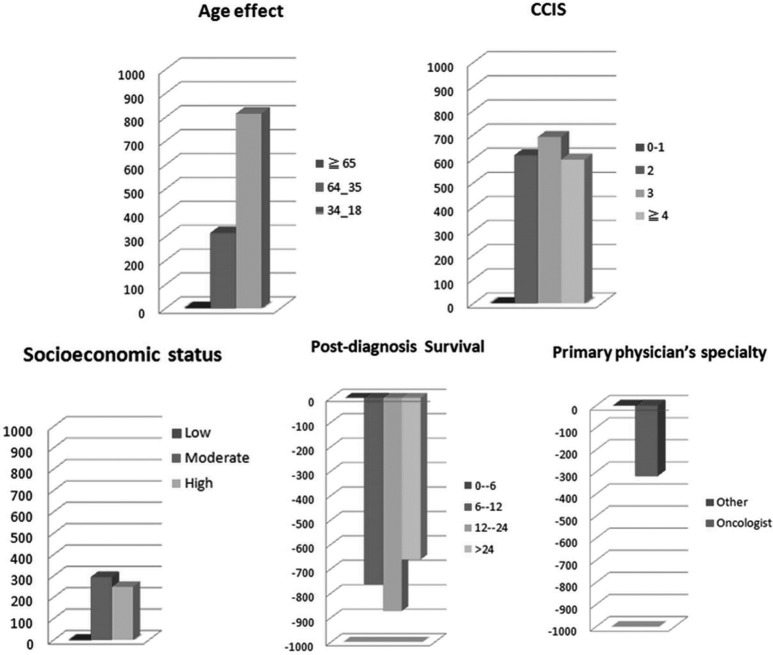
Fig 1 shows that younger age, increased CCIS, higher SES, shorter post-diagnosis survival, or treatment by the non-oncologists were associated with higher end-of-life expenditure. Patients younger than 35 years had the most substantial medical cost. In HGLM using a random-intercept model, we found several correlations of higher cost, listed in Table 2. The mean medical cost in the last month of life was $2,611 ± 3,329 (U.S. dollars). Patients younger than 35 years and those aged 35–64.99 years had a additional cost of $819 and $316, respectively, over those aged ≥65 years (p<0.001). Patients who had a CCIS of 2, 3 and 4 had an additional cost of $616, $692 and $597, respectively, over those with lower CCIS ( p<0.001). Compared with those with post-diagnosis survival shorter than 6 months, those with longer post-diagnosis surival incurred less expense.
Fig 1. Factors associated with increased or decreased EOL expenditure in oral cancer decedents.
Table 2. Medical cost in the last one month of life of Taiwanese oral cancer decedents from 2009 to 2011 by hierarchical generalized linear model using a random-intercept model.
| Parameter | Estimated cost (U.S. dollars) | P value |
|---|---|---|
| Intercept | 2,470 | <0.001 |
| Socioeconomic status | ||
| Low | Reference | |
| Moderate | 292 | 0.007 |
| High | 249 | 0.037 |
| Gender | ||
| Male | Reference | |
| Female | -292 | 0.117 |
| Age group, years | ||
| More than 65 | Reference | |
| 35–64.99 | 316 | 0.004 |
| 18–34.99 | 819 | 0.018 |
| Charlson Comorbidity Index Score | ||
| 0 or 1 | Reference | |
| 2 | 616 | <0.001 |
| 3 | 692 | <0.001 |
| > = 4 | 597 | <0.001 |
| Cancer group | ||
| Local disease | Reference | |
| Advanced disease | -2 | 0.984 |
| Post-diagnosis survival, months | ||
| 0–6 | Reference | |
| 6.01–12 | -761 | <0.001 |
| 12.01–24 | -871 | <0.001 |
| >24 | -659 | <0.001 |
| Primary physician’s specialty | ||
| Other | Reference | |
| Oncologist | -319 | 0.014 |
| Hospital characteristics | ||
| Medical center | Reference | |
| Regional | -31 | 0.848 |
| District | -656 | 0.085 |
| Caseload group | ||
| High | Reference | |
| Medium | -92 | 0.549 |
| Low | -217 | 0.292 |
| Urbanization | ||
| Urban | Reference | |
| Suburban | -197 | 0.116 |
| Rural | -252 | 0.086 |
| Geographic Region | ||
| Northern | Reference | |
| Central | 230 | 0.129 |
| Southern | 181 | 0.168 |
| Eastern | 126 | 0.603 |
| Year | ||
| 2009 | Reference | |
| 2010 | 294 | 0.013 |
| 2011 | 75 | 0.520 |
*Medical cost of aggressive care in the last one month of life US dollars 2,611±3,329.
**95% CI, 95% confidence interval.
Patients with a medium or high individual SES had an additional cost of $292 and $249, versus to low individual SES (p = 0.007 and p = 0.037, respectively). Medical cost was $319 more in those who treated by the non-oncologists (p = 0.014).
Discussion
From 2009 to 2011, our study identified 3,887 patients with oral cancer in Taiwan. We found significantly increased end-of-life expenditure in patients younger than 65 years of age, those with high CCIS, those treated by the non-oncologists, those of medium to high individual SES and those who survived less than 6 months after diagnosis.
The major strengths of this study are its characteristic as a nationwide population-based retrospective cohort study, with complete follow-up information captured by Taiwan’s NHIRD and NHI. This scheme provides comprehensive health care benefits with a moderate cost sharing and uses a uniform fee schedule to control health care costs. In 1995 it covered 92% of the population and had covered 99% by 2003. Medical cost is a reliable parameter of care because of the universal health insurance in Taiwan [14]. Hospital billing does not vary widely from one hospital to another. Furthermore, we assessed the impact of different variables on the medical expenditure with a hierarchical generalized linear model. There were obvious violations of the linearity and equal-variance assumptions in multiple linear regression. Significant clustering effect of medical expenditure was observed in hospital levels in our series. Due to above mentioned features in dataset, hierarchical generalized linear model, which extended generalized linear model with a hospital-level random intercept, was suggested [15,16]. Without using multi-level analysis and considering group-level variables, psychologistic fallacy which assumed the individual-level outcomes that could be totally explained by the individual-level variables may develop and the impact of main variables may be diluted [12].
Older patients receive much less aggressive end-of-life care. A meta-analysis [17] reported that non-treatment decisions occur more frequently in patients older than 80 years. Most publications also report less aggressiveness of end-of life care in the elderly [5, 6, 18]. In our study, patients varied greatly in medical cost by age. Medical cost was higher in patients younger than 65 years, especially those younger than 35 years. A possible explanation is that younger patients receive more aggressive end-of-life care. It is worth to investigate predictors for this group in the future.
Others have reported a trend of aggressiveness of end-of life cancer care for patients who were primarily cared for by an oncologist [19]. Patients cared for by oncologists were more likely to receive chemotherapy. However, we found higher medical cost in patients cared for by the non-oncologists. This discrepancy in results may be due to differences in the delivery of medical service among countries. In Taiwan, any practice of doctors can order administration of chemotherapy and hospitalize the patient.
Patients who had high levels of CCIS had higher medical cost than those with low levels of CCIS. Higher comorbidity scores were associated with higher rates of treatment complications and lower survival rates [20–22]. Nao et al. [23] found that high levels of comorbidity had a significant impact on the general complications rate after major surgery. In a study of patient group, Fiorentino et al. [24] found that acute toxicity after radiotherapy was mild and not influenced by the comorbidity score. Singh et al. [22] suggested that the presence of advanced comorbid conditions was not associated with an increase in the rate of treatment-associated complications, but comorbid condition might be more severe in such patients. Moreover, Lang et al. [4] found that patients diagnosed with advanced squamous cell carcinoma of the head and neck (SCCHN) had shorter survival time and higher medical cost than those with less advanced SCCHN. Clearly, there is an obvious correlation among shorter survival time, advanced disease, and higher medical cost. However, we did not find a significant association between advanced disease and higher medical cost. Contrary, we found that patients who survived less than 6 months post-diagnosis had higher medical cost than those who survived longer. Theoretically, a more severe disease usually requires more intense care, indirectly resulting higher expense. Perhaps, being aware of not living long due to serious diseases, also with the increasing popularity of hospice care, doctors and patients choose to have hospice care over intense care. Besides and in general, shorter post-diagnosis survival is more unlikely to be predicted which results the lack of intervention for the help of hospice care, and this might be the cause of high medical expense. We provide this information for doctors or patients to understand end-of-life expenditures in the years following their diagnosis, especially in the countries without universal health coverage. Although we can’t find out the predictors associated with survival in this study, for patients concerned about medical expenditures or who have longer life, their end-of-life expenditures will have no increase when compared to that of patients who have shorter life.
In our study, after controlling for other variables, low SES patients had lower medical costs, a result consistent with previous studies. Al-Refaie et al. [25] examined the impact of sociodemographic factors on receipt of complex cancer surgery at low-volume hospitals. Patients without private insurance were more likely to receive their cancer surgery at low-volume hospitals. Kong et al. [26] found that patients with lower household income or with residence in a rural location were more likely to be treated at low-volume hospitals. In such cases, patients with low SES have less access to medical resources. However, theoretically, in the system of universal health coverage, the levels of SES should not be the reason in affecting the use of medical resources. It appears that unknown reason exists. Perhaps, we could employ some approaches to enhance the use of medical resources in patients who have low SES, such as to advance accessibility, to reinforce the managerial skill in cases, and to invigorate the communication channel with patients. Nevertheless, our study meant to remind people that patients like this kind should receive special attention and care.
Our study has several limitations. First, the diagnosis of oral cancer and record of comorbid conditions are dependent on ICD codes. However, the NHI program in Taiwan reviews charts and interviews patients to verify the accuracy of diagnosis and treatment coding. The diagnosis of oral cancer was further verified by its inclusion in the catastrophic illness file. Second, we chose hierachical linear regression to analyze our data due to the clustering effect of the end-of-life expenditures among the hospital level. The interquartile range for median end-of-life expenditures was $ 1,132 ($923–2056) between different hospitals, and it represented the clustering effect of hospitals (S2 Fig). Third, medical expenditures resulting from malpractice or overtreatment were deducted. Although the “true” expenditure might not be obtainable from the present database, the results provide reasonably reliable information of the medical cost of this condition, according to the general standard of medical care after review of the NHI Program.
In the modern era, decision makers must not only provide optimal medical treatment but also consider the cost of futile medical care. Decision makers always follow the principle of providing appropriate cancer treatment. However, it can be difficult for them to determine when to begin hospice care. In this study, we provide decision makers information on medical cost for end-of-life expenditures in patients with oral cancer.
Conclusion
We found significantly increased end-of-life expenditure in patients who were aged younger than 65 years, those with high CCIS, those treated by the non-oncologists, those with medium to high levels of individual SES and those who survived less than 6 months after diagnosis. These parameters may help healthcare providers and those responsible for health policy better understand this patient population so as to make informed decisions on how to reduce healthcare expenditures in the future.
Supporting Information
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2. Amonkar MM, Chastek B, Samant N, Teitelbaum A. Economic burden of resected squamous cell carcinoma of the head and neck in a US managed-care population. J Med Econ. 2011;14:421–432. 10.3111/13696998.2011.584096 [DOI] [PubMed] [Google Scholar]
- 3. Kim K, Amonkar MM, Högberg D, Kasteng F. Economic burden of resected squamous cell carcinoma of the head and neck in an incident cohort of patients in the UK. Head Neck Oncol. 2011;3:47 10.1186/1758-3284-3-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lang K, Menzin J, Earle CC, Jacobson J, Hsu MA. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Head Neck Surg. 2004;130:1269–1275. [DOI] [PubMed] [Google Scholar]
- 5. Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. 10.1200/JCO.2007.15.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang ST, Wu SC, Hung YN, Chen JS, Huang EW, Liu TW. Determinants of aggressive end-of-life care for Taiwanese cancer decedents, 2001 to 2006. J Clin Oncol. 2009;27:4613–4618. 10.1200/JCO.2008.20.5096 [DOI] [PubMed] [Google Scholar]
- 7.Bureau of National Health Insurance (2011). Available: http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=296&WD_ID=296&webdata_id=4229. Accessed 2012 December 14.
- 8. Chang TS, Chang CM, Hsu TW, Lin YS, Lai NS, Su YC, et al. The combined effect of individual and neighborhood socioeconomic status on nasopharyngeal cancer survival. PLoS One. 2013;8:e73889 10.1371/journal.pone.0073889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 10. Lin CC, Lin HC. Effects of surgeon and hospital volume on 5-year survival rates following oral cancer resections: the experience of an Asian country. Surgery. 2008;143:343–351. 10.1016/j.surg.2007.09.033 [DOI] [PubMed] [Google Scholar]
- 11. Chen LF, Ho HC, Su YC, Lee MS, Hung SK, Chou P, et al. Association between provider volume and healthcare expenditures of patients with oral cancer in Taiwan: a population-based study. PLoS One. 2013;8(6) pe65077 10.1371/journal.pone.0065077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998. 88(2):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–192. [DOI] [PubMed] [Google Scholar]
- 14. Chiang TL. Taiwan's 1995 health care reform. Health Policy. 1997;39:225–239. [DOI] [PubMed] [Google Scholar]
- 15. Lee Y, Nelder JA. Hierarchical generalized linear models. J. R. Statist. Soc. B. 1996;58:619–678. 17476647 [Google Scholar]
- 16. Stryhn H, Christensen J. The analysis—hierarchical models: past, present and future. Prev Vet Med. 2014;113:304–312. 10.1016/j.prevetmed.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 17. Rietjens JA, Deschepper R, Pasman R, Deliens L. Medical end-of-life decisions: does its use differ in vulnerable patient groups? A systematic review and meta-analysis. Soc Sci Med. 2012;74:1282–1287. 10.1016/j.socscimed.2011.12.046 [DOI] [PubMed] [Google Scholar]
- 18. Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587–1591. 10.1200/JCO.2010.31.9897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Setoguchi S, Earle CC, Glynn R, Stedman M, Polinski JM, Corcoran CP, et al. Comparison of prospective and retrospective indicators of the quality of end-of-life cancer care. J Clin Oncol. 2008;26:5671–5678. 10.1200/JCO.2008.16.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boje CR, Dalton SO, Grønborg TK, Primdahl H, Kristensen CA, Andersen E, et al. The impact of comorbidity on outcome in 12 623 Danish head and neck cancer patients: a population based study from the DAHANCA database. Acta Oncol. 2013;52:285–293. 10.3109/0284186X.2012.742964 [DOI] [PubMed] [Google Scholar]
- 21. Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 22. Singh B, Bhaya M, Zimbler M, Stern J, Roland JT, Rosenfeld RM, et al. Impact of comorbidity on outcome of young patients with head and neck squamous cell carcinoma. Head Neck. 1998;20:1–7. [DOI] [PubMed] [Google Scholar]
- 23. Nao EE, Dassonville O, Chamorey E, Poissonnet G, Pierre CS, Riss JC, et al. Head and neck free-flap reconstruction in the elderly. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:47–51. 10.1016/j.anorl.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 24. Fiorentino A, Chiumento C, Fusco V. Do comorbidity influences acute toxicity and outcome in elderly patients with endometrial cancer treated by adjuvant radiotherapy plus brachytherapy? Clin Transl Oncol. 2013;15:665–669. 10.1007/s12094-012-0986-9 [DOI] [PubMed] [Google Scholar]
- 25. Al-Refaie WB, Muluneh B, Zhong W, Parsons HM, Tuttle TM, Vickers SM, et al. Who receives their complex cancer surgery at low-volume hospitals? J Am Coll Surg. 2012;214:81–87. 10.1016/j.jamcollsurg.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 26. Kong AL, Yen TW, Pezzin LE, Miao H, Sparapani RA, Laud PW, et al. Socioeconomic and racial differences in treatment for breast cancer at a low-volume hospital. Ann Surg Oncol. 2011;18:3220–3227. 10.1245/s10434-011-2001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.