Abstract
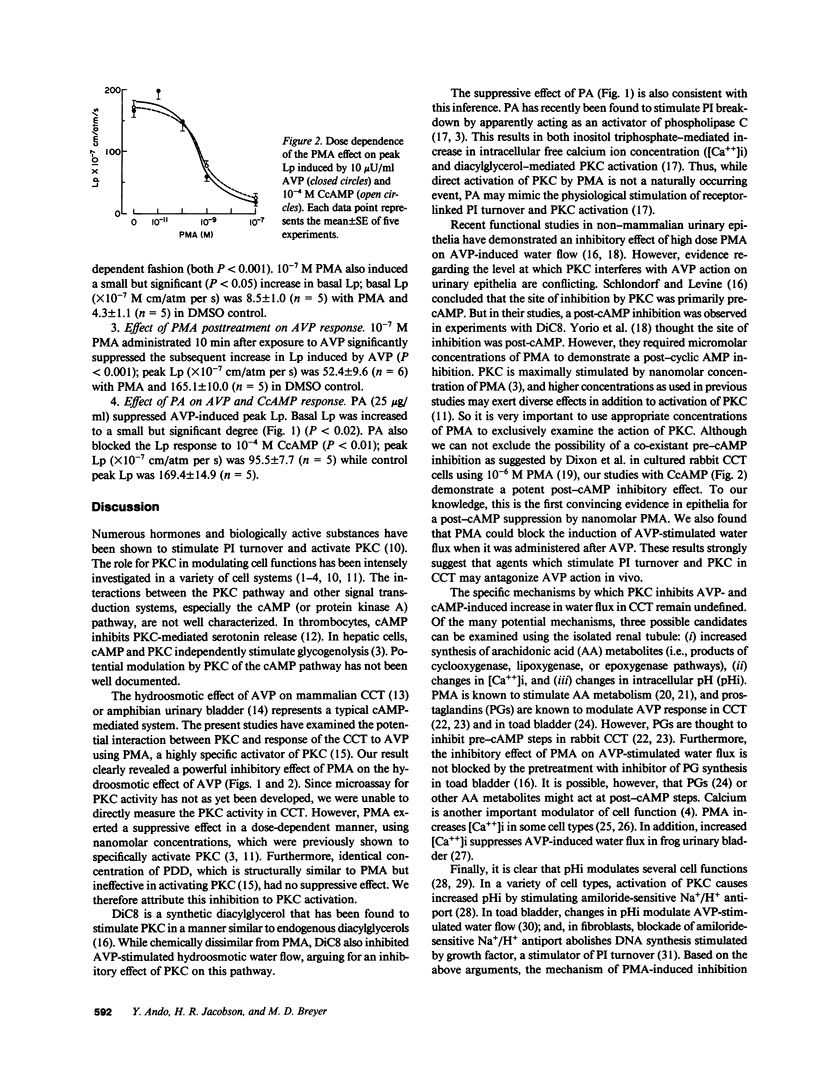
We explored the role for protein kinase C (PKC) in modulating vasopressin (AVP)-stimulated hydraulic conductivity (Lp) in rabbit cortical collecting tubule (CCT) perfused in vitro at 37 degrees C. In control studies, 10 microU/ml AVP increased Lp (mean +/- SE, X 10(-7) centimeters/atmosphere per second) from 4.4 +/- 0.9 to 166.0 +/- 10.4. Pretreatment with dioctanoylglycerol (DiC8) suppressed AVP stimulated peak Lp (peak Lp, 21.9 +/- 3.1). Pretreatment with 10(-9) and 10(-7) M 4 beta-phorbol 12 beta-myristate 13 alpha-acetate (PMA) also blocked the increase in Lp in a dose-dependent fashion (peak Lp, 59.3 +/- 7.5 and 18.6 +/- 4.8, respectively). Inactive phorbol ester, 4 alpha-phorbol 12 beta,13 alpha-didecanoate (10(-7) M), had no effect. PMA also suppressed the increase in Lp induced by 10(-4) M 8-p-chlorophenylthio-cyclic AMP (CcAMP): peak Lp was 169.4 +/- 14.9 in control, 79.2 +/- 5.5 with 10(-9) M PMA, and 25.7 +/- 2.9 with 10(-7) M PMA. Furthermore, when 10(-7) M PMA was added to the bath 10 min after exposure to AVP, the Lp response to AVP was blocked. Peak Lp was 52.4 +/- 9.6 with PMA vs. 165.1 +/- 10.0 in control. Phosphatidic acid (PA), which is thought to stimulate phosphatidylinositol (PI) turnover, produced similar inhibitory effects on AVP as well as CcAMP-stimulated Lp: PA suppressed 10-microU/ml AVP-induced peak Lp from a control value of 159.6 +/- 7.9 to 88.9 +/- 15.8, and 10(-4) M CcAMP induced peak Lp from 169.4 +/- 14.9 to 95.5 +/- 7.7. We conclude that PMA, at concentrations known to specifically activate PKC, suppresses the hydroosmotic effect of AVP on CCT; This suppression is primarily a post-cAMP event; Inhibition of AVP-stimulated Lp by DiC8 and PA also suggests an inhibitory role for the PKC system; The ability of pre- and post-AVP administration of PMA to blunt the AVP response suggests that agents that act through modulation of PI turnover in CCT may regulate the hydroosmotic effect of AVP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brem A. S., Eich E., Pearl M., Taylor A. Anion transport inhibitors: effects on water and sodium transport in the toad urinary bladder. Am J Physiol. 1985 Apr;248(4 Pt 2):F594–F601. doi: 10.1152/ajprenal.1985.248.4.F594. [DOI] [PubMed] [Google Scholar]
- Breyer M. D., Kokko J. P., Jacobson H. R. Regulation of net bicarbonate transport in rabbit cortical collecting tubule by peritubular pH, carbon dioxide tension, and bicarbonate concentration. J Clin Invest. 1986 May;77(5):1650–1660. doi: 10.1172/JCI112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Du Bois R., Vernoiry A., Abramow M. Computation of the osmotic water permeability of perfused tubule segments. Kidney Int. 1976 Dec;10(6):478–479. doi: 10.1038/ki.1976.135. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. A. Intracellular calcium as a modulator of transepithelial permeability to water in frog urinary bladder. J Cell Biol. 1978 Mar;76(3):787–791. doi: 10.1083/jcb.76.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson H. R. Medullary collecting duct acidification. Effects of potassium, HCO3 concentration, and pCO2. J Clin Invest. 1984 Dec;74(6):2107–2114. doi: 10.1172/JCI111635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Hutter A., Bührle C., Nobiling R., Taugner R., Hackenthal E., Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986 Apr;250(4 Pt 1):C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- Levine L., Fujiki H. Stimulation of arachidonic acid metabolism by different types of tumor promoters. Carcinogenesis. 1985 Nov;6(11):1631–1634. doi: 10.1093/carcin/6.11.1631. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Polyphosphoinositide breakdown as the initiating reaction in receptor-stimulated inositol phospholipid metabolism. Life Sci. 1983 May 2;32(18):2083–2085. doi: 10.1016/0024-3205(83)90095-4. [DOI] [PubMed] [Google Scholar]
- Minakuchi R., Takai Y., Yu B., Nishizuka Y. Widespread occurrence of calcium-activated, phospholipid-dependent protein kinase in mammalian tissues. J Biochem. 1981 May;89(5):1651–1654. doi: 10.1093/oxfordjournals.jbchem.a133362. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Nadler S. P., Hebert S. C., Brenner B. M. PGE2, forskolin, and cholera toxin interactions in rabbit cortical collecting tubule. Am J Physiol. 1986 Jan;250(1 Pt 2):F127–F135. doi: 10.1152/ajprenal.1986.250.1.F127. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi K., Levine L. Stimulation of prostaglandin synthesis by tumor-promoting phorbol-12, 13-diesters in canine kidney (MDCK) cells. Cycloheximide inhibits the stimulated prostaglandin synthesis, deacylation of lipids, and morphological changes. J Biol Chem. 1978 Jul 10;253(13):4783–4790. [PubMed] [Google Scholar]
- Rasmussen H., Kojima I., Kojima K., Zawalich W., Apfeldorf W. Calcium as intracellular messenger: sensitivity modulation, C-kinase pathway, and sustained cellular response. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:159–193. [PubMed] [Google Scholar]
- Schlondorff D., Carvounis C. P., Jacoby M., Satriano J. A., Levine S. D. Multiple sites for interaction of prostaglandin and vasopressin in toad urinary bladder. Am J Physiol. 1981 Dec;241(6):F625–F631. doi: 10.1152/ajprenal.1981.241.6.F625. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., Levine S. D. Inhibition of vasopressin-stimulated water flow in toad bladder by phorbol myristate acetate, dioctanoylglycerol, and RHC-80267. Evidence for modulation of action of vasopressin by protein kinase C. J Clin Invest. 1985 Sep;76(3):1071–1078. doi: 10.1172/JCI112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabei K., Levenson D. J., Brenner B. M. Early enhancement of fluid transport in rabbit proximal straight tubules after loss of contralateral renal excretory function. J Clin Invest. 1983 Sep;72(3):871–881. doi: 10.1172/JCI111058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Sano K., Nishizuka Y. Counteraction of calcium-activated, phospholipid-dependent protein kinase activation by adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in platelets. J Biochem. 1982 Jan;91(1):403–406. doi: 10.1093/oxfordjournals.jbchem.a133700. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kikkawa U., Kaibuchi K., Nishizuka Y. Membrane phospholipid metabolism and signal transduction for protein phosphorylation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:119–158. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Yorio T., Quist E., Masaracchia R. A. Calcium/phospholipid-dependent protein kinase and its relationship to antidiuretic hormone in toad urinary bladder epithelium. Biochem Biophys Res Commun. 1985 Dec 17;133(2):717–723. doi: 10.1016/0006-291x(85)90963-5. [DOI] [PubMed] [Google Scholar]



