Abstract
INTRODUCTION
Gastric bypass surgery (GBP) leads to sustained weight loss and significant improvement in type 2 diabetes (T2DM). Bile acids (BAs), signaling molecules which influence glucose metabolism, are a potential mediator for the improvement in T2DM after GBP. This study sought to investigate the effect of GBP on BA levels and composition in individuals with T2DM.
METHODS
Plasma BA levels and composition and fibroblast growth factor (FGF)-19 levels were measured during fasting and in response to an oral glucose load before and at 1 month and 2 years post GBP in 13 severely obese women with T2DM.
RESULTS
A striking temporal change in BA levels and composition was observed after GBP. During the fasted state, BA concentrations were generally reduced at 1 month, but increased 2 years post GBP. Postprandial BA levels were unchanged 1 month post GBP, but an exaggerated postprandial peak was observed 2 years after the surgery. A significant increase in the 12α-hydroxylated/non12α-hydroxylated BA ratio during fasting and postprandially at 2 years, but not 1 month, post GBP was observed. Significant correlations between BAs vs FGF-19, body weight, the incretin effect and peptide YY (PYY) were also found.
CONCLUSIONS
This study provides evidence that GBP temporally modifies the concentration and composition of circulating BAs in individuals with T2DM, and suggests that BAs may be linked to the improvement in T2DM after GBP.
INTRODUCTION
Gastric bypass surgery (GBP) results in large, sustained weight loss and significant improvement in obesity-related comorbidities including type 2 diabetes (T2DM);1,2 however, it is still debated if the improvement in T2DM occurs independently of caloric restriction and weight loss. Bile acids (BAs) are signaling molecules that modulate a number of metabolic processes, including glucose and lipid metabolism3,4 and energy expenditure.5 Several studies have reported that fasted and postprandial circulating BA levels are increased after GBP in cohorts without T2DM or with mixed T2DM status;6–15 however, this has not been investigated longitudinally in an exclusively T2DM cohort. Moreover, the relationship between BAs vs body weight, glucose-related parameters and gut hormones is unclear. Thus, there are gaps in understanding the effects of GBP on BA metabolism and its association with obesity and T2DM.
BAs have been linked to glucose metabolism. BAs are ligands for TGR-5 and farnesoid X receptor (FXR), which influence lipid and glucose homeostasis.16,17 Activation of TGR-5 has been shown to stimulate the release of the incretin glucagon-like peptide (GLP)-1,18 and is important for maintaining normoglycemia.18,19 BA activation of FXR in the intestine stimulates synthesis of fibroblast growth factor (FGF)-19,20 a secreted factor that leads to the inhibition of CYP7A1 expression in the liver.21 FXR activity is important for maintaining normolipidemia, normoglycemia and BA homeostasis.3,22–24 12α-hydroxylated (12α-OH) BAs in particular associate with insulin resistance in humans25 and are increased in human26 and rodent models27,28 of diabetes.
We sought to investigate the effect of GBP on circulating BA levels and composition and circulating FGF-19 levels during both the fasted and postprandial states in severely obese individuals with T2DM who experienced significant improvement or remission of T2DM after GBP. We also investigated relationships between BAs vs body weight, glucose metabolism and gut hormones in this population. This study is unique as it focuses exclusively on subjects with T2DM, follows subjects during both an acute (1 month) and chronic time point (2 years), carefully characterizes changes in BA composition, and measures the postprandial response to an oral glucose load. We hypothesized that fasted and postprandial total BAs and FGF-19 levels would increase after GBP, with a reduction in the 12α-OH/non-12α-OH BA ratio. We also hypothesized that a significant relationship between BAs vs FGF-19, body weight, glucose metabolism and gut hormones, would emerge.
MATERIALS AND METHODS
Thirteen obese women with T2DM were studied before, 1 month and 2 years after GBP with a 3-h oral glucose tolerance test (OGTT, 50 g glucose in 200 ml noncarbonated total volume) and an isoglycemic intravenous glucose infusion to calculate the incretin effect on insulin as previously described.29 Two subjects had a cholecystectomy before surgery and one subject had one 2 months after surgery. Blood samples were collected during fasting and at 0, 30, 60, 90, 120 and 180 min after the oral glucose load in chilled EDTA tubes with aprotinin and dipeptidiyl peptidase-4 inhibitor. Samples were centrifuged at 4 °C before long-term storage at − 80 °C. Blood glucose concentrations were measured by the glucose oxidase method (Analox Instruments USA, Lunenberg, MA, USA). Total GLP-1, peptide YY (PYY3–36) and insulin were measured by RIA (Millipore, St Charles, MO, USA), glucose-dependent insulinotropic polypeptide (GIP) and total ghrelin by ELISA (Millipore), and FGF-19 by ELISA (R&D, Minneapolis, MN, USA) at the Hormone Core laboratory of the New York Obesity Nutrition Research Center. Fifteen fractionated BAs were measured by high-performance liquid chromatography tandem mass spectrometry at the King’s Imperial College in London, UK, as previously described.30 BAs analyzed include chenodeoxycholic acid (CDCA), cholic acid (CA), deoxycholic acid (DCA), lithocholic acid (LCA), ursodeoxycholic acid (UDCA) and each, respective, glycine (G-) and taurine (T-) conjugate. Owing to undetectable plasma concentrations of tauroursodeoxycholic acid (TUDCA) and taurocholic acid (TCA) at certain time points, these BAs were removed from all calculations and analyses.
Calculations
Composite Variables
BAs were grouped according to their site of synthesis (primary vs secondary), conjugation (conjugated vs unconjugated) or 12α-hydroxylation (12α-OH vs non12α-OH) to create composite variables. Composite variables included: (1) Total BAs = all 13 BAs and conjugates; (2) Primary BAs = CA, CDCA and conjugates; (3) Secondary BAs = DCA, UDCA, LCA and conjugates; (4) 12α-OH BAs = CA, DCA and conjugates; (5) non12α-OH BAs = CDCA, LCA, UDCA and conjugates; (6) Conjugated BAs = all glycine and taurine-conjugated BAs; (7) Unconjugated BAs = all unconjugated BAs; (8) Glycine-conjugated; (9) Taurine-conjugated; (10) Primary conjugated; (11) Primary unconjugated; (12) Secondary conjugated; (13) Secondary unconjugated. Concentration values of each composite variable were determined by calculating the molar sum of BA concentrations in each category.
Statistical analysis
Linear mixed model analysis was used to detect changes in all variables over time relative to surgery. Repeated measures analysis of variance with simple contrasts was used to detect changes: (1) over the time course of the OGTT (fasted vs postprandial); (2) over longitudinal time relative to surgery (that is, pre, 1 month, 2 years); and (3) for the interaction of OGTT time course × longitudinal time for all outcome variables. Paired t-tests were used to calculate change from presurgery at 1 month vs 2 years. Linear mixed model analysis was used to test for omnibus correlations between sets of variables measured longitudinally (for example, body weight measured pre, 1 month and 2 years vs BAs measured at the same time points). R values were estimated for predictors in mixed model analyses based on improvements in log-likelihoods between baseline and more complex models (for example, model containing one or more predictors compared with model with slope only).31 Data were log-transformed as necessary to correct for skewness. Data are expressed as mean ± s.d. in the tables and mean ± s.e.m. in the figures. Statistical significance was set at P < 0.05 (two tailed). SPSS 19.0, 21.0 and 22.0 were used for data analysis.
RESULTS
Clinical characteristics
Subject characteristics are presented in Table 1. Body weight loss was 11% at 1 month and 31% at 2 years. As expected, glucose levels and insulin sensitivity improved at 1 month and either improved further and/or normalized by 2 years. As shown previously, circulating gut hormone concentrations increased significantly in response to oral glucose after GBP, with an ~ 300% and 50% increase in GLP-1 and PYY area under the curve (AUC), respectively, and an 80% increase in peak GIP (Table 1, Supplementary Figure 1). No significant change in ghrelin was observed, although levels tended to be higher 2 years after surgery.
Table 1.
Subject characteristics
| Pre-GBP | 1 Month post GBP | 2 Years post GBP | |
|---|---|---|---|
| n | 13 | 13 | 13 |
| Gender (men/women) | 0/13 | 0/13 | 0/13 |
| Age (years) | 49.5± 8.5 | ||
| HbA1c (%) | 6.8± 0.7 | 5.6± 0.8* | |
| Weight (kg) | 111.5± 15.2 | 99.4± 12.5* | 77.0± 11.2*† |
| BMI (kg m−2) | 43.3± 4.9 | 38.7± 4.8* | 29.9± 3.4*† |
| Weight loss (kg) | 12.2± 5.2 | 34.5± 11.9† | |
| Weight loss (%) | 10.7± 3.7 | 30.6± 7.9† | |
| Fasting glucose (mmol l−1) | 7.4± 1.6 | 6.5± 1.6 | 5.2± 0.8*† |
| Glucose 120 min (mmol l−1) | 10.1± 2.3 | 6.9± 2.2* | 4.6± 1.8*† |
| Glucose AUC (mmol l−1min−1) | 10.4± 2.0 | 8.3± 2.2* | 6.7± 1.5*† |
| Fasting insulin (pmol l−1) | 164.9± 64.1 | 123.9± 51.2 | 65.5± 19.5*† |
| HOMA-IR | 7.6± 3.3 | 5.0± 2.6* | 2.2± 0.9*† |
| ISI composite (Matsuda) | 2.2± 0.9 | 3.0± 1.4 | 5.1± 1.8*† |
| Incretin effect on insulin (%) | 20.4± 20.3 | 52.6± 13.5* | 54.7± 12.6* |
| Fasting GLP-1 (pmol l−1) | 6.0± 3.6 | 5.8± 3.0 | 7.9± 5.8 |
| GLP-1 peak (pmol l−1) | 17.1± 11.5 | 80.5± 34.0* | 79.4± 46.0* |
| GLP-1 AUC (pmol l−1min−1) | 6.7± 3.2 | 25.4± 8.9* | 20.7± 17.4* |
| Fasting GIP (pmol l−1) | 36.0± 12.2 | 37.1± 13.2 | 45.9± 13.0 |
| GIP peak (pmol l−1) | 181.0± 45.7 | 279.6± 88.7* | 319.3± 130.8* |
| GIP AUC (pmol l−1min−1) | 43.5± 10.8 | 51.4± 17.9 | 48.7± 23.6 |
| Fasting PYY (pmol l−1) | 57.4± 17.6 | 49.2± 26.3 | 70.7± 26.4† |
| PYY peak (pmol l−1) | 70.4± 22.6 | 113.1± 38.4* | 103.3± 33.8* |
| PYY AUC (pmol l−1min−1) | 49.9± 24.2 | 79.1± 30.2* | 72.8± 26.1* |
| Fasting ghrelin (pg ml−1) | 577.9± 255.2 | 572.7± 387.9 | 780.4± 635.8 |
| Ghrelin nadir (pg ml−1) | 492.7± 232.7 | 434.5± 273.9 | 602.7± 473.0 |
| Ghrelin AUC (pg ml−1min−1) | 552.0± 251.7 | 498.6± 304.1 | 705.9± 567.1 |
Abbreviations: AUC, area under the curve; BMI, body mass index; GBP, gastric bypass surgery; GLP-1, glucagon-like peptide -1; GIP, gastric inhibitory peptide; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model of insulin resistance; ISI, insulin sensitivity index; PYY, peptide YY. Data are mean ± s.d.
P < 0.05 vs Pre-GBP.
P < 0.05 vs 1 month post GBP.
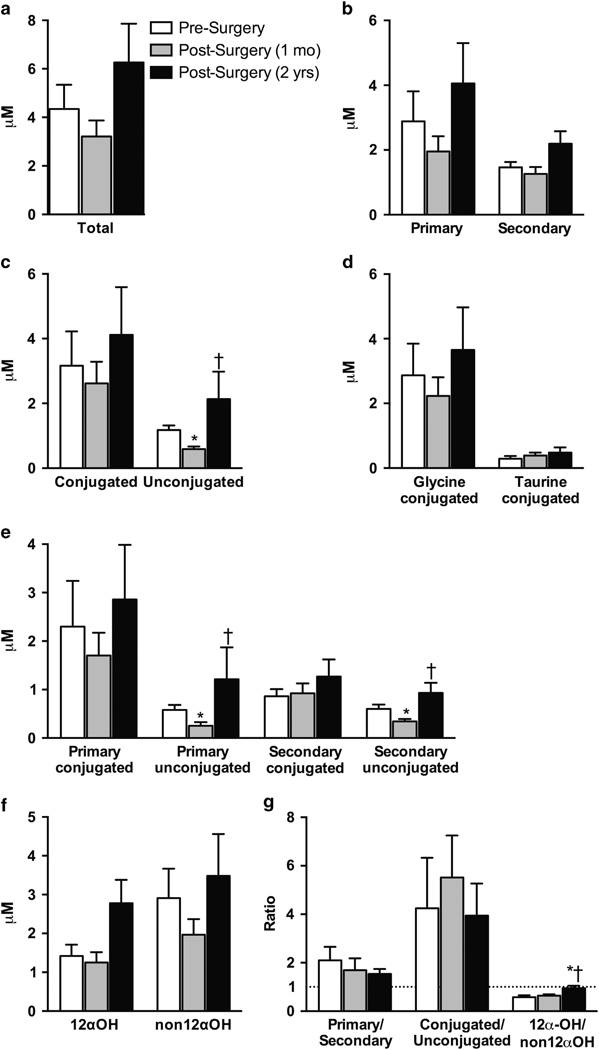
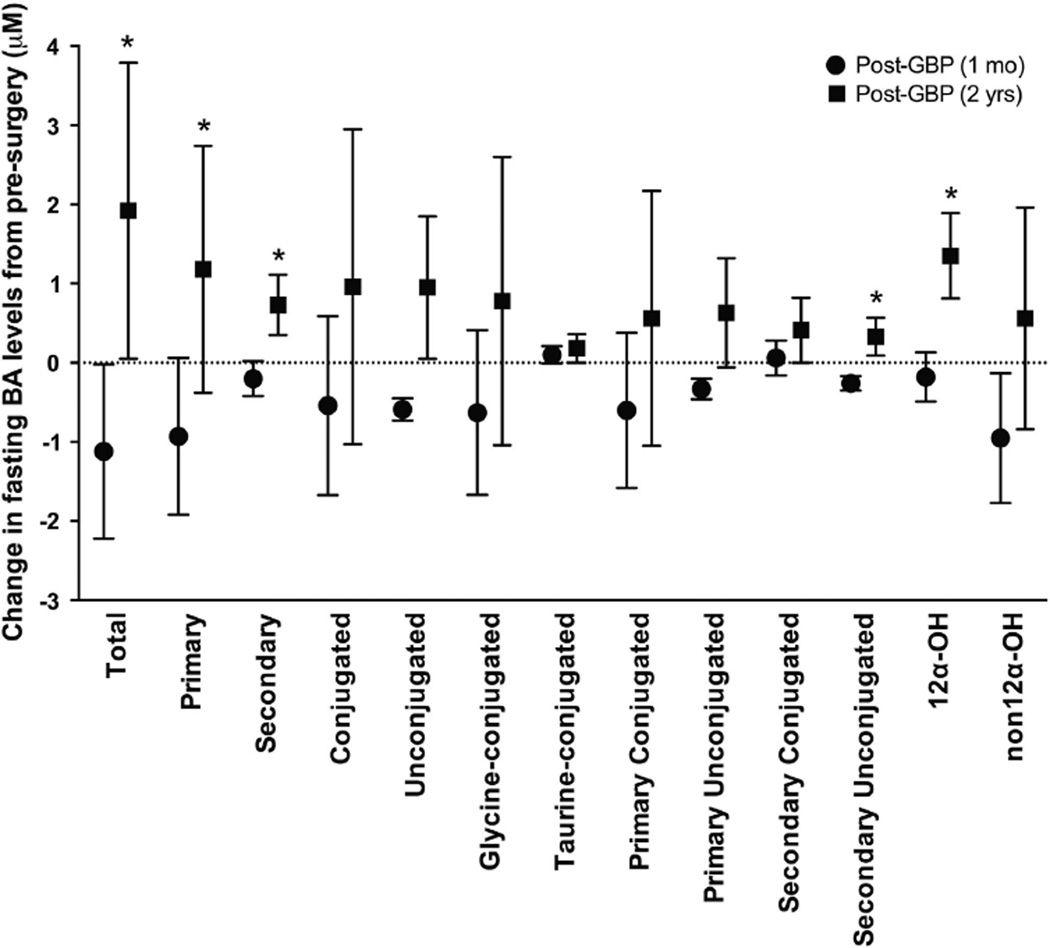
Effect of GBP on circulating fasted BA levels and composition During the fasted state, most circulating individual and nearly all composite BA levels followed the same trend; a small reduction at 1 month, followed by an increase 2 years after GBP (Figure 1; Supplementary Table 1). However, only the unconjugated BAs reached significance (Figures 1c–e; Supplementary Table 1). The change in fasted BA levels was significantly different at 1 month vs 2 years for the composite variables total, primary, secondary, secondary unconjugated and 12α-OH (Figure 2).
Figure 1.
Effect of GBP on circulating BA levels and ratios during fasting. (a–f) Composite BA values tended to be reduced at 1 month and increased at 2 years. Unconjugated, primary unconjugated and secondary unconjugated BA levels were significantly lower at 1 month vs presurgery, and significantly higher at 2 years post surgery vs 1 month post surgery. (g) The primary/secondary ratio tended to decrease progressively after surgery, the conjugated/unconjugated ratio remained relatively unchanged after surgery, and there was a significant increase in the 12α-OH/non12α-OH ratio at 2 years post surgery vs presurgery and vs 1 month post surgery. Mean ±s.e.m. *P < 0.05 vs presurgery, †P < 0.05 vs 1 month post surgery.
Figure 2.
Effect of GBP on the absolute change in circulating BA levels at 1 month vs 2 years. A significant difference in the change in BA concentration at 1 month vs 2 years was observed for the composite variables total, primary, secondary, secondary unconjugated and 12α-OH BAs. Mean±s.e.m. *P < 0.05 vs 1 month post surgery.
Effect of GBP on circulating postprandial BA levels and composition
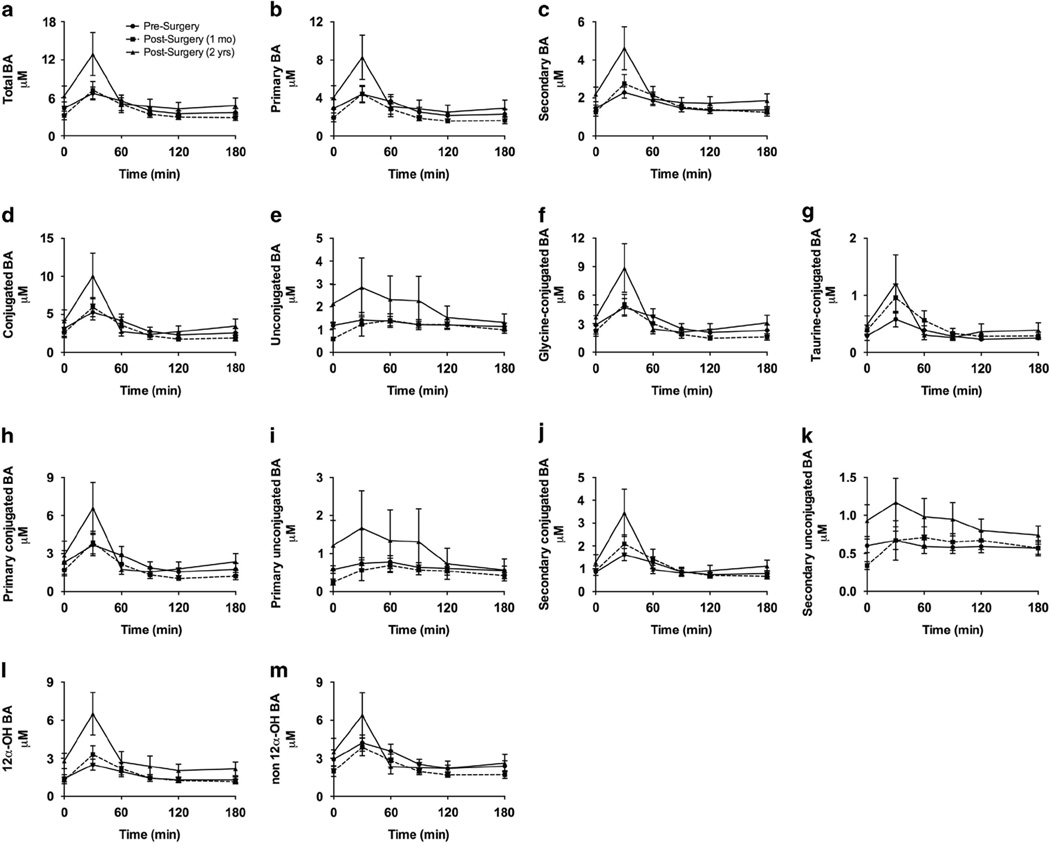
The postprandial BA curves show an increase in BA levels in response to glucose at 30 min for all composite variables except primary unconjugated BAs (P < 0.05; Figure 3), regardless of longitudinal time relative to surgery. A significant OGTT time course × longitudinal time interaction was observed for the unconjugated, primary unconjugated and secondary unconjugated BAs (P < 0.05; Figure 3). The rise in peak BA levels 30 min after ingestion was more exaggerated 2 years vs 1 month after surgery. We observed a significant difference between the change at 1 month vs 2 years post GBP (at 30 min and from 0 to 60 min AUC after oral glucose) for 12α-OH BAs (P < 0.05) as well as trends for total BAs (P = 0.07), primary BAs (P = 0.08) and secondary BAs (P = 0.08; Figure 3; Supplementary Table 2). No difference in absolute AUC from 0 to 180 min was observed (Supplementary Table 3).
Figure 3.
Effect of GBP on circulating BA levels in response to a 50-g oral glucose load. (a–m). A significant increase in postprandial vs fasted BA levels was observed at the 30-min time point for all BAs except primary unconjugated bile acids (P < 0.05). Overall, a trend for an increase in postprandial BA levels was observed 30 min after glucose ingestion at 2 years after surgery, compared with 1 month post surgery and presurgery. Mean ±s.e.m.
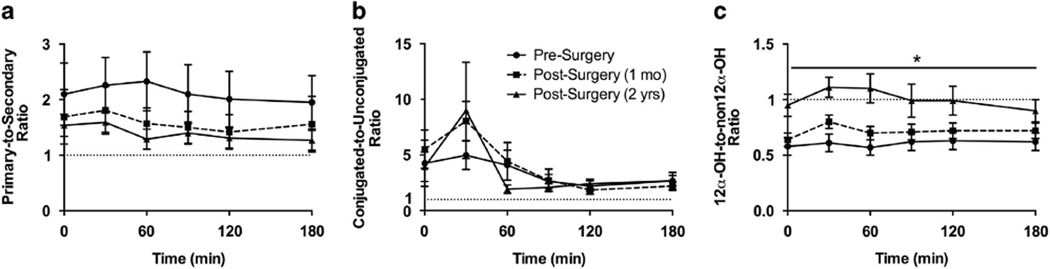
Effect of GBP on ratios of circulating BA levels during fasting and postprandially
Computed ratios of the circulating composite BA variables revealed a predominance of primary and conjugated BAs, compared with secondary and unconjugated BAs, respectively (Figures 1g and 4a and b; Supplementary Table 1). During fasting and postprandially, there was a trend for the primary/secondary BA ratio to progressively decline after surgery. During fasting, the conjugated/unconjugated ratio appeared relatively unchanged post GBP. However, during the postprandial period, a non-significant spike in the conjugated/unconjugated ratio was evident at 30 min during the post-surgery conditions compared to presurgery (Figure 4b). During both the fasted and postprandial states, the 12α-OH/non-12α-OH ratio increased significantly at the 2-year time point post surgery relative to presurgery and 1 month post surgery (P < 0.05; Figure 1g and Figure 4c).
Figure 4.
Effect of GBP on BA ratios in response to a 50-g oral glucose load. (a) The primary/secondary ratio showed a progressive but nonsignificant increase after GBP. The primary/secondary ratio was relatively unchanged in response to glucose. (b) Before surgery, the conjugated/unconjugated ratio appeared relatively unchanged 30min after glucose ingestion, with a decline thereafter. After surgery, a more dramatic and exaggerated, but non-significant, increase in the conjugated/unconjugated ratio was observed 30min after glucose ingestion versus presurgery, with a steep decline thereafter. (c) The 12α-OH/non12α-OH ratio was significantly increased 2 years after surgery compared with presurgery and 1 month post surgery. Mean±s.e.m. *P < 0.05 vs presurgery and 1 month post surgery (longitudinal time relative to surgery).
Effects of GBP on circulating FGF-19 levels and correlation between BAs and FGF-19
FGF-19 levels were significantly increased in the postprandial state, compared with fasted levels (P < 0.05; Supplementary Figure 2a). Although there appeared to be a progressive increase in FGF-19 fasted, peak and AUC levels from presurgery to 2 years post surgery, these did not reach significance (Supplementary Figure 2a,b). We also observed a striking correlation between total BA AUC and FGF-19 AUC (P < 0.001; Supplementary Figure 2c).
Correlations between BAs and body weight, glucose-related parameters and gut hormones
Body weight, but not weight loss, was negatively correlated with both total BAs (r = 0.345, P = 0.049) and secondary BAs (r = 0.303, P = 0.024) during fasting. The incretin effect on insulin was positively correlated with the AUC of total BAs (r = 0.265, P = 0.032), primary BAs (r = 0.304, P = 0.017), conjugated BAs (r = 0.304, P = 0.031), 12α-OH BAs (r = 0.253, P = 0.032) and non12α-OH BAs (r = 0.251, P = 0.04). However, fasted and postprandial glucose and insulin, and insulin sensitivity (HOMA-IR, ISI) were not significantly correlated with BAs. PYY AUC was positively correlated with the AUC of primary BAs (r = 0.343, P = 0.04), unconjugated BAs (r = 0.482, P = 0.004) and non12α-OH BAs (r = 0.364, P = 0.04). There appeared to be a trend for GIP and GLP-1 AUC to correlate with BA composite variables, but none of these reached significance (data not shown).
DISCUSSION
It remains unclear whether the mechanisms independent of weight loss have a role in the remarkable improvement in T2DM after GBP. Altered BA metabolism has been linked to T2DM,16 and it has been suggested that changes in BA metabolism after GBP could mediate improvements in T2DM. In our cohort of subjects with T2DM, we observed a temporal pattern of change in BA levels and composition during both the fasted and postprandial states. We found a reduction or no change at 1 month and increase at 2 years, for most BA composite variables and the 12α-OH/non12α-OH BA ratio. We also identified significant relationships between BAs vs FGF-19, body weight, the incretin effect on insulin, and PYY.
Our study confirms that the temporal change in fasted and postprandial total BAs after GBP, documented in obese populations without T2DM,13,14 also occurs in a population with T2DM. Total BAs do not rise immediately after GBP (4–7 days),7,13,14 and it is not entirely clear when this increase begins. Some studies report a significant increase in total BAs 1–3 months post GBP,7,14,15 whereas other studies (including ours) do not.13 Although most studies show a general increase in BAs long term after GBP,6,8–14 the pattern of change in BA composition after GBP is unclear. Some studies have observed that the postprandial increase in BAs is driven by conjugated BAs,10,14 but others did not observe this effect.8,10,12 We observed a non-significant spike in the conjugated/unconjugated ratio 30 min after glucose ingestion at both timepoints after GBP. Moreover, this observed increase has been reported to be due to the glycine-conjugated BAs,10,14 and the taurine/glycine ratio during fasting is lower after GBP.8 Primary BAs (CDCA, CA) are synthesized and conjugated in the liver. After export to the small intestine, these primary BAs are deconjugated and dehydroxylated by gut microbiota to secondary BAs (DCA, LCA).20 Thus, it is tempting to speculate that altered gut microbiota abundance and diversity, which has been reported after GBP,32–35 could have a role in the shift in BA composition.
In our study, the most striking change in BA composition after GBP was with respect to the 12α-OH/non12α-OH ratio. This ratio is predominately determined by the expression of Cyp8b1 in the liver, responsible for the synthesis of CA and its derivatives. Contrary to our hypothesis, we observed a significant and preferential increase in the 12α-OH/non12α-OH ratio 2 years after GBP. These findings were somewhat unexpected, considering that 12α-OH BAs are reported to be higher in human and rodent models of insulin resistance and T2DM25–28 and that the genetic deletion of Cyp8b1 in mice leads to improved insulin secretion and glucose tolerance.36 In addition, caloric restriction has been shown to increase 12α-OH BAs in a rodent model,37 and caloric restriction would be expected to be more stringent at 1 month, compared with 2 years, after surgery.
The relationship between BAs, obesity and T2DM is complex. Total BAs are reported to be blunted in obese persons compared with lean controls during fasting and/or postprandially.13,14,38 This blunting appears to be driven by conjugated BAs, specifically, glycine-conjugated BAs.14,38 T2DM is associated with altered BA metabolism.9,25,39,40 Total BAs in obese persons with T2DM are reported to be higher compared with normoglycemic obese controls during fasting and postprandially;9,39,40 some studies showed that this was driven by an increase in either glycine and/or taurine-conjugated BAs.39,40 Another study showed that plasma CA and CDCA were inversely associated with insulin sensitivity.41 The increase in BAs after GBP is unlikely to be secondary to glycemic control, as a recent report showed that intensive glycemic control with insulin did not affect circulating BA levels or composition.40 Recently, total urinary BA excretion has been shown to be significantly greater in subjects with T2DM compared with lean subjects without T2DM.42 Interestingly, stratification based on BMI showed that this was driven by lean and overweight subjects with T2DM, and not evident in obese subjects with T2DM, suggesting that the obese state and T2DM have independent and potentially opposing effects on BA metabolism.42
Blunted total BA levels in obese subjects are increased 10–12 months post GBP.13,14 Ahmad et al.14 found that this effect appeared to be due to an increase in glycine-conjugated BAs, and was more pronounced in the postprandial, rather than the fasted state. Weight loss has been speculated as a mechanism for the altered BA response after GBP; however, evidence for this is unclear. Kohli et al.12 observed that after 20% weight loss, fasted and postprandial total BA levels were significantly higher post GBP, but lower after gastric banding. Steinert et al.13 showed that GBP and vertical sleeve gastrectomy increased circulating fasted total BA levels similarly, but only GBP significantly increased postprandial BA levels. On the other hand, we observed a negative relationship between body weight vs total BAs, similar to what was reported by Steinert et al.13 Future studies should clarify the role of weight and weight loss to changes in BA metabolism.
FGF-19 has an important role in the regulation of BA synthesis in humans43 and has been linked to improvements in lipid and glucose metabolism44,45 and the regulation of food intake.46 A recent study in mice with genetic manipulation of FXR activity showed that this target is critical for the reduction in body weight, food intake, and improvement in glucose control after vertical sleeve gastrectomy.47 Two studies have observed an increase in fasted FGF-19 levels early (days–3 months)7,15 after GBP in humans; however, Patti et al.6 did not observe this in post-GBP subjects compared with morbidly obese or overweight controls. Moreover, Pournaras et al.7 observed that this effect was found exclusively after GBP and not after AGB. Gerhard et al.9 observed that fasted FGF-19 levels were significantly lower in subjects with T2DM compared with subjects without T2DM, and that FGF-19 levels were increased to a greater degree after GBP in subjects that underwent T2DM remission compared with subjects without T2DM or who did not undergo remission. We did not observe any significant increase in FGF-19 levels after GBP. Yet, we observed a strong, positive correlation between postprandial total BAs vs FGF-19. This may be regarded as surprising, as the net effect of FGF-19 activation of FGF receptor-4 is to downregulate BA synthesis.20 However, these increased FGF-19 levels could reflect a response to increased circulating BA levels, as BAs bind to FXR and stimulate FGF-19 synthesis.20 This correlation, although supportive of a role for FGF-19 in human BA physiology, prompts further investigation of this relationship in humans.
BAs stimulate GLP-1 and PYY secretion via the activation of the TGR-5 receptor18,48–51 on enteroendocrine L-cells.52 Previous studies including post-GBP subjects reported a relationship between peak GLP-1 levels and total BAs6,12 and another study in healthy individuals reported correlations between specific BAs and GLP-1 and PYY.53 Yet, a relationship between total BAs and GLP-1 was not observed in Steinert et al.,13 and was not significant in our study either. However, we did observe a positive relationship between PYY vs multiple BA composite variables during the postprandial state. In humans, vertical sleeve gastrectomy enhances postprandial GLP-1 and PYY release as well, without any increase in circulating postprandial BAs.13 In addition, the fact that the rise in PYY and GLP-1 shortly after the surgery (< 1 week) does not parallel the rise in BAs which is much later,13 suggests that the link between BAs and gut hormones is still murky. Future studies are required to clarify the contribution of increased BA levels to GLP-1 and PYY release.
GBP leads to several anatomic changes to the gastrointestinal tract, which could alter BA metabolism. The distal mixing of undigested nutrients with gallbladder and pancreatic excretions in lower sections of the jejunum, the change in pH, and accelerated nutrient transit time, all may all alter BAs.54–57 Furthermore, gut hypertrophy58 could potentially alter the site or efficiency of BA reabsorption. Another potentially attractive hypothesis is that changes in the gut microbiome, reported after GBP,33–35 modulate BA metabolism, as the gut microbiota have a key role in BA deconjugation, dehydroxylation and dehydrogenation.59 There are also numerous other potential contributors to changes in BA levels after GBP, including alterations in hepatic insulin sensitivity, BA synthesis or excretion, enterohepatic cycling and gut permeability. Clearly, circulating BAs are a complex variable reflecting a number of processes—synthesis, excretion, trafficking—that may each be modulated differentially by GBP.
There are several limitations to this study. First, our sample size was small, and BAs, particularly in the postprandial state, were highly variable, as also shown by others.60,61 Several factors may contribute to this variability including surgical technique, T2DM status,9 timing of sampling in relation to surgery, energy balance status,13,14 insulin sensitivity,27 diet composition, and others. Second, our population was exclusively women, which could limit interpretation of the data, as gender differences in BA metabolism have been reported.62 In addition, in this study, 3/13 subjects underwent a cholecystectomy, two were before surgery and one occurred 2 months after surgery. As this may alter BA kinetics or composition,63–66 we analyzed the data during the fasted state, excluding these three subjects, and observed very similar results (data not shown). Moreover, our measurements were limited to peripheral blood concentrations of BAs. Although portal blood concentrations have been shown to correlate with peripheral values,67 measurements of BA synthesis would be more telling. Finally, we used an OGTT, rather than a mixed meal test, which although providing a unique perspective, may not be the most appropriate representation of BA response in a human GBP model.
This study provides a significant and unique contribution to the literature. It is the only study to report the effects of GBP on fasted and postprandial BA levels in individuals with T2DM, up to 2 years after surgery, and the only to measure BA response to an oral glucose load in a GBP population. We observed a general reduction or no change in BA levels or composition 1 month post GBP, but an overall increase in BAs and the 12α-OH/non12α-OH ratio 2 years after surgery. BAs appeared to be related to FGF-19, body weight, the incretin effect on insulin, and PYY. Further work is necessary to understand the etiology and implications of the temporal change in circulating BA levels after GBP in subjects with T2DM.
Supplementary Material
ACKNOWLEDGEMENTS
We thank our participants, Yim Dam and Ping Zhou (technicians from the NYONRC Hormonal Core laboratory), and the entire GCRC-CTSA staff. This work was funded by grants from the American Diabetes Association (CR-7-05 CR-18) and NIH (R01-DK067561, P30-DK26687, P30-DK063608). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000040, formerly the National Center for Research Resources, grant number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. RD was supported by the NYONRC training grant 5T32DK007559-22. ClR received funding from Science Foundation Ireland 12/YI/B2480.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smushkin G, Sathananthan M, Piccinini F, Dalla Man C, Law JH, Cobelli C, et al. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes. 2013;62:1094–1101. doi: 10.2337/db12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 6.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–1864. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werling M, Vincent RP, Cross GF, Marschall HU, Fandriks L, Lonroth H, et al. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2013;48:1257–1264. doi: 10.3109/00365521.2013.833647. [DOI] [PubMed] [Google Scholar]
- 11.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891–902. doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity. 2013;21:E660–E668. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013;37:1553–1559. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48–51. doi: 10.1159/000324128. [DOI] [PubMed] [Google Scholar]
- 16.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011;11:160–166. doi: 10.1007/s11892-011-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362:793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- 20.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 23.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 24.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brufau G, Stellaard F, Prado K, Bloks VW, Jonkers E, Boverhof R, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–1464. doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- 27.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012;15:65–74. doi: 10.1016/j.cmet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida K, Makino S, Akiyoshi T. Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes. 1985;34:79–83. doi: 10.2337/diab.34.1.79. [DOI] [PubMed] [Google Scholar]
- 29.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di Ilio C, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41:1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 31.Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Am Stat. 1990;44:250–253. [Google Scholar]
- 32.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 33.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur A, Patankar JV, de Haan W, Ruddle P, Wijesekara N, Groen AK, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2014 doi: 10.2337/db14-0716. [DOI] [PubMed] [Google Scholar]
- 37.Fu ZD, Klaassen CD. Increased bile acids in enterohepatic circulation by short-term calorie restriction in male mice. Toxicol Appl Pharmacol. 2013;273:680–690. doi: 10.1016/j.taap.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glicksman C, Pournaras DJ, Wright M, Roberts R, Mahon D, Welbourn R, et al. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem. 2010;47:482–484. doi: 10.1258/acb.2010.010040. [DOI] [PubMed] [Google Scholar]
- 39.Vincent RP, Omar S, Ghozlan S, Taylor DR, Cross G, Sherwood RA, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50:360–364. doi: 10.1177/0004563212473450. [DOI] [PubMed] [Google Scholar]
- 40.Wewalka M, Patti ME, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442–1451. doi: 10.1210/jc.2013-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cariou B, Chetiveaux M, Zair Y, Pouteau E, Disse E, Guyomarc'h-Delasalle B, et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab. 2011;8:48. doi: 10.1186/1743-7075-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor DR, Alaghband-Zadeh J, Cross GF, Omar S, le Roux CW, Vincent RP. Urine bile acids relate to glucose control in patients with type 2 diabetes mellitus and a body mass index below 30kg/m2. PLoS One. 2014;9:e93540. doi: 10.1371/journal.pone.0093540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Int Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 45.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 46.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adrian TE, Gariballa S, Parekh KA, Thomas SA, Saadi H, Al Kaabi J, et al. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–2347. doi: 10.1007/s00125-012-2593-2. [DOI] [PubMed] [Google Scholar]
- 49.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 50.Ullmer C, Alvarez Sanchez R, Sprecher U, Raab S, Mattei P, Dehmlow H, et al. Systemic bile acid sensing by G protein-coupled bile acid receptor 1 (GPBAR1) promotes PYY and GLP-1 release. Br J Pharmacol. 2013;169:671–684. doi: 10.1111/bph.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab. 2013;15:474–477. doi: 10.1111/dom.12043. [DOI] [PubMed] [Google Scholar]
- 52.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts RE, Glicksman C, Alaghband-Zadeh J, Sherwood RA, Akuji N, le Roux CW. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin Endocrinol. 2011;74:67–72. doi: 10.1111/j.1365-2265.2010.03886.x. [DOI] [PubMed] [Google Scholar]
- 54.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity. 2014;22:2003–2009. doi: 10.1002/oby.20791. [DOI] [PubMed] [Google Scholar]
- 56.Wang G, Agenor K, Pizot J, Kotler DP, Harel Y, Van Der Schueren BJ, et al. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP) Obes Surg. 2012;22:1263–1267. doi: 10.1007/s11695-012-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dirksen C, Damgaard M, Bojsen-Moller KN, Jorgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25 doi: 10.1111/nmo.12087. 346–e255. [DOI] [PubMed] [Google Scholar]
- 58.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252:50–56. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- 59.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Galman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Int Med. 2011;270:580–588. doi: 10.1111/j.1365-2796.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 61.Steiner C, Othman A, Saely CH, Rein P, Drexel H, von Eckardstein A, et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS One. 2011;6:e25006. doi: 10.1371/journal.pone.0025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trottier J, Caron P, Straka RJ, Barbier O. Profile of serum bile acids in noncholestatic volunteers: gender-related differences in response to fenofibrate. Clin Pharmacol Ther. 2011;90:279–286. doi: 10.1038/clpt.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimball A, Pertsemlidis D, Panveliwalla D. Composition of biliary lipids and kinetics of bile acids after cholecystectomy in man. Am J Dig Dis. 1976;21:776–781. doi: 10.1007/BF01073029. [DOI] [PubMed] [Google Scholar]
- 64.Almond HR, Vlahcevic ZR, Bell CC, Jr, Gregory DH, Swell L. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N Engl J Med. 1973;289:1213–1216. doi: 10.1056/NEJM197312062892302. [DOI] [PubMed] [Google Scholar]
- 65.Berr F, Stellaard F, Pratschke E, Paumgartner G. Effects of cholecystectomy on the kinetics of primary and secondary bile acids. J Clin Invest. 1989;83:1541–1550. doi: 10.1172/JCI114050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kullak-Ublick GA, Paumgartner G, Berr F. Long-term effects of cholecystectomy on bile acid metabolism. Hepatology. 1995;21:41–45. doi: 10.1002/hep.1840210109. [DOI] [PubMed] [Google Scholar]
- 67.Angelin B, Bjorkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.