Abstract
Enhanced startle during exposure to unpleasant cues (aversive startle potentiation; ASP) appears in the RDoC matrix as a physiological index of acute threat response. Increased ASP has been linked to focal fear disorders and to scale measures of dispositional fearfulness (i.e., threat sensitivity; THT+). However, some studies have reported reduced ASP for fear pathology accompanied by major depressive disorder (MDD) or pervasive distress. The current study evaluated whether (a) THT+ as indexed by reported dispositional fearfulness mediates the relationship between fear disorders (when unaccompanied by depression) and ASP, and (b) depression moderates relations of THT+ and fear disorders with ASP. Fear disorder participants without MDD showed enhanced ASP whereas those with MDD (or other distress conditions) showed evidence of reduced ASP. Continuous THT+ scores also predicted ASP, and this association: (a) was likewise moderated by depression/distress, and (b) accounted for the relationship between ASP and fear pathology without MDD. These findings point to a role for the RDoC construct of acute threat, operationalized dispositionally, in enhanced ASP shown by individuals with fear pathology unaccompanied by distress pathology.
Keywords: fear, anxiety disorders, startle, threat sensitivity, depression
1) Introduction
The National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative calls for progress toward alternative neurobiologically informed conceptions of psychiatric disorders (Sanislow et al., 2010). The initiative encourages investigation of core biobehavioral constructs with relevance to multiple clinical problems across differing levels of analysis, from genetic and neuro-cellular to brain systems/processes to domains of observable behavior and perceived experience. However, empirical examples are needed of how constructs specified in the RDoC framework can serve as bridges between neurophysiology and clinical problems. The current study addresses this need by demonstrating a role for dispositional threat sensitivity (THT+; the trait counterpart to acute threat sensitivity in RDoC) as indexed by scores on a self-report dimension of fear/fearlessness (Kramer, Patrick, Krueger, & Gasperi, 2012) in mediating the relationship between fear disorders and aversive startle potentiation (ASP), a physiological index of activation of the brain’s defensive system. Extending prior research, the current work also demonstrates a moderating impact of major depression on associations of fear pathology and threat sensitivity with ASP—suggesting that the co-occurrence of depression with fear symptomatology may signify the presence of a distinct pathophysiological condition (Rosen & Schulkin, 1998; Lang & McTeague, 2009).
1.1) Aversive Startle Potentiation: Associations with Fear Disorders and Dispositional Threat Sensitivity
Excessive emotional responding to discrete stimuli perceived as harmful or threatening is a core feature of focal fear disorders such as specific and social phobia, agoraphobia, and panic disorder. Negative emotional reactivity to such discrete aversive cues, reflecting activation of the brain’s core defensive system, can be measured in terms of enhancement (potentiation) of the noise-elicited blink-startle reflex (Bradley, Codispoti, Cuthbert, & Lang, 2001; Lang, 1995)— and individuals high in reported fearfulness show increased startle reflex potentiation during viewing or imagery of aversive compared to neutral scenes (Cook et al., 1991; Cook, Davis, Hawk, Spence, & Gautier, 1992; Vaidyanathan, Patrick, & Bernat, 2009a; see also Lissek & Powers, 2003). Similarly, individuals with specific phobias show measurably greater startle potentiation than controls when viewing or imaging scenes related to their phobias (Hamm, Cuthbert, Globisch, & Vaitl, 1997; Globisch, Hamm, Esteves, & Ohman, 1999). Enhanced startle potentiation during fear-relevant cuing (i.e., increased ASP) has also been reported in individuals diagnosed with social phobia, particularly of the circumscribed (performance-related) type (Lang, McTeague, & Cuthbert, 2007; McTeague et al., 2009). Findings for panic disorder have been more mixed. Cuthbert et al. (2003) reported that panic disorder patients (relative to healthy controls and other anxiety patients) showed diminished rather than enhanced ASP during imagery of personalized fear scenes, while showing a trend toward enhanced general reactivity as indexed by startle magnitude during non-imagery (intertrial) intervals. Somewhat in contrast with this, Lang et al. (2007) found ASP during personal aversive imagery to be reduced in panic patients relative to phobic patients, but greater for panic patients than for GAD or controls.1
Regarding the role of threat sensitivity in fear pathology, it has been hypothesized that high dispositional reactivity of the brain’s core defensive system, encompassing the amygdala and affiliated structures, constitutes a liability factor for focal fear disorders (Rosen & Schulkin, 1998). In RDoC terms, the dispositional liability for disorders of this type corresponds to the construct of “acute threat” in the “Negative Valence Systems” domain. Importantly, a quantitative-structural model exists for measures of reported fear versus fearlessness in relation to specific objects/situations, social contexts, danger/uncertainty, and other stressful circumstances (Kramer et al., 2012). The model, formulated using data from a large adult twin sample, specifies a broad common factor on which all scale measures load substantially; this factor can be viewed as reflecting individual differences along a dimension of dispositional threat sensitivity (THT+). Consistent with this perspective, scores on this factor predict degree of ASP (i.e., compared to intermediate scorers, individuals with low scores on this factor showed reduced ASP, and those with high scores show enhanced ASP (Kramer et al., 2012; Vaidyanathan et al., 2009a). Additionally, scores on the THT+ factor are appreciably heritable (Kramer et al., 2012)—as would be expected of an underlying liability factor. In sum, existing research has demonstrated associations for focal fear disorders and reported fearful tendencies with ASP in different cuing contexts, including picture viewing. Based on the idea of dispositional threat sensitivity (THT+) as a liability for fear-related disorders, the current study evaluated the possibility that individual differences in this dispositional variable (operationalized as scores on the common factor shown to underlie various scale measures of fear/fearlessness) might account for the relationship between fear disorder diagnoses and ASP.
1.2) Moderating Impact of Depression on Fear/Startle Associations
Data from a number of studies have produced evidence that the presence of major depression, or perhaps distress pathology more broadly, moderates the relationship between fear pathology and ASP. In work examining startle modulation during imaginal processing of fear-relevant and neutral scenarios, Lang et al. (2007) found that patients with focal fear disorders showed greater ASP than patients with diffuse anxiety conditions, and that patients of either type with co-morbid depression showed reduced startle potentiation compared to those without co-morbid depression. Additionally, these investigators reported that diffuse-anxiety patients with comorbid depression displayed the greatest levels of pervasive distress (negative affectivity) as indexed by multiple self-report measures. Subsequent work by Taylor-Clift, Morris, Rottenberg, and Kovacs (2011) evaluated the moderating impact of comorbid depression on the relationship between anxiety conditions and emotion modulated startle in a picture viewing paradigm. These investigators found that healthy controls and individuals with current anxiety disorders unaccompanied by depression exhibited robust startle potentiation during aversive scenes (relative to neutral), whereas individuals with anxiety disorders and co-morbid depression failed to show such potentiation. Similar to this, a more recent study by Vaidyanathan, Welo, Malone, Burwell, and Iacono (2014) found that subjects with recurrent depression (relative to single-episode or never-depressed subjects) exhibited a flattened affect-startle pattern, providing further evidence that depression exerts a suppressive effect on startle modulation.
In sum, available evidence indicates that the expected increase in ASP during aversive picture or image processing in individuals with anxiety disorders, and focal fear conditions in particular, may be moderated by the presence of co-morbid depression—which operates to dampen startle potentiation. Extending beyond findings for depression, work by Lang and colleagues (e.g., Lang et al., 2007; Lang & McTeague, 2009; McTeague, Lang, Wangelin, Laplante, & Bradley, 2012) suggests that it may be pervasive distress and dysphoria more broadly, rather than depression per se, that accounts for this suppressive effect on ASP. The current study was conducted to further address questions pertaining to affective individual differences, internalizing psychopathology, and startle reactivity.
1.3) Current Study Hypotheses
Building on prior published work as described, the current study tested the following specific hypotheses:
Fear disorder participants will show increased ASP relative to non-fear-disorder participants, but only in the absence of a history of major depression, or (per work by Lang et al.) distress conditions more broadly;
ASP will covary positively with dispositional threat sensitivity (THT+) as indexed by scores on a self-report based measure of variations in fear/fearlessness;
THT+ will mediate the observed relationship between ASP and fear disorder diagnosis (when not accompanied by major depressive disorder [MDD] or other distress pathology).
2) Materials and Methods
2.1) Participants
The base sample for the study consisted of 508 adult twins (257 female) recruited from the greater Twin Cities metro area who participated for a payment of $100. Most participants were tested concurrently with their co-twin (of the same gender, in all cases, by design), within the same scheduled session but by different experimenters in separate lab rooms. Participants were selected for testing based on levels of THT+ (see next section), and were free of visual or hearing impairments as assessed by a screening questionnaire. From among the full base sample, 55 participants were excluded from analyses due to unstable/noisy blink EMG signals or excessive zero-amplitude trials (see “Data Reduction” section), and 32 were excluded due to missing physiological or self-report data. Data for the remaining participants with valid blink startle data and relevant questionnaire/diagnostic information (N = 421; 222 female, 199 male) were utilized in the analyses describe below.
2.2) Dispositional and Diagnostic Measures
2.2.1) Dispositional threat sensitivity (THT+): Trait Fear Inventory
Participants were assessed for levels of THT+ using an inventory developed to index a broad dimension of fear/fearlessness identified through structural modeling analyses (Kramer et al., 2012; see also Vaidyanathan et al., 2009a; Vizueta, Patrick, Jiang, Thomas, & He, 2012). The inventory consists of 55 items drawn from various established self-report inventories of fear and fearlessness, including the Fear Survey Schedule-III (Arrindell et al., 1984), the Fearfulness subscale of the EAS Temperament Survey (Buss and Plomin, 1984), the Harm Avoidance subscale of the Temperament and Personality Questionnaire (Cloninger, 1987), subscales comprising Factor 1 of the Psychopathic Personality Inventory (Lilienfeld and Andrews, 1996), and the Thrill/Adventure Seeking subscale of the Sensation Seeking Scale (Zuckerman, 1979). Scores on the 55-item Trait fear measure used in the current study correlate very highly (r>.9) with scores on the general fear/fearlessness factor that these various inventories assess in common (Kramer et al., 2012; see also: Patrick, Durbin, & Moser, 2012; Vaidyanathan et al., 2009a). The items of this inventory are inherently dispositional in nature and designed to index stable trait-like tendencies as opposed to transitional states. Scores on the inventory were quantified in terms of an average score across items, each scored 0 to 3; descriptive statistics for this average fear score variable in the current sample were: M = 1.13, SD = .47, range = .04 to 2.51.
Participants were recruited from a larger sample prescreened using the TF-55 (N = 2,511; Kramer, et al. 2012). Half of the sample (one member of each twin pair) was pre-selected based on TF-55 scores to ensure effective representation of individuals at high and low levels of THT+, and the other half represented unselected co-twins. Specifically, about one-third were chosen to be high in THT+ (i.e., highest 15% of screening sample), one third low (lowest 15%), and the remaining third intermediate (16th to 84th percentile of scorers). The TF-55 was re-administered at time of testing and scores from this administration were utilized in all analyses.
2.2.2) Fear Disorders and Depression: Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I)
All participants were assessed for the full range of lifetime Axis I psychiatric disorders, including anxiety disorders and MDD, using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First et al., 2002). Each participant was interviewed by a PhD-level clinical psychologist or advanced clinical psychology graduate student trained in administration and scoring of the SCID-I diagnostic interview. Interviewers had no knowledge of other assessment data collected from interviewees. Symptom ratings were assigned through a consensus process entailing meetings attended by the interviewers (cf. Iacono, Carlson, Taylor, Elkins, & McGue, 1999), along with the project PI (Christopher Patrick) and a licensed clinical psychologist who provided expert consultation on ratings and diagnostic decisions. Of the 421 participants for whom startle data were available, 74 (18%) met for at least one fear disorder (specific phobia, n = 20; social phobia, n = 40; panic disorder, n = 14; agoraphobia, n = 5; Krueger, 1999), 84 (20%) met full criteria for lifetime history of major depression, and 92 (22%) met criteria for one or more distress disorders (MDD, n = 84; dysthymia, n = 7; or generalized anxiety disorder [GAD], n = 14).
2.3) Stimulus Materials and Design
Each participant viewed a series of 90 pictures consisting of 30 pleasant, 30 neutral, and 30 aversive scenes selected from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999). Each picture stimulus was presented for 6 s, followed by an intertrial interval of 12 s preceding the next picture presentation. Pleasant pictures included erotic, nurturant (babies and small animals), and adventure scenes (10 each). Neutral pictures included household objects, buildings, and neutral faces (also 10 each). Aversive scenes included 20 threat pictures (aimed guns and attacking animals) and 10 mutilation pictures (injured bodies, limbs, faces). Stimuli within each picture category were selected to be gender matched in terms of mean IAPS normative ratings for valence and arousal. Because affective ratings for some of the IAPS pictures differ by gender, there were some differences in the picture sets presented to men and women; specifically, 44 of the 90 pictures comprising the stimulus set differed between genders while the remaining pictures were the same.
Mean normative valence and arousal ratings (respectively) for the picture valence categories were: pleasant: 7.56, 6.15; neutral 4.94, 2.61; aversive 2.30, 6.55. During 81 of the 90 picture stimuli, noise probes (50 ms, 105 dB, 10 μs rise time) were presented at varying points during the 6 s presentation interval to elicit startle blink responses. The probes occurred 3, 4, or 5 s after stimulus onset. For 6 of the remaining 9 pictures, probes were delivered 1, 1.5, or 2 s following picture offset. As a prelude to the main picture presentation sequence, participants viewed a practice picture followed by a noise probe to provide familiarity with the task stimuli, and then (after a recovery interval of ~ 1 min) viewed four probed pictures (IAPS numbers 7508, 7110, 9252, 7233) that served as habituation trials for the startle blink measure. These habituation pictures were presented in the same order for all participants regardless of gender or counterbalancing order. Eight picture presentation orders were used for the main viewing sequence for each gender subgroup. Within and between orders, pictures and noise probes were counterbalanced such that all valence categories (pleasant, neutral, and aversive) were represented equally across orders at each serial position, with the following constraints: no more than two pictures of the same valence occurred consecutively within any stimulus order; pictures of the same content category never appeared consecutively or across orders; and pictures were rotated so as to serve in both probed and unprobed conditions.
2.4) Stimulus Delivery and Physiological Measures
During the test procedure, participants sat in a padded recliner at a distance of 100 cm from a 21-in computer monitor on which the picture stimuli were displayed. Blink responses to noise probes were recorded from a pair of Med Associates 0.25 cm Ag-AgCl electrodes filled with electrolyte paste and positioned over the orbicularis oculi muscle under the left eye. Data collection was performed using two IBM compatible computers, one running E-Prime software for stimulus delivery (Psychology Software Tools, Inc) and the other running Neuroscan Acquire software for physiological data acquisition. Blink EMG responses were recorded at a sampling rate of 1000 Hz using a Neuroscan SynAmps amplifier with a 200 Hz low pass and 0.05 Hz high pass analog filter applied before digitization to prevent aliasing (Blumenthal et al., 2005). Data were then digitally high passed at 10 Hz to remove artifacts due to movement (van Boxtel, Boelhouwer, & Bos, 1998). Lastly, the signals were rectified and integrated using a digital single-pole recursive filter (implemented using Matlab software; Mathworks, Inc.) to simulate a Coulbourn contour-following filter with a 30 ms time constant.
2.5) Procedure
The data for the picture-viewing task were collected as part of a larger test protocol. Prior to testing, participants provided written informed consent and completed a biographical information form, which assessed for physical ailments, medication use, and visual and auditory impairments. Participants also completed a set of questionnaires that included the TF-55 index of THT+, as described above.
While participants completed the foregoing measures, electrodes were attached for physiological response measurement and participants were advised they would be presented with a series of neutral and emotional pictures, each to be viewed for its entire time of presentation. Participants were also informed that they would hear brief noises at times through insert earphones, which they were instructed to disregard.
2.6) Data Reduction
The noise probes delivered during the first four habituation pictures were excluded from analyses due to these initial responses being disproportionately large compared with responses to subsequent probes (cf. Patrick, Bradley, & Lang, 1993). To quantify the magnitude of the startle blink response to each probe thereafter, a Matlab-based scoring algorithm was used in which the peak of the startle blink was defined as the highest level of EMG activity reached between 30 and 120ms following noise probe onset, relative to the median activity during the 50ms period preceding probe onset.
After algorithm scoring all trials were visually inspected by two independent reviewers to identify unstable or no-response trials. Trials identified as unstable included those in which blink onset occurred earlier than 20 ms, trials in which startle blinks overlapped with a preceding spontaneous eye blink, and trials with highly variable pre-probe baseline. No-response trials were defined as those in which no discernable blink response occurred within the peak scoring window. Trials that both raters identified as unstable were excluded from analyses; no-response trials identified as such by both raters were scored as zero. In cases where raters disagreed on more than 15 trials, a third rater re-evaluated the participant’s blink data to finalize decisions about unstable and no-response trials.
Based on the foregoing criteria, the number of unstable and zero response trials was tallied for each participant. Participants with more than 30% trials (i.e., ≥ 27 out of 87) classified as unstable or no-response by the independent raters were flagged for removal from analyses. As a further check, all trials for subjects who had been flagged for removal were re-evaluated through consensus to ensure that at least 30% of trials were in fact unstable or zeroed. These evaluative criteria led to approximately 18.44% of trials being rejected due to unstable baseline and 4.06% scored as zero-amplitude. 55 participants were excluded entirely from analyses, 54 due to excessive unstable trials and 1 due to excessive no-response trials. Data for another 32 participants were not analyzed due to either missing startle data (n = 10) or missing individual difference or diagnostic information (n = 22), resulting in a total of 421 participants for the analyses reported below.
To establish a common response metric for all participants in the evaluation of relations between startle reflex modulation and trait fear, raw startle magnitude values were converted to T score units (cf. Bradley et al., 2001; Levenston et al., 2000) by standardizing raw values across trials within subject as follows: z score = (raw trial magnitude value - M of raw values across all trials) / SD for raw values across all trials; T score value= (z score value × 10) + 50. This transformation yielded standardized blink magnitude scores with M = 50 and SD = 10 for each participant.
2.7) Data Analysis
To test the above-mentioned study hypotheses, we utilized mixed model ANOVAs in which average startle blink response during viewing of neutral and aversive picture categories was treated as a within-subjects factor (Valence).2 The presence or absence of fear psychopathology (Fear DO+/Fear DO-) and lifetime history of depression (MDD+/MDD−) were treated as dichotomous between-subjects factors in one analysis to assess for unique and interactive effects of each. A similar analysis was conducted to evaluate the effect of THT+ on ASP. In this analysis, THT+ was treated as a continuous covariate in place of DSM based fear diagnoses, with depression (or distress) diagnosis included as a fixed factor. Our major a priori hypotheses of positive associations for ASP with presence of fear psychopathology and higher THT+ (Cook et al, 1992; Hamm et al, 1996; Vaidyanathan et al., 2009a; Kramer et al, 2012) were evaluated at one-tailed p<.05; all other effects were evaluated at two-tailed p<.05.3 Complementary analyses focusing on presence versus absence of lifetime distress psychopathology more broadly (i.e., MDD, dysthymia, and/or GAD) in place of MDD per se were conducted as well to assess for unique and interactive effects of this set of conditions.
Finally, the hypothesized mediating role of THT+ and moderating effect of major depression (or distress psychopathology more broadly) on ASP were formally assessed using the MODMED macro in SPSS (Preacher, Rucker, & Hayes, 2007), which yields bootstrapped confidence intervals for the indirect effect of a mediator (in this case, THT+) at specified levels of a moderator (MDD+/MDD−). More specifically, confidence intervals computed using the bias-corrected and accelerated (BCa) bootstrap method (Efron, 1987) are reported for tests of moderated mediation effects.
3) Results
3.1) Aversive Startle Potentiation: Relations with Fear Disorders and Depression
Table 1 displays descriptive statistics for the startle magnitude variable by picture valence category and diagnostic status. The analysis of picture valence effects as a function of diagnostic status yielded a significant main effect of Valence, F(1, 417) =124.33, p<.001, and a significant Valence × Fear × Depression interaction, F(1, 417) = 5.50, p<.05. Figure 1 depicts the nature of this interaction. Among MDD participants, those with a fear disorder diagnosis displayed enhanced ASP (i.e., Aversive-Neutral difference scores) relative to those without fear disorders, F(1, 335) = 2.93, p<.05. For MDD+ participants, results trended in the opposite direction—i.e., participants exhibiting fear disorders with comorbid depression tended to show reduced ASP, F(1, 82) = 3.35, p = .07.
Table 1.
Startle Magnitude M (SD) by Picture Valence and Diagnostic Status
| Diagnostic Status | Picture Valence | ||
|---|---|---|---|
|
| |||
| Neutral | Aversive | ||
| No MDD | No Fear Disorder (n = 292) | 49.46 (1.86) | 52.02 (2.19) |
| Fear Disorder (n = 45) | 49.22 (1.83) | 52.77 (2.72) | |
|
| |||
| MDD | No Fear Disorder (n = 55) | 49.12 (1.85) | 52.29 (1.67) |
| Fear Disorder (n = 29) | 49.57 (1.82) | 51.40 (2.17) | |
|
| |||
| No Distress Disorder | No Fear Disorder (n = 287) | 49.45 (1.86) | 52.02 (2.12) |
| Fear Disorder (n = 42) | 49.22 (1.87) | 52.82 (2.79) | |
|
| |||
| Distress Disorder | No Fear Disorder (n = 60) | 49.18 (1.85) | 52.25 (1.68) |
| Fear Disorder (n = 32) | 49.53 (1.77) | 51.47 (2.11) | |
Note. Startle magnitude Ms and SDs are in standardized (T-score) units. MDD = meets criteria for major depressive disorder (lifetime). Distress Disorder = meets criteria for major depressive disorder, dysthymia, and/or generalized anxiety disorder.
Figure 1.
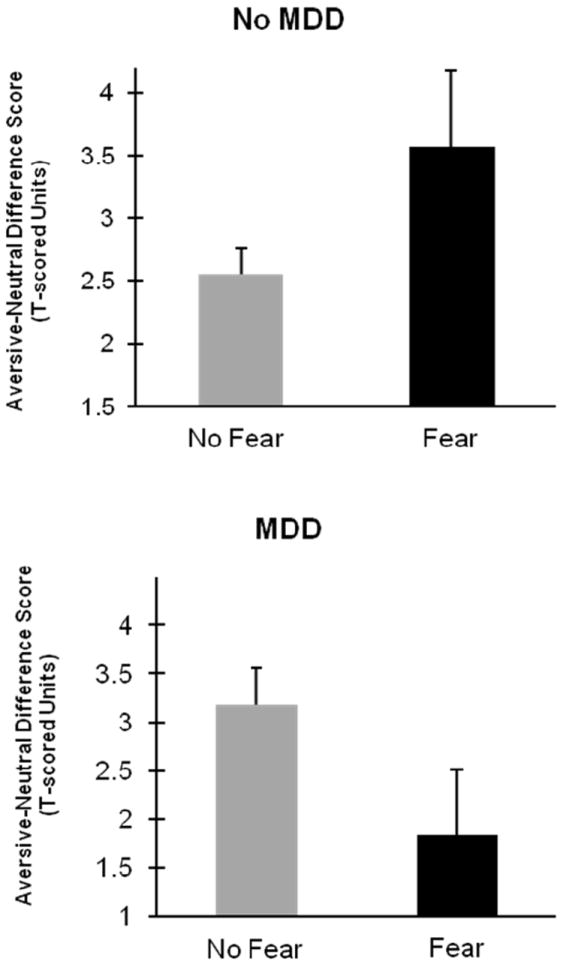
Bar graphs depicting average aversive startle potentiation as indexed by unpleasant-picture minus neutral-picture difference scores. Upper plot displays comparison between no-fear and fear disorder groups within non-depressed portion of study sample (i.e., no lifetime diagnosis of major depressive episode [MDD]). Lower plot displays same comparison for depressed (lifetime MDD present) portion of sample.
As a supplement to the foregoing, a further analysis was conducted in which presence versus absence of any broad distress condition (i.e., MDD, dysthymia, or GAD; cf. Krueger, 1999; Watson, 2005) was included as a covariate in place of presence/absence of depression per se. The results of this analysis were quite similar: A significant Valence × Fear × Distress interaction emerged F(1, 417) = 4.88, p<.05, with participants meeting criteria for a fear disorder alone showing enhanced startle potentiation F(1, 327) = 2.90, p<.05, and those exhibiting comorbid fear and distress conditions showing a trend toward reduced startle potentiation F(1, 90) = 2.66, p = .11.
3.2) Aversive Startle Potentiation: Associations with Dispositional Threat Sensitivity
For the analysis utilizing continuous THT+ scores in place of DSM-IV based fear diagnoses, a significant main effect of Valence was evident F(1, 417) =19.41, p<.001. A significant Valence × Depression interaction was also observed, F(1, 417) = 4.97, p<.05, but this two-way interaction was subsumed by a significant three-way (Valence × THT+ × Depression) interaction, F(1, 417) = 6.05, p<.05.4 Follow-up analyses focusing on Aversive-Neutral potentiation scores for MDD+ and MDD− subsamples separately revealed a robust positive r between THT+ and ASP in the MDD− subsample, r = .15, p <.005, contrasted with a trend in the opposite direction for the MDD+ subsample, r = -.15, p = .08. Associations for these two subsamples are depicted in Figure 2. A follow-up analysis including presence/absence of any distress disorder in place of MDD status likewise yielded a significant Valence × THT+ × Distress interaction, F(1, 417) = 5.84, p<.05, with follow-up correlations revealing a positive association between THT+ and ASP for participants without distress psychopathology, r = .16, p < .005, and a trend-level negative association for participants with distress psychopathology, r = -.17, p = .11.
Figure 2.
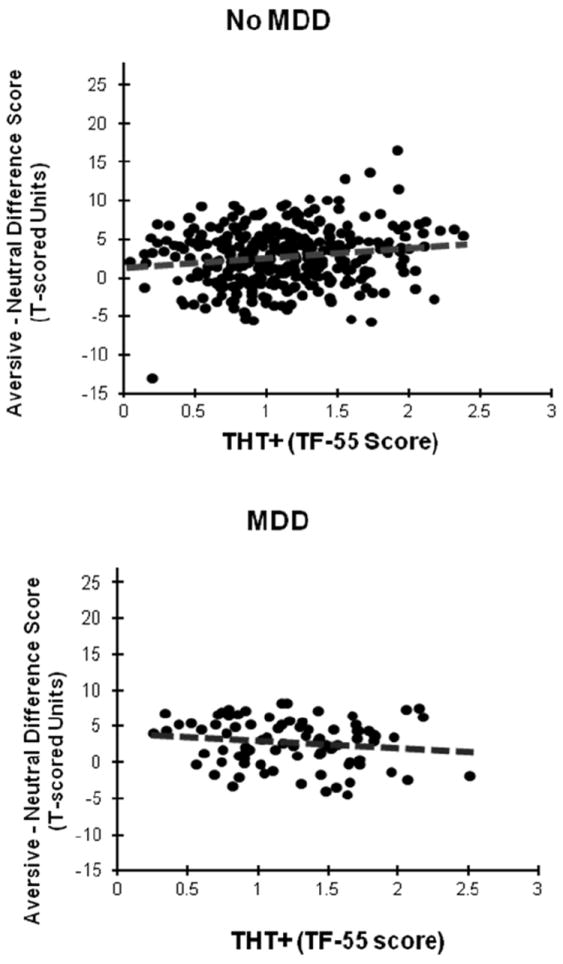
Scatter plots illustrating relationship between aversive-neutral startle potentiation scores and threat sensitivity THT+ as indexed by 55-item Trait Fear inventory (TF-55) scores for never-depressed (No MDD; upper) and depressed subsamples (MDD; lower) separately.
3.3) Evidence for a Mediating role of Threat Sensitivity and a Moderating effect of Depression on the Fear Pathology/ASP relationship
Based on the aforementioned findings, we tested for the presence of moderated mediation effects involving depression (or distress psychopathology more broadly) and threat sensitivity, in relation to the ASP/fear pathology association—i.e., a moderating impact of presence versus absence of depression or distress psychopathology on the indirect (mediating) effect of threat sensitivity. Formal analysis (Preacher et al., 2007) confirmed that THT+ mediated the relationship between fear disorder status and ASP in the absence of MDD (95% BCa CI = {.17, 1.12}), but not in participants who exhibited a history of MDD (95% BCa CI = {-1.23, .28}). A corresponding analysis for distress conditions yielded similar results: THT+ mediated the fear psychopathology/ASP relationship for participants without a history of distress pathology (95% BCa CI = {.17, 1.15}), but mediation was not evident for participants exhibiting comorbid fear and distress pathology (95% BCa CI = {-1.17, .27}). Notably, within the MDD- subsample, the effect of fear disorder status on ASP fell below significance after accounting for THT+ as a mediating variable in a regression model (95% CI = {-0.76, 1.69}); the same was true for the subsample without history of any distress pathology (95% CI = {-0.80, 1.74}). By contrast, the predictive relationship for THT+ with ASP within these subsamples remained significant even after accounting for presence versus absence of fear psychopathology (95% CIs = {.19, 2.03} and {.19, 2.07}, respectively).
4) Discussion
Results from the current study replicate and extend findings from prior work investigating relationships between ASP and anxiety-based disorders. In line with previous reports (Hamm et al, 1996; Lang et al, 2007; McTeague et al, 2009), participants in the current study with focal fear diagnoses displayed greater ASP on average than participants without a fear disorder—when free from comorbid depression or other pervasive distress pathology. When coupled with a history of major depression or distress pathology, the presence of fear pathology was associating with blunted rather than augmented startle potentiation. This pattern dovetails with findings reported by other groups (Taylor-Clift et al, 2011; Vaidyanathan et al., 2014) and appears consistent with the conceptual perspective on anxiety pathology advanced by Lang and McTeague (2009). These authors posited that anxiety conditions marked by pervasive distress or negative affectivity, entailing anxious apprehension and hyperarousal along with prominent dysphoria, are associated with dysregulation in core defensive circuitry of the brain that results in diminished priming of protective responses (including startle) in aversive cuing contexts. In contrast with circuitry dysfunction of this type in pervasive distress conditions, circumscribed fear conditions appear to involve intact defensive circuitry operating at a heightened level of sensitivity in relation to specific perceived threats.
Current findings suggest further that threat sensitivity as indexed by a continuous scale measure of dispositional fear/fearlessness (cf. Kramer et al., 2012) effectively captures the heightened defense-system sensitivity associated with focal fear problems—such that scores on the THT+ measure correlated robustly with ASP and accounted for the observed positive relationship between ASP and fear disorder diagnosis when unaccompanied by major depression or distress pathology. The fact that THT+ did not account for the contrasting negative relationship between fear disorder status and ASP in the depressed/distressed subsample raises the possibility that alternative mechanisms (e.g., defense system dysfunction arising from persistent intense distress [Lang & McTeague, 2009] and/or overgeneralization of conditioned fear responding [Lissek, 2012]) may account for the attenuation of defensive response priming in this subsample.
It is conceivable that this impairment in defensive reflex priming observed for individuals with comorbid distress and fear pathology could reflect an underlying state or process akin to learned helplessness (Seligman, 1975). That is, chronically high levels of worry and anxious arousal may be associated with an underlying condition of motivational/motoric disengagement that is reflected in blunted ASP (Vaidyanathan, Patrick, & Cuthbert, 2009b). Based on animal neuroscience research demonstrating differing roles for the amygdala and affiliated structures extending beyond it (e.g., bed nucleus of the stria terminalis) in phasic, cue-elicited fear as compared to more ‘tonic anxiety’ (e.g., Davis, 1998; Davis, Walker, & Lee, 1997), it has been postulated that the progression from high trait fear (THT+) to pervasive distress pathology arises from adverse experiences that produce chronic sensitization of the amygdala and extended amygdale—giving rise to symptoms of persistent negative arousal, hypervigilance, and aversive anticipation (Rosen & Schulkin, 1998; see also Grillon & Davis, 1997). Further systematic research will be needed to confirm the role of chronic defense-system sensitization in human psychological disorders marked by pervasive distress, and to test the hypothesis that this sensitization is associated with a condition of helpless disengagement that accounts for deficits in ASP.
4.1) Mechanisms of Anxiety Pathology: Liability versus Expression
While complementary to previous published research on ASP in anxiety-related conditions, the current work also differs in some notable ways from prior work. In particular, ASP was assessed in relation to IAPS picture stimuli selected for normative aversiveness, as opposed to pictorial or imaginal stimuli of distinct relevance to participants’ anxiety problems (i.e., depictions of phobic objects or specific anxiety-provoking scenarios). The reason is that the current work sought to examine threat sensitivity as a broad disposition contributing to fear-related conditions, rather than focusing on processes unique to specific variants of fear pathology. Given this objective, the participant selection strategy for the current study also differed from past work on ASP in anxiety disorders. Rather than selecting expressly for diagnosable anxiety pathology, we selected participants ranging across the continuum of dispositional threat sensitivity, with overrepresentation of the high (as well as low) extreme—under the presumption that individuals high in threat sensitivity would show increased rates of fear-related pathology.
This supposition was borne out by the finding that THT+ scores covaried systematically with fear disorder status and accounted for the relationship between fear disorder status and ASP. Notably, the converse was not true (i.e, fear disorder status did not fully account for the relationship between THT+ and ASP). This finding, which suggests primacy of the association for THT+ with ASP over that for fear disorder status, highlights the importance of understanding the basic dispositional dimension underlying associations between fear disorders and ASP. While operationalized here as self-report fear/fearlessness, THT+ has clear neurophysiological and behavioral referents and thus has been characterized as a neurobehavioral construct (Patrick et al., 2012a). From an RDoC standpoint, this dispositional dimension—particularly when indexed through use of physiological and behavioral indicators in conjunction with report-based indicators (cf. Patrick et al., 2013)—can be expected to exhibit clearer connections to neurobiological systems and processes than diagnoses rooted in clinical consensus. As such, focusing on neurobehavioral dispositions has the potential to move the field toward conceptions of psychological problems more closely linked to brain circuitry and function.
Findings of the current work also highlight the importance of studying liability along with active expression in seeking to understand psychopathology. As emphasized in the developmental literature, clinical conditions arise from the dynamic interplay between dispositional liabilities and adverse, pathology-promoting experiences across time. Full understanding of fear and anxiety related conditions will require clarification of the nature of dispositions that confer risk for such conditions, along with delineation of the types of adverse experiences that operate at particular points in development to spawn and maintain active pathology. Relevant to this, the current work highlights the fact that biobehavioral constructs such as acute threat from the RDoC framework can be operationalized both as processes, and as dispositions. When studied as processes (e.g., brain states involved with detection and responsivity to cues), the focus of research on constructs of this type is more naturally on their role as mediators of current clinical symptoms. When studied as dispositions, the emphasis can instead be on their role as liability factors for the emergence of psychopathology. While informative in this respect, the current study is limited in that THT+ was assessed concurrently with fear and anxiety symptomatology. A more compelling case for the role of heightened threat sensitivity in anxiety-related conditions could be made by demonstrating significant prospective prediction of clinical problems later in life from THT+ at earlier ages. It is also worth noting that although findings from our analyses of distress conditions more broadly (i.e., including dysthymia and GAD, along with MDD) paralleled those for depression alone, base rates for these other distress conditions were somewhat limited. Follow-up studies employing samples with greater representation of other such disorders along with MDD are needed to clarify the role of broad distress pathology (as opposed to depression per se) in moderating ASP. Additionally, although the current study utilized a scale measure of THT+ with known physiological correlates (Patrick et al., 2012a; Vaidyanathan et al., 2009a), it will be valuable in future work to operationalize THT+ in a more explicitly biobehavioral manner—through inclusion of physiological and/or behavioral indicators along with report-based indicators (cf. Patrick et al., 2013). Work of this kind can be expected to lead to improved methods for early identification of individuals at high biological risk for fear- and anxiety-related problems, while at the same time advancing knowledge of brain circuitry variations underlying dispositional differences in THT+.
4.2) Conclusions
Notwithstanding its limitations, the current study serves to illustrate—in line with the aims of NIMH’s RDoC initiative—how a basic biobehavioral construct (acute threat sensitivity) can help account for observed relations between a clinical symptom variable (phobic fear) and a key measure from the domain of neurobiology (ASP). By extension, constructs like acute threat sensitivity from the RDoC framework can serve as crucial points of reference for systematic research on the neurobiology of anxiety-related conditions and affiliated dispositional liabilities. Relatedly, current findings corroborate prior work (Kramer et al., 2012) demonstrating that THT+ as indexed by scale measures of fear/fearlessness and protective response readiness operationalized via ASP operate as indicators of a common dispositional dimension entailing variations in sensitivity/responsiveness of the brain’s core defensive system. These results point to the possibility of operationalizing THT+ as a composite of physiological and trait-scale indicators that can serve as a direct bridge between neural circuits/processes and clinical symptomatology (cf. Patrick et al., 2013). Conceptualizing and measuring tendencies toward pervasive distress and depression in a parallel manner (i.e., as aggregates of relevant physiological and scale indicators) should also be valuable for clarifying the basis of their moderating impact on the relationship between fearful tendencies and ASP.
Highlights.
Aversive Startle Potentiation (ASP) was enhanced in participants exhibiting fear disorders without a history of comorbid depression or other pervasive distress disorders
ASP was positively associated with dispositional threat sensitivity (THT+) in participants without a history of depression or distress pathology
THT+ mediated the relationship between fear disorders and ASP in participants without depressive/distress pathology
Acknowledgments
This work was supported by grant W911NF-14-1-0027 from the US Army and grants MH072850 and MH089727 from the National Institute of Mental Health. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Government, Department of Defense, Department of the Army, Department of Veterans Affairs, or U.S. Recruiting Command.
Footnotes
Notably, studies utilizing threat of shock as opposed to aversive pictures or imagery have also reported somewhat mixed findings for panic disorder. For example, Grillon et al. (2008) reported enhanced startle potentiation in an unpredictable aversive condition (uncued delivery of shock) among patients with panic disorder selected to be depression-free, and Melzig et al. (2007) similarly found that panic patients without comorbid depression showed enhanced ASP under conditions of shock-threat, whereas those with comorbid depression did not. By contrast, Nelson et al. (2013) reported enhanced ASP during shock-threat in individuals with a family history of panic disorder, regardless of history of comorbid depression.
The current report focuses on aversive startle potentiation (ASP) because major study hypotheses pertained to this modulatory effect. However, consistent with findings from many prior studies, current participants as a whole showed significant inhibition of blink startle during viewing of pleasant as compared to neutral pictures, F(1,420) = 50.10, p<.0001. In contrast with ASP, no significant moderating effects were evident for fear psychopathology, MDD, or THT+ on pleasant startle inhibition.
We conducted supplemental analyses including male/female as a factor to test for possible effects of gender. No main effects or interactions involving gender were evident, so reported analyses focus on effects for men and women combined.
Supplemental two-way (THT+ × Depression) analyses revealed that the three-way interaction involving Valence was driven mainly by startle reactivity to unpleasant pictures, F(1, 417)= 7.32, p<.05 (for neutral pictures, F(1, 417)= 2.34, p = .13). The same was true for the corresponding analysis (above) incorporating fear disorder status together with depression (Fs for unpleasant and neutral = 7.22 and 1.77, respectively).
Financial Disclosures: We have no conflicts of interest or financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrindell W, Emmelkamp P, Van der Ende J. Phobic dimensions: I. Reliability and generalizability across samples, gender and nations. Advances in Behaviour Research and Therapy. 1984;6:207–254. [Google Scholar]
- Bradley M, Codispoti MM, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley SA, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Buss AH, Plomin R. Temperament: Early developing personality traits. Hillsdale: Lawrence Erlbaum Associates; 1984. [Google Scholar]
- Center for the Study of Emotion and Attention (CSEA-NIMH) The International Affective Picture System: Digitized photographs. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Hawk LW, Jr, Davis TL, Stevenson VE. Affective individual differences and startle reflex modulation. Journal of Abnormal Psychology. 1991;100:5–13. doi: 10.1037//0021-843x.100.1.5. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the strial terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Annals of the New York Academy of Sciences. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Efron B. Better bootstrap confidence intervals. Journal of the American Statistical Association. 1987;82:171–185. [Google Scholar]
- First MB, Spitzter RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, non-patient edition (SCID-I/NP) New York, NY: Biometrics; 2002. [Google Scholar]
- Globisch J, Hamm AO, Esteves F, Ohman A. Fear appears fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 1999;36:66–75. doi: 10.1017/s0048577299970634. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses; Evidence for enhanced anxious anticipation. PLoS ONE. 2013;8(8):e70969. doi: 10.1371/journal.pone.0070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;108:134–142. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Patrick CJ, Krueger RF, Gasperi M. Delineating physiological defensive reactivity in the domain of self-report: Phenotypic and etiologic structure of dispositional fear. Psychological Medicine. 2012;42:1305–1320. doi: 10.1017/S0033291711002194. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Cuthbert BN. Fear, anxiety, depression, and the anxiety disorder specturm: A psychophysiological analysis. In: Treat RBT, Baker T, editors. Psychological clinical science: Papers in honor of Richard M McFall. New York, NY: Psychology Press; 2007. pp. 167–195. [Google Scholar]
- Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety, Stress & Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: Emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;109:373–385. [PubMed] [Google Scholar]
- Lilienfeld S, Andrews B. Development and preliminary validation of a self-report measure of psychopathic personality traits in a noncriminal population. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicted on basic, neurally mapped mechanisms of pavolovian fear learning: The case for conditioned overgernalization. Depression and Anxiety. 2012;29:257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS. Sensation seeking and startle modulation by physically threatening images. Biological Psychology. 2003;63:179–197. doi: 10.1016/s0301-0511(03)00053-x. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Strauss CC, Bradley MM. Fearful Imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Wangelin BC, Laplante MC, Bradley MM. Defensive mobilization in specific phobia: fear specificity, negative affectivity, and diagnostic prominence. Biological Psychiatry. 2012;72:8–18. doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Zimmermann J, Hamm AO. Startle reflex modulation and autonomic responding during anxious apprehension in panic disorder patients. Psychophysiology. 2007;44:846–854. doi: 10.1111/j.1469-8986.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Gorka SM, Katz AC, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122:662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath-startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Psychoneurometrics: A paradigm for grounding psychological assessment in neurophysiology; Paper presented at the 52nd Annual Meeting of the Society for the Psychophysiological Research; New Orleans, LA. 2012a. [Google Scholar]
- Patrick CJ, Durbin CE, Moser JS. Conceptualizing proneness to antisocial deviance in neurobehavioral terms. Development and Psychopathology. 2012b;24:1047–1071. doi: 10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122:902–916. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Rosen J, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Sanislow CS, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. Developing constructs for psychopathology research: Research Domain Criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Helplessness: On depression, development, and death. San Francisco, CA: Freeman; 1975. [Google Scholar]
- Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychological Medicine. 2011;41:129–139. doi: 10.1017/S003329171000036X. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009a;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing pathology to neurobiological systems: Affect-Modulated Startle as an indicator of fear and distress disorders and affiliated traits. Psychological Bulletin. 2009b;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Welo EJ, Malone SM, Burwell SJ, Iacono WG. The effects of recurrent depression on startle responses. Psychophysiology. 2014;51:103–109. doi: 10.1111/psyp.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel A, Boelhouwer AJW, Bos A. Optimal EMG signal bandwidth and interelectrode distance for recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology. 1998;35:690–697. [PubMed] [Google Scholar]
- Vizueta N, Patrick CJ, Jiang Y, Thomas KM, He S. Trait fear and negative affectivity as predictors of neuroimaging response to invisible fear faces. NeuroImage. 2012;59:761–771. doi: 10.1016/j.neuroimage.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1979. A new theory of sensation seeking. [Google Scholar]


