Abstract
The use of current source density (CSD), the Laplacian of the scalp surface voltage, to map the electrical activity of the brain is a powerful method in studies of cognitive and affective phenomena. During the last few decades, mapping of CSD has been successfully applied to characterize several neuropsychiatric conditions such as alcoholism, schizophrenia, depression, anxiety disorders, childhood/developmental disorders, and neurological conditions (i.e., epilepsy and brain lesions) using electrophysiological data from resting state and during cognitive performance. Use of CSD and Laplacian measures has proven effective in elucidating topographic and activation differences between groups: i) patients with a specific diagnosis vs. healthy controls, ii) subjects at high risk for a specific diagnosis vs. low risk or normal controls, and iii) patients with specific symptom(s) vs. patients without these symptom(s). The present review outlines and summarizes the studies that have employed CSD measures in investigating several neuropsychiatric conditions. The advantages and potential of CSD-based methods in clinical and research applications along with some of the limitations inherent in the CSD-based methods are discussed in the review, as well as future directions to expand the implementation of CSD to other potential clinical applications. As CSD methods have proved to be more advantageous than using scalp potential data to understand topographic and source activations, its clinical applications offer promising potential, not only for a better understanding of a range of psychiatric conditions, but also for a variety of focal neurological disorders, including epilepsy and other conditions involving brain lesions and surgical interventions.
Keywords: Current source density (CSD), surface Laplacian, EEG, event-related potentials (ERPs), neural generators, neurocognitive deficits, neuropsychiatric disorders, alcohol use disorders, schizophrenia, depression, childhood disorders, epilepsy, brain lesions
Introduction
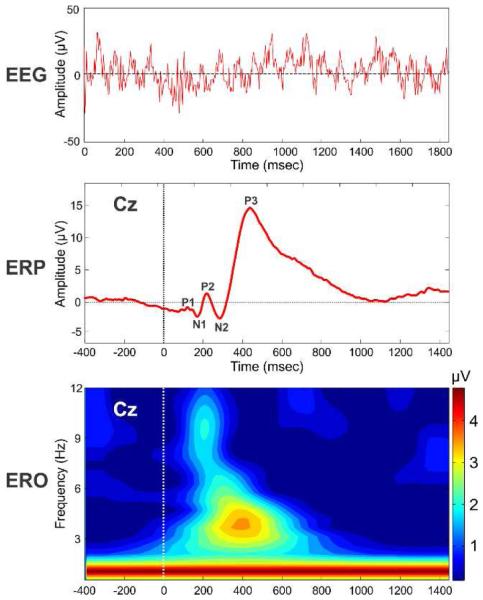
Ever since Hans Berger reported his discovery of brain electrical activity in humans as measured by the electroencephalogram (EEG) (Berger, 1929), scientific and clinical applications of EEG has proliferated and achieved several milestones. One of the main advantages of EEG measures is its time resolution in milliseconds, a scale at which many of the key sensory, motor and cognitive phenomena take place at the neural level. The neuroelectric potentials as recorded from the scalp are measured and analyzed depending upon whether the recordings are made during task-related activity or in situations which are not characterized by the occurrence of a specific event. The ongoing (stimulus- and time independent) neuroelectric activity using scalp electrodes in a continuous fashion during a specific mental state (i.e., eyes-closed relaxed state, eyes-open steady state, meditation, hypnosis, sleep, coma, and other normal/altered states of consciousness) is the EEG (cf. Kamarajan and Porjesz, 2012) [see Fig. 1, top panel]. Common measures of EEG include, but not limited to, absolute and relative power as well as coherence in specific frequency bands, such as delta (1-4 Hz), theta (4-8 Hz), alpha (8-13), beta (13-30), and gamma (above 30 Hz). On the other hand, event-related potentials (ERPs) are voltage fluctuations (i.e., positive and negative components) that are associated in time (time-locked) in response to some physical or mental occurrence (i.e., sensory, motor, or cognitive event), and are extracted from the EEG data by means of filtering and signal/trial averaging (Picton et al., 2000). Generic ERP components include but are not limited to P1, N1, P2, N2, and P3 (with ‘P’ and ‘N’ referring to polarity) as they occur in temporal sequence (see Fig. 1, middle panel), and typically represent specific neural or neurocognitive event. For example, N1 represents physical stimulus characteristics (e.g., brightness or loudness) (Coles and Rugg, 1995; Johannes et al., 1995) as well as the selective attentional processing of the stimulus (e.g., attended/unattended) (Haider et al., 1964; Hansen and Hillyard, 1980; Naatanen and Picton, 1987; Rugg and Coles, 1996), while P3 (also called P300), the most robust and widely studied ERP component, represents context specific cognitive processing such as stimulus/feature/target identification or discrimination, response selection or inhibition, reward evaluation, etc., based on the task requirements and conditions (Sutton et al., 1965; Sutton and Ruchkin, 1984; Donchin and Coles, 1988; Verleger, 1988; Salisbury et al., 2004; Kamarajan et al., 2009). Finally, event-related oscillations (EROs), obtained by time-frequency analysis of the ERP recordings, represent both time and frequency information for a specific sensory, motor or cognitive event, and brain oscillations of different frequency bands are related to various cognitive functions (Basar, 1999a, b; Basar et al., 1999, 2000, 2001). ERO measures often include power and coherence/synchrony for specific time and frequency ranges, and are considered to be involved in the generation of ERPs (see Sauseng et al., 2007 for a critical discussion).
Fig. 1.
Illustration of typical EEG trace (top panel), ERP waveform (middle panel), and ERO time-frequency map at Cz electrode in a visual oddball paradigm, recorded with nose tip as reference and forehead as ground electrode placements. EROs were computed using the S-transform algorithm as described in Jones et al. (2006). The horizontal axis in all panels represent time in milliseconds, and the color scale in bottom panel indicate amplitude in μV. In the middle and bottom panels, zero (0) millisecond represent the stimulus onset during a task.
Despite the fact that EEG has excellent temporal resolution, its spatial resolution (i.e., the information about the specific location or source of the recorded neuroelectric activity) is poor, due to the “blurring effects” of volume conduction, as the head acts like a low-pass spatial filter, transmitting to the scalp broad, as opposed to focal, spatial patterns of activity (Srinivasan et al., 1998b). The EEG activity recorded from each scalp electrode does not represent the specific activity of local brain sources (i.e. directly underneath the electrode), but the “volume-conducted” activity from multiple spatially dispersed sources. While this problem of removing or reducing the volume conduction effects has been a huge challenge for EEG technology, several methods have been devised to spatially represent the local effects of the brain sources responsible for the recorded scalp potentials. One such method is to calculate the current source density (CSD) or the Laplacian (second spatial derivative) of the scalp voltage using one of a number of specific algorithms.
Mapping of the CSD is often used to identify the neuronal generator patterns contributing to scalp-recorded EEG by providing a global empirical and biophysical context for generator localization (Tenke and Kayser, 2012). CSD transformations are implemented by algorithms that compute estimates of the current projected radially towards the skull and scalp from the underlying neuronal tissue at a given surface location, from a spatially weighted sum of the potential gradients directed at this site from some or all recording sites (cf. Kayser and Tenke, 2006b). In essence, the CSD maps represent the magnitude of the radial (transcranial) current flow from the brain to the scalp (source) and to the brain from the scalp (sink) (Perrin et al., 1989a; Kayser et al., 2012). In the CSD-converted scalp potentials, source and sink may correspond to the positive and negative (going) activity respectively. For instance, positive-going CSD activity related to a P3 potential is termed as P3 source while the negative-going CSD activity to an N1 component is described as N1 sink (Kayser et al., 2006). In effect, the CSD transformation functions as a high-pass spatial filter that minimizes the electrical distortions produced by the mediums between cortical surface and sensor (electrode) such as skull and scalp, thus facilitating spatial separation of temporally overlapping components (Turetsky et al., 2000). Therefore, the benefits of a CSD transform are a reference-free, spatially enhanced representation of the direction, location, and intensity of current generators that underlie the recorded scalp potentials (cf. Kayser and Tenke, 2006b), and provides topographies with more sharply localized peaks than those of the scalp potential, while eliminating volume-conducted contributions from distant regions and sources (Tenke and Kayser, 2005; Kayser et al., 2006).
There are many methods for computing the surface Laplacian of brain potentials, which include: 1) local methods (e.g., Hjorth, 1975), 2) global methods (e.g., Perrin et al., 1987a), 3) ‘realistic geometry’ or ‘realistic Laplacian estimator’ (Babiloni et al., 1996), and 4) local polynomial approximations (Wang and Begleiter, 1999). Each of these methods has its own strengths and weaknesses. Some local methods compute the surface Laplacian by using only the potentials at "nearest neighbor" electrodes in the algorithm, such as a finite difference scheme (Hjorth, 1975; Katznelson, 1981) and a least squares solution of fitting a local quadratic representation of the potentials (Le et al., 1994). However, the surface Laplacian of brain potentials computed by local methods are said to be very sensitive to noise, with amplified high spatial frequencies (Le et al., 1994). Given a small number of electrodes, noise in any of them (including the spatial precision of their location) may be exaggerated, while good (artifact-free) data can yield reliable CSD estimates via local methods. Further, the surface Laplacian for the peripheral electrodes cannot be estimated by these local methods (cf. Wang and Begleiter, 1999). On the other hand, global methods compute the surface Laplacian of the global potential function by using the potentials at all electrodes in interpolation functions, such as the spherical spline (Perrin et al., 1987a, 1989b). Realistic Laplacian estimator approaches assume that the best surface Laplacian estimates are computed by second order spline along with a smoothing parameter, and can be applied to any arbitrarily shaped scalp (Babiloni et al., 1995, 1996, 1998). The local polynomial approximations method proposed by Wang and Begleiter (1999) is: 1) a local method which assigns larger weight for closer electrodes; (2) able to estimate surface Laplacian at peripheral locations; (3) able to estimate potential and surface Laplacian simultaneously so that direct differentiation on an interpolated function is unnecessary; and (4) robust to noise. Compared to the second order spline estimation (Babiloni et al., 1995), the local polynomial approximations is better for cleaner data with high signal-to-noise-ratio (SNR), such as averaged ERPs, but this method fares poorly for very noisy data with poor SNR, such as single trials (cf. Wang and Begleiter, 1999).
CSD methods have been widely used to determine differential topographic patterns of cortical activations in clinical conditions as distinguished from healthy controls in order to develop potential correlates and/or bio-markers for the disorder or condition. As described in the following sections, this review focuses only on the CSD methods as applied in several neuropsychiatric conditions in human participants. While the utility of CSD has been established in electrophysiological studies in animals as well as humans, it may be surprising to note that this method has not been adequately applied in some common clinical domains, such as substance use disorders (other than alcoholism), obsessive-compulsive disorder, and post-traumatic stress disorder. By summarizing the studies that have successfully used CSD measures, this review attempts to present an overview of salient findings and utility of CSD (in terms of activation patterns and topography) in several neuropsychiatric conditions, while listing out some of the limitations as well as future prospects.
Alcoholism
CSD methods have been effectively applied in alcoholism to elucidate topographical features and source activations distinguishing alcoholics or those at risk from control subjects (see Table 1, section 1), although there have been no such studies for other substance use disorders. CSD methodology has been used in combination with resting EEG, ERP, and ERO measures in a number of studies of individuals with alcohol use disorder (AUD), as well as in studies of high-risk offspring of alcoholic parents. Many of these studies of AUDs have used the local polynomial approximations method of calculating the surface Laplacian as proposed by Wang and Begleiter (1999), which is described in the Introduction section above.
Table 1.
List of studies that have used CSD or surface Laplacian to differentiate a clinical group from control subjects.
| Citation | Sample / Groups |
Measures and Task |
CSD Method [# Scalp Electrodes] |
CSD / surface Laplacian Findings |
|---|---|---|---|---|
| 1. Alcoholism | ||||
| Ramachandran et al. (1996) | 48 young sons of alcoholics and 23 age and gender- matched controls |
P3 during auditory oddball paradigm |
Spline Laplacian (Nunez and Pilgreen, 1991) [31 electrodes] |
Reduced CSD in the high-risk group over the posterior central, parietal, and occipital areas bilaterally and in the right frontal area |
| Ji et al. (1999) | 36 sober adult alcoholics and 28 healthy controls |
P1, N1 and P2 during visual category matching paradigm |
Spline Laplacian (Nunez and Pilgreen, 1991) [61 electrodes] |
Suppressed P2 related CSD activation of left temporal-occipital brain regions in alcoholics during both matching and nonmatching conditions (around 250 ms) |
| Rodriguez Holguin et al. (1999b) | 26 adult offspring of alcoholics and 23 healthy controls |
P3a during visual oddball paradigm |
Spline Laplacian (Nunez and Pilgreen, 1991) [31 electrodes] |
Sources of current in the HR group were smaller than in the control group, along with topographic differences in CSD activations |
| Rodriguez Holguin et al. (1999a) | 44 adult male alcoholics and 28 controls |
P3a and P3b during visual oddball paradigm |
Spline Laplacian (Nunez and Pilgreen, 1991) [31 electrodes] |
Alcoholic group produced smaller P3 sources than the control group in all scalp regions |
| Hada et al. (2000) | 27 male alcoholics and 25 male controls |
P3a and P3b during auditory oddball paradigm |
Surface spline interpolation (Perrin et al., 1987b) [61 electrodes] |
Weaker P3 sources and less topographic specificity in the CSD maps of alcoholics |
| Hada et al. (2001) | 24 male high- risk subjects and 17 low- risk controls |
P3a and P3b during auditory oddball paradigm |
Surface spline interpolation (Perrin et al., 1987b) [61 electrodes] |
High risk subjects manifested weaker current density and more P3 sources and sinks than low risk subjects for the rare non-target (novel) condition |
| Bauer and Hesselbrock (2002) | 151 adolescent females: individuals with a lifetime history of a major depressive episode and subjects with family history of alcoholism |
Resting EEG | Realistic head-shape boundary element method (Fuchs et al., 1998) [31 electrodes] |
CSD activations over the left frontal area differentiated the effects of a family history of alcoholism |
| Cohen et al. (2002) | 30 sober male alcoholics and 39 normal controls |
N1 and P3 during auditory and visual oddball paradigms |
Spherical splines (Perrin et al., 1989b) [61 electrodes] |
P3 sources were weaker in alcoholics than in controls anteriorly in the auditory modality and posteriorly in the visual paradigm |
| Kamarajan et al. (2005) | 30 alcoholics and 30 healthy controls |
P3 during visual Go/No-Go task |
Local polynomial estimate (Wang and Begleiter, 1999) [61 electrodes] |
No-Go condition: Alcoholics had a more diffused and weaker P3 source without the prefrontal sink observed in the controls Go condition: Controls manifested three distinct posterior P3 sources, while the alcoholics showed a single parietal- occipital source |
| Roopesh et al. (2010) | 87 alcohol dependent subjects and 57 community controls |
N400 from a lexical decision task |
Local polynomial estimate (Wang and Begleiter, 1999) [61 electrodes] |
Alcoholics, compared to controls, showed a weaker anterior sink for the primed words and a weaker posterior source for the unprimed words, along with lateral shifts in the posterior sources of both conditions. |
| Kamarajan et al. (2012) | 38 abstinent alcoholics and 38 healthy controls |
Event-related theta power around N2-P3 complex (200- 500 ms) during a single outcome gambling task |
Local polynomial estimate (Wang and Begleiter, 1999) [61 electrodes] |
During the loss condition, controls had a single and stronger midline prefrontal source related to theta EROs, and alcoholics showed bilateral and weaker prefrontal sources. Further, the alcoholic group showed more diffuse source activity compared to controls, especially during gain conditions |
| 2. Schizophrenia | ||||
| Turetsky et al. (1998a) | 18 schizophrenic patients at two time points and baseline normative data from 48 controls. |
P3 subcomponents during auditory oddball paradigm |
Weighted average with neighbors [14 electrodes] |
Reduced amplitudes in CSD-converted P3 were seen in schizophrenics at baseline and follow-up. |
| Turetsky et al. (1998b) | 65 schizophrenic patients (42 male, 23 female) and 48 controls (30 male, 18 female) |
P3 subcomponents during auditory oddball paradigm |
Weighted average with neighbors [14 electrodes] |
Schizophrenic patients had reduced CSD- converted P3 amplitudes relative to healthy controls, especially in the left hemisphere |
| Turetsky et al. (2000) | 11 schizophrenic patients, 12 healthy siblings and 23 matched control subjects |
P3 subcomponents during auditory oddball paradigm |
Weighted average with neighbors [14 electrodes] |
Patients had reduced parietal and frontal P3 amplitudes of CSD waveforms. The healthy siblings of the schizophrenic probands had an isolated reduction of the frontal P3. |
| Klein et al. (1999) | 14 participants with low schizotypal scores and 13 participants with high scores |
P3b during auditory oddball paradigm |
Spherical spline interpolation (Perrin et al., 1989b) [32 electrodes] |
Individuals with high schizotypal scores showed decreased CSD- converted P3 amplitude compared to low- scoring participants |
| Kayser et al. (2006) | 13 schizophrenic patients and 17 healthy adults |
N1, P3, and slow waves during a visual word serial position test |
Spherical spline surface Laplacian (Perrin et al., 1989b) [30 electrodes] |
Patients showed poorer performance and reduced left inferior parietal-temporal P3 source |
| Kayser et al. (2010a) | 23 schizophrenic patients and 23 age- and gender- matched healthy controls |
N2 and P3 during tonal and phonetic oddball tasks |
Spherical spline surface Laplacian (Perrin et al., 1989b) [30 electrodes] |
Frontal-central N2 sinks and parietal P3 sources were bilaterally reduced in patients |
| Kayser et al. (2009) | 20 schizophrenic patients and 20 age-, gender-, and handedness- matched healthy adults |
N1, N2, and P3 components during a recognition memory task (auditory and visual modalities) |
Spherical spline surface Laplacian (Perrin et al., 1989b) [30 electrodes] |
Reduction in left- parietal P3 sources, vertex N2 sinks, and mid-frontal sink (50 ms post-response) in patients, more prominently for the auditory stimuli |
| Kayser et al. (2010b) | 57 schizophrenic patients and 44 healthy adults |
P1, N1, N2, and P3 components during a recognition memory task using common words or unknown faces |
Spherical spline surface Laplacian (Perrin et al., 1989b) [66 electrodes] |
Left parietal old/new effects at later component (around 700 ms) were markedly reduced in patients over lateral temporal-parietal region, particularly for words |
| Kayser et al. (2012) | 26 schizophrenic patients with and 49 patients without auditory hallucinations; 46 healthy controls |
N1 sinks during recognition and working memory paradigms with words or faces |
Spherical spline surface Laplacian (Perrin et al., 1989b) [66 electrodes] |
For words and faces in both paradigms, N1 was substantially reduced in schizophrenic patients with auditory hallucinations compared with other two groups. |
| Kayser et al. (2010c) | 32 schizophrenic patients and 35 healthy adults |
Olfactory (sensory) detection of hydrogen sulfide |
Spherical spline surface Laplacian (Perrin et al., 1989b) [30 electrodes] |
N1 sink and P2 source were markedly reduced in patients for high intensity stimuli |
| Kayser et al. (2013) | 21 clinical high-risk (CHR) subjects at prodromal phase of psychosis and 20 healthy controls |
Olfactory ERP/CSD measures (N1 and P2 potentials) during an odor detection task |
Spherical spline surface Laplacian (Perrin et al., 1989b) [49 electrodes] |
Three patients who later developed psychosis had poorer odor detection and thresholds, and marked reductions of N1 and P2, although there were no group differences. |
| Kayser et al. (2014) | 22 clinical high-risk subjects at prodromal phase of psychosis and 20 healthy controls |
Alpha event- related desynchronizati on during a three-stimulus novelty oddball task |
Spherical spline surface Laplacian (Perrin et al., 1989b) [49 electrodes] |
Markedly reduced alpha desynchronization was observed in high-risk subjects compared to controls |
| Whitford et al. (2011) | 33 schizophrenic patients and 22 matched controls |
Inter- hemispheric transmission times (IHTTs) computed by ipsilateral minus contralateral latency in P1 and N1 components during a visual target detection task |
Spherical spline surface Laplacian (Perrin et al., 1989b) [71 electrodes] |
There was no difference in IHTT between schizophrenic patients and controls |
| 3. Depression, Anxiety, and Internalizing Disorders | ||||
| Bauer and Hesselbrock (2002) | 151 adolescent females: individuals with a lifetime history of a major depressive episode and subjects with family history of alcoholism |
Resting EEG | Realistic head-shape boundary element method (Fuchs et al., 1998) [31 electrodes] |
Laplacian transformed right frontal activations in alpha and fast beta power differentiated the effects of depression |
| Stewart et al. (2010) | 143 subjects with and 163 subjects without lifetime major depressive disorder |
Resting EEG | Spherical spline surface Laplacian (Perrin et al., 1989b) [64 electrodes] |
Lifetime depression was linked to relatively less left frontal activity of the CSD |
| Tenke et al. (2011) | 41 unmedicated depressed patients and 41 healthy control subjects |
Resting EEG | Spherical spline surface Laplacian (Perrin et al., 1989b) [67 electrodes] |
Patients who did not respond to treatment had significantly less posterior alpha CSD compared with responders or healthy control subjects |
| Stewart et al. (2014) | 143 individuals with and 163 subjects without lifetime major depressive disorder |
EEG during resting and during a facial emotion task |
Spherical spline surface Laplacian (Perrin et al., 1989b) [64 electrodes] |
CSD-transformed asymmetry was indicative of lifetime depression status under resting and during emotion processing. |
| Houston et al. (2003) | 29 female subjects who met DSM-III- R diagnostic criteria for a lifetime history of a major depressive episode and 101 subjects without the history |
P3 during a complex visual oddball task |
Realistic head-shape boundary element method (Fuchs et al., 1998) [31 electrodes] |
Decreased CSD activations in depressed group as compared to subjects with no history of depression, and this difference was maximal over the right prefrontal region |
| Bruder et al. (1998) | 40 depressed outpatients and 22 normal controls |
N1, N2, and P3 during an auditory oddball task |
Nearest neighbor (local Laplacian) method (Hjorth, 1980) [30 electrodes] |
Controls showed stronger P3 source over right than over left central regions. While patients with high anhedonia did not show this asymmetry, patients with low anhedonia showed an intermediate asymmetry. |
| Tenke et al. (2008) | 38 depressed patients and 26 healthy controls |
N1 and P3 during tonal and phonetic oddball tasks |
Spherical spline surface Laplacian (Perrin et al., 1989b) [30 electrodes] |
Patients showed reductions in P3 source for both targets and nontargets |
| Tenke et al. (2010) | 49 depressed patients and 49 healthy controls |
P3 during an auditory novelty oddball task |
Spherical spline surface Laplacian (Perrin et al., 1989b) [66 electrodes] |
A novelty vertex source at 241 ms, found during novel condition, was reduced in patients |
| Pause et al. (2010) | 28 individuals without and 16 subjects with social anxiety |
N1 and P3 components in response to chemosensory anxiety signals |
Spherical spline surface Laplacian (Perrin et al., 1989b) [60 electrodes] |
Females without social anxiety showed increased P3 related CSD activity component in response to chemosensory anxiety signals. |
| 4. Childhood and Developmental Disorders | ||||
| Bauer and Hesselbrock (2001) | 89 subjects with and 69 subjects without symptoms of conduct disorder (CD) in childhood |
P3 during a memory scanning task |
Realistic head-shape boundary element method (Fuchs et al., 1998) [31 electrodes] |
P3 related surface Laplacian for matching minus non-matching trials showed a weaker left frontal activation in those with CD symptoms than those without |
| Milne (2011) | 13 high functioning adolescents with autism spectrum disorders (ASD) and 12 matched controls |
P1 component and inter-trial alpha coherence |
Spherical spline surface Laplacian (Perrin et al., 1989b) [64 electrodes] |
P1 related CSD as well as alpha phase coherence was lower in the participants with ASD compared to controls |
| Duffy and Als (2012) | 463 children with ASD and 571 neuro- typical controls |
Resting EEG coherence |
Spherical spline surface Laplacian (Perrin et al., 1989b) [24 electrodes] |
CSD transformed EEG spectral coherence factors successfully discriminated ASD from the controls. Coherence values for the short- distance pairs were lower while the longdistance pairs showed both increase and decrease in the ASD groups compared to the controls |
| Duffy et al. (2013) | 430 children with ASD, 26 children with Asperger syndrome, and 554 neuro- typical controls |
Resting EEG coherence |
Spherical spline surface Laplacian (Perrin et al., 1989b) [24 electrodes] |
CSD transformed coherence factors separated Asperger syndrome from the ASD population as well as from the controls with significant classification success. |
| Ponomarev et al. (2014) | 96 adults with retrospective childhood ADHD and 376 healthy adults |
Resting EEG | Spherical spline surface Laplacian (Perrin et al., 1989b) [19 electrodes] |
Reduction in CSD spectral power in the ADHD group for delta, theta, alpha and beta1 bands |
| 5. Neurological Conditions | ||||
| Rodin (1999) | 5 children with absence seizures |
electrical fields of spikes and waves from the spontaneous EEG |
CSD method (using Bio-logic Ceegraph system) is not specified [30 electrodes] |
CSD maps delineated the propagation of potentials and ictal events better than raw EEG tracings |
| Ulbert et al. (2004) | 4 patients with epilepsy |
Intra-cortical EEG activity during interictal phase |
CSD method is not specified [24 microelectrode sites] |
CSD transformed interictal spikes were useful to identify that interictal epileptiform events in humans are initiated by large postsynaptic depolarizations |
| Fabo et al. (2008) | 11 drug- resistant epilepsy patients undergoing temporal lobectomy |
Intra-cortical EEG recordings at subiculum and lateral temporal lobe using laminar multielectrodes under anesthesia |
CSD method is not specified [24 or 48 channels depending on single or dual multielectrode recording] |
CSD transformed spike activities revealed multiple spike generator mechanisms of subiculum and further discharges to other cortical regions |
| Harmony et al. (1993) | 33 patients with space- occupying brain lesions |
Absolute and relative power of resting EEG |
Spherical harmonic expansion (Pascual-Marqui et al., 1988) [19 electrodes] |
The volume and density of the brain edema showed a significant correlation with CSD transformed absolute power of theta and alpha band in the Laplacian |
| Fernandez-Bouzas et al. (1995) | 61 patients with space- occupying brain lesions |
Absolute and relative power of resting EEG |
Spherical harmonic expansion (Pascual-Marqui et al., 1988) [19 electrodes] |
CSD maps of the delta and theta bands characterized brain tumors and edema respectively |
| Fernandez-Bouzas et al. (1997) | 10 patients with space- occupying brain lesions before and after treatment |
Absolute power of resting EEG |
Spherical harmonic expansion (Pascual-Marqui et al., 1988) [19 electrodes] |
CSD maps characterized the site of the lesion and of the edema better than voltage maps |
Alcoholism has been described as a disinhibitory disorder as individuals with AUD and their high risk offspring often display impulsivity and dysfunctions in several electrophysiological measures, more prominently demonstrated by lower P3 amplitude in several tasks (for a review, see Porjesz et al., 2005). Using a three stimulus auditory oddball paradigm, Hada et al. (2000) found that CSD topographic maps served better to discriminate alcoholics from controls than ERP potential maps. They reported that alcoholics produced lower P3a amplitudes to target stimuli, along with distinct topographic distributions of P3a-related CSD maps, characterized by more but weaker sources in alcoholics compared to controls. In another study with a similar task, Cohen et al. (2002) found that significant P3 reductions in alcoholics were pronounced over the posterior regions, and the group differentiation was more obvious in the CSD maps wherein the sources and sinks were fewer and weaker in alcoholics. In a sample of individuals at high risk (HR) for alcoholism during the performance of an auditory oddball task, Ramachandran et al. (1996) reported significant reductions in both P3 amplitudes and P3-related CSD activations in the HR group over the posterior central, parietal, and occipital areas, along with prominent topographic differences in the CSD maps. Similarly, in a three stimuli auditory oddball paradigm, Hada et al. (2001) found that the HR group manifested significantly lower P3a amplitudes as well as a highly differentiated CSD map characterized by more but weaker posterior sources for P3a compared to low-risk subjects.
Differences in both P3 amplitude and CSD topography were also reported in visual oddball tasks in alcoholics (Rodriguez Holguin et al., 1999a; Cohen et al., 2002) as well as in their high risk offspring (Rodriguez Holguin et al., 1999b), although the group differences were topographically different in the visual task compared to the auditory mode. For example, Cohen et al. (2002) reported a modality-specific pattern in that alcoholics manifested weaker P3 sources mainly over the frontal region in the auditory modality and primarily over the parietal-occipital region in the visual modality.
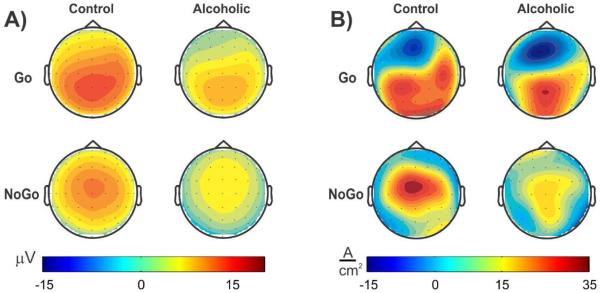
In order to demonstrate deficits in inhibitory processing in alcoholics, the Go-NoGo paradigm has been used in several studies. In a visual Go-NoGo task, Kamarajan et al. (2005) reported that controls manifested two bilateral sources, while alcoholics exhibited only a midline source during the Go condition that had a button press response. During the NoGo condition which involved inhibitory processing (and no button press), controls showed a stronger and focused source over the central region, while the alcoholics showed a weaker and diffuse source over the central and posterior regions (see Fig. 2). The CSD maps were statistically compared between controls and alcoholics using a randomization technique described in Srebro (1996), and found significant group differences in several brain regions.
Fig. 2.
Comparison of P3 response between the Alcoholic and Control group shown in ERP amplitudes (panel A) and in CSD transformed data (panel B) during a Go-NoGo task. A) Alcoholics showed lower P3 amplitude in both Go and NoGo conditions without any topographic difference; and B) alcoholics manifested differences in both intensity (‘Go’ sinks and ‘NoGo’ sources) and topography of CSD activations (mainly in ‘Go’ sources). The CSD maps provide sharper and more interpretable activation features (sources and sinks) to compare between groups and conditions. In the Go condition with a button press response, controls manifested two bilateral sources, while the alcoholics exhibited only a midline source. In the NoGo condition with inhibitory processing (and no button press), controls showed a stronger and focused source over the central region, while the alcoholics exhibited a weaker and diffused source over the central and posterior regions. The physical units for the scalp potentials and CSD data are microvolt (μV) and ampere per square centimeter (A/cm2) respectively [Adapted from: Kamarajan et al. (2005)]
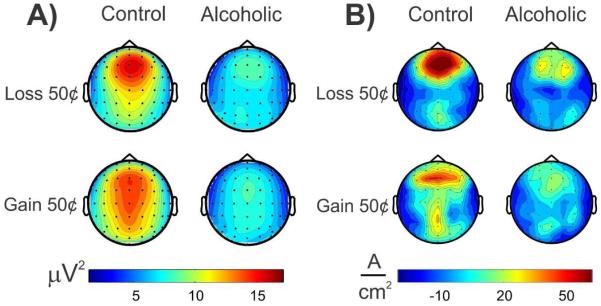
Using a monetary gambling task, Kamarajan et al. (2012) found that during the loss condition controls showed a single and stronger midline prefrontal source related to theta EROs around N2-P3 complex (200-500 ms), while alcoholics showed bilateral and weaker prefrontal sources. During the gain condition, controls had well-defined anterior and posterior sources while the alcoholics showed weaker and diffused sources (see Fig. 3). Although this study has not explicitly described how exactly the CSD was computed, as cited by the references in the Method section, we (the same authors) confirm that the CSD was computed using local polynomial approximations method proposed by Wang and Begleiter (1999). In this study, the authors found that the CSD topographic maps, in comparison to the activity patterns of the potential data, were more advantageous to interpret the activation patterns during loss and gain and to differentiate alcoholics from control group.
Fig. 3.
Topographic patterns of ERO theta activity representing total theta power (panel A) and of CSD maps computed from theta amplitude data (panel B) as compared between alcoholic and control group. These values were extracted within the time interval of 200-500 ms during the feedback of loss and gain of 50φ in a monetary gambling task. A) Alcoholics showed lower theta power in both loss and gain conditions; and B) alcoholics manifested lower CSD activations as well as topographic differences during loss and gain conditions. The CSD maps provided more prominent and useful information regarding the activation profiles across groups and conditions [Adapted from: Kamarajan et al. (2012)]
Region specific activations and altered topographic features of CSD have also been reported in other tasks and during the resting state with EEG. Ji et al. (1999) found suppressed activations over the left temporal-occipital areas in alcoholics during both matching and nonmatching conditions (around 250 ms) with prominent left hemispheric activation in controls in a visual category matching task (i.e., match/mismatch S1–S2 paradigm). Roopesh et al. (2010) investigated the N400 component in 87 alcohol dependent subjects and 57 community controls using a lexical decision task which required a button press response to indicate the presented visual stimulus was a word or non-word. It was found that control subjects, as expected, revealed a significant “priming effect” which is attenuation of the N400 response to the primed word in comparison to the unprimed word, whereas alcoholic subjects showed significantly less N400 attenuation. The CSD topography revealed a centroparietal and right hemisphere predominant source only for the control group. In alcoholics, by contrast, the posterior source was largely at the midline region and the sink shifted to the left for the primed condition, while the controls showed a strong source that was predominant in the right hemisphere, suggesting possible hemispheric asymmetry during linguistic processing in alcoholics. Using the measures of resting EEG, Bauer and Hesselbrock (2002) reported that individuals with family history of alcoholism showed increased activations of fast beta at both anterior and posterior locations while having decreased theta (anterior and posterior) as well as alpha (anterior) activations. However, as the measure used in the study was not a formal CSD, the results cannot be interpreted based on the radial current flow since the surface Laplacian has been computed from the EEG relative power data rather than from the actual amplitude data of the (frequency filtered) EEG waveforms.
In sum, these findings demonstrate the utility of CSD in differentiating not only the alcoholics from controls but also the high-risk offspring from low risk controls in terms of strength of activations and topographic patterns derived from various paradigms. It is also clear that alcoholism is one of the clinical entities wherein CSD methods have been successfully applied in alcoholism to understand the neurocognitive deficits in AUD as reported in the literature, and also to predict risk status to develop alcoholism.
Schizophrenia
Abnormal topographic features and decreased activations of CSD have been reported in schizophrenic subjects in specific ERP components and in time-frequency measures under various task conditions (See Table 1, section 2). Significantly reduced CSD activations of P3 component over the central and parietal regions of the auditory oddball paradigm have been reported in schizophrenic patients during baseline (Time 1) (Turetsky et al., 1998a) and follow up (Time 2) recordings (Turetsky et al., 1998b) , although there were no significant CSD differences between Time 1 and Time 2. Further, CSD transformation enabled to identify five spatially distinct subcomponents of the P300 (mean integrated amplitude between 280 and 400 ms poststimulus): bilateral parietal (P3pL and P3pR), bilateral temporal (P3tL and P3tR), and midline frontal (P3f) (Turetsky et al., 1998a, b). These studies pioneered the application of the spatial CSD-PCA method; this also enabled comparison of the amplitudes of hemisphere-related components in two or more groups (Spencer et al., 1999, 2001; Tenke et al., 2008). Extending the study to include unaffected siblings, Turetsky et al. (2000) reported that both schizophrenic patients and unaffected siblings showed significant P3-related CSD reductions at the parietal and frontal P300 subcomponents compared to the healthy controls (patients < unaffected siblings < healthy controls). Interestingly, undiagnosed individuals with high schizotypal scores showed decreased P3-related CSD activation at the parietal (Pz) region for the target and novel conditions in an auditory oddball task as compared to the low-scoring participants (Klein et al., 1999).
Kayser and colleagues have implemented CSD methods in a series of studies on schizophrenic patients in a variety of task conditions based on a rationale (for each study as mentioned below) derived from the findings of the schizophrenia literature. In order to unravel the subprocesses of verbal working memory deficits in schizophrenia, Kayser et al. (2006) used the CSD method in a visual word serial position task and found that schizophrenic patients showed reduced left inferior parieto-temporal P3 sources. Using tonal and phonetic oddball tasks, Kayser et al. (2010a) studied ERP generator patterns as elicited by temporal PCA to examine the earlier findings showing left-lateralized dysfunction in schizophrenics with greater left than right reductions of P3 amplitude, and found a bilateral reduction in fronto-central N2 sinks and parietal P3 sources. In another study, using visual continuous recognition memory tasks with common words or unknown faces, Kayser et al. (2010b) investigated whether the visual recognition memory deficits in schizophrenics were restricted to only words. This study involved the episodic memory effect, also known as ‘old/new’ effect, as indexed by an early mid-frontal negativity (FN400) reflecting item familiarity as well as a late P3-like left-parietal positivity (parietal P600) indexing explicit memory-retrieval processes. Results showed that CSD activations of the P600-like component were markedly reduced in schizophrenic patients over the left lateral temporo-parietal region for words, however, not for faces. This study was motivated by their previous CSD/PCA study (Kayser et al., 2009), where old/new effects for words were investigated in schizophrenic patients using the continuous word recognition memory paradigm involving the serial presentation of words in both auditory and visual modalities, and found more prominent reductions (of left-parietal P3 sources, vertex N2 sinks, and mid-frontal sink at 50-ms post-response) in the auditory modality. Further, in order to understand neurophysiological processes underlying olfactory dysfunction in schizophrenia, Kayser et al. (2010c) employed a nose-referenced EEG recording in a sensory detection study. This involved 200 ms presentations of olfactory stimuli (hydrogen sulfide) to which participants responded whether they perceived a low or high intensity odor. Given that linked-mastoids or linked-ears are frequently employed as EEG reference for chemosensory ERP studies, these investigators changed the original nose-referenced ERPs to a mastoid reference in order to compare the resulting ERP waveforms to CSDs, which revealed that using a linked-mastoid reference can dramatically mask an olfactory N1 component. Results showed that the N1 sink and P2 source were markedly reduced in schizophrenic patients for high intensity stimuli, thus confirming olfactory dysfunction in schizophrenia. Further, the study demonstrated that similar results were obtained for CSDs derived from different recording montages and systems. In a replication study using the same task, Kayser et al. (2013) investigated clinical high-risk (CHR) subjects at prodromal phase of psychosis and healthy controls and obtained similar olfactory CSD findings for N1 sink and P2 source, and thus closely replicated the previous findings of Kayser et al., (2010c). In addition, the results showed that three patients who later developed psychosis had poorer odor detection and thresholds, along with marked reductions of N1 and P2 (in the absence of significant group differences between CHR and control subjects), suggesting that olfactory measures may be of utility in predicting the transition from prodromal phase to psychosis among CHR subjects. Furthermore, in a follow-up study using a different task (three-stimulus novelty oddball task) for essentially the same cohort of CHR (prodromal) patients and healthy controls, Kayser et al. (2014) found that, similar to their previous study (Kayser et al., 2013), the same three prodromal individuals who later developed psychosis had marked reductions of novelty MMN whereas prodromal patients as a group did not show this reduction. In addition, CHR patients showed reduced ERO (alpha desynchronization at 9 Hz) over the right posterior regions for the target stimuli (at 610 ms) compared to healthy controls, suggesting a deficit of alpha-mediated cognitive control processes, and these ERO abnormalities were also most pronounced for the three converters. This series of studies confirmed that CSD measures are both reliable (as they could be replicated across studies with similar measures and subjects) and robust (as they provide identifiable and sensitive electrophysiological indices across groups and conditions). Thus, it has been demonstrated by these studies that employing CSD methods during specific cognitive/sensory tasks to assess neurocognitive deficits in schizophrenia yielded results that could be easily interpreted as well as identified with brain function than the conventional ERP methods.
Most interestingly, the CSD method has also been found to be sensitive in differentiating two types of patient groups. Kayser et al. (2012) found that CSD related to the N1 component during a recognition and working memory paradigm was substantially reduced in schizophrenic patients with auditory hallucinations compared to patients without auditory hallucinations and healthy controls. However, CSD activation deficits observed in schizophrenics did not have any impact on inter-hemispheric transmission times (IHTT) as observed by Whitford et al. (2011); they reported that there was no difference between schizophrenic patients and controls in the IHTT (computed by ipsilateral minus contralateral latency) in P1 and N1 components of the CSD transformed ERP waveforms during a visual target detection task, suggesting that schizophrenics may not have problems related to neural conduction speed. Taken together, it is clear that the CSD method has been successfully applied in schizophrenia to understand the neurocognitive deficits reported in the literature, to predict transition from prodromal stage to psychosis, and to differentiate two sets of symptomatology within the schizophrenic diagnosis.
Internalizing Disorders (Depression and Anxiety)
Internalizing disorders, such as depression and anxiety, have been linked to relative right-sided resting frontal EEG asymmetry among adults and infants of afflicted mothers (for a meta-analytic review, see Thibodeau et al., 2006), and therefore this abnormality has been suggested to be an endophenotype for these disorders. Although a few studies have identified anomalous CSD features of EEG and ERP activations in depression, such studies in other internalizing disorders such as anxiety disorders are rare (See Table 1, section 3). Using a surface Laplacian method, Bauer and Hesselbrock (2002) attempted to discern whether abnormalities related to frontal EEG asymmetry in depressed individuals are due to depression per se or the result of an interaction between depression and either of two family history variables—a family history of alcoholism or a family history of depression. While no significant main or interactive effects of a family history of depression, topographic analyses of surface Laplacian showed that the effects of depression could be localized to the right frontal region, whereas the effects of a family history of alcoholism were localized to the left frontal area. Stewart et al. (2010) reported that lifetime depression was linked to lower CSD alpha activity of the resting EEG in left compared to right hemisphere, and suggested that asymmetry of the CSD activity derived from the resting EEG could be an endophenotype for depression. Further, as patients with depressive disorder were reported to have greater EEG alpha power (Pollock and Schneider, 1989) and abnormal regional hemispheric asymmetries of alpha (Bruder et al., 1997), Tenke et al. (2011) investigated CSD profiles for antidepressant treatment response and found that depressed patients who did not respond to treatment had significantly less posterior alpha CSD related to resting EEG compared with responders or healthy control subjects. Recently, Stewart et al. (2014) examined the capability model of frontal EEG asymmetry (Coan et al., 2006), which suggests that brain activity during emotional challenge is a more powerful indicator of predispositions toward psychopathology than activity observed at rest. They assessed EEG during a resting baseline and a facial emotion task, and found that EEG asymmetry during emotional challenge was a more powerful indicator of major depression than resting asymmetry for average and linked mastoid references, thus supporting the capability model. Further, CSD-transformed asymmetry was indicative of lifetime depression status under resting and task-elicited conditions. These findings further suggest that CSD-transformed data are more robust indicators of trait frontal EEG asymmetry than spectra stemming from EEG potentials referenced to vertex, linked mastoids, or all recording sites (common average).
Using ERPs during a complex visual oddball task, Houston et al. (2003) computed CSD using the realistic head-shape boundary element method to study laterality effects (frontal asymmetry) of depression and of a family history of alcohol or substance dependence on P3 component in young women, and found decreased P3-related CSD activations in the depressed group, maximally over the right prefrontal region, compared to subjects with no history of depression, suggesting laterality effects during P3 processing. Based on their prior auditory P3 findings of P3 asymmetry in depressed patients (Bruder et al., 1995), Bruder et al. (1998) tested the hypothesis in depressed outpatients and also examined the CSD topography using the nearest neighbor (local Laplacian) method (Hjorth, 1980). P3 asymmetry was observed in control participants and in depressed patients with low anhedonia, but not for patients with high anhedonia scores. Similarly, Laplacian maps corresponding to P3 showed greater radial current flow over right than over left central regions in control participants and in patients with low anhedonia, suggesting that depression (with low anhedonia) may be associated with hemispheric asymmetry during auditory processing. Using the spatial CSD-PCA method, Tenke et al. (2008) examined ERP waveforms from tonal and phonetic oddball tasks in a group of unmedicated depressed patients and found that the patients showed reductions in P3 source for both targets and nontargets, more prominently at lateral sites of the left hemisphere. In another study, Tenke et al. (2010) improved on the prior ERP findings reported during a novelty oddball paradigm in depression (Bruder et al., 2009) by discovering a new novelty component, termed novelty vertex source (NVS), which contributes to the frequently-described frontal P3a. They reported that depressed patients, in comparison to healthy controls, showed significantly reduced NVS at 241 ms during the novel condition. However, to our knowledge, there has been only a single CSD study on anxiety. In a group of individuals with social anxiety, Pause et al. (2010) examined N1 and P3 components of the olfactory potentials in response to chemosensory anxiety signals (i.e., smelling sweat samples donated from either female or male donors) and reported these findings: 1) socially non-anxious females showed much stronger P3-related CSD activation (around 800 ms) at central-parietal regions than males in response to both chemosensory stimuli; and 2) socially anxious female participants showed stronger N1-related CSD activity (435–440 ms) across left and right frontal scalp areas in response to chemosensory anxiety signals than to the control stimuli. However, there are a number of factors that limit the generalizability of these findings: 1) the CSD maps are widely inconsistent across study groups; 2) the N1 sink topography does not correspond with existing findings in the literature; 3) only the source (i.e., positivity) part of the CSD topography is considered and the sink (negativity) has not been explained in the study; and 4) ERP waveforms are filtered with successive 40-Hz and 7 Hz low pass filters at 24 dB, and the noise evident in these (heavily-smoothed) waveforms adversely impact on the CSD transform (order of splines used in the spherical spline model: m = 4, only 20 iterations, no lambda mentioned). For these reasons, this study is limited in its effectiveness as an implementation of the CSD method.
In summary, while there are studies showing CSD activation correlates of depressive disorder, there is a dearth of such studies for other internalizing disorders such as generalized anxiety disorder, phobic disorder, panic disorder and obsessive compulsive disorder. Further studies are essential to demonstrate and validate the potential utility of CSD methods in this clinical spectrum.
Childhood and Developmental Disorders
CSD measures have the potential to be applied to childhood disorders, as they provide enhanced spatial resolution, sensitivity to the regional brain electrical activity, and reference independence to the raw EEG potentials (Perrin et al., 1987a; Srinivasan et al., 1998a). Although few in number, studies have reported CSD activation profiles in conduct disorder (CD), autism spectrum disorders (ASD), and attention deficit hyperactivity disorder (ADHD). Bauer and Hesselbrock (2001) examined the performance of individuals with CD in a memory scanning task and found that P3 related CSD activations for matching minus non-matching trials were markedly weaker over the left frontal region in individuals with CD symptoms compared to those without these symptoms. Milne (2011) studied early ERP components during the presentation of visual stimuli (Gabor patches) and found that high functioning adolescents with ASD showed lower P1 related CSD activation as well as decreased alpha phase coherence compared to controls. Duffy and Als (2012) employed CSD transformed spectral coherence of the resting EEG in 463 children with ASD and 571 neuro-typical controls in order to see the classification accuracy based on the diagnosis. They found that coherence factors successfully discriminated ASD from the controls. Coherence values for the short-range pairs were lower while the values in the long-range pairs were both higher and lower in the ASD groups compared to the controls. In their follow-up study, Duffy et al. (2013) used the same measure (i.e., CSD transformed spectral coherence) in 430 children with ASD, 26 children with Asperger syndrome, and 554 neuro-typical controls in order to determine the classification pattern among all three groups, especially to see whether the 26 children with ASD were systematically separable from the larger population of 430 subjects with ASD. They found that coherence factors as used in discriminant function analysis could successfully separate Asperger syndrome from the ASD population and from the controls with significant classification success. In these studies, although the authors have not directly compared the raw EEG potentials with CSD measures, they state that they have employed surface Laplacian methods in order to minimize the effect of volume conduction on coherence estimates by emphasizing sources at smaller spatial scales than unprocessed scalp potentials (EEG). Lastly, Ponomarev et al. (2014) found significant reduction in CSD spectral power in the ADHD group compared to healthy subjects in delta, theta, alpha and beta1 bands, although no significant CSD differences were observed for the subgroups of patients with ADHD. In the same study, it was also evident that transformation of the raw EEG into the CSD data significantly increased the sensitivity of the spectral analysis in detecting differences between ADHD patients and healthy subjects. Overall, these studies provide evidence that CSD can provide more useful and interpretable measures than the raw EEG potentials in childhood and developmental disorders as well, although there is an obvious dearth of studies in this important clinical domain. Therefore, more CSD studies during different task conditions are necessary in order to elicit, understand and establish the neurocognitive markers for various childhood and developmental disorders.
Neurological Conditions
There are studies showing the application of CSD measures in neurological conditions, such as epilepsy and space occupying brain lesions, reporting the advantages of CSD over the recorded potentials. Rodin (1999) examined the electrical fields of spikes and waves from the spontaneous EEG in children with absence seizures and found that CSD maps delineated the propagation of potentials and ictal events better than the EEG potentials. While it is beyond the scope of the present review to include studies that have applied intracranial CSD methods as it would require additional description of its method and techniques (Tenke and Kayser, 2012), two of the intra-cortical studies on epilepsy patients are worth a brief mention here. In an effort to track the ictal spread in cortical regions, Ulbert et al. (2004) recorded intra-cortical multiple unit activity (MUA) during interictal phase in epilepsy patients who were undergoing subdural grid implantation for seizure localization and chronically implanted with multicontact microelectrodes. They found that two distinct intracortical CSD/MUA patterns were related to the propagated interictal spikes: 1) a granular pattern with layer IV CSD sink and MUA onset, delayed CSD sink and MUA in layer III, and also delayed sources and MUA in layers V–VI; and 2) a supragranular pattern with a major layer I–III CSD sink with enhanced MUA, and later sinks and sources in the deep layers. In another intra-cortical EEG study by the same group of researchers, Fabo et al. (2008) implanted laminar microelectrodes at subiculum and lateral temporal lobe under anesthesia in drug-resistant epilepsy patients undergoing temporal lobectomy in order to understand mechanisms underlying generation, maintenance and propagation of epileptic activity. They identified two types of interictal spikes in the subiculum that were related to epileptic activity: 1) initial excitatory currents (CSD sink) in the pyramidal cell layer; and 2) later inhibitory currents (CSD source) along with decreased MUA. Finally, in a series of studies, it was found that CSD measures were more useful than traditional EEG measures to localize brain lesions as well as to characterize brain edema in patients with space-occupying brain lesions (Harmony et al., 1993; Fernandez-Bouzas et al., 1995, 1997). Harmony et al. (1993) found that the volume and density of the brain edema showed a significant correlation with Laplacian estimates of the absolute power of theta and alpha band, and further observed that spectral parameters obtained from the Laplacian estimates showed higher correlations with neuroimaging measures (computed tomography) than those calculated from the EEG potentials. They also found that Canonical correlations with the volume and the density of the edema showed a significant correlation with delta, theta and alpha power of scalp voltage and only with theta and alpha power computed from CSD. The authors explained that the absence of correlation with CSD delta power may be due to the fact that the Laplacian acts as a spatial filter which provides more weightage to local generators over distant ones. In another study by the same group of researchers (Fernandez-Bouzas et al., 1995), lesions (brain tumors) were better detected by CSD maps of the delta band, while brain edema was better represented by CSD maps in the theta range. Furthermore, in their follow-up study, Fernandez-Bouzas et al. (1997) measured the changes in the CSD and voltage measures of the EEG in relation to the volume of the lesions as well as the edema as measured by the CT scans in patients with space-occupying lesions before and after treatment. They found that the volume, site, and size of the lesion and edema were better represented by CSD maps than by voltage maps and suggested that more precise characterization (e.g., localization) of brain lesions can be obtained by CSD than by voltage estimates.
Taken together, it is obvious that CSD and Laplacian measures can aid in identifying and understanding the brain lesions in some of the neurological conditions and thus serve as powerful and sensitive measures of cortical activation. However, as is the case with many other clinical domains, several neurological conditions, including epilepsy and specific brain lesions, need more CSD studies in order to elicit and establish functional electrophysiological markers in these conditions.
Summary and Discussion
As described in the previous sections and listed in Table 1, it is very clear that CSD measures have shown remarkable advantages compared to scalp potentials in terms of topographic patterns, strength of activations, and more reliable spatial localization of neuroelectric activity during resting EEG as well as during cognitive tasks by elucidating subtle differences across and within several neuropsychiatric conditions, such as, alcoholism, schizophrenia, depression, anxiety, CD, ASD, ADHD, epilepsy, and space-occupying brain lesions. CSD has also proven to successfully differentiate individuals at high risk to develop certain clinical conditions, more successfully in alcoholism (Ramachandran et al., 1996; Rodriguez Holguin et al., 1999b; Hada et al., 2001) and also in psychosis (Kayser et al., 2014) from that of healthy controls. Further, presence and absence of a specific symptom (e.g., hallucination) within a particular diagnostic category or domain (e.g., psychosis) could also be differentiated using the CSD method, namely CSD measure could elicit differences in activation patterns between schizophrenic subjects with and without hallucinations (Kayser et al., 2012). Additionally, CSD has been found useful to understand neuroelectric activations in patients with brain lesions (Harmony et al., 1993; Fernandez-Bouzas et al., 1995, 1997) and epilepsy (Rodin, 1999; Ulbert et al., 2004; Fabo et al., 2008). Specifically, Harmony et al. (1993) interpreted the finding that significant correlations of the volume and the density of the brain edema were with only theta and alpha power (representing local activity) of the CSD measure but additionally included delta power (representing long-range activity) of the voltage measure could be due to the fact that the Laplacian acts as a spatial filter emphasizing local sources over distant sources, while the non-transformed EEG potential tends to produce a more extensive and diffuse picture of neuroelectric abnormality and hence lacks the information about its source activation. Follow-up studies from the same researchers also compared both measures and further confirmed the superiority of CSD measure in localizing brain lesions than the traditional voltage measure (Fernandez-Bouzas et al., 1995, 1997). However, it should also be mentioned that even though the CSD method has amply demonstrated numerous merits in terms of clinical/diagnostic applications, electrophysiological studies on several clinical conditions, (e.g., substance use disorders other than alcoholism, obsessive-compulsive disorder, post-traumatic stress disorder, etc.), have yet to exploit the benefits of this technique and hence there are no CSD studies available in the literature for these disorders.
While the advantages of CSD measures over the raw scalp potentials are well-established, there are some limitations in the CSD technique, which are outlined here so that further research efforts may be directed to address these issues. One of the limitations is the observation that there are no individually identifiable markers available for distinct clinical conditions in terms of specific CSD activation patterns and topography. Differences in subject groups and task paradigms may be one of the major reasons for the lack of disorder-specific CSD markers. On the other hand, seemingly inconsistent topographic patterns even between studies with similar clinical groups and ERP tasks [e.g., Cohen et al. (2002) and Rodriguez Holguin et al. (1999a) in alcoholics using visual oddball task; Hada et al. (2001) and Ramachandran et al. (1996) in children of alcoholics using auditory oddball task] could be due to variations in sample characteristics and other methodological factors (e.g., sample matching, signal processing parameters, etc.). Replication studies with identical methodology are required to validate the phenomena under study (e.g., sensory, cognitive, emotional phenomena) in a given neuropsychiatric condition and to arrive at CSD-based clinical markers. Further, most of the studies have made only a visual, qualitative comparison rather than a statistical or computational procedure to compare the CSD topographies between groups or conditions; however, some studies have used ANOVA to compare specific regions, and a few studies have used randomization tests to compare the whole topographies, e.g., Kamarajan et al. (2005) who have statistically compared whole CSD topographies between controls and alcoholics using randomization tests. It is suggested that application of statistical methods to compare topographic maps can provide objective and quantitative measures for comparison (e.g., effect size, cross-validation, etc.) and to help evaluate relative merits of CSD methods in comparison to traditional scalp potential maps. Furthermore, methodological improvements for the CSD techniques have been very rare and slow. One obvious exception to this limitation is the work by Kayser, Tenke and Colleagues, who have innovated some methods, for example, to extract PCA components of the CSD (Kayser and Tenke, 2003; Tenke and Kayser, 2005) and successfully implemented this approach to study clinical domains (Kayser et al., 2006, 2009, 2010a,b,c, 2012, 2013, 2014; Tenke et al., 2008, 2010, 2011). Other concerns of CSD are related to its methodological limitations, which may be addressed by future studies: 1) the transformation of the EEG potentials into the CSD leads to a partial data loss, since the low-spatial-frequency (LSF) components are attenuated (Hjorth, 1980; cf. Ponomarev et al., 2014), although it can be argued that it amounts to ‘data loss’ only if the attenuated/removed LSF contains ‘neural signal’ rather than volume conduction effects; 2) the requirement of an adequate number of scalp electrodes (32 electrodes or above) for reliable computation of CSD (Pizzagalli, 2007), although helpful CSD topography has been obtained from 19 scalp electrodes (Harmony et al., 1993; Fernandez-Bouzas et al., 1995, 1997; Ponomarev et al., 2014) or even less (Turetsky et al., 1998a, 1998b, 2000); and 3) it is often assumed that CSD mainly represents the activation patterns of the cortical surface and is relatively insensitive to the deep brain sources (e.g., Duffy and Als 2012), although it is suggested that this concern could be methodologically addressed by using a low resolution CSD (Tenke and Kayser, 2012).
Despite these reported and often unsupported limitations, the proven and potential utilities of CSD are well-recognized for several key reasons. The fact that the surface Laplacian mapping approach does not require knowledge of conductivity distribution inside the subject's head provides an easy-to-use methodology by improving the spatial resolution of the conventional EEG (He et al., 2001). CSD, as a conservative description of neural current generators (Tenke and Kayser, 2012), has been implemented for all electrophysiological methods (EEG, ERPs, and EROs) and measures (amplitude, phase, power, and coherence), and provides a unique and important tool for understanding brain sources in a variety of contexts, ranging from basic research to clinical applications. For example, Srinvasan et al. (2007) suggest that moderate to large EEG coherence can also arise simply by the volume conduction of current through the tissues of the head, and therefore the coherence measure appears to result from a mixture of volume conduction effects and genuine source coherence. Therefore, surface Laplacian methods are the available best options to minimize the effect of volume conduction on coherence estimates. Further, according to Tenke and Kayser (2005), the simple fact that CSD provides a reference-independent (i.e., the waveforms at different electrodes are not influenced by activity at a common reference electrode) measure of EEG topographies is by itself sufficient to endorse its use over traditional qEEG measures which are reference-dependent (see Fig. 1 in Tenke and Kayser, 2005). Further, it has been empirically shown in the intracranial laminar recordings that CSD,derived from the local field potential (LFP) profile, virtually eliminates volume conduction at the spatial scales that are of interest to most in vivo LFP studies, and also improves the precision of inferences that can be made about underlying synaptic processes (Kajikawa and Schroeder, 2011). Thus, CSD methodology provides a global empirical and biophysical context for generator localization, spanning scales from cortical laminae to scalp topographies (cf. Tenke and Kayser, 2012).
In conclusion, this review has addressed the merits of using CSD and Laplacian measures in comparison to raw potential data obtained from either resting state or task conditions as applied to several neuropsychiatric conditions, such as alcoholism, schizophrenia, depression, conduct disorder, autism, ADHD, epilepsy, and space-occupying brain lesions. This review has also provided evidence for the possibility that CSD and Laplacian measures can serve as effective and sensitive neurocognitive markers in several neuropsychiatric conditions. It is observed that there is a perceptible dearth of CSD studies in some of the major clinical domains, such as internalizing disorders, childhood and developmental disorders, and neurological conditions. Several limitations and concerns of present CSD methods have also been discussed, with possible solutions from future studies. As clearly outlined in this review, proven merits and advantages of CSD measures, including enhanced spatial resolution, sensitivity to the regional brain electrical activity, reference independence, and enhanced interpretability of the neuroelectric signals, will potentially render this method as one of the most powerful and promising tools for future electrophysiological research as well as for a variety of clinical applications including diagnosis and treatment of neuropsychiatric disorders.
Highlights.
### All published studies using current source density (CSD) or surface Laplacian (SL) methods in a neuropsychiatric condition have been reviewed and several merits of these methods have been highlighted.
### Evidence suggests that SL/CSD measures can serve as effective and sensitive neurocognitive markers in several neuropsychiatric conditions.
### A perceptible dearth of CSD studies in some of the major clinical domains has been observed.
### Some limitations of present CSD methods have also been discussed, while possible solutions to circumvent these limitations have also been suggested.
Acknowledgements
This study was supported by the Grants # U10 AA008401-26, RO1 AA05524-29, and RO1 AA02686-37 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). In memory of Dr. Henri Begleiter, founder and longtime mentor of the Neurodynamics Laboratory, we acknowledge with great admiration his seminal scientific contributions to the field. We are sincerely indebted to his charismatic leadership and luminous guidance, truly inspired by his scientific mission and vision, and highly motivated to carry forward the work he fondly cherished.
We are grateful to Jürgen Kayser and Craig E. Tenke for their thorough preview and thoughtful comments, which were very useful to improve the manuscript of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babiloni F, Babiloni C, Carducci F, Fattorini L, Onorati P, Urbano A. Spline Laplacian estimate of EEG potentials over a realistic magnetic resonance-constructed scalp surface model. Electroencephalogr. Clin. Neurophysiol. 1996;98:363–373. doi: 10.1016/0013-4694(96)00284-2. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Babiloni C, Fattorini L, Carducci F, Onorati P, Urbano A. Performances of surface Laplacian estimators: a study of simulated and real scalp potential distributions. Brain Topogr. 1995;8:35–45. doi: 10.1007/BF01187668. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Carducci F, Babiloni C, Urbano A. Improved realistic Laplacian estimate of highly-sampled EEG potentials by regularization techniques. Electroencephalogr. Clin. Neurophysiol. 1998;106:336–343. doi: 10.1016/s0013-4694(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Basar E. Principles and Approaches. I. Springer Verlag; Berlin: 1999a. Brain Function and Oscillations. [Google Scholar]
- Basar E. Integrative brain function, neurophysiology and cognitive processes. II. Springer Verlag; Berlin: 1999b. Brain Function and Oscillations. [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Eng. Med. Biol. Mag. 1999;18:56–66. doi: 10.1109/51.765190. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int. J. Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents "at-risk": evidence of frontal cortex dysfunction in conduct disorder. Biol. Psychiatry. 2001;50:600–608. doi: 10.1016/s0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Lateral asymmetries in the frontal brain: effects of depression and a family history of alcoholism in female adolescents. Alcohol. Clin. Exp. Res. 2002;26:1662–1668. doi: 10.1097/01.ALC.0000036283.60525.B3. [DOI] [PubMed] [Google Scholar]
- Berger H. Uber das Elecktroenzephalogramm des Menschen. Arch Psychiatry (Nervenk) 1929;87:527–570. [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol. Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kroppmann CJ, Kayser J, Stewart JW, McGrath PJ, Tenke CE. Reduced brain responses to novel sounds in depression: P3 findings in a novelty oddball task. Psychiatry Res. 2009;170:218–223. doi: 10.1016/j.psychres.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Towey JP, Leite P, Fong R, Stewart JE, McGrath PJ, Quitkin FM. Brain ERPs of depressed patients to complex tones in an oddball task: relation of reduced P3 asymmetry to physical anhedonia. Psychophysiology. 1998;35:54–63. [PubMed] [Google Scholar]
- Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B. Alcohol-related ERP changes recorded from different modalities: a topographic analysis. Alcohol. Clin. Exp. Res. 2002;26:303–317. [PubMed] [Google Scholar]
- Coles MGH, Rugg MD. Event-related brain potentials: an introduction. In: Rugg MD, Coles MGH, editors. Electrophysiology of mind: Event-related brain potentials and cognition. Oxford University Press; New York, NY: 1995. pp. 1–26. [Google Scholar]
- Donchin E, Coles GH. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988;11:357–374. [Google Scholar]
- Duffy FH, Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med. 2012;10:64. doi: 10.1186/1741-7015-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, Shankardass A, McAnulty GB, Als H. The relationship of Asperger's syndrome to autism: a preliminary EEG coherence study. BMC Med. 2013;11:175. doi: 10.1186/1741-7015-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabo D, Magloczky Z, Wittner L, Pek A, Eross L, Czirjak S, Vajda J, Solyom A, Rasonyi G, Szucs A, Kelemen A, Juhos V, Grand L, Dombovari B, Halasz P, Freund TF, Halgren E, Karmos G, Ulbert I. Properties of in vivo interictal spike generation in the human subiculum. Brain. 2008;131:485–499. doi: 10.1093/brain/awm297. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bouzas A, Harmony T, Galan L, Marosi E, Fernandez T, Reyes A, Silva J, Rodriguez M, Bernal J, Alonso M. Comparison of Z and multivariate statistical brain electromagnetic maps for the localization of brain lesions. Electroencephalogr. Clin. Neurophysiol. 1995;95:372–380. doi: 10.1016/0013-4694(95)00111-b. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bouzas A, Harmony T, Marosi E, Fernandez T, Silva J, Rodriguez M, Bernal J, Reyes A, Casian G. Evolution of cerebral edema and its relationship with power in the theta band. Electroencephalogr. Clin. Neurophysiol. 1997;102:279–285. doi: 10.1016/s0013-4694(96)96049-6. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Drenckhahn R, Wischmann HA, Wagner M. An improved boundary element method for realistic volume-conductor modeling. IEEE Trans. Biomed. Eng. 1998;45:980–997. doi: 10.1109/10.704867. [DOI] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biol. Psychiatry. 2000;48:276–286. doi: 10.1016/s0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Chorlian DB, Begleiter H, Polich J. Auditory P3a deficits in male subjects at high risk for alcoholism. Biol. Psychiatry. 2001;49:726–738. doi: 10.1016/s0006-3223(00)01049-0. [DOI] [PubMed] [Google Scholar]
- Haider M, Spong P, Lindsley DB. Attention, Vigilance, and Cortical Evoked-Potentials in Humans. Science. 1964;145:180–182. doi: 10.1126/science.145.3628.180. [DOI] [PubMed] [Google Scholar]
- Hansen JC, Hillyard SA. Endogenous brain potentials associated with selective auditory attention. Electroencephalogr. Clin. Neurophysiol. 1980;49:277–290. doi: 10.1016/0013-4694(80)90222-9. [DOI] [PubMed] [Google Scholar]
- Harmony T, Fernandez-Bouzas A, Marosi E, Fernandez T, Bernal J, Rodriguez M, Reyes A, Silva J, Alonso M, Casian G. Correlation between computed tomography and voltage and current source density spectral EEG parameters in patients with brain lesions. Electroencephalogr. Clin. Neurophysiol. 1993;87:196–205. doi: 10.1016/0013-4694(93)90019-r. [DOI] [PubMed] [Google Scholar]
- He B, Lian J, Li G. High-resolution EEG: a new realistic geometry spline Laplacian estimation technique. Clin. Neurophysiol. 2001;112:845–852. doi: 10.1016/s1388-2457(00)00546-0. [DOI] [PubMed] [Google Scholar]
- Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr. Clin. Neurophysiol. 1975;39:526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Hjorth B. Source derivation simplifies topographical EEG interpretation. Am. J. EEG Technol. 1980;20:121–132. [Google Scholar]
- Houston RJ, Bauer LO, Hesselbrock VM. Depression and familial risk for substance dependence: a P300 study of young women. Psychiatry Res. 2003;124:49–62. doi: 10.1016/s0925-4927(03)00074-x. [DOI] [PubMed] [Google Scholar]
- Ji J, Porjesz B, Begleiter H. Event-related potential index of semantic mnemonic dysfunction in abstinent alcoholics. Biol. Psychiatry. 1999;45:494–507. doi: 10.1016/s0006-3223(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Johannes S, Munte TF, Heinze HJ, Mangun GR. Luminance and spatial attention effects on early visual processing. Brain Res. Cogn. Brain Res. 1995;2:189–205. doi: 10.1016/0926-6410(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin. Neurophysiol. 2006;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder CE. How local is the local field potential? Neuron. 2011;72:847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B. Brain waves in Impulsivity Spectrum Disorders. In: Cyders MA, editor. Psychology of Impulsivity. Nova Science Publishers; Hauppauge, NY: 2012. pp. 20–93. [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol. Psychol. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Rangaswamy M, Tang Y, Chorlian DB, Padmanabhapillai A, Saunders R, Pandey AK, Roopesh BN, Manz N, Stimus AT, Begleiter H. Brain signatures of monetary loss and gain: outcome-related potentials in a single outcome gambling task. Behav. Brain Res. 2009;197:62–76. doi: 10.1016/j.bbr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Manz N, Chorlian DB, Pandey AK, Roopesh BN, Porjesz B. Topography, power, and current source density of theta oscillations during reward processing as markers for alcohol dependence. Hum. Brain Mapp. 2012;33:1019–1039. doi: 10.1002/hbm.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katznelson RD. EEG recording, electrode placement, and aspects of generator localization. In: Nunez PL, editor. Electric Fields of the Brain: The Neurophysics of EEG. Oxford University Press; New York: 1981. pp. 176–213. [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin. Neurophysiol. 2003;114:2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin. Neurophysiol. 2006b;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43:237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gil R, Bruder GE. ERP generator patterns in schizophrenia during tonal and phonetic oddball tasks: effects of response hand and silent count. Clin. EEG Neurosci. 2010a;41:184–195. doi: 10.1177/155005941004100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gil RB, Bruder GE. Stimulus- and response-locked neuronal generator patterns of auditory and visual word recognition memory in schizophrenia. Int. J. Psychophysiol. 2009;73:186–206. doi: 10.1016/j.ijpsycho.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Alschuler DM, Ben-David S, Fekri S, Bruder GE, Corcoran CM. Olfaction in the psychosis prodrome: electrophysiological and behavioral measures of odor detection. Int. J. Psychophysiol. 2013;90:190–206. doi: 10.1016/j.ijpsycho.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Alschuler DM, Fekri S, Ben-David S, Corcoran CM, Bruder GE. Auditory event-related potentials and alpha oscillations in the psychosis prodrome: Neuronal generator patterns during a novelty oddball task. Int. J. Psychophysiol. 2014;91:104–120. doi: 10.1016/j.ijpsycho.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Alschuler DM, Fekri S, Gil R, Jarskog LF, Harkavy-Friedman JM, Bruder GE. A neurophysiological deficit in early visual processing in schizophrenia patients with auditory hallucinations. Psychophysiology. 2012;49:1168–1178. doi: 10.1111/j.1469-8986.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Fekri S, Alschuler DM, Gates NA, Gil R, Harkavy-Friedman JM, Jarskog LF, Bruder GE. Current source density (CSD) old/new effects during recognition memory for words and faces in schizophrenia and in healthy adults. Int. J. Psychophysiol. 2010b;75:194–210. doi: 10.1016/j.ijpsycho.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Malaspina D, Kroppmann CJ, Schaller JD, Deptula A, Gates NA, Harkavy-Friedman JM, Gil R, Bruder GE. Neuronal generator patterns of olfactory event-related brain potentials in schizophrenia. Psychophysiology. 2010c;47:1075–1086. doi: 10.1111/j.1469-8986.2010.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Berg P, Rockstroh B, Andresen B. Topography of the auditory P300 in schizotypal personality. Biol. Psychiatry. 1999;45:1612–1621. doi: 10.1016/s0006-3223(98)00254-6. [DOI] [PubMed] [Google Scholar]
- Le J, Menon V, Gevins A. Local estimate of surface Laplacian derivation on a realistically shaped scalp surface and its performance on noisy data. Electroencephalogr. Clin. Neurophysiol. 1994;92:433–441. doi: 10.1016/0168-5597(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front. Psychol. 2011;2:51. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J. Clin. Neurophysiol. 1991;8:397–413. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Gonzalez-Andino SL, Valdes-Sosa PA, Biscay-Lirio R. Current source density estimation and interpolation based on the spherical harmonic Fourier expansion. Int. J. Neurosci. 1988;43:237–249. doi: 10.3109/00207458808986175. [DOI] [PubMed] [Google Scholar]
- Pause BM, Lubke K, Laudien JH, Ferstl R. Intensified neuronal investment in the processing of chemosensory anxiety signals in non-socially anxious and socially anxious individuals. PLoS One. 2010;5:e10342. doi: 10.1371/journal.pone.0010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Scalp current density mapping: value and estimation from potential data. IEEE Trans. Biomed. Eng. 1987a;34:283–288. doi: 10.1109/tbme.1987.326089. [DOI] [PubMed] [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Topographic brain mapping of EEG and evoked potentials. Springer; 1989a. Early cortical somatosensory and N1 auditory evoked responses: analysis with potential maps, scalp current density maps and three-concentric-shell head models; pp. 390–395. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989b;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr. Clin. Neurophysiol. 1987b;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr., Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Cambridge University Press; New York: 2007. pp. 56–84. [Google Scholar]
- Pollock VE, Schneider LS. Topographic electroencephalographic alpha in recovered depressed elderly. J. Abnorm. Psychol. 1989;98:268–273. doi: 10.1037//0021-843x.98.3.268. [DOI] [PubMed] [Google Scholar]
- Ponomarev VA, Mueller A, Candrian G, Grin-Yatsenko VA, Kropotov JD. Group Independent Component Analysis (gICA) and Current Source Density (CSD) in the study of EEG in ADHD adults. Clin. Neurophysiol. 2014;125:83–97. doi: 10.1016/j.clinph.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin. Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol. Clin. Exp. Res. 1996;20:9–15. doi: 10.1111/j.1530-0277.1996.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Rodin E. Decomposition and mapping of generalized spike-wave complexes. Clin. Neurophysiol. 1999;110:1868–1875. doi: 10.1016/s1388-2457(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcohol. Clin. Exp. Res. 1999a;23:582–591. [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male subjects at high risk for alcoholism. Biol. Psychiatry. 1999b;46:281–291. doi: 10.1016/s0006-3223(98)00247-9. [DOI] [PubMed] [Google Scholar]
- Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Pandey AK, Porjesz B. Reduced resource optimization in male alcoholics: N400 in a lexical decision paradigm. Alcohol. Clin. Exp. Res. 2010;34:1905–1914. doi: 10.1111/j.1530-0277.2010.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Coles MGH. Electrophysiology of mind: event-related brain potentials and cognition. Oxford University Press; 1996. p. New York. [Google Scholar]
- Salisbury DF, Griggs CB, Shenton ME, McCarley RW. The NoGo P300 'anteriorization' effect and response inhibition. Clin. Neurophysiol. 2004;115:1550–1558. doi: 10.1016/j.clinph.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Srebro R. A bootstrap method to compare the shapes of two scalp fields. Electroencephalogr. Clin. Neurophysiol. 1996;100:25–32. doi: 10.1016/0168-5597(95)00205-7. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans. Biomed. Eng. 1998a;45:814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Tucker DM, Murias M. Estimating the spatial Nyquist of the human EEG. Behav. Res. Methods Instrum. Comput. 1998b;30:8–19. [Google Scholar]
- Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJ. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJ. Resting and task-elicited prefrontal EEG alpha asymmetry in depression: support for the capability model. Psychophysiology. 2014;51:446–455. doi: 10.1111/psyp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Sutton S, Ruchkin DS. The late positive complex. Advances and new problems. Ann. N. Y. Acad. Sci. 1984;425:1–23. doi: 10.1111/j.1749-6632.1984.tb23520.x. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin. Neurophysiol. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clin. Neurophysiol. 2012;123:2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, Alschuler DM, Stewart JW, McGrath PJ, Bruder GE. Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol. Psychiatry. 2011;70:388–394. doi: 10.1016/j.biopsych.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Shankman SA, Griggs CB, Leite P, Stewart JW, Bruder GE. Hemispatial PCA dissociates temporal from parietal ERP generator patterns: CSD components in healthy adults and depressed patients during a dichotic oddball task. Int. J. Psychophysiol. 2008;67:1–16. doi: 10.1016/j.ijpsycho.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Stewart JW, Bruder GE. Novelty P3 reductions in depression: characterization using principal components analysis (PCA) of current source density (CSD) waveforms. Psychophysiology. 2010;47:133–146. doi: 10.1111/j.1469-8986.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J. Abnorm. Psychol. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: I. Physiological evidence for gender and subtype specific differences in regional pathology. Biol. Psychiatry. 1998a;43:84–96. doi: 10.1016/S0006-3223(97)00258-8. [DOI] [PubMed] [Google Scholar]
- Turetsky B, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: II. Longitudinal stability and relationship to symptom change. Biol. Psychiatry. 1998b;43:31–39. doi: 10.1016/s0006-3223(97)00261-8. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Cannon TD, Gur RE. P300 subcomponent abnormalities in schizophrenia: III. Deficits In unaffected siblings of schizophrenic probands. Biol. Psychiatry. 2000;47:380–390. doi: 10.1016/s0006-3223(99)00290-5. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45(Suppl 4):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Verleger R. Event-related potentials and cognition: a critique of the context updating hypothesis and an alternative interpretation of P3. Behav. Brain Sci. 1988;11:343–427. [Google Scholar]
- Wang K, Begleiter H. Local polynomial estimate of surface Laplacian. Brain Topogr. 1999;12:19–29. doi: 10.1023/a:1022225522447. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Ghorashi S, Schneiderman JS, Hawley KJ, McCarley RW, Shenton ME, Spencer KM. Predicting inter-hemispheric transfer time from the diffusion properties of the corpus callosum in healthy individuals and schizophrenia patients: a combined ERP and DTI study. Neuroimage. 2011;54:2318–2329. doi: 10.1016/j.neuroimage.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]