Abstract
Background
We explored the relationship between virologic response in the first year of treatment and long-term outcomes in the BENCHMRK studies.
Methods
Patients failing ART with 3-class resistant HIV-1 received double-blinded raltegravir (or placebo) with optimized background therapy (OBT) until week 156, then open-label raltegravir with OBT up to week 240. In this exploratory analysis of patients randomized to raltegravir, virologic response over weeks 16–48 was categorized as�continuous suppression (CS: vRNA always <50 copies/mL), low-level viremia�(LLV: vRNA always < 400 copies/mL; <50 copies/mL at least once), or not suppressed (NS: vRNA <400 copies/mL at least once). The association between these first-year vRNA response categories and baseline factors was analyzed with univariate and multivariate models. Virologic and immunologic outcomes for years 2–5 were assessed by first-year vRNA response category (observed failure approach).
Results
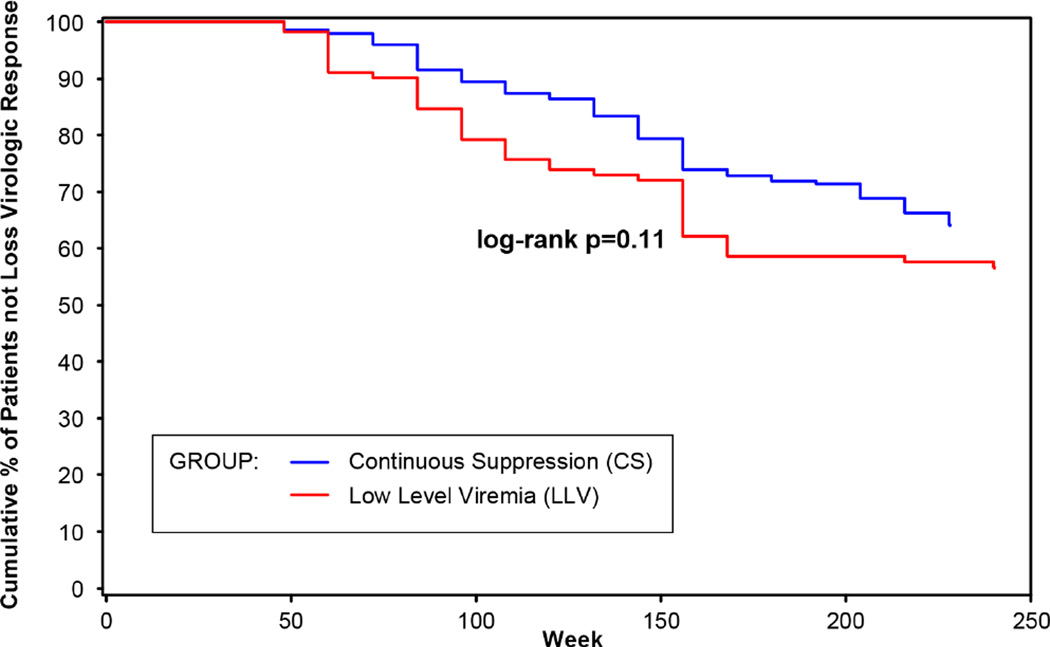
Baseline vRNA, baseline CD4 count, and rapid viral decay (vRNA <50 copies/mL between weeks 2–12) correlated with first-year vRNA response (p<0.001); only rapid viral decay remained significant by multiple regression. Virologic response rates were similar in the LLV and CS groups and lowest in the NS group. CD4 increased through week 240 in the CS and LLV groups. Time to loss of virologic response (confirmed vRNA ≥400 copies/mL) through Week 240 did not support as strong a difference between the LLV and CS groups (log-rank p=0.11) as previously reported through Week 156 and 192 (p<0.05).
Conclusions
Treatment-experienced patients on a raltegravir-based regimen with early LLV may have long-term virologic and immunologic benefit when their therapy is maintained.
Keywords: raltegravir, HIV-1 infection, BENCHMRK studies, low-level viremia
INTRODUCTION
Raltegravir is an HIV-1 integrase strand-transfer inhibitor [1] approved for use in combination regimens for the treatment of HIV-1 infection in treatment-naïve and treatment-experienced patients [2]. We have previously reported the final results of the combined BENCHMRK Phase III studies of raltegravir plus an optimized background regimen (OBT) in treatment-experienced patients infected with multi-drug resistant HIV-1 [3]. In the BENCHMRK studies, raltegravir with OBT demonstrated superiority over the control group of OBT plus placebo through 3 years of double-blind treatment. At week 48, approximately 10% of patients in the raltegravir group had HIV RNA below 400 but greater than 50 copies/mL [4]. According to current treatment recommendations, there is a lack of consensus on the management of patients with HIV-1 RNA levels between 50 and 200 copies/mL [5]. The PLATO collaboration has shown that increases in CD4+ T-cell count can be achieved even when virus is not completely suppressed [6]. Several studies have shown that transient low-level viremia (vRNA between 50 and 400 copies/ml) has a limited effect on virologic and immunologic responses and on the development of drug resistance [7–10], while others have found that low-level viremia was associated with virologic failure and the emergence of drug resistance [11–14].
The predictors of and the long-term virologic and immunologic consequences of low-level viremia in the first year after initiation of therapy in highly treatment-experienced patients have not been described. Whether this pattern of response is associated frequently with subsequent virologic failure and resistance, as might be predicted, could influence treatment decisions. To gain a better understanding of the long-term clinical implications of low-level viremia, we explored the relationship between first-year vRNA response (at weeks 16–48) and long-term virologic and immunologic outcomes (from week 96 through week 240) among patients assigned to the raltegravir group in the BENCHMRK studies.
METHODS
Study Design
BENCHMRK-1 (Protocol 018; NCT 00293267) and BENCHMRK-2 (Protocol 019; NCT 00293254) were multicenter, double-blind, randomized, placebo-controlled studies to evaluate the safety, tolerability, and efficacy of raltegravir 400 mg b.i.d. compared with placebo, each in combination with OBT for 156 weeks [3,4]. After completing 156 weeks of double-blind therapy, patients were eligible to receive open-label raltegravir 400 mg b.i.d. plus OBT for an additional 84 weeks, for a total treatment duration of up to 240 weeks. The protocols were approved by the Institutional Review Board or Ethical Review Committee at each site, and all participants provided written informed consent.
HIV-seropositive patients who had failed prior antiretroviral therapy and had documented resistance to at least one protease inhibitor (PI), one nucleoside reverse transcriptase inhibitor, and one non-nucleoside reverse transcriptase inhibitor were enrolled at 61 sites in Europe, Asia, Australia, and Peru (BENCHMRK-1) and 53 sites in North and South America (BENCHMRK-2). Each patient’s OBT regimen was determined at study entry prior to randomization; the site investigator selected the best available combination of agents based on the patient’s antiretroviral treatment history, available drug-resistance testing, and laboratory data. Patients were randomly assigned in a 2:1 ratio to receive raltegravir 400 mg b.i.d. or matching placebo b.i.d. in addition to their OBT. Randomization was stratified by enfuvirtide use in OBT and degree of viral resistance to commercially available PIs. Investigators, study site and sponsor personnel, patients, and laboratory personnel remained blinded to treatment allocation until all patients had completed or discontinued the study, protocol violators had been identified, and data were declared complete.
HIV-1 RNA (vRNA) levels were measured by the standard COBAS Amplicor HIV-1 Monitor™ assay (version 1.5; Roche Diagnostics, Branchburg, NJ; LLQ 400 copies/mL) and the Ultrasensitive Amplicor HIV-1 Monitor™ assay (version 1.5; Roche Diagnostics; LLQ 50 copies/mL) for samples with vRNA levels < 400 copies/mL by the standard assay. vRNA levels were measured at entry, every 4 weeks through week 16, every 8 weeks through week 48, and every 12 weeks thereafter. Resistance testing (PhenoSense GT™, Monogram Biosciences, San Francisco, CA) was performed at baseline and at the time of virologic failure.
Statistical Methods
These exploratory analyses utilized a modified-intention-to-treat (mITT) population. Patients were included in the treatment group to which they were randomized (provided they received at least one dose of study drug), regardless of adherence to the entry criteria, treatment actually received, and deviations from the protocol. The observed failure (OF) approach was used; only discontinuations due to lack of efficacy were counted as failures, and baseline values were carried forward for these patients.
Patients assigned to the raltegravir group who completed 48 weeks of double-blind treatment and continued in the study were categorized based on their observed vRNA response at five time points between weeks 16 and 48 (with no imputation for missing data) as follows: continuous suppression (CS) was defined as vRNA <50 copies/mL at all time points; low-level viremia (LLV) was defined as vRNA < 400 copies/mL at all time points and <50 copies/mL at least once; and not suppressed (NS) was defined as vRNA <400 copies/mL at least once. The association between these first-year vRNA response categories and the following prognostic factors was analyzed with univariate and multivariate models: baseline vRNA (≤100,000 vs <100,000 copies/mL), baseline CD4 cell count (≤50 vs <50 cells/mm3, and ≤200 vs <200 cells/mm3), baseline genotypic sensitivity score (<2 vs ≥2), baseline phenotypic sensitivity score (<2 vs ≥2), and rapid viral decay, defined as vRNA <50 copies/mL at least once between weeks 2 and 12 (yes vs no). A k-by-k contingency table using each prognostic factor was constructed, and p-values were calculated by the Mantel-Haenszel chi-square test.
The following outcome measures were plotted for each of the first-year vRNA response categories: the proportion of patients with vRNA <50 copies/mL, the proportion with vRNA < 400 copies/mL, and the change from baseline in CD4 cell count (cells/mm3). Among patients with virologic failure, the proportion who developed integrase resistance-associated mutations was tabulated for the three vRNA response categories.
Kaplan-Meier curves were plotted for time to loss of virologic response (TLOVR) for the CS and LLV groups. For each patient, TLOVR was defined as the visit when vRNA exceeded 400 copies/mL and was subsequently confirmed (or the patient was discontinued); TLOVR was restricted to the first loss after week 48, which is the latest time point to determine the first-year vRNA response category. Cox regression was utilized to detect prognostic factors which could predict TLOVR; the prognostic factors mentioned above and the first-year vRNA response category (CS vs LLV) were included in this analysis.
By definition, patients in the LLV group could have had vRNA <50 copies/mL at a single time point or at multiple time points between weeks 16 and 48. To explore the impact of having one vs multiple vRNA responses in the LLV range (50–400 copies/mL), the LLV group was further divided into 2 subgroups: Group 1 (LLV1) included patients with only one vRNA response <50 copies/mL during weeks 16–48, and Group 2 (LLV2) included patients with multiple vRNA responses <50 copies/mL during the same period. The following analyses were repeated for the LLV1 vs LLV2 subgroups: virologic and immunologic responses over time, Kaplan-Meier curves for TLOVR, and Cox regression to detect factors predicting TLOVR (see above).
RESULTS
Of the 373 patients who completed 48 weeks of treatment with raltegravir, 199 (53%) were continuously suppressed during weeks 16–48, 111 (30%) had low-level viremia, and 63 (17%) were not suppressed. Baseline vRNA levels were lowest among patients with continuous suppression and highest among those who were not suppressed, while baseline CD4 cell count, CD4%, CD8 cell count, and CD4/CD8 ratio were highest in the CS group and lowest in the NS group (Table 1). The CS and LLV groups had slightly more patients with at least one active PI in the OBT (65% and 69%, respectively) compared with the NS group (59%). Phenotypic sensitivity scores were similar for the CS and LLV groups (≥2 in 54% and 56%, respectively, vs 48% of the NS group), while genotypic sensitivity scores were similar for the LLV and NS groups (≥2 in 33%, vs 44% of the CS group). Rapid viral decay (vRNA <50 copies/mL at least once between weeks 2 and 12) was observed in 94% of the CS group, 64% of the LLV group, and 43% of the NS group. In the univariate analysis, baseline vRNA level, baseline CD4 count, and rapid viral decay were correlated with first-year vRNA response category (p<0.001), while genotypic and phenotypic sensitivity scores were not (p=0.072 and 0.592, respectively). In the multiple regression analysis, only rapid viral decay was significantly associated with first-year vRNA response category (p<0.001).
Table 1.
Baseline Characteristics by First-Year vRNA Response
| Baseline Characteristic |
Continuous Suppression (N = 199) n (%) |
Low Level Viremia (N = 111) n (%) |
Not Suppressed (N = 63) n (%) |
|---|---|---|---|
| Plasma HIV RNA (copies/mL) | |||
| Geometric Mean | 22770 | 60592 | 82652 |
| Median (range) | 25800 (200-574000) | 78900 (441-750000) | 102000 (619-750000) |
| ≤ 50,000 | 126 (63.3) | 40 (36.0) | 23 (36.5) |
| 50,000 – 100,000 | 36 (18.1) | 24 (21.6) | 7 (11.1) |
| > 100,000 | 37 (18.6) | 47 (42.3) | 33 (52.4) |
| CD4 Cell Counts (cells/mm3) | |||
| Mean | 188 | 138 | 110 |
| Median (range) | 166 (2 to 792) | 101 (1 to 740) | 69 (1 to 469) |
| ≤ 50 | 33 (16.6) | 41 (36.9) | 29 (46.0) |
| > 50 and ≤ 200 | 81 (40.7) | 43 (38.7) | 22 (34.9) |
| > 200 | 84 (42.2) | 27 (24.3) | 12 (19.0) |
| CD4%, median (range) | 11 (0 to 46) | 9 (0 to 30) | 5 (0 to 26) |
| CD8 Cell Counts (cells/mm3) | |||
| Median (range) | 887 (167 to 2605) | 729 (46 to 2296) | 642 (69 to 2530) |
| CD8%, median (range) | 62 (26 to 89) | 61 (11 to 86) | 62 (21 to 83) |
| CD4/CD8 Ratio | |||
| Median (range) | 0.18 (0.01 to 1.23) | 0.14 (0.01 to 0.66) | 0.07 (0.01 to 0.91) |
| Number of ARTs in OBT | |||
| Median (range) | 4.0 (1 to 6) | 4.0 (2 to 6) | 4.0 (2 to 7) |
| Number of Active PI in OBT by Phenotypic Resistance Test† | |||
| 0 | 63 (31.7) | 31 (27.9) | 23 (36.5) |
| 1 or more | 129 (64.8) | 77 (69.4) | 37 (58.7) |
| Phenotypic Sensitivity Score (PSS)‡ | |||
| 0 | 25 (12.6) | 8 (7.2) | 8 (12.7) |
| 1 | 58 (29.1) | 37 (33.3) | 21 (33.3) |
| 2 | 66 (33.2) | 47 (42.3) | 12 (19.0) |
| 3 or more | 41 (20.6) | 15 (13.5) | 18 (28.6) |
| Genotypic Sensitivity Score (GSS)‡ | |||
| 0 | 33 (16.6) | 21 (18.9) | 16 (25.4) |
| 1 | 76 (38.2) | 52 (46.8) | 24 (38.1) |
| 2 | 60 (30.2) | 31 (27.9) | 10 (15.9) |
| 3 or more | 27 (13.6) | 6 (5.4) | 11 (17.5) |
For patients missing baseline genotypic and/or phenotypic test results, "number of active PI in OBT determined by phenotypic resistance test" =1 or more if first time use darunavir in OBT.
PSS GSS were defined as the total oral ARTs in OBT to which a patient's viral isolate showed phenotypic sensitivity and genotypic sensitivity, respectively, based upon phenotypic and genotypic resistance tests. Enfuvirtide use in OBT in enfuvirtide-naïve patients was counted as one active drug in OBT and added to the GSS and PSS. Darunavir use in OBT in darunavir-naïve patients was counted as one active drug in OBT and added to the PSS and GSS.
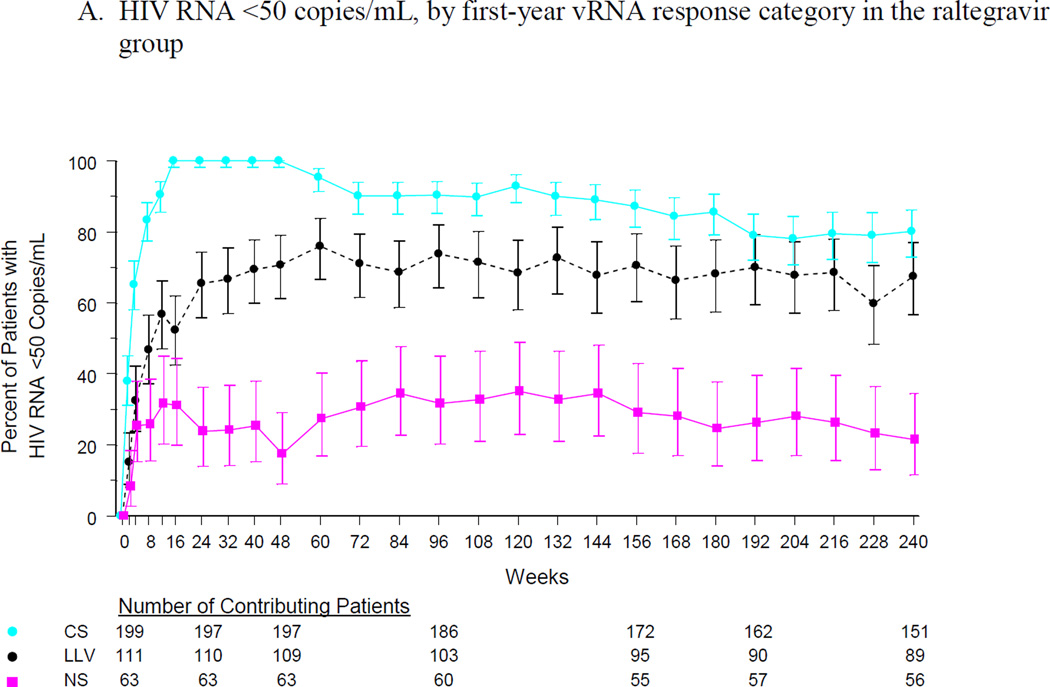
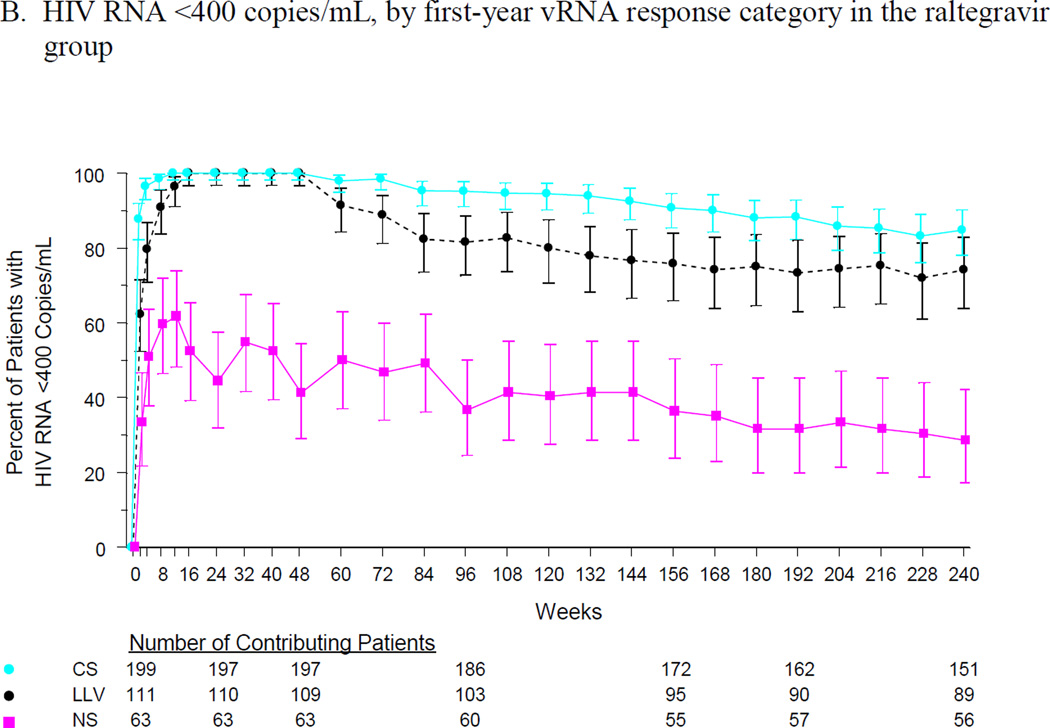
Long-term virologic response rates in the LLV group were similar to those in the CS group and were substantially higher than those in the NS group. At week 240, 80% of the CS group and 67% of the LLV group had vRNA < 50 copies/mL, compared with 21% of the NS group (Figure 1A). At the same time point, vRNA < 400 copies/mL was maintained in 85%, 74% and 29% of the CS, LLV, and NS groups, respectively (Figure 1B). The mean change in CD4 cell count continued to increase in the CS and LLV groups, reaching 244 and 286 cells/mm3, respectively, at week 240. In the NS group, the mean change in CD4 cell count peaked at 125 cells/mm3 at week 72 and remained relatively constant thereafter (Figure 1C).
Figure 1.
Efficacy outcomes by first-year vRNA response category: (A) % of patients with vRNA <50 copies/mL, (B) % of patients with vRNA <400 copies/mL, and (C) change from baseline in CD4 cell count.
Patients in the CS and LLV groups had higher rates of self-reported medication adherence than patients in the NS group (Table 2). Approximately 6% of patients in the CS group and 12% in the LLV group discontinued treatment due to virologic failure or lack of treatment efficacy; substantially more patients in the NS group (44%) discontinued for these reasons (Table 3). Among patients�with virologic failure�between weeks 48 and 240 who had resistance testing performed, one or more�raltegravir signature mutations (AA 143, 148, or 155) were�detected in 43% (9/21) from the CS group, 29% (4/14) from the LLV group, and 55% (26/47) from the NS group. Unlike prior analyses out to Week 156 (3 years) and Week 192 (4 years), the TLOVR analysis using data through Week 240 did not support as strong a difference (log-rank p=0.11) between the LLV and CS groups (Figure 2). Furthermore, none of the factors examined in the Cox regression analysis were significant predictors of TLOVR, including initial rapid viral decay.
Table 2.
Self-reported Medication Adherence by First-Year vRNA Response
| CS Group (N = 199) |
LLV Group (N = 111) |
NS Group (N = 63) |
|
|---|---|---|---|
| Percent Compliance† | n (%) | n (%) | n (%) |
| 100% | 158 (79.4) | 88 (79.3) | 44 (69.8) |
| 99 to 90% | 39 (19.6) | 21 (18.9) | 15 (23.8) |
| 89 to 80% | 2 (1.0) | 2 (1.8) | 1 (1.6) |
| 79 to 70% | 0 | 0 | 2 (3.2) |
| <70% | 0 | 0 | 1 (1.6) |
Percent compliance was calculated as: [number of days on therapy / number of days should be on therapy] * 100.
Table 3.
Patient Disposition by First-Year vRNA Response
| CS Group (N=199) n (%) |
LLV Group (N=111) n (%) |
NS Group (N=63) n (%) |
|
|---|---|---|---|
| Completed 240 weeks of treatment | 133 (66.8) | 69 (62.2) | 19 (30.2) |
| Discontinued due to virologic failure† | 7 (3.5) | 10 (9.0) | 15 (23.8) |
| Discontinued due to lack of efficacy‡ | 4 (2.0) | 3 (2.7) | 13 (20.6) |
| Discontinued for other reasons | 55 (27.6) | 29 (26.1) | 16 (25.4) |
| Clinical adverse event | 10 (5.0) | 6 (5.4) | 3 (4.8) |
| Consent withdrawn | 12 (6.0) | 8 (7.2) | 7 (11.1) |
| Lost to follow-up | 6 (3.0) | 2 (1.8) | 2 (3.2) |
| Other reasons†† | 27 (13.6) | 13 (11.7) | 4 (6.3) |
Virologic failure was considered to have occurred if vRNA did not decrease to <400 copies/mL or by >1 log10 copies/mL from baseline; if vRNA increased by >1 log10 copies/mL from the nadir level on two consecutive measurements; or if vRNA was ≥400 copies/mL on two consecutive measurements after having been <400 copies/mL.
Lack of efficacy was determined by the site investigator.
Includes the following: protocol deviation, did not enter extension phase, moved or relocated, or clinical trial was terminated at the site.
Figure 2.
Time to loss of virologic response (TLOVR, ≥400 copies/mL) by first-year vRNA response category
To further assess the prognostic nature of the LLV group, the impact of the number of times patients had vRNA <50 copies was explored. Of the LLV group, 63 patients had only one vRNA response <50 copies/mL during weeks 16–48 (LLV1), and 48 patients had multiple vRNA responses <50 copies/mL during the same period (LLV2; the number of responses <50 copies/mL was 2 in 21 patients, 3 in 17 patients, 4 in six patients, and 5 in four patients). Virologic response rates (after week 48) and the mean change from baseline in CD4 counts through week 240 were higher in the LLV1 group than in the LLV2 group, although there was substantial overlap in the 95% confidence intervals (Suppl. Figures S1-S3). The TLOVR analysis also suggested there may be a difference favoring the LLV1 group (Suppl. Figure S4), although LLV subgroup was not a significant predictor of TLOVR in the Cox regression (LLV1 vs. LLV2, log-rank, p=0.07). The other factors examined in this analysis also were not significant predictors of TLOVR.
DISCUSSION
We explored the relationship between late outcomes and early virologic response among patients who received raltegravir for at least 48 weeks in the BENCHMRK studies. Patients were categorized by their early virologic response (vRNA levels at weeks 16–48) as continuously suppressed (CS, all measurements <50 copies/mL), low-level viremia (LLV, all measurements < 400 copies/mL and at least one <50 copies/mL), or not suppressed (NS, at least one measurement <400 copies/mL). Patients with low-level viremia had higher vRNA levels and lower CD4 cell counts at baseline than patients with continuous suppression. However, rapid viral decay (defined as vRNA <50 copies/mL at least once before week 12) was the only factor found to be significantly associated with first-year viral response category in the multiple regression analysis. Patients with continuous suppression were significantly more likely to have demonstrated rapid virologic decay (94%) compared to those with low-level viremia (64%) and those who were not suppressed (43%). Patients with low-level viremia demonstrated durable efficacy responses through 5 years of treatment, showing favorable virologic and immunologic outcomes that were similar to those in patients with continuous suppression, including no significant difference in TLOVR (vRNA ≥400 copies/mL) and a low rate of treatment-emergent resistance mutations (4 of 14 patients with virologic failure). Although previous analyses [15,16] suggested a significant difference (p<0.05) in TLOVR between the CS and LLV groups at week 156 (3 years) and week 192 (4 years), the overall data through week 240 did not support as strong a difference (p=0.11).
Low-level viremia may not be a marker of incomplete suppression of ongoing viral replication in highly treatment-experienced patients after initiation of combination therapy that includes at least one fully active agent. The fact that only a minority of these raltegravir-treated patients with low-level viremia during the first year had subsequent overt virologic failure despite limited activity of the OBT regimen suggests that ongoing viral replication may not be the source of the viremia in many of these patients. Emergence of resistance to raltegravir was also uncommon and similar to the continually suppressed group, supporting the hypothesis that ongoing rounds of replication are not the predominant source of early low-level viremia.
Raltegravir-containing regimens are associated with significantly longer first-phase viral decay (d1) and transition to second-phase decay (d2) at lower vRNA levels than efavirenz-containing regimens [17,18]. Andrade and colleagues conclude that the rapid viral decay observed with raltegravir is due to the longer duration of d1, which may reflect a reduction in the pool of cells that contribute to d2 [17]. In our study, the observation that rapid viral decay was the only significant predictor of vRNA response category (CS>LLV>NS) suggests that patients with low-level viremia may have a larger reservoir contributing to viral decay, as the transition from the first to second phase decay was more likely to occur at a vRNA level <50 copies/mL and therefore lead to a longer time to suppression to <50 copies/mL. In these patients, release of virus during the 2nd or 3rd phase of viral decay may result in vRNA levels <50 copies/mL that do not represent ongoing replication. The substantially higher median baseline viral load in the LLV group compared to the CS group, with a larger percentage with vRNA <100,000 copies/mL (42.3 vs 18.6%), also supports the concept that early low-level viremia may represent release from a larger reservoir, as pre-therapy vRNA has been clearly associated with reservoir size on therapy [19–22]. The fact that rapid viral decay was associated with initial complete suppression but not long-term response may be because patients in both the CS and LLV categories had full suppression of HIV-1 replication. However, given our small sample size and retrospective analysis design, an association between rapid viral decay and longer term suppression may have been missed.
Differences in adherence could provide an explanation as to why some patients have continuous suppression and others have low-level viremia. Acknowledging the limitations of self-reported adherence, the adherence rates in this study were remarkably similar between the CS and LLV groups (Table 2) and are therefore an unlikely explanation for the differences observed in the first 48 weeks between these two groups. In contrast, patients in the NS group had poorer self-reported adherence and were substantially more likely to have virologic or efficacy failure and evolution of raltegravir resistance, all suggestive of ongoing rounds of replication as a cause of the higher level of early viremia in this group. Finally, it is possible that the difference between the CS group and the LLV group is based predominantly on the imprecision of the assays at these low levels [8]. Certainly assay variability at low levels of vRNA may contribute to single episodes of vRNA <50 copies/mL. However, the LLV group in this study seems biologically different from the CS group, with higher baseline vRNA, lower baseline CD4 cells counts, and a lower likelihood of rapid vRNA suppression. Within the LLV group, patients who had more than one vRNA measurement <50 copies/mL during weeks 16–48 did not appear to have substantially different outcomes than those with a single vRNA value <50 copies/mL, although small numbers make these estimates less precise and proportion of patients with loss of virologic response was higher in this group.
The clinical implications of our post hoc analysis should be interpreted cautiously. Our observations raise the possibility that low-level viremia that occurs in treatment-experienced patients in the year following initiation of therapy may not be evidence of incomplete suppression of ongoing HIV-1 replication and impending virologic failure in all patients. This level of vRNA may not require a change in therapy, especially in patients who have limited further treatment options. In this study raltegravir was frequently the only fully active agent in the treatment regimen and ongoing replication would be expected to lead to emergence of integrase inhibitor resistance, an uncommon result in our patients with early low-level viremia. In addition, long-term CD4 increases were very similar in patients with low-level viremia who continued the same therapy and those with continued suppression. Our results reflect the DHHS guidelines which suggest that a change in therapy may not be necessary in patients with low-level viremia [23], although the DHHS guidelines use a confirmed viral load <200 copies/mL as the definition for virologic failure, while we used an upper level of 400 copies/mL in our definition of low-level viremia. A more stringent cutoff of 200 copies/mL would likely further minimize the differences between the CS and LLV groups. In the context of large clinical cohorts, others have shown that low-level viremia is associated with subsequent virologic failure, although the timing of the low-level viremia in relationship to when therapy was initiated may be important [24–27]. Resistance testing during periods of low-level viremia may help to understand the consequences of the viremia [13,28,29] and allow for optimization of therapy [30,31], but resistance testing when vRNA is < 400 copies/mL may not be available to all clinicians. Therefore, treatment-experienced patients with low-level viremia in the year following a change in therapy prompted by virologic failure should be followed carefully with serial vRNA measurements. Our data suggest that treatment-experienced patients on a raltegravir-based regimen with early low-level viremia may have long-term virologic and immunologic benefit when their therapy is maintained.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Hong Wan (formerly with Merck & Co., Inc.) and Zijiang Yang (of Merck & Co., Inc.) for performing the statistical analyses.
Footnotes
DISCLOSURES
The BENCHMRK studies were sponsored and funded by Merck, which markets raltegravir under the brand name ISENTRESS. The study was designed, managed, and analyzed by the sponsor in conjunction with external investigators. Authors had access to all study data upon request. All authors approved the final version of the manuscript for submission. The manuscript was also reviewed by the sponsor. The opinions expressed in the manuscript represent the collective views of the authors and do not necessarily reflect the official position of Merck or the institutions affiliated with the academic authors.
Potential Conflicts of Interest
JJE: research support from Merck, GSK/ViiV and BMS; consulting fees from Merck, BMS, GSK/ViiV, Gilead, and Janssen.
DAC: research support, speaker fees, and consulting fees from Merck.
RTS: research support and consulting fees from Merck.
BC: consultant on advisory boards, participated in speakers’ bureaus, or conducted clinical trials with Roche, Boehringer-Ingelheim, Abbott, BMS, GSK, Gilead, Tibotec, Janssen, Merck, Pfizer, Siemens, Monogram Biosciences, and Panacos.
PY: no conflicts to declare.
KMS, AR, RJB, BTN, and HT are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and may own stock or stock options in the company.
REFERENCES
- 1.Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646–650. doi: 10.1126/science.287.5453.646. [DOI] [PubMed] [Google Scholar]
- 2.Merck & Co., Inc. Prescribing information for ISENTRESS (raltegravir) tablets [Google Scholar]
- 3.Eron JJ, Cooper DA, Steigbigel RT, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13:587–596. doi: 10.1016/S1473-3099(13)70093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steigbigel RT1, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008 Jul 24;359(4):339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MA, et al. Antiretroviral Treatment of Adult HIV Infection 2012 Recommendations of the International Antiviral Society–USA Panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 6.Ledergerber B, et al. Predictors of CD4+ T-Cell Counts of HIV Type 1–Infected Persons After Virologic Failure of All 3 Original Antiretroviral Drug Classes. Journal of Infectious Diseases Advance Access published January 8, 2013. doi: 10.1093/infdis/jis752. [DOI] [PubMed] [Google Scholar]
- 7.Sklar PA, Ward DJ, Baker RK, et al. Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS. 2002;16:2035–2041. doi: 10.1097/00002030-200210180-00008. [DOI] [PubMed] [Google Scholar]
- 8.Nettles RE, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 9.Lee PK, Kieffer TL, Siliciano RF, Nettles RE. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother. 2006;57:803–805. doi: 10.1093/jac/dkl092. [DOI] [PubMed] [Google Scholar]
- 10.van Sighem A, Zhang S, Reiss P, et al. Immunologic, virologic, and clinical consequences of episodes of transient viremia during suppressive combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;48:104–108. doi: 10.1097/QAI.0b013e31816a1d4f. [DOI] [PubMed] [Google Scholar]
- 11.Nettles RE, et al. Genotypic resistance in HIV-1-infected patients with persistently detectable low-level viremia while receiving highly active antiretroviral therapy. Clin. Infect. Dis. 2004;39:1030–1037. doi: 10.1086/423388. [DOI] [PubMed] [Google Scholar]
- 12.Raboud JM, RAE S, Woods R, Harris M, Montaner JS. Consecutive rebounds in plasma viral load are associated with virological failure at 52 weeks among HIV-infected patients. AIDS. 2002;16:1627–1632. doi: 10.1097/00002030-200208160-00008. [DOI] [PubMed] [Google Scholar]
- 13.Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis. 2011;204(4):515–520. doi: 10.1093/infdis/jir353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Wyl V, Yerly S, Böni J, et al. Incidence of HIV-1 drug resistance among antiretroviral treatment-naive individuals starting modern therapy combinations. Clin Infect Dis. 2012;54(1):131–140. doi: 10.1093/cid/cir728. [DOI] [PubMed] [Google Scholar]
- 15.Eron JJ, Cooper DA, Steigbigel RT, et al. Sustained Antiretroviral Effect of Raltegravir at Week 156 in the BENCHMRK studies, and Exploratory Analysis of Late Outcomes based on Early Virologic Responses. Presented at CROI 2010, abstract K-128 [Google Scholar]
- 16.Eron JJ, Cooper DA, Steigbigel RT, et al. Exploratory Analysis in the BENCHMRK studies at Week 192: Late Outcomes based on Early Virologic Responses. Presented at IAS 2011, abstract MOPE225 [Google Scholar]
- 17.Andrade A, Rosenkranz SL, Cillo AR, et al. Three distinct phases of HIV-1 RNA decay in treatment-naïve patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis. 2013;208:884–891. doi: 10.1093/infdis/jit272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21(17):2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 19.Fourati S, Flandre P, Calin R, et al. Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother. 2014;69:753–756. doi: 10.1093/jac/dkt428. [DOI] [PubMed] [Google Scholar]
- 20.Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hocqueloux L1, Avettand-Fènoël V, Jacquot S, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68(5):1169–1178. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 22.Williams JP, Hurst J, Robinson N, et al. HIV-1 DNA levels after antiretroviral therapy in primary infection predict disease progression: the SPARTAC Trial; Abstracts of the Seventh IAS Conference on HIV Pathogenesis, Treatment and Prevention, Kuala Lumpur; 2013. Abstract WELBA04. [Google Scholar]
- 23.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011:1–166. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 24.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57(10):1489–1496. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 25.Greub G, Cozzi-Lepri A, Ledergerber B, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16:1967–1969. doi: 10.1097/00002030-200209270-00017. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Gasco P, Maida I, Blanco F, et al. Episodes of low-level viral rebound in HIV-infected patients on antiretroviral therapy: frequency, predictors and outcome. J Antimicrob Chemother. 2008;61:699–704. doi: 10.1093/jac/dkm516. [DOI] [PubMed] [Google Scholar]
- 27.Geretti AM, Smith C, Haberl A, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–936. [PubMed] [Google Scholar]
- 28.Swenson LC, Min JE, Woods CK, et al. HIV drug resistance detected during low-level viremia is associated with subsequent virologic failure. AIDS. 2014 Jan 21; doi: 10.1097/QAD.0000000000000203. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Serna A, Min JE, Woods C, et al. Performance of HIV-1 Drug Resistance Testing at Low-Level Viremia and Its Ability to Predict Future Virologic Outcomes and Viral Evolution in Treatment-Naive Individuals. Clin Infect Dis. 2014;58(8):1165–1173. doi: 10.1093/cid/ciu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConnell MJ, Mier-Mota J, Flor-Parra F, et al. Improved viral suppression after treatment optimization in HIV-infected patients with persistent low-level viremia. J Acquir Immune Defic Syndr. 2011;58:446–449. doi: 10.1097/QAI.0b013e3182364513. [DOI] [PubMed] [Google Scholar]
- 31.Santoro MM, Fabeni L, Armenia D, et al. Reliability and Clinical Relevance of the HIV-1 Drug Resistance Test in Patients With Low Viremia Levels. Clin Infect Dis. 2014;58(8):1156–1164. doi: 10.1093/cid/ciu020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.