Abstract
Tenascins are a family of extracellular matrix molecules that are mainly expressed in embryonic development and down-regulated in adulthood. A re-expression in the adult occurs under pathological conditions such as inflammation, regeneration or neoplasia. As the most prominent member of the tenascin family, TN-C, is highly expressed in glioma tissue and rising evidence suggests that TN-C plays a crucial role in cell migration or invasion – the most fatal characteristics of glioma – also the other members of this protein family have been investigated with regard to their impact on glioma biology. For all tenascins correlations between the expression levels of the different family members and the degree of malignancy and invasiveness of glial tumors could be detected. Overall, the former and recent results in the research on glioma and tenascins point at distinct roles of each of the molecules in glioma biology and the devastating properties of these tumors.
Keywords: extracellular matrix, extracellular matrix receptors, glial stem and progenitors cells, tumor stem cells; angiogenesis, cell migration, central nervous system
Glioma
The most common type of primary brain tumors is represented by the class of glioma with an incidence of ∼5/100,000 patients.1,2 Despite intensive research, advanced diagnostics and improved therapy strategies the prognosis of high-grade glioma remains devastating.3 The survival time still varies between 15 months (high-grade glioma) and 3 y (low-grade glioma), depending on the grade of malignancy which is categorized by the World Health Organization (WHO) as follows: Tumors of grade I and II were referred to as low grade tumors with a 5-years survival rate of 58–72% as shown by the NCCTG trial, EORTC 22844 and EORTC 22845.4 Glial malignancies of grade III and IV are classified as high-grade glioma, with the worst survival rate indicated above.2 Particularly, patients diagnosed with the severest glial tumor glioblastoma multiforme (GBM, grade IV) who survive more than 3 y after diagnosis were referred to as long time survivors. Certainly, this group comprises only 3–5% of patients with a GBM5 and this underlines that the GBM is not only the most frequently recognized glioma (>51% of all glioma), but also the malignant endpoint of this cancer type.6 Comparing primary and secondary glioblastoma both GBM-types share similarities concerning histologic and morphologic features, but differ in their genetic profiles. Primary glioblastoma arise de novo and are characterized by EGFR amplification, mutations in rb1, cdkn2a, p14arf and PTEN and monosomia 10.7-9 Secondary glioblastoma develop from former low-grade glioma and show unique alterations such as mutations of TP53 or IDH1, or the loss of chromosome 19q and 13q and the overexpression of PDGFRα.8,10-12
Histologically primary and secondary glioblastoma display characteristic attributes, including high mitotic activity, cellular and nuclear atypia, strong microvascular proliferation and extended areas of necrosis.6,10 Even though these atypias arise from different genomic alterations both GBM types respond similarly to therapeutic approaches13 with a slightly better prognosis for patients suffering from secondary glioblastomas.8 The high heterogeneity within the classification of glioma tumors gained renewed attention in conjunction with the highly promising research field of glioma-initiating cells14,15 in order to develop new, personalized therapy strategies.
Additionally, the highly invasive behavior of glioma cells leads to quick spreading of the tumor throughout both hemispheres. This feature dramatically shortens the lifespan of glioma patients. Despite accurate surgery it is impossible to remove all malignant cells6 and the recurrent tumors exhibit explicit resistance to chemotherapy and radiation.16,17 The invasion of glioma cells is characterized by their ability to migrate as single cells even to distant parts of the brain. With regard to migration pathways, the tumor cells display a preference for white matter tracts, subependymal layers and blood vessel basement membranes as leading structures.10,18,19 To initiate this migration process glioma cells degrade the ECM into a migration favorable microenvironment.
The Extracellular Matrix
Cells of each tissue are surrounded by a dynamic molecular meshwork filling the extracellular space. This extracellular matrix (ECM) provides a scaffold for the organization of tissues and supports the cohesion of cells.20 While the structural task represents an important function of the ECM, numerous additional features of the ECM have been uncovered in recent years. The maintenance of cell-cell-communications21,22 and the construction of favorable substrates for cell migration20 illustrate central tasks of the ECM which could be observed ubiquitously in the context of differentiation, proliferation, survival and polarity in the regulation of embryonic development as well as in the homeostasis and tissue remodeling in pathological incidents.23,24
The ECM is composed of a complex mixture of matrix molecules. Glycoproteins like fibronectin, laminins or tenascins contribute to it, as well as glycosaminoglycans.24,25 With regard to the content of collagens the ECM of the central nervous system (CNS) differs from the classical matrix in other organs. Whereas the ECM of numerous tissues contains a high amount of collagen fibers this element is rigorously restricted to blood vessels and the glia limitans26 in the brain. The proteins of the ECM interact with each other and their neighboring cells mainly via the specialized matrix receptors of the integrin superfamily. As a result of this interaction the ECM is able to influence different signaling pathways and to give impulses to the behavior of cells by varying its mechanical properties.27-29 During this remodeling process the ability of sulfated proteoglycans to bind growth factors allows the ECM to function as a pool of growth factors which can be released if possible.30
Especially the involvement of the ECM in the regulation of cell motility constitutes an area of interest in the glioma research field because of the known high motility of these cells that results in invasion and recurrence of the tumor. It has been discovered long ago that proteins of the ECM such as laminins, fibronectin or tenascins influence the behavior of glioma cells.31-35 Several ECM components including collagens I, II and IV as well as laminin, fibronectin and tenascin-c (TN-C) could be detected within the basal lamina of tumor blood vessels.23,36-38 With exception of tenascin-c the ECM molecules are not synthesized by the tumor cells themselves. Rather, the tumor cells induce cells of the surrounding brain tissue to produce these proteins.39 In contrast, tenascin-c is autonomously expressed by the glioma cells.35 Hereby the tumor is able to generate an individual overexpression of matrix components whose secretion alters the ECM in various manners. On the one hand it could lead to the stimulation of adhesion and migration of the glioma cells, but on the other hand the ECM could be condensed in a way that a highly concentrated ECM might diminish the diffusion of neuroactive molecules or therapeutical agents.40,41
Tenascins
Tenascin-C
Tenascin-C (TN-C) is a member of the glycoprotein family of tenascins (Fig. 1) mainly expressed during embryonic development, downregulated in the adult and re-expressed under pathological conditions.42,43 The discovery of TN-C occurred in parallel in different fields of research (e.g., embryonic development, tumor biology, neurobiology). From this follows that TN-C was originally known under various names. In 1983 it was introduced by Bourdon & Wikstrand as glial/mesenchymal extracellular matrix protein GMEM.44 Shortly thereafter TN-C was named myotendinous antigen,45,46 Hexabrachion,47 Cytotactin,48 J1220/20049 and neuronectin.50 Erickson & Inglesias introduced the name hexabrachion, refering to the structural composition of the TN-C molecule. TN-C consists of 6 polypeptide monomers which are combined into the hexamer at their N-termini.47 Each monomer of human TN-C comprises a cysteine-rich domain on the N-terminus followed by 14.5 EGF-like repeats. The EGF-like repeats are connected to 8 constitutively expressed fibronectin type III (FNIII) domains (1–8). In between the fifth and sixth FNIII domain up to 9 alternatively spliced FNIII domains (A1-A4, B, AD2, AD1, C, D) may be integrated. At the C-terminus a fibrinogen-like globe terminates each monomer (Fig. 1A).51-53 The alternative splicing of the FNIII domains leads to various isoforms which influence different cell types in varying manners, depending on the individual set up of FNIII domains.54,55
Figure 1.
Monomer structure of tenascin family members. A Tenascin-C, B Tenascin-R, C Tenascin-W, D Tenascin-X EGF/R epidermal growth factor; N-Cad, N-Cadherin; α/β, α/β-Integrin; GPC-1, Glypican-1; FGF/R, fibroblast growth factor; Syn-4, Syndecan-4; ANXII, Annexin II; Cont, Contactin, CASP; Contactin associated Protein; Cat, Catenin; PI3K, Phospho-Inositol-3-Kinase; GPα, G-Protein α; FAK, Focal adhesion kinase; PAX, Paxillin; PIP2, Phosphatidyl-Inositol-Biphosphate, α-Act, α-Actinin; PKC, Proteinkinase C.
Whereas in the adult the smallest isoform with only one alternatively spliced FNIII domain is found in static tissues (e.g., cartilage),56 the embryonic development57,58,59,60 as well as pathological situations like inflammation, regeneration or tumorigenesis61,62,42,63,60 is marked by the dominant expression of large isoforms.
By binding to integrins as their main receptor type TN-C affects the cell behavior in a direct way.43 Indirectly, TN-C acts via binding to other ECM molecules like brevican or neurocan.54,64 This leads to a variety of processes TN-C could be effective in: cell migration,65,66 inhibition of focal adhesion assembly,67 promotion of angiogenesis,43 increase in proliferation68,69 and changes in gene expression to modulate the composition of the ECM.70
The discovery in 1983 of TN-C as GMEM in glioblastoma tissue and glioma cell lines by Bourdon44 highlighted the interest of this protein as a characteristic component of tumors. Nearly all kinds of solid tumors express high levels of TN-C but the highest concentrations were found in glioma (Fig. 2).71 72 This is coupled to the correlation of a high TN-C expression with elevated malignancy and poor patients' survival.18,73,74
Figure 2.
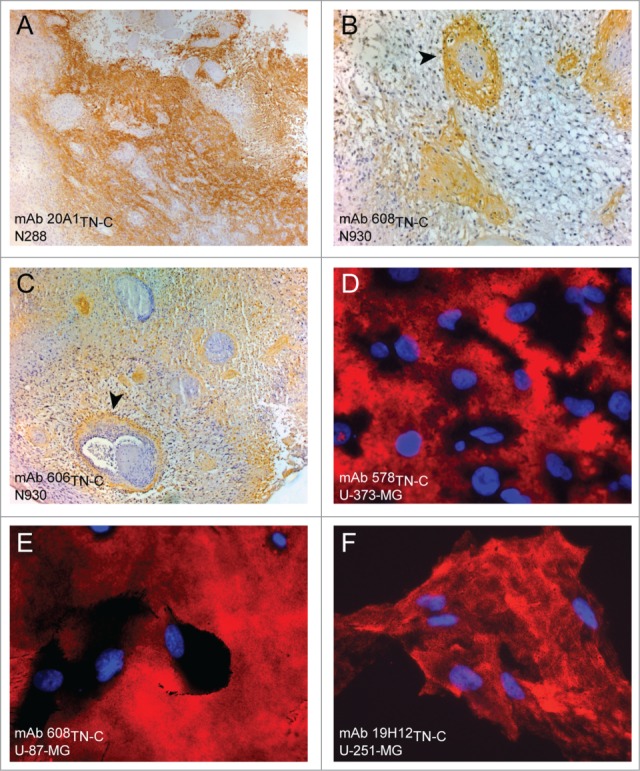
Tenascin-C strongly expressed in cells and tissues of glioblastoma multiforme (A–C) Staining of glioblastoma tissue specimen probed for Tenascin-C with monoclonal antibodies mAb 20A1TN-C, mAb 608TN-C and mAb606TN-C (D–F) Staining of glioblastoma cell lines examined with monoclonal anti-tenascin-C antibodies mAb 578TN-C, mAB 608TN-C and mAb 19H12TN-C.
The deleterious influence of TN-C could be associated with 3 main areas TN-C plays a crucial role in: angiogenesis, proliferation and cell migration. Each of these stands for prospects of high malignancy and in combination they represent the “evil face” of glioma.
TN-C in tumor angiogenesis
Glioma – like all other cancer types – are in need of receiving nutrients and disposing metabolic waste. Therefore they trigger the generation of new blood vessels from pre-existing vessels. In the last years it could be concordantly revealed that TN-C is highly associated with tumor blood vessels (Fig. 2A–C).38,75,76 The amount of TN-C in tumor blood vessels is correlated with the malignancy of the tumor as it is found in higher concentration in high-grade glioma than in low-grade glioma.75 Certainly, the blood vessels of glioma do not resemble the architecture of normal blood vessels. Within one tumor different phenotypes like incipient proliferation of endothelial cells or sarcomatous structures are found.77
Especially the alternatively spliced domains TNfnC78,79 and TNfnA238 could be found highly up-regulated in glioma blood vessels, but also the EGF-type repeats as well as the fibrinogen globe are supposed to play a role in the formation of tumor blood vessels.80 The TN-C-induced generation of tumor blood vessels could be related to the binding to endothelial cells81 and its stimulating effect on this cell type.82,83 Besides TGFbeta as an expected candidate involved in the underlying pathway,84 it could be revealed that the expression of VEGF is strongly correlated with the expression of tenascin-C in perivascular zones. Tanaka et al. discovered that TN-C regulates the expression of VEGF85 and Behrem et al. showed that TN-C has an influence on VEGF action and that the microvascular density shows a dependency of TN-C expression.86 Additionally to the high expression of TN-C in glioma blood vessels the known receptor for TN-C, integrin α v, is found in elevated levels in glioma tissue, as well as the protein periostin.77 Periostin was detected as a promoter of TN-C incorporation into the ECM and to organize the architecture of the ECM.87 For murine pancreatic neuroendocrine tumorigenesis it could be recently shown that the composition of upregulated matrisomal genes in pericytes could be correlated to genes overexpressed also in glioma. Furthermore an abrogation of TN-C from this matrix diminishes the number and proportion of angiogenic islets formed during the progression of this tumor. 88
TN-C in tumor cell proliferation
It has been convincingly proven that TN-C stimulates the proliferation of different cell types.86,89 Among them are not only endothelial cells with their importance for the tumor angiogenesis82,83 mentioned above, but also the glioma cells themselves.90-92 Until now only little is known about the detailed signaling pathways TN-C contributes to proliferation. Much less information has been obtained about the involvement of distinct domains of TN-C and their impact on individual signaling.86,93 Nonetheless some signaling pathways could be revealed that are involved in TN-C-stimulated proliferation. One known way to induce proliferation in glioma cells concerns the interaction between TN-C and fibronectin. By blocking the adhesion of cells to fibronectin the activation of syndecan-4 is impaired, which leads to proliferation of the cells caused by prevention of cell adhesion and spreading.94
In the alternatively spliced region of the FNIII-domains the highly overexpressed domains C, AD1 and AD2 in glioma79,95,96 correlate with the proliferation rate of cancer cells.97 More detailed information about underlying mechanisms of glioma proliferation has been obtained for the N-terminal EGF type domains69,98 and the C-terminal fibrinogen knob.97 These pathways including phospholipase Cγ1, Ras/MAPK, phosphatidyl inositol 3-kinase/Akt could be activated by binding of EGF domains to the EGF receptor69,99-101 and increase cell proliferation. In line with this observation the inhibition of the phosphatidyl-inositol-3-kinase/Akt pathway via modulated suppression or inhibition of the EGFR leads to a decrease in the proliferation of glioma cells.102,103 Additionally, the fibrinogen domain of TN-C plays a pivotal role in stimulating the proliferation of chondrocytes via the ERK/MAPK-pathway.104 In contrast, the combination of all FNIII-domains decreases the proliferative effect of intact TN-C on glioma cells.38
TN-C and tumor cell migration
The most fatal attribute of glioma is the capability to invade into healthy brain parenchyma as single cells to build new tumors.10 This leads to severe problems for therapeutic approaches because the secondary tumors could arise even in distant parts of the opposite hemisphere.1,10 Cell migration in general can be separated into 4 distinct steps. After an initial polarization step the cells generate cell protrusions and in the third step create new contacts with the surrounding matrix. Finally, the previous cell matrix contacts are disrupted.105 The ECM modulates this sequential mechanism.106,107 As known for other cells types also in glioma cells TN-C leads to a highly motile and invasive phenotype.108,109
The alternatively spliced domains of TN-C are thought to play a crucial role in the migratory behavior of glioma cells. Thus, the domain TNfnA2 has been reported to induce the generation of stress fibers and focal adhesion sites in dependency of β1 integrin110 – 2 highly important processes in the context of motility. Whereas the decrease of stress fibers and focal adhesion sites leads to weakening of the contractile forces, inducing a decrease in cell migration.111,112 For example, the initiation of contractile forces due to strengthened stress fibers and an augmentation of focal adhesion sites by TNfnA2 could support an increase in cell motility. The underlying pathways of these morphological changes influencing migration are related to the activation of the focal adhesion kinase (FAK) and the GTPases of the RhoA-subfamily.113
Another mechanism by which TNfnA2 may boost cell migration could be mediated by the transmembrane heparan sulfate proteoglycan syndecan-4. This proteoglycan favors the activation of a cryptic binding site in TNfnA2 resulting from the action of MMP-2110 and leads to changes in stress fibers and focal adhesion sites which can produce alterations in cell migration in mouse embryonic stem cells.114,115 Considering the high expression of TN-C71,72 and MMP-2116 in glioma cells this pathway is a possible candidate to induce glioma migration.
Not only the alternatively spliced domains contribute to cell migration but also the constitutively expressed domains such as TNfn3 are involved. This domain contains an RGD-sequence and could mediate cell adhesion via different integrins to modulate cell motility (reviewed in43). Yokosaki et al. have reported that the integrin α9β1 increases cell migration independently of the RGD-sequence.117
Another prominent pathway involved in cell migration includes signaling via the α2β1-integrin and can be neutralized by blocking this receptor.109 Possibly, this integrin represents a potential candidate for the still unknown signaling cascade that is activated by the EGF-type domains of TN-C and induces glioma cells migration.69 It is noteworthy that a HER2-specific phosphorylation of the EGFR could lead to an activation of this receptor and an N-Cadherin mediated stimulation of glioma cell migration.118 As the fibrinogen domain plays a role in the migration of vascular smooth muscle and bladder cancer cells through an ICAM-1-mediated pathway119,120 including Akt- and MAPK-dependent signaling, it is conceivable that the fibrinogen domain of TN-C exhibits analogous properties. This hypothesis, however, remains to be examined in future studies.
Tenascin-R
TN-R as a member of the tenascin family121,122 was first discovered in 1985 as “low molecular weight J1 glycoprotein (J1–160/ 80)”.49 Similar to TN-C also TN-R has originally been independently discovered in different species and designated with different names, that is janusin123,124 and restrictin.125 Structurally TN-R appears in 2 variants of the glycoprotein: TN-R 160 (160 kDa) and TN-R 180 (180 kDa) that differ by one alternatively spliced FNIII-domain.126 Each molecule starts at the N-terminus with a cysteine-rich region followed by 4.5 EGF-like repeats and 8 constitutively expressed FNIII domains, possibly supplemented with one alternatively spliced FNIII domain. At the C-terminus the molecule is completed with a fibrinogen like domain (Fig. 1B).127 The expression of TN-R is restricted to the nervous system. It could be found in motor neurons, on motor axons,125 in the hippocampus, cerebellum, olfactory bulb, myelinating oligodendrocytes as well as type-2 astrocytes.127
The function of TN-R depends on distinct ligands and receptors, for example chondroitinsulfate proteoglycans (CSPGs) of the lectican-family, the membrane-based part-time proteoglycan CALEB, or the Ig-superfamily-based receptor F3/contactin.128 The resulting effects comprise influences on cell adhesion, neural cell migration, the size of the extracellular space, regulation of cell-matrix interaction and axon outgrowth (extensively reviewed in128). Additionally the CS-GAG chains of TN-R are involved in an interaction with TN-C in a Ca2+-dependent manner and lead to a regulation of cell-matrix interactions in cases of tissue repair and neoplasms.128-130 Although studies on TN-R-deficient mice reveal that they have a normal life span and display only few histological aberrations with mild behavioral changes124,131,132 the outcome of EEG-examinations support the hypothesis that TN-R could play a crucial role in the development of epilepsy.128 A possible role for TN-R in other CNS diseases is suggested by the observation that TN-R is reduced in tissue samples from patients suffering from multiple sclerosis.133 Already in 1996 Carnemolla et al. have reported that TN-R is expressed in the healthy brain. Furthermore, TN-R was found in samples of human astrocytoma and meningioma, where the small isoform with one FNIII-repeat less amounted for 10% of the whole TN-R content.127
Studies on TN-R in glioma have in common that tumors with non-invasive behavior and/or grading into WHO I or II (e.g. pilocytic astrocytoma,134 medulloblastoma in children135) show a high expression of TN-R. In contrast, a decrease of TN-R expression has been observed in correlation with increasing malignancy. At the endpoint of glioma progression, that is in the glioblastoma of WHO grade IV only a weak TN-R expression could be detected. Whether there is an inverse correlation between the grade of malignancy and the expression level of TN-R and whether TN-R plays a role in the non-invasive behavior of tumors is still unclear and remains to be clarified further.134
Tenascin-W
Cloned from zebrafish and mice in the late 1990s136 and early 2000s,137,138 human TN-W was cloned and characterized in 2007.139 Like TN-C it appears in hexamers composed of 2 × 3 monomers. Each monomer starts with the typical cysteine-rich region at the N-terminus. 3.5 EGF-like domains are connected to this region, followed by varying FNIII-repeats (in mice: 12, in human: 9 repeats). The sequence ends with a fibrinogen-like globe at the C-terminus (Fig. 1C).138 Like the other tenascins TN-W is expressed during embryonic development and partially co-expressed with TN-C.136,138 In adulthood it is nearly absent from most types of tissues but could be found re-expressed under pathological conditions, especially in tumor development. In human cancer TN-W was found highly enriched in colon carcinoma and breast tumors, whereas the healthy tissue is devoid of any TN-W.139 In the special case of highly destructive brain tumors TN-W has been reported in elevated levels in astrocytoma, glioblastoma and oligodendroglioma while the healthy adult brain is devoid of TN-W expression.140
In contrast to the best-known tenascin family member TN-C, TN-W is not expressed by glioma cells, but exclusively by endothelial cells in tumor blood vessels, where it colocalizes with von-Willebrand-factor.140 TN-W could be associated with different cellular activities. Distinct from TN-C, TN-W is not able to mediate any adhesion to cells138-140 or to induce proliferation.141 TN-W-dependent signaling could partially be attributed to the activation of the β1-integrin subunit138,139 that is highly expressed in glioma tumors.142,143 Therefore, it is not surprising that TN-W is able to induce the migration of tumor139 as well as of endothelial cells.140 Concerning tumors of the glioma type investigations of the influence of TN-W on cell motility or angiogenesis are still outstanding but the previous results point to a potential role of TN-W also in this cancer type.
Tenascin-X
The latest discovered member of the tenascin family TN-X possesses the same molecular organization than the other family members TN-C, TN-R and TN-W. Initiated by a N-terminal cysteine-rich assembly domain, 18.5 EGF-like domains are attached and followed by 32 FNIII domains (human). The C-terminus is formed by a fibrinogen like globe (Fig. 1D). In contrast to the other tenascins, the TN-X molecule integrates a proline-rich stretch of about 100 amino acids.144 Via disulphide bonds TN-X is assembled into trimers145 and achieved prominence as it is related to the human heritable disorder named Ehlers-Danlos syndrome.146 The research on this syndrome has led to insights that TN-X is involved in network formation of the ECM as patients suffering from this disease show joint laxity and skin hyperextensibility.146,147
Found in many adult tissues TN-X plays a crucial role in the modulation of cell-matrix communication.145 By interacting with numerous ECM molecules like collagens148,149 or decorin150 it is involved in the adhesion and spreading of cells. The mechanisms behind these effects are still mostly unclear but 2 structures are thought to be part of the used signaling pathways: the C-terminal fibrinogen-like globe could interact with a still unknown integrin containing the β1 subunit whereas the signaling via heparansulfate proteoglycans might use the heparin binding site contained in the FNIII domains 10 and 11.151
Which of these signaling pathways – if any – is responsible for the inhibition of tumor cell invasion and metastasis152 is presently unknown. But considering the results by Hasegawa et al. showing that there is an inverse correlation between the expression level of TN-X and malignancy of glioma tumors153 there is rising evidence that TN-X could play a role in the invasion of glioma cells. Comparing the expression patterns of TN-C which is found in the intercellular spaces and in tumor blood vessels of high grade glioma38,153 and TN-X which is restricted to the tumor stroma and surrounds blood vessels153 the question remains whether TN-X is a potential key for the restricted invasiveness of low-grade glioma.
Conclusion
Considering the data discussed above there is no doubt that the family of tenascin proteins is involved in regulating the behavior of glioma cells and/or the development of glial tumors. Although TN-C is so far the most intensely examined member of the tenascin family, a substantial portion of its mechanisms of action and signaling pathways still remains unclear. In particular, the effects of the FNIII-domains on tumor cell migration and invasion as well as on angiogenesis remain to be clarified. Ongoing research in this direction is very encouraging and seems on the right track toward elucidation. Concerning the other members of the family only few studies have been published and further investigations are required to understand the contribution to glioma development and inhibition of increasing malignancy due to expression of TN-R, TN-W and TN-X.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
We acknowledge grant support by the Stem Cell Network Northrhine Westphalia, the German Research Foundation (DFG: SPP 1109, Fa 159/16-1, SFB 642, SPP 1757, GRK 736, GSC 98/1), The German Ministry of Education, Research and Technology (BMBF 01GN0503) and the Ruhr-University (President's special program call 2008).
References
- 1. Wen PY, Kesari S. Malignant gliomas in adults. New Engl J Med 2008; 359:492-507; PMID:18669428; http://dx.doi.org/ 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114:97-109; PMID:17618441; http://dx.doi.org/ 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol (Berl) 2005; 109:93-108; PMID:15685439; http://dx.doi.org/ 10.1007/s00401-005-0991-y [DOI] [PubMed] [Google Scholar]
- 4. Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist 2006; 11:681-93; PMID:16794247; http://dx.doi.org/ 10.1634/theoncologist.11-6-681 [DOI] [PubMed] [Google Scholar]
- 5. Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, et al. Long-term survival with glioblastoma multiforme. Brain 2007; 130:2596-606; PMID:17785346; http://dx.doi.org/ 10.1093/brain/awm204 [DOI] [PubMed] [Google Scholar]
- 6. Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 2009; 18:1061-83; PMID:19555299; http://dx.doi.org/ 10.1517/13543780903052764 [DOI] [PubMed] [Google Scholar]
- 7. Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res 2004; 64:6892-9; PMID:15466178; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1337 [DOI] [PubMed] [Google Scholar]
- 8. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol 2007; 170:1445-53; PMID:17456751; http://dx.doi.org/ 10.2353/ajpath.2007.070011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujisawa H, Reis RM, Nakamura M, Colella S, Yonekawa Y, Kleihues P, Ohgaki H. Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest 2000; 80:65-72; ; http://dx.doi.org/ 10.1038/labinvest.3780009 [DOI] [PubMed] [Google Scholar]
- 10. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol 2007; 114:443-58; PMID:17805551; http://dx.doi.org/ 10.1007/s00401-007-0293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H, Adamson C. Glioblastoma multiforme oncogenomics and signaling pathways. Clin Med Oncol 2009; 3:39-52; PMID:19777070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riemenschneider MJ, Reifenberger G. Molecular neuropathology of gliomas. Int J Mol Sci 2009; 10:184-212; PMID:19333441; http://dx.doi.org/ 10.3390/ijms10010184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jovcevska I, Kocevar N, Komel R. Glioma and glioblastoma – how much do we (not) know? Mol Clin Oncol 2013; 1:935-41; PMID:24649273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulte A, Gunther HS, Martens T, Zapf S, Riethdorf S, Wulfing C, Stoupiec M, Westphal M, Lamszus K. Glioblastoma stem-like cell lines with either maintenance or loss of high-level EGFR amplification, generated via modulation of ligand concentration. Clin Cancer Res 2012; 18:1901-13; PMID:22316604; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3084 [DOI] [PubMed] [Google Scholar]
- 15. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res 2013; 19:764-72; PMID:23209033; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3002 [DOI] [PubMed] [Google Scholar]
- 16. Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol: Off J Am Soc Clin Oncol 2003; 21:1624-36; PMID:12697889; http://dx.doi.org/ 10.1200/JCO.2003.05.063 [DOI] [PubMed] [Google Scholar]
- 17. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444:756-60; PMID:17051156; http://dx.doi.org/ 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 18. Rascher G, Fischmann A, Kröger S, Duffner F, Grote E-H, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol (Berl) 2002; 104:85-91; PMID:12070669; http://dx.doi.org/ 10.1007/s00401-002-0524-x [DOI] [PubMed] [Google Scholar]
- 19. Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery 1996; 39:235-50; discussion 50-2; PMID:8832660; http://dx.doi.org/ 10.1097/00006123-199608000-00001 [DOI] [PubMed] [Google Scholar]
- 20. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326:1216-9; PMID:19965464; http://dx.doi.org/ 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aumailley M, Gayraud B. Structure and biological activity of the extracellular matrix. J Mol Med 1998; 76:253-65; PMID:9535559; http://dx.doi.org/ 10.1007/s001090050215 [DOI] [PubMed] [Google Scholar]
- 22. Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol 2003; 200:488-99; PMID:12845616; http://dx.doi.org/ 10.1002/path.1415 [DOI] [PubMed] [Google Scholar]
- 23. Chintala SK, Gokaslan ZL, Go Y, Sawaya R, Nicolson GL, Rao JS. Role of extracellular matrix proteins in regulation of human glioma cell invasion in vitro. Clin Exp Metast 1996; 14:358-66; PMID:8878410; http://dx.doi.org/ 10.1007/BF00123395 [DOI] [PubMed] [Google Scholar]
- 24. Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol 2007; 213:565-73; PMID:17680633; http://dx.doi.org/ 10.1002/jcp.21237 [DOI] [PubMed] [Google Scholar]
- 25. Rauch U. Brain matrix: structure, turnover and necessity. Biochem Soc Trans 2007; 35:656-60; PMID:17635114; http://dx.doi.org/ 10.1042/BST0350656 [DOI] [PubMed] [Google Scholar]
- 26. Mentlein R, Hattermann K, Held-Feindt J. Lost in disruption: role of proteases in glioma invasion and progression. Biochim Biophys Acta 2012; 1825:178-85; PMID:22209868 [DOI] [PubMed] [Google Scholar]
- 27. Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol 2003; 200:423-8; PMID:12845610; http://dx.doi.org/ 10.1002/path.1437 [DOI] [PubMed] [Google Scholar]
- 28. Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009; 324:1673-7; PMID:19556500; http://dx.doi.org/ 10.1126/science.1171643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 2009; 10:21-33; PMID:19197329; http://dx.doi.org/ 10.1038/nrm2593 [DOI] [PubMed] [Google Scholar]
- 30. Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 2010; 341:126-40; PMID:19854168; http://dx.doi.org/ 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giese A, Rief MD, Loo MA, Berens ME. Determinants of human astrocytoma migration. Cancer Res 1994; 54:3897-904; PMID:8033113 [PubMed] [Google Scholar]
- 32. Berens ME, Rief MD, Loo MA, Giese A. The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Metast 1994; 12:405-15; PMID:7923993; http://dx.doi.org/ 10.1007/BF01755884 [DOI] [PubMed] [Google Scholar]
- 33. Giese A, Loo MA, Norman SA, Treasurywala S, Berens ME. Contrasting migratory response of astrocytoma cells to tenascin mediated by different integrins. J Cell Sci 1996; 109 (Pt 8):2161-8; PMID:8856512 [DOI] [PubMed] [Google Scholar]
- 34. Mahesparan R, Tysnes BB, Read TA, Enger PO, Bjerkvig R, Lund-Johansen M. Extracellular matrix-induced cell migration from glioblastoma biopsy specimens in vitro. Acta Neuropathol 1999; 97:231-9; PMID:10090669; http://dx.doi.org/ 10.1007/s004010050979 [DOI] [PubMed] [Google Scholar]
- 35. Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol (Berl) 2003; 105:49-57. [DOI] [PubMed] [Google Scholar]
- 36. Rutka JT, Myatt CA, Giblin JR, Davis RL, Rosenblum ML. Distribution of extracellular matrix proteins in primary human brain tumours: an immunohistochemical analysis. Can J Neurol Sci 1987; 14:25-30; PMID:3028590 [DOI] [PubMed] [Google Scholar]
- 37. Giordana MT, Bradac GB, Pagni CA, Marino S, Attanasio A. Primary diffuse leptomeningeal gliomatosis with anaplastic features. Acta Neurochir (Wien) 1995; 132:154-9; PMID:7754854; http://dx.doi.org/ 10.1007/BF01404866 [DOI] [PubMed] [Google Scholar]
- 38. Brosicke N, van Landeghem FK, Scheffler B, Faissner A. Tenascin-C is expressed by human glioma in vivo and shows a strong association with tumor blood vessels. Cell Tissue Res 2013; 354:409-30; PMID:23963648; http://dx.doi.org/ 10.1007/s00441-013-1704-9 [DOI] [PubMed] [Google Scholar]
- 39. Knott JC, Mahesparan R, Garcia-Cabrera I, Bolge Tysnes B, Edvardsen K, Ness GO, Mork S, Lund-Johansen M, Bjerkvig R. Stimulation of extracellular matrix components in the normal brain by invading glioma cells. Int J Cancer J Int Du Cancer 1998; 75:864-72; PMID:9506531; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19980316)75:6%3c864::AID-IJC8%3e3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- 40. Saito Y, Shiota Y, Nishisaka M, Owaki T, Shimamura M, Fukai F. Inhibition of angiogenesis by a tenascin-c peptide which is capable of activating beta1-integrins. Biol Pharm Bull 2008; 31:1003-7; PMID:18451535; http://dx.doi.org/; http://dx.doi.org/ 10.1248/bpb.31.1003 [DOI] [PubMed] [Google Scholar]
- 41. Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathol 2005; 110:435-42; PMID:16175354; http://dx.doi.org/ 10.1007/s00401-005-1078-5 [DOI] [PubMed] [Google Scholar]
- 42. Latijnhouwers MA, de Jongh GJ, Bergers M, de Rooij MJ, Schalkwijk J. Expression of tenascin-C splice variants by human skin cells. Arch Dermatol Res 2000; 292:446-54; PMID:11000288; http://dx.doi.org/ 10.1007/s004030000152 [DOI] [PubMed] [Google Scholar]
- 43. Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett 2006; 244:143-63; PMID:16632194; http://dx.doi.org/ 10.1016/j.canlet.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 44. Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res 1983; 43:2796-805; PMID:6342760 [PubMed] [Google Scholar]
- 45. Chiquet M, Fambrough DM. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol 1984; 98:1926-36; PMID:6725406; http://dx.doi.org/ 10.1083/jcb.98.6.1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiquet M, Fambrough DM. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol 1984; 98:1937-46; PMID:6202699; http://dx.doi.org/ 10.1083/jcb.98.6.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erickson HP, Inglesias JL. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature 1984; 311:267-9; PMID:6482952; http://dx.doi.org/ 10.1038/311267a0 [DOI] [PubMed] [Google Scholar]
- 48. Grumet M, Hoffman S, Crossin KL, Edelman GM. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A 1985; 82:8075-9; PMID:2415980; http://dx.doi.org/ 10.1073/pnas.82.23.8075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kruse J, Keilhauer G, Faissner A, Timpl R, Schachner M. The J1 glycoprotein-a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature 1985; 316:146-8; PMID:2409452; http://dx.doi.org/ 10.1038/316146a0 [DOI] [PubMed] [Google Scholar]
- 50. Rettig WJ, Triche TJ, Garin-Chesa P. Stimulation of human neuronectin secretion by brain-derived growth factors. Brain Res 1989; 487:171-7; PMID:2752284; http://dx.doi.org/ 10.1016/0006-8993(89)90954-2 [DOI] [PubMed] [Google Scholar]
- 51. Aukhil I, Slemp CC, Lightner VA, Nishimura K, Briscoe G, Erickson HP. Purification of hexabrachion (tenascin) from cell culture conditioned medium, and separation from a cell adhesion factor. Matrix 1990; 10:98-111; PMID:1695709; http://dx.doi.org/ 10.1016/S0934-8832(11)80176-9 [DOI] [PubMed] [Google Scholar]
- 52. Jones PL, Jones FS. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol 2000; 19:581-96; PMID:11102748; http://dx.doi.org/ 10.1016/S0945-053X(00)00106-2 [DOI] [PubMed] [Google Scholar]
- 53. Tsunoda T, Inada H, Kalembeyi I, Imanaka-Yoshida K, Sakakibara M, Okada R, Katsuta K, Sakakura T, Majima Y, Yoshida T. Involvement of large tenascin-C splice variants in breast cancer progression. Am J Pathol 2003; 162:1857-67; PMID:12759243; http://dx.doi.org/ 10.1016/S0002-9440(10)64320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adams M, Jones JL, Walker RA, Pringle JH, Bell SC. Changes in tenascin-C isoform expression in invasive and preinvasive breast disease. Cancer Res 2002; 62:3289-97; PMID:12036947 [PubMed] [Google Scholar]
- 55. Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, Shaw JA, Walker RA, Pringle JH, Jones JL. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res: BCR 2009; 11:R24; PMID:19405959; http://dx.doi.org/ 10.1186/bcr2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mackie EJ, Tucker RP. Tenascin in bone morphogenesis: expression by osteoblasts and cell type-specific expression of splice variants. J Cell Science 1992; 103 (Pt 3):765-71; PMID:1282516 [DOI] [PubMed] [Google Scholar]
- 57. Joester A, Faissner A. Evidence for combinatorial variability of tenascin-C isoforms and developmental regulation in the mouse central nervous system. J Biol Chem 1999; 274:17144-51; PMID:10358070; http://dx.doi.org/ 10.1074/jbc.274.24.17144 [DOI] [PubMed] [Google Scholar]
- 58. Maseruka H, Ridgway A, Tullo A, Bonshek R. Developmental changes in patterns of expression of tenascin-C variants in the human cornea. Invest Ophthalmol Visual Sci 2000; 41:4101-7; PMID:11095602 [PubMed] [Google Scholar]
- 59. Sahlberg C, Aukhil I, Thesleff I. Tenascin-C in developing mouse teeth: expression of splice variants and stimulation by TGFbeta and FGF. Eur J Oral Sci 2001; 109:114-24; PMID:11347655; http://dx.doi.org/ 10.1034/j.1600-0722.2001.00990.x [DOI] [PubMed] [Google Scholar]
- 60. Galoian KA, Garamszegi N, Garamszegi SP, Scully SP. Molecular mechanism of tenascin-C action on matrix metalloproteinase-1 invasive potential. Exp Biol Med (Maywood) 2007; 232:515-22; PMID:17392487 [PubMed] [Google Scholar]
- 61. Borsi L, Carnemolla B, Nicolo G, Spina B, Tanara G, Zardi L. Expression of different tenascin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer J Int Du Cancer 1992; 52:688-92; PMID:1385335; http://dx.doi.org/ 10.1002/ijc.2910520504 [DOI] [PubMed] [Google Scholar]
- 62. Hindermann W, Berndt A, Borsi L, Luo X, Hyckel P, Katenkamp D, Kosmehl H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol 1999; 189:475-80; PMID:10629546; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199912)189:4%3c475::AID-PATH462%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 63. Ghert MA, Jung ST, Qi W, Harrelson JM, Erickson HP, Block JA, Scully SP. The clinical significance of tenascin-C splice variant expression in chondrosarcoma. Oncology 2001; 61:306-14; PMID:11721178; http://dx.doi.org/ 10.1159/000055338 [DOI] [PubMed] [Google Scholar]
- 64. Day JM, Olin AI, Murdoch AD, Canfield A, Sasaki T, Timpl R, Hardingham TE, Aspberg A. Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J Biol Chem 2004; 279:12511-8; PMID:14722076; http://dx.doi.org/ 10.1074/jbc.M400242200 [DOI] [PubMed] [Google Scholar]
- 65. Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 2007; 35:695-7; PMID:17635125; http://dx.doi.org/ 10.1042/BST0350695 [DOI] [PubMed] [Google Scholar]
- 66. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009; 17:153-62; PMID:19320882; http://dx.doi.org/ 10.1111/j.1524-475X.2009.00466.x [DOI] [PubMed] [Google Scholar]
- 67. Murphy-Ullrich JE, Lightner VA, Aukhil I, Yan YZ, Erickson HP, Höök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol 1991; 115:1127-36; PMID:1720121; http://dx.doi.org/ 10.1083/jcb.115.4.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell 1996; 7:883-92; PMID:8816995; http://dx.doi.org/ 10.1091/mbc.7.6.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 2001; 154:459-68; PMID:11470832; http://dx.doi.org/ 10.1083/jcb.200103103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tremble P, Chiquet-Ehrismann R, Werb Z. The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol Biol Cell 1994; 5:439-53; PMID:7519905; http://dx.doi.org/ 10.1091/mbc.5.4.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zalutsky MR, Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, McLendon RE, Wong TZ, Bigner DD. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med: Off Pub, Soc Nucl Med 2008; 49:30-8; PMID:18077533; http://dx.doi.org/ 10.2967/jnumed.107.046938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M, Mergenthaler S, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res 2009; 69:458-65; PMID:19147558; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2610 [DOI] [PubMed] [Google Scholar]
- 73. Leins A, Riva P, Lindstedt R, Davidoff MS, Mehraein P, Weis S. Expression of tenascin-C in various human brain tumors and its relevance for survival in patients with astrocytoma. Cancer 2003; 98:2430-9; PMID:14635078; http://dx.doi.org/ 10.1002/cncr.11796 [DOI] [PubMed] [Google Scholar]
- 74. Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner HH. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer J Int Du Cancer 2002; 98:362-9; PMID:11920587; http://dx.doi.org/ 10.1002/ijc.10233 [DOI] [PubMed] [Google Scholar]
- 75. Kim CH, Bak KH, Kim YS, Kim JM, Ko Y, Oh SJ, Kim KM, Hong EK. Expression of tenascin-C in astrocytic tumors: its relevance to proliferation and angiogenesis. Surg Neurol 2000; 54:235-40; PMID:11118570; http://dx.doi.org/ 10.1016/S0090-3019(00)00307-4 [DOI] [PubMed] [Google Scholar]
- 76. Martina E, Chiquet-Ehrismann R, Brellier F. Tenascin-W: an extracellular matrix protein associated with osteogenesis and cancer. Int J Biochem Cell Biol 2010; 42:1412-5; PMID:20541035; http://dx.doi.org/ 10.1016/j.biocel.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 77. Mustafa DA, Dekker LJ, Stingl C, Kremer A, Stoop M, Sillevis Smitt PA, Kros JM, Luider TM. A proteome comparison between physiological angiogenesis and angiogenesis in glioblastoma. Mol Cell Proteomics 2012; 11:M111 008466; PMID:22278369; http://dx.doi.org/ 10.1074/mcp.M111.008466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Silacci M, Brack SS, Spath N, Buck A, Hillinger S, Arni S, Weder W, Zardi L, Neri D. Human monoclonal antibodies to domain C of tenascin-C selectively target solid tumors in vivo. Protein Eng Des Sel 2006; 19:471-8; PMID:16928692; http://dx.doi.org/ 10.1093/protein/gzl033 [DOI] [PubMed] [Google Scholar]
- 79. Carnemolla B, Castellani P, Ponassi M, Borsi L, Urbini S, Nicolo G, Dorcaratto A, Viale G, Winter G, Neri D, et al. Identification of a glioblastoma-associated tenascin-C isoform by a high affinity recombinant antibody. Am J Pathol 1999; 154:1345-52; PMID:10329587; http://dx.doi.org/; http://dx.doi.org/ 10.1016/S0002-9440(10)65388-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol 2000; 50:121-37; PMID:11245272; http://dx.doi.org/ 10.1023/A:1006436624862 [DOI] [PubMed] [Google Scholar]
- 81. Prieto AL, Andersson-Fisone C, Crossin KL. Characterization of multiple adhesive and counteradhesive domains in the extracellular matrix protein cytotactin. J Cell Biol 1992; 119:663-78; PMID:1383239; http://dx.doi.org/ 10.1083/jcb.119.3.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol 1994; 126:539-48; PMID:7518469; http://dx.doi.org/ 10.1083/jcb.126.2.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci 1995; 108 (Pt 2):797-809; PMID:7539439 [DOI] [PubMed] [Google Scholar]
- 84. Orlova VV, Liu Z, Goumans MJ, ten Dijke P. Controlling angiogenesis by two unique TGF-beta type I receptor signaling pathways. Histol Histopathol 2011; 26:1219-30; PMID:21751154 [DOI] [PubMed] [Google Scholar]
- 85. Tanaka K, Hiraiwa N, Hashimoto H, Yamazaki Y, Kusakabe M. Tenascin-C regulates angiogenesis in tumor through the regulation of vascular endothelial growth factor expression. Int J Cancer J Int Du Cancer 2004; 108:31-40; PMID:14618612; http://dx.doi.org/ 10.1002/ijc.11509 [DOI] [PubMed] [Google Scholar]
- 86. Behrem S, Zarkovic K, Eskinja N, Jonjic N. Distribution pattern of tenascin-C in glioblastoma: correlation with angiogenesis and tumor cell proliferation. Pathol Oncol Res 2005; 11:229-35; PMID:16388320; http://dx.doi.org/ 10.1007/BF02893856 [DOI] [PubMed] [Google Scholar]
- 87. Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 2010; 285:2028-39; PMID:19887451; http://dx.doi.org/ 10.1074/jbc.M109.051961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Langlois B, Saupe F, Rupp T, Arnold C, van der Heyden M, Orend G, Hussenet T. AngioMatrix, a signature of the tumor angiogenic switch-specific matrisome, correlates with poor prognosis for glioma and colorectal cancer patients. Oncotarget 2014; 5:10529-45; PMID:25301723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bronner-Fraser M. Distribution and function of tenascin during cranial neural crest development in the chick. J Neurosci Res 1988; 21:135-47; PMID:2464073; http://dx.doi.org/ 10.1002/jnr.490210206 [DOI] [PubMed] [Google Scholar]
- 90. Lange K, Kammerer M, Hegi ME, Grotegut S, Dittmann A, Huang W, Fluri E, Yip GW, Gotte M, Ruiz C, et al. Endothelin receptor type B counteracts tenascin-C-induced endothelin receptor type A-dependent focal adhesion and actin stress fiber disorganization. Cancer Res 2007; 67:6163-73; PMID:17616673; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-3348 [DOI] [PubMed] [Google Scholar]
- 91. Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell 2004; 15:5670-7; PMID:15483051; http://dx.doi.org/ 10.1091/mbc.E04-08-0759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Erickson HP, Bourdon MA. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Ann Rev Cell Biol 1989; 5:71-92; PMID:2480799; http://dx.doi.org/ 10.1146/annurev.cb.05.110189.000443 [DOI] [PubMed] [Google Scholar]
- 93. Orend G. Potential oncogenic action of tenascin-C in tumorigenesis. Int J Biochem Cell Biol 2005; 37:1066-83; PMID:15743679; http://dx.doi.org/ 10.1016/j.biocel.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 94. Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res 2001; 61:8586-94; PMID:11731446 [PubMed] [Google Scholar]
- 95. Derr LB, Chiquet-Ehrismann R, Gandour-Edwards R, Spence J, Tucker RP. The expression of tenascin-C with the AD1 variable repeat in embryonic tissues, cell lines and tumors in various vertebrate species. Differentiation 1997; 62:71-82; PMID:9404002; http://dx.doi.org/ 10.1046/j.1432-0436.1997.6220071.x [DOI] [PubMed] [Google Scholar]
- 96. Mighell AJ, Thompson J, Hume WJ, Markham AF, Robinson PA. Human tenascin-C: identification of a novel type III repeat in oral cancer and of novel splice variants in normal, malignant and reactive oral mucosae. Int J Cancer J Int Du Cancer 1997; 72:236-40; PMID:9219826; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970717)72:2%3c236::AID-IJC6%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 97. Dueck M, Riedl S, Hinz U, Tandara A, Möller P, Herfarth C, Faissner A. Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. Int J Cancer J Int Du Cancer 1999; 82:477-83; PMID:10404058; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19990812)82:4%3c477::AID-IJC2%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 98. Iyer AK, Tran KT, Borysenko CW, Cascio M, Camacho CJ, Blair HC, Bahar I, Wells A. Tenascin cytotactin epidermal growth factor-like repeat binds epidermal growth factor receptor with low affinity. J Cell Physiol 2007; 211:748-58; PMID:17311283; http://dx.doi.org/ 10.1002/jcp.20986 [DOI] [PubMed] [Google Scholar]
- 99. Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 1998; 10:262-7; PMID:9561851; http://dx.doi.org/ 10.1016/S0955-0674(98)80149-X [DOI] [PubMed] [Google Scholar]
- 100. Pennock S, Wang Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol Cell Biol 2003; 23:5803-15; PMID:12897150; http://dx.doi.org/ 10.1128/MCB.23.16.5803-5815.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Benzil DL, Finkelstein SD, Epstein MH, Finch PW. Expression pattern of alpha-protein kinase C in human astrocytomas indicates a role in malignant progression. Cancer Res 1992; 52:2951-6; PMID:1316231 [PubMed] [Google Scholar]
- 102. Liu H, Zhou L, Shi S, Wang Y, Ni X, Xiao F, Wang S, Li P, Ding K. Oligosaccharide G19 inhibits U-87 MG human glioma cells growth in vitro and in vivo by targeting epidermal growth factor (EGF) and activating p53/p21 signaling. Glycobiology 2014; 24:748-65; PMID:24799378; http://dx.doi.org/ 10.1093/glycob/cwu038 [DOI] [PubMed] [Google Scholar]
- 103. Ramis G, Thomas-Moya E, Fernandez de Mattos S, Rodriguez J, Villalonga P. EGFR inhibition in glioma cells modulates Rho signaling to inhibit cell motility and invasion and cooperates with temozolomide to reduce cell growth. PLoS One 2012; 7:e38770; PMID:22701710; http://dx.doi.org/ 10.1371/journal.pone.0038770 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104. Murphy LI, Fischer D, Chiquet-Ehrismann R, Mackie EJ. Tenascin-C induced stimulation of chondrogenesis is dependent on the presence of the C-terminal fibrinogen-like globular domain. FEBS Lett 2000; 480:189-92; PMID:11034326; http://dx.doi.org/ 10.1016/S0014-5793(00)01936-0 [DOI] [PubMed] [Google Scholar]
- 105. Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704-9; PMID:14657486; http://dx.doi.org/ 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- 106. Heino J, Kapyla J. Cellular receptors of extracellular matrix molecules. Curr Pharm Des 2009; 15:1309-17; PMID:19355970; http://dx.doi.org/ 10.2174/138161209787846720 [DOI] [PubMed] [Google Scholar]
- 107. Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res 2009; 339:83-92; PMID:19921267; http://dx.doi.org/ 10.1007/s00441-009-0892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hirata E, Arakawa Y, Shirahata M, Yamaguchi M, Kishi Y, Okada T, Takahashi JA, Matsuda M, Hashimoto N. Endogenous tenascin-C enhances glioblastoma invasion with reactive change of surrounding brain tissue. Cancer Sci 2009; 100:1451-9; PMID:19459858; http://dx.doi.org/ 10.1111/j.1349-7006.2009.01189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Deryugina EI, Bourdon MA. Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci 1996; 109 (Pt 3):643-52; PMID:8907709 [DOI] [PubMed] [Google Scholar]
- 110. Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, et al. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J Biol Chem 2007; 282:34929-37; PMID:17901052; http://dx.doi.org/ 10.1074/jbc.M705608200 [DOI] [PubMed] [Google Scholar]
- 111. Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res 2005; 65:10930-7; PMID:16322240; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1890 [DOI] [PubMed] [Google Scholar]
- 112. Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol 2009; 185:357-70; PMID:19364917; http://dx.doi.org/ 10.1083/jcb.200809110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Midwood KS, Schwarzbauer JE. Tenascin-C modulates matrix contraction via focal adhesion kinase- and rho-mediated signaling pathways. Mol Biol Cell 2002; 13:3601-13; PMID:12388760; http://dx.doi.org/ 10.1091/mbc.E02-05-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest 2001; 107:R9-R14; PMID:11160142; http://dx.doi.org/ 10.1172/JCI10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, Mercurio AM, Couchman JR, Wewer UM. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J Biol Chem 2003; 278:9576-84; PMID:12509413; http://dx.doi.org/ 10.1074/jbc.M208937200 [DOI] [PubMed] [Google Scholar]
- 116. Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Brit J Cancer 1999; 79:1828-35; PMID:10206300; http://dx.doi.org/ 10.1038/sj.bjc.6990291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, Shigeto N, Chen J, Sheppard D. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem 1998; 273:11423-8; PMID:9565552; http://dx.doi.org/ 10.1074/jbc.273.19.11423 [DOI] [PubMed] [Google Scholar]
- 118. Rappl A, Piontek G, Schlegel J. EGFR-dependent migration of glial cells is mediated by reorganisation of N-cadherin. J Cell Sci 2008; 121:4089-97; PMID:19033391; http://dx.doi.org/ 10.1242/jcs.027995 [DOI] [PubMed] [Google Scholar]
- 119. Rauch BH, Muschenborn B, Braun M, Weber AA, Schror K. ICAM-1 and p38 MAPK mediate fibrinogen-induced migration of human vascular smooth muscle cells. Eur J Pharmacol 2007; 577:54-7; PMID:17904546; http://dx.doi.org/ 10.1016/j.ejphar.2007.08.041 [DOI] [PubMed] [Google Scholar]
- 120. Roche Y, Pasquier D, Rambeaud JJ, Seigneurin D, Duperray A. Fibrinogen mediates bladder cancer cell migration in an ICAM-1-dependent pathway. Thromb Haemost 2003; 89:1089-97; PMID:12783123 [PubMed] [Google Scholar]
- 121. Erickson HP. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr Opin Cell Biol 1993; 5:869-76; PMID:7694605; http://dx.doi.org/ 10.1016/0955-0674(93)90037-Q [DOI] [PubMed] [Google Scholar]
- 122. Chiquet-Ehrismann R. Tenascins, a growing family of extracellular matrix proteins. Experientia 1995; 51:853-62; PMID:7556567; http://dx.doi.org/ 10.1007/BF01921736 [DOI] [PubMed] [Google Scholar]
- 123. Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron 1993; 10:69-82; PMID:7678967; http://dx.doi.org/ 10.1016/0896-6273(93)90243-K [DOI] [PubMed] [Google Scholar]
- 124. Pesheva P, Probstmeier R. The yin and yang of tenascin-R in CNS development and pathology. Prog Neurobiol 2000; 61:465-93; PMID:10748320; http://dx.doi.org/ 10.1016/S0301-0082(99)00061-1 [DOI] [PubMed] [Google Scholar]
- 125. Rathjen FG, Wolff JM, Chiquet-Ehrismann R. Restrictin: a chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development 1991; 113:151-64; PMID:1764992 [DOI] [PubMed] [Google Scholar]
- 126. Woodworth A, Pesheva P, Fiete D, Baenziger JU. Neuronal-specific synthesis and glycosylation of tenascin-R. J Biol Chem 2004; 279:10413-21; PMID:14681222; http://dx.doi.org/ 10.1074/jbc.M312466200 [DOI] [PubMed] [Google Scholar]
- 127. Carnemolla B, Leprini A, Borsi L, Querze G, Urbini S, Zardi L. Human tenascin-R. Complete primary structure, pre-mRNA alternative splicing and gene localization on chromosome 1q23-q24. J Biol Chem 1996; 271:8157-60; PMID:8626505; http://dx.doi.org/ 10.1074/jbc.271.14.8157 [DOI] [PubMed] [Google Scholar]
- 128. Anlar B, Gunel-Ozcan A. Tenascin-R: role in the central nervous system. Int J Biochem Cell Biol 2012; 44:1385-9; PMID:22634605; http://dx.doi.org/ 10.1016/j.biocel.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 129. Aukhil I, Joshi P, Yan Y, Erickson HP. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem 1993; 268:2542-53; PMID:7679097 [PubMed] [Google Scholar]
- 130. Weber P, Zimmermann DR, Winterhalter KH, Vaughan L. Tenascin-C binds heparin by its fibronectin type III domain five. J Biol Chem 1995; 270:4619-23; PMID:7533163; http://dx.doi.org/ 10.1074/jbc.270.9.4619 [DOI] [PubMed] [Google Scholar]
- 131. Bruckner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 2000; 428:616-29; PMID:11077416; http://dx.doi.org/ 10.1002/1096-9861(20001225)428:4%3c616::AID-CNE3%3e3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 132. Gurevicius K, Gureviciene I, Valjakka A, Schachner M, Tanila H. Enhanced cortical and hippocampal neuronal excitability in mice deficient in the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci 2004; 25:515-23; PMID:15033179; http://dx.doi.org/ 10.1016/j.mcn.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 133. Gutowski NJ, Newcombe J, Cuzner ML. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol Appl Neurobiol 1999; 25:207-14; PMID:10417662; http://dx.doi.org/ 10.1046/j.1365-2990.1999.00176.x [DOI] [PubMed] [Google Scholar]
- 134. El Ayachi I, Baeza N, Fernandez C, Colin C, Scavarda D, Pesheva P, Figarella-Branger D. KIAA0510, the 3'-untranslated region of the tenascin-R gene, and tenascin-R are overexpressed in pilocytic astrocytomas. Neuropathol Appl Neurobiol 2010; 36:399-410; PMID:20202125; http://dx.doi.org/ 10.1111/j.1365-2990.2010.01074.x [DOI] [PubMed] [Google Scholar]
- 135. Park PC, Taylor MD, Mainprize TG, Becker LE, Ho M, Dura WT, Squire J, Rutka JT. Transcriptional profiling of medulloblastoma in children. J Neurosurg 2003; 99:534-41; PMID:12959442; http://dx.doi.org/ 10.3171/jns.2003.99.3.0534 [DOI] [PubMed] [Google Scholar]
- 136. Weber P, Montag D, Schachner M, Bernhardt RR. Zebrafish tenascin-W, a new member of the tenascin family. J Neurobiol 1998; 35:1-16; PMID:9552162; http://dx.doi.org/ 10.1002/(SICI)1097-4695(199804)35:1%3c1::AID-NEU1%3e3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- 137. Neidhardt J, Fehr S, Kutsche M, Lohler J, Schachner M. Tenascin-N: characterization of a novel member of the tenascin family that mediates neurite repulsion from hippocampal explants. Mol Cell Neurosci 2003; 23:193-209; PMID:12812753; http://dx.doi.org/ 10.1016/S1044-7431(03)00012-5 [DOI] [PubMed] [Google Scholar]
- 138. Scherberich A, Tucker RP, Samandari E, Brown-Luedi M, Martin D, Chiquet-Ehrismann R. Murine tenascin-W: a novel mammalian tenascin expressed in kidney and at sites of bone and smooth muscle development. J Cell Sci 2004; 117:571-81; PMID:14709716; http://dx.doi.org/ 10.1242/jcs.00867 [DOI] [PubMed] [Google Scholar]
- 139. Degen M, Brellier F, Kain R, Ruiz C, Terracciano L, Orend G, Chiquet-Ehrismann R. Tenascin-W is a novel marker for activated tumor stroma in low-grade human breast cancer and influences cell behavior. Cancer Res 2007; 67:9169-79; PMID:17909022; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0666 [DOI] [PubMed] [Google Scholar]
- 140. Martina E, Degen M, Ruegg C, Merlo A, Lino MM, Chiquet-Ehrismann R, Brellier F. Tenascin-W is a specific marker of glioma-associated blood vessels and stimulates angiogenesis in vitro. Faseb J 2010; 24:778-87; PMID:19884327; http://dx.doi.org/ 10.1096/fj.09-140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres A-C, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-alpha induced expression in vitro. Oncogene 2005; 24:1525-32; PMID:15592496; http://dx.doi.org/ 10.1038/sj.onc.1208342 [DOI] [PubMed] [Google Scholar]
- 142. Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol 1993; 143:154-63; PMID:8317546 [PMC free article] [PubMed] [Google Scholar]
- 143. Gingras MC, Roussel E, Bruner JM, Branch CD, Moser RP. Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. J Neuroimmunol 1995; 57:143-53; PMID:7535788; http://dx.doi.org/ 10.1016/0165-5728(94)00178-Q [DOI] [PubMed] [Google Scholar]
- 144. Tucker RP, Drabikowski K, Hess JF, Ferralli J, Chiquet-Ehrismann R, Adams JC. Phylogenetic analysis of the tenascin gene family: evidence of origin early in the chordate lineage. BMC Evol Biol 2006; 6:60; PMID:16893461; http://dx.doi.org/ 10.1186/1471-2148-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J Cell Biol 2014; 205:409-28; PMID:24821840; http://dx.doi.org/ 10.1083/jcb.201308031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schalkwijk J, Zweers MC, Steijlen PM, Dean WB, Taylor G, van Vlijmen IM, van Haren B, Miller WL, Bristow J. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. New Engl J Med 2001; 345:1167-75; PMID:11642233; http://dx.doi.org/ 10.1056/NEJMoa002939 [DOI] [PubMed] [Google Scholar]
- 147. Margaron Y, Bostan L, Exposito JY, Malbouyres M, Trunfio-Sfarghiu AM, Berthier Y, Lethias C. Tenascin-X increases the stiffness of collagen gels without affecting fibrillogenesis. Biophys Chem 2010; 147:87-91; PMID:20089348; http://dx.doi.org/ 10.1016/j.bpc.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 148. Veit G, Hansen U, Keene DR, Bruckner P, Chiquet-Ehrismann R, Chiquet M, Koch M. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem 2006; 281:27461-70; PMID:16861231; http://dx.doi.org/ 10.1074/jbc.M603147200 [DOI] [PubMed] [Google Scholar]
- 149. Egging D, van den Berkmortel F, Taylor G, Bristow J, Schalkwijk J. Interactions of human tenascin-X domains with dermal extracellular matrix molecules. Arch Dermatol Res 2007; 298:389-96; PMID:17033827; http://dx.doi.org/ 10.1007/s00403-006-0706-9 [DOI] [PubMed] [Google Scholar]
- 150. Elefteriou F, Exposito JY, Garrone R, Lethias C. Binding of tenascin-X to decorin. FEBS Lett 2001; 495:44-7; PMID:11322944; http://dx.doi.org/ 10.1016/S0014-5793(01)02361-4 [DOI] [PubMed] [Google Scholar]
- 151. Lethias C, Elefteriou F, Parsiegla G, Exposito JY, Garrone R. Identification and characterization of a conformational heparin-binding site involving two fibronectin type III modules of bovine tenascin-X. J Biol Chem 2001; 276:16432-8; PMID:11278641; http://dx.doi.org/ 10.1074/jbc.M010210200 [DOI] [PubMed] [Google Scholar]
- 152. Matsumoto K, Takayama N, Ohnishi J, Ohnishi E, Shirayoshi Y, Nakatsuji N, Ariga H. Tumour invasion and metastasis are promoted in mice deficient in tenascin-X. Genes Cells 2001; 6:1101-11; PMID:11737270; http://dx.doi.org/ 10.1046/j.1365-2443.2001.00482.x [DOI] [PubMed] [Google Scholar]
- 153. Hasegawa K, Yoshida T, Matsumoto K, Katsuta K, Waga S, Sakakura T. Differential expression of tenascin-C and tenascin-X in human astrocytomas. Acta Neuropathol 1997; 93:431-7; PMID:9144580; http://dx.doi.org/ 10.1007/s004010050636 [DOI] [PubMed] [Google Scholar]



