Abstract
It is generally agreed that a bacteriophage-associated phenomenon was first unambiguously observed one-hundred years ago with the findings of Twort in 1915. This was independently followed by complementary observations by d'Hérelle in 1917. D'Hérelle's appreciation of the bacteriophage phenomenon appears to have directly led to the development of phages as antibacterial agents within a variety of contexts, including medical and agricultural. Phage use to combat nuisance bacteria appears to be especially useful where targets are sufficiently problematic, suitably bactericidal phages exist, and alternative approaches are lacking in effectiveness, availability, safety, or cost effectiveness, etc. Phage development as antibacterial agents has been strongest particularly when antibiotics have been less available or useful, e.g., such as in the treatment of chronic infections by antibiotic-resistant bacteria. One relatively under-explored or at least not highly reported use of phages as therapeutic agents has been to combat bacterial infections of the lungs and associated tissues. These infections are diverse in terms of their etiologies, manifestations, and also in terms of potential strategies of phage delivery. Here I review the literature considering the phage therapy of pulmonary and pulmonary-related infections, with emphasis on reports of clinical treatment along with experimental treatment of pulmonary infections using animal models.
Keywords: lung infection, phage therapy, pharmacology, pleural infection, pulmonary infection
Introduction
There exists a diversity of efforts to employ phages as antibacterial agents within a variety of contexts. In general terms these efforts can be described as a form of antibacterial biological control,1 a.k.a., biocontrol. Within especially clinical or veterinary contexts, however, the phrase phage therapy is prefered.2 Historically, phage use to combat bacterial infections has displayed a negative association with antibiotic availability, with phage therapy development most robust when antibiotics either did not exist, could provide less than satisfactory efficacy, or have been deemed to be unsuitable for other reasons. For example, phage therapy development was particularly robust in the Soviet Union, first as a consequence of limited access to Western-developed antibiotics and later perhaps as a means of distinguishing Soviet medical practices from those of the West. For a history of phage use particularly to combat human disease, see for example.3-6
A resurgence of interest in the use of bacterial viruses to combat bacterial infections has occurred over the past 20 or so years, stemming especially from concerns over bacterial evolution of antibiotic resistance.7,8 For over 50 years, half the time that phages have been known, phages have been suggested as alternatives to antibiotics in light of the evolution of antibiotic resistance in bacteria;9 see also Krestovikova.10 Hoeflmayr, more specifically, was reporting on a clinical study of phage treatment of pulmonary infections. In another publication that considers in part phage treatment of pulmonary infections, and citing mainly French-language publications from the early 1960s, “a revised interest in phage therapy” is reported.11 Here I review generally the use of phages as treatments of lung and lung-associated bacterial infections. This is in terms of reports of phage use to treat infections within clinical settings as well as their application within the context of animal disease models. Though not covered here, there exist two related areas of study, that of phage delivery as aerosols into lungs12,13 and the use of phages to combat the air sac-associated bacterial disease of poultry, colibacillosis, e.g., 14; see also section 2.2 of 15.
Pulmonary Phage Therapy, General Principles
Phage therapy is the application of phages essentially as drugs to treat bacterial infections. Phage use as antibacterial agents consequently can benefit from a pharmacological perspective, which I introduce here generally before delving into considerations that are more specific to pulmonary treatment using phages. For further detailed presentation of the more general aspects of phage therapy pharmacology, see 15-21.
In pharmacology one can speak of body “compartments”. Pharmacokinetically, the entrance of a drug into the blood (absorption) involves drug movement into the body compartment that is the blood. Since blood mixes as it circulates throughout the body, it is a fairly homogeneous compartment with regard to drug concentration. Movement of a drug out of the blood, and into non-blood tissues, known pharmacokinetically as distribution, is movement from one compartment to another. As an alternative to this movement into and then out of the blood, it is possible in many instances to treat an infection topically, that is with drug application directly to the site of infection rather than first into the blood.
The utility of topical application is at least 3-fold. First, it is not always possible to deliver drugs by other means. Second, direct application of a drug to its targets can more efficiently increase drug concentrations within the vicinity of those targets than less direct strategies of drug application. Third, the drug levels attained via distribution to specific targets, following systemic application, can be lower than those found within the vicinity of topical treatments or instead that are supplied to infections via direct injection. Higher drug densities at the point of drug utility given more localized drug application in particular can result in a greater potential for successful treatment and may be achieved without simultaneously building up densities systemically to toxic levels.
Inundative densities of virions may thus be more easily as well as more safely achieved given topical application. These inundative densities may be attained particularly in the course of more “passive” phage therapy strategies16-21 versus dosing strategies that rely instead on active, in situ phage replication to achieve inundative phage densities. Contrasting topical phage dosing, parenteral or per os dosing can be more reliant on the latter, that is, so-called “active” treatment strategies, since achievement of inundative phage densities “passively” can be difficult without direct physical application of phages to target bacteria. See Figure 1 for summary particularly of the pharmacokinetic justifications for topical vs. systemic phage application.
Figure 1.
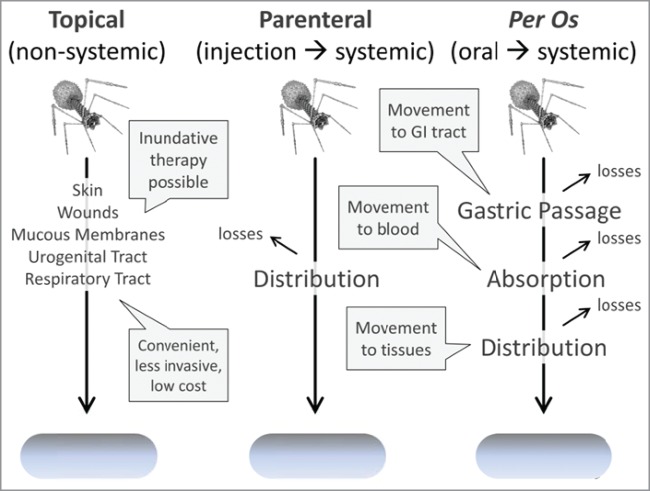
Pharmacokinetics of different phage application strategies. Inefficiencies of phage movement from one body compartment to another can serve to limit the potential for phages to effect an inundative therapy. Both parenteral and per os dosing result initially in systemic circulation. This absorption is expected to be more efficient given application into the body via parenteral dosing, that is, there is an expectation of less phage loss with parenteral application in comparison to oral delivery. With per os dosing, by contrast, phages must first survive and then move out the gastrointestinal tract, both of which are not straightforward processes. Per os in the hands of different researchers also does not appear to consistently occur with phages, so possesses some controversy. Topical application by contrast avoids losses associated with absorption and distribution, increasing the potential for local achievement of inundative phage densities. Direct injection of phages into sites of bacterial infection (not shown) similarly avoids issues of adsorption and distribution though is more invasive than topical application.
Treatment of pulmonary infections using phages is interesting in part because there can exist a potential for topical phage delivery, systemic phage delivery, or both, and, in some instances, direct injection of phages into bacterial infections as well. As noted, with topical delivery there is an expectation that passive, inundation therapy will at least be possible. With systemic delivery, by contrast, there is an expectation that phages not only must be able to pass out of the blood and into lung tissue to reach sites of infection but also that phages, once in contact with target bacteria, will be able to replicate to sufficient densities that subsequent inundation is possible. Both of these latter properties of systemic phage delivery are not mechanistically well understood for lung infections but nonetheless are likely consequences, at least in part, of the the to-be-phage-treated bacterial infection itself. Bacterial infection of lungs, that is, may give rise to increased tissue permeability, e.g., as can be seen in terms of the blood-brain barrier22 (see also 20 and, as summarized below, particularly by 23). This may potentially allow for more effective phage distribution from the blood into infection-damaged lungs, and the presence of sufficient bacterial hosts within the lungs also may support phage population growth to higher densities once phages have reached those bacteria.
With topical delivery to the lungs such as may be achieved in the course of phage inhalation therapy,12,13 both of these factors – modified phage penetration properties along with phage population growth – also can come into play. Modification of phage penetration properties, however, may not occur in the same manner as with systemic delivery. In particular, phage delivery into the lumen of the lungs, unless phages are delivered directly into the lungs by injection, may be less efficient within diseased versus healthy lungs.24,25 This potentially lower prospect for distribution within diseased lungs given inhalation therapy contrasts with instead the potentially increased possibility of phage movement out of the blood into diseased vs. healthy lung tissue (above). Delivered phages as a result could be more spatially heterogeneously present within the lungs, relative to the location of target bacteria, given inhalation versus systemic phage delivery. Even given topical delivery of substantial phage numbers into lungs via inhalation therapy, successful bacterial eradication thus may still require in situ phage replication to locally achieve inundative densities, including toward deeper phage penetration into more bacteria-laden, diseased portions of lungs. Topical application to wounds or direct phage injection into the sites of bacterial infection, by contrast, should supply phages more directly to target bacteria than aerosol penetration into the lungs, so may not display equivalent pharmacokinetic idiosyncrasies.
In any case, it is a reasonable expectation that greater phage penetration into the lungs to infecting bacteria may be achieved given repeated phage dosing rather than relying on a single phage application, given phage application via multiple routes rather than relying on just one, given phage in situ replication once they have reached target bacteria, and perhaps also with phages exogenously supplied to bacterial infections over longer time spans (e.g., weeks). These various concepts – including requirements for phage penetration to target bacteria, achievement of sufficient phage numbers within the vicinity of target bacteria, and also adequate phage bactericidal activity against target bacteria once reached – are worth keeping in mind as one considers examples of treatment of pulmonary infections in both humans and in terms of disease models in animals.
Pulmonary Phage Therapy, Examples of Human Treatment
The literature on phage treatment of pulmonary infections in humans as well as that using animal disease models is not extensive. Here I consider this literature, dividing it into 2 categories, human treatment and, in the section that follows, animal experimentation. Universally these studies are ones in which at least some positive effect had been noted, and though no publications presenting solely negative phage therapy results targeting pulmonary infections were identified, this does not imply that all phage therapy efforts by all researchers and clinicians have been successful. Nonetheless, the studies reviewed present results that to at least a first approximation are consistent with phage therapy-associated, anti-bacterial infection treatment efficacy. Note that, in cases of non-English-language publications, I review as based on a combination of secondary sources as indicated and, as possible, employing machine translations of the actual publications, with checks by a speaker in the case of French and Russian translations (and with original passages provided as footnotes). I present a summary of a number of these studies in Table 1.
Table 1.
Summary of Clinical Phage Therapy of Pulmonary Infections
| Reference | Year | Treatment of… | Etiology | Delivery | Efficacya (# cases) |
|---|---|---|---|---|---|
| Morrison and Gardner26 | 1936 | Bronchial fistula | “a colon bacillus” | Topical | 100% (1) |
| Shishenko28 | 1938 | Newborns and infants | Staphylococcus (other?) | Topical | b |
| Cevey and Schwiez29 | 1958 | Pleuropulmonary perforation-associated pleural empyema | Staphylococcus | Topical | 100% (1) |
| Delacoste33 | 1959 | Refractory coughs | N.A.c | Inhalation | 100% (19)d |
| Hoeflmayr9 | 1962 | Bronchitis | Streptococci (2/3); Staphylococci (1/3) | Inhalation | 90% (29) |
| Garsevanishvili34 | 1974 | Pneumonia | Staphylococci, streptococci, and enterics were targeted | Inhalation | e (189) |
| Nikolaeva35 | 1974 | Lung infection and pneumonia | Staphylococci | Inhalation | f |
| Sakandelidze and Meipariani36 | 1974 | Lung abscesses and bronchiectasis | Staphylococcus aureus, Proteus, and Streptococci | Subcutaneous or topical | 92% |
| Ioseliani et al.38 | 1980 | Lung infections | Staphylococci and/or others | Inhalation, topicalg | >90% (45) |
| Meladze et al.40 | 1982 | Parenchyma and pleura infections | Staphylococcus | Inhalation, topical, parenteral | >90% (223) |
| Chkhetia41 | 1984 | Recovery from lung operations | N.A. | Topical, parenteral | >90% (107)h |
| Clinical Trials27 | 1985 | Various lung infections | N.A. | N.A. | 77% (60); 90% (61)i |
| Ślopek et al.42 | 1987 | Various lung infections | Escherichia, Klebsiella, Proteus, Pseudomonas, Staphylococcus, and Streptococcus | Topical, per os | 87% (202) |
| Weber-Dabrowska et al.47 | 2000 | Various lung infections | E. coli, Klebsiella, Proteus, Pseudomonas, S. aureus | Topical, per os | >90% (376) |
| Kvachadze et al.32 | 2011 | Cystic fibrosis-associated infections | Staphylococcus and Pseudomonas | Inhalation | j |
Approximately equivalent to percentage “++” or higher as found in Figure 2 through Figure 5.
At least one apparent success of 3 cases of pleuritis.
Information not available.
No total failures.
Degree of success is not easily discerned from the publication of this study.
There was “general improvement in the condition” in treatment of lung abscesses.
“Applying bacteriophage with antibiotics by inhalation, catheterization, bronchoscopy and to the pleural cavity…” (p. 66)
Phages in combination with antibiotics versus 80% (45) for antibiotic treatment alone.
Phage treatment along (“ISVP”) versus phages plus antibiotics (“ISVP + ABP”), respectively.
50% reduction in required ongoing antibiotic treatment.
Morrison and Gardner26 published in 1936 a case study of phage treatment of a lung infection that followed an appendectomy (p. 33): “A bronchial fistula developed shortly after the rib resection and the patient was expectorating the same kind of material as that which drained from the resection wound. The appearance of the area around the resection opening was necrotic and ‘mossy’ and failed to show any improvement on local irrigations with 1,000 cc of saline solution twice a day.” Phage treatment resulted in remarkable improvement and eventual cure, with phage dosing directly to the infection rather than via inhalation or other means of phage application. Given the level of detail supplied, I quote extensively (p. 33):
After a cutaneous test September 20 of 0.1 cc of the lytic filtrate twelve hours previously had given little or no reaction, and after irrigating the chest with 1 liter of physiologic solution of sodium chloride, 1 ounce (30 cc) of the phage was instilled and allowed to remain for 2 h. This was followed by saline irrigation and the wound covered by a dressing saturated with the bacteriophage. The following day the observation was made that the discharge had become thin and watery and had lost its offensive character for the first time since the resection was done five days before, even though saline irrigations had been administered twice daily during this five day period. A second and equally remarkable change had occurred at the resection wound itself, where the mossy, necrotic character was entirely changed to a clean, fresh, healthy appearing incision. Since the first use of bacteriophage had given such excellent results, a second application seemed indicated, and therefore the procedure was repeated. However, within ten minutes a violent generalized rose-colored urticaria appeared and the patient complained of nausea and vomited. The bacteriophage was drained immediately and the chest irrigated with large quantities of saline solution. Epinephrine was administered and the eruption cleared within an hour. The patient, among others known to one of the authors, reacted to the epinephrine with a thready pulse and cold and clammy skin. The epinephrine reaction, however, completely subsided in the course of another hour. After such a marked allergic reaction to the bacteriophage had occurred it was decided to discontinue bacteriophage instillations and continue only with saline irrigations and external dressings saturated with bacteriophage. The dressings of bacteriophage were continued for a week along with irrigations of physiologic solution of sodium chloride. Throughout this period the resection wound maintained its healthy normal appearance and the discharge remained clear, watery and nonodorous. The temperature reached 102.2 F. each day for the thirteen days prior to the urticarial reaction. On that day the reading was 103.2 F. after the reaction. After this reaction the temperature did not go above 102.2 F. ¶ The patient's general condition was remarkably improved and within six weeks she was able to leave the hospital. The appendiceal wound had healed but the fever, less hectic in type, continued as well as the thin nonodorous drainage. At home the fever gradually subsided as well as the drainage, and healing was practically complete toward the end of December.
Note the use of multiple, topical dosings along with overall phage treatment duration of approximately one week, but nevertheless the rapid, positive impact of treatment on infection signs following the initial phage application.
Chanishvili27 (p. 42) discusses an article by Shishenko28 published in 1938: “According to his observations the application of phages even for newborns and infants in high doses (3.0-5.0 ml per injection into the pleural cavity or around the abscesses repeated 3-5 times) did not result in any side effects, such as increase in temperature, development of infiltrations at the sites of injection, etc. … The author recommended that special attention be given to the application of phage therapy for the treatment of suppurative pleuritis since this disease predominated in newborns and was fatal in up to 90% of cases. Shishenko assumed that application of phages by lung puncture directly into the site of infection would mean no further surgical procedure would be required.” One case history of treatment of Shishenko's treatment of pleuritis is presented. A Staphylococcus infection following thoracotomy was treated thus (p. 43): “3.0 ml of Staphylo-phage was introduced through the drainage. The effect was outstanding. Since the next day no pus was evacuated any longer.” Subsequent infection approximately 2 weeks later with Streptococcus was treated with the same phage but without success, which presumably would be expected given the use of a “Staphylo” phage. Note the use of topical dosings along with the rapid, positive impact on infection signs.
Cevey and Schwiez29 described in 1958 the phage treatment of a pleural empyema due to antibiotic-resistant bacteria in a patient who previously had active tuberculosis. The patient had been treated with a number of antibiotics and other procedures (from p. 37)A : “almost all regular and specific antibiotics, left-side thoracoplasty, perforation, drainage, lavages, etc.” The patient's condition was described as “very bad” with fever between 38°and 39.5°C. A pleurectomy and additional thoracoplasty were performed to attempt to close the pleuropulmonary perforation. Despite continuing antibiotic treatment, the patient remained febrile with approximately 300 ml/day of pus drainage. Phage treatment was then commenced with an instillation of 2 ml of phage active against the infecting staphylococci, following washing of the pleural pocket with sterile saline. This was followed by an increase in the patient's fever to 39.6°C, as also occurred during the following 2 instillations, but not during ensuing phage application. Over subsequent days the volume of phage applied was increased, the patient felt better, declined in fever, and displayed less drainage of pus, down to roughly 50 ml/day after 15 d of treatment. By three weeks the amount of pus was less than 10 ml/d. Improvement was slow but steady over the following months (p. 38)B : “the patient gradually regained weight and could get up.” They note that (pp. 38–39)C , “In conclusion we believe that in these cases of pyogenic infection resistant to antibiotics, it is worthwhile to immediately consider a combined treatment of antibiotics and stock phage that should be replaced later by an autophage whose action is more effective.” “Autophage” generally is defined as a bacteriophage that has been isolated against a bacterium derived from a patient's own flora, i.e., the specific to-be-treated pathogen.30–32 Note the use of topical phage application, use in association with antibiotics, and multiple dosing along with the multi-week duration of phage treatment.
Delacoste33 described in 1959 the successful treatment of refractory cough, delivering phages using a nebulizer.12 Phage treatment was attempted in part because it was felt that phage therapy at least would not be expected to harm the patient but also, I infer, because (p. 562)D “cough[s were] more or less refractory to conventional therapy”. It is not apparent, however, that conventional treatments in fact were performed prior to phage application. Cases were reported in moderate detail and in all but one case a commercial phage preparation was employed throughout the reported treatment. From p. 562E : “The results, as regards the cough may be characterized as very favorable. In a series of 19 cases, I have observed no total failure. There was complete cure in 15 cases; one case had a bronchial reinfection that resolved by the same method (case 17); and there was significant improvement in three cases. By cure, I mean complete resolution of the cough and resolution of the purulent character of sputum when it existed. In all cases, concomitant symptoms have disappeared…” In terms of side effects (p. 562)F : “On one occasion, the product triggered febrile reactions with urticaria (case 15). This reaction was probably allergic in nature, to the components of the drug, maybe the albumin in the medium… but could be prevented by prophylactic administration of antihistamines. I did not notice other intolerances.” Note the use of inhalation therapy for phage application.
Hoeflmayr9 achieved 90% cure rates, in 1962, against bronchitis also using a nebulizer.12 A 1963 partial translation of this paper is available here: http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U-2&docGetTRDoc.pdf&AD=AD0837021. Employed was a polyvalent phage lysate reportedly containing 180 to 200 “different phage strains” (p. 404)G . Also present in these formulations were what are described as (p. 404)H “so-called targeted antimicrobials added to combat those bacteria which have a primary phage resistance” and which presumably should be viewed as non-phage antibacterial substances. As noted, treatment was of bronchitis, particularly chronic bronchitis that had not responded to non-phage treatments. Infecting bacteria consisted of either streptococci (2 thirds of cases) or staphylococci (one third). Treatments were daily for 10 to 15 minutes and were repeated for what I interpret as up to 40 d (with an average of 11 d). It is indicated that 55% of patients were “cured” while another 35% were “improved”, for 90% cure or improvement rate (of a total of 29 cases). In terms of side effects, the author notes that (p. 408)I “We have initially aerosolized [phages] undiluted and occasionally observed after the first session low-grade fever. We assume that it is a response to decomposition products of the bacteria. … Later we used a dilution with physiological NaCl to 1: 5 and saw no side effects.” Note the use of inhalation therapy for phage application as well as multiple dosing and week or more overall durations of phage treatments.
Garsevanishvili34 employed, in 1974, a nebulizer as well.12 Specifically, polyvalent phages were used to treat pneumonia via inhalation therapy in children (153 with more severe and 36 with more localized pneumonia). The phages targeted staphylococci (55% of phages present), streptococci (30% of phages), and enterics (i.e., 15% coliphages). The author in conclusion recommends phage therapy for treatment of staphylococcal pneumonia, and does so in part from the perspective of a perceived lack of associated side effects. Also recommended was phage therapy as a means of eliminating coccal secondary infections associated with viral pneumonia. Note the use of inhalation therapy for phage application, which is reported to have taken place over a total of 10 sessions.
An additional summary provided by Chanishvili27 (p. 39) is that of Nikolaeva,35 also as published in 1974: “…the author summarizes experience in the application of polyvalent Staphylococcal phage in clinics in Gorkij (now Nizhniy Novgorod, Russia) and Moscow. Phage preparations were applied in 430 cases of staphylococcal infection. … ¶ The phage was applied by inhalation in cases of staphylococcal destruction of lungs and in cases of staphylococcal pneumonia. … ¶ Phage therapy of lung abscesses led to general improvement in the condition with normalization of temperature and lessening of pus in the sputum. After 7-10 d of phage therapy, infiltration of lung tissue was reduced and abscess became dry and decreased in size. ¶ Nikolaeva35 indicated that best therapeutic effect was achieved when phage therapy was started early.” Note the use of inhalation therapy for phage application along with the at-least one week or more durations of phage treatment.
Sakandelidze and Meipariani,36 as described in their 1974 publication, delivered phages to infections that included lung abscesses, inflammation as associated with bronchiectasis, and also a variety of other, non-pulmonary infections and inflammatory conditions. Infections were associated with Staphylococcus aureus in 98 patients, Proteus in 88, and streptococci in 16. Sulakvelidze et al.37 provide this summary (p. 652): “Phages administered subcutaneously or through surgical drains in 236 patients having antibiotic-resistant infections eliminated the infections in 92% of the patients.” Sakandelidze and Meipariani conclude that (p. 136)J “The data obtained suggest that the bacteriophage is an effective drug that is active against the vast majority of strains of Streptococcus, Staphylococcus and Proteus, and it can be widely used in medical practice in mixed diseases.” Sakandelidze and Meipariani36 state further (p. 136)K that phage use, “doesn't give rise to any side effects that are observed when antibiotic is used (dysbacteriosis, allergic reactions, the formation of the resistant cultures, etc.).”
Ioseliani et al.38 used combinations of polyvalent phages and antibiotics to treat lung infections, as published in 1980.12,37,39 Among other methods, mention is made of application of phages and/or antibiotics via “inhalation”. An English-language abstract is included with the article, from which I quote (p. 67):
A total of 45 patients with chronic pulmonary suppurations who had been subjected to resection were examined. A specific bacteriophage with antibiotics was applied for preoperative preparation and prophylaxis of postoperative acute empyemas. The inoculated flora was sensitive to bacteriophage in 86.6 % and to 14 antibiotics in 72 %. After intrapleural administration of the phage with antibiotics the authors noted a decrease in the pathogenicity of microbes, an increase in the sensitivity to antibiotics, a reduced number of microbes associations, but in certain cases — sterility of a pleural exudate. The results of prevention of acute postresectional pleural empyemas with bacteriophage combined with antibiotics were compared with those obtained in the treatment of patients who had not used bacteriophage. A fall in the percentage of purulent pleural complications from 18.7 to 6.7 % was noted. Intrapleural administration of 5–50 ml of a specific bacteriophage with antibiotics did not produce side-effects.
Noted as well is that upon surgical treatment, of 107 patients not treated with bacteriophage, 23 (21.49%) experienced complications and 8 (7.47%) died, whereas among 45 patients who were phage treated (in addition to treatment with antibiotics), 5 (11.11%) experienced complications and none died. Overall for the 45 treated with phages and antibiotics (p. 64)L , “28 (62.2%) patients achieved stable remission with positive clinical and radiographic shifts and removal of purulent intoxication. In 14 (31.1%) patients significantly decreased the number of purulent sputum, and there was a noticeable positive radiological dynamics. In 3 (6.6%) patients sputum although diminished, but it was fetid…”
Meladze et al.40 treated parenchyma and pleura Staphylococcus infections in 1982.39 Their description of phage administration is informative (p. 53)M :
Staphylococcal bacteriophage used both separately and in combination with antibiotics. … Bacteriophage administered topically as — to form endobronchial sanitation (inhalation, catheterization of the trachea, bronchoscopy) directly into purulent lesion in the lung or pleural cavity (transthoracic puncture and catheterization [of] purulent foci) and parenterally — intramuscularly, intravenously, through prolonged perfusion in bronchial or pulmonary artery. [The] quantity of bacteriophage was limited by the place of administration: for endobronchial sanitations — 10–30 ml, directly in suppurative lesion in the lung parenchyma — 10–50 ml in empyema cavity — 20–100 ml parenteral — 0.5–1.0 ml/kg. Duration of phage therapy depended on clinical and radiological effect and is 2–4 weeks.
A total of 223 patients were treated with bacteriophages vs. 117 patients who received only antibiotics. What the authors describe as (p. 54)N “Stable remission and, in some cases, recovery” was achieved in 82.9% of phage-treated patients versus 64.1% without phages. A “relative remission” (p. 54)O was seen in a further 12.5% of patients with phage treatment vs. 29.1% without. Either “death or aggravation of purulent process” (p. 54)P occurred in 4.5% of phage-treated patients versus 12% without. The authors note as well that (p. 56)Q “Topical and parenteral use of staphylococcal bacteriophage with antibiotics does not cause negative side effects. When intravenous infusions of staphylococcal bacteriophages [were administrated] at a dose of 0.5–1 ml/kg no allergic or pyrogenic reactions were observed, only in rare cases the temperature increased by 0.3–0.6 ° C.” A substantial amount of immunological characterization during treatment also was undertaken. Chanishvili 27, p. 179, provides a translation of specific results that I present in Figure 2. Note the multi-week duration of treatments.
Figure 2.
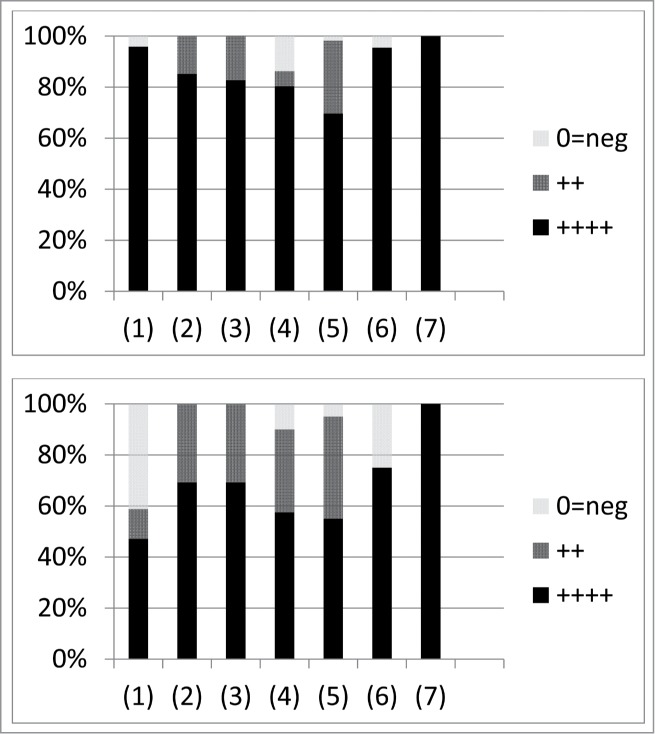
Results of lung treatment by Meladze et al.40 as translated and summarized by Chanishvili.27 Numbers found along the x axis correspond to (1) “Acute lung abscess” (24 and 17 cases for the phage therapy experimental group and the control group, respectively), (2) “Bronchoextatic [sic] disease” (27 and 13), (3) “Chronic festering bronchitis” (29 and 13), (4) “Chronic lung abscess” (51 and 40), (5) “Chronic pneumonia” (56 and 20), (6) “Pleural empyema” (22 and 8), and (7) “Suppurating lung cyst” (11 and 9). The top panel shows phage treatment (both with and without antibiotic treatment) and the bottom antibiotic therapy only. In the key, “++++” corresponds to “Stable remission”, “++” to “Comparative remission”, and “0=neg” to “Consequences, lethal outcome”.
Associated with the work of Meladze et al.40 is a 1984 dissertation which Chanishvili27 summarizes (p. 181):
Dr. Nugzar Chkhetia41 described the results of treating 152 patients recovering from lung operations. A control group (Gr. I) of 107 patients was treated with antibiotics alone and 45 patients (Gr. II) received antibiotics together with phages. Remission and stabilization of the suppuration process in the experimental group (Gr. II) was observed in 93.3% of cases, whilst in the control group (Gr. I) it was 80.4%. The frequency of the post operational re-infection of the pleural cavity was 23.7% in the experimental group (Gr. II), compared to the control group (Gr. I) of patients where the re-infection frequency was as high as 67.3%. No lethal outcomes were observed in the experimental Group II, while in the group treated with antibiotics alone a lethal outcome was observed in 8.4% of cases… ¶ Administration of the phage preparation was performed through various routes including local administration (tampons, bathing of cavities), inhalation, oral administration and also via the parenteral mode, including intramuscular and intravenous injections. Phage preparations were administered as liquid preparations or as aerosols. The phage dose varied between 10ml to 150ml. The author recommended performing post-operational treatment by intra-pleural administration of phages. In this case the phage could be administered either via a drainage tube or by puncture. … Prior to phage administration the pleural cavity should be released of exudates by aspiration and then washed with a sterile saline solution mixed with painkiller. Topical application of bacteriophage therapy did not cause any side effects. The duration of the topical phage therapy depended on the speed of the healing process, however according to the author it should not exceed 20 days.41
Chanishvili27 also notes (p. 175) with regard to Chkhetia,41 “For treatment of lung infections intravenous administration was combined with local treatment (washing of lungs)”. Note the diversity of application strategies employed. In addition, an approximately 3-week limit on the duration of treatments is suggested.
On page 176 of Chanishvili27 there is a table that is titled, “Summarized results of phage-therapy of different types of diseases (according to the Report of Clinical Trials, 1985).” This is found in the chapter titled, “Phage therapy against septic infections”. The description from the text indicates that the table “summarizes the results of treatment performed by application of IVSP [IntraVenous Staphylococcal Phage]. As the table shows, the results of phage therapy alone were better than those of antibiotic therapy. However, the best therapeutic outcome was achieved in the case of complex therapy performed with phages and antibiotics.” The lung-associated treatments include “Acute lung abscess”, “Chronic lung abscess”, “Chronic pneumonia”, “Chronic bronchitis”, and “Bronchiectasis”. In Figure 3 I summarize the results of ISVP treatment alone, ISVP + ABP treatment, and ABP treatment alone. ABP in Chanishvili27 stands for (p. 174) “antibacterial preparations commonly used in the medical practice (antibiotics, etc.).” These practices are not otherwise specified.
Figure 3.
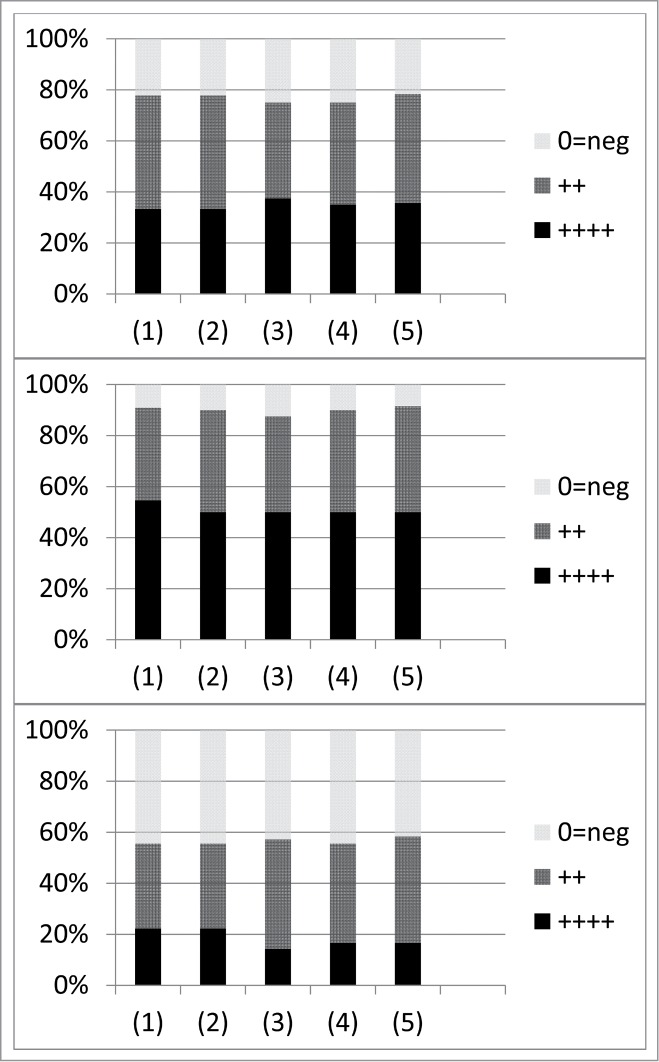
Summary of lung treatment of Staphylococcus aureus provided by Chanishvili27 (p. 176). Numbers found along the x axis correspond to (1) “Acute lung abscess” (9, 11, and 9 cases for IVSP, IVSP + ABP, and ABP, respectively), (2) “Bronchiectasis” (9, 10, 9), (3) “Chronic bronchitis” (8, 8, 7), (4) “Chronic lung abscess” (20, 20, 18), and (5) “Chronic pneumonia” (14, 12, 12). The top panel shows phage therapy only (IVSP), the middle combination of phage and antibiotic therapy (IVSP + ABP), and the bottom antibiotic therapy only (ABP). Phage application appears to have been intravenous. In the key, “++++” corresponds to “Complete cure”, “++” to “Improvement”, and “0=neg” to “No effect”.
Ślopek et al.42 published, in 1987, a summary of numerous phage therapy case studies as provided by a variety of Polish physicians working in association with the Hirszfeld Institute of Immunology and Experimental Therapy in Wrocław, Poland. A variety of conditions were treated including lung abscess, bronchitis, pneumonia, etc. Etiologies consisted of a number of pathogens, both individually and in combination including Escherichia, Klebsiella, Proteus, Pseudomonas, Staphylococcus, and Streptococcus. Phages generally were matched to what bacteria were present within individual patients. For infections corresponding to (1) “Inflammation of the upper and lower respiratory tract”, (2) “Pneumonia, pneumonia abscedens, bronchopneumonia”, (3) “Suppurative pneumonia, empyema with thoracic fistula”, and (4) “Pleuritis with fistula”, the reported percent of successful treatments was 90.5, 82.5, 85.2, and 86.4, respectively. These are summarized in Figure 4. For the majority of cases, “Antibiotic treatment was ineffective.”
Figure 4.
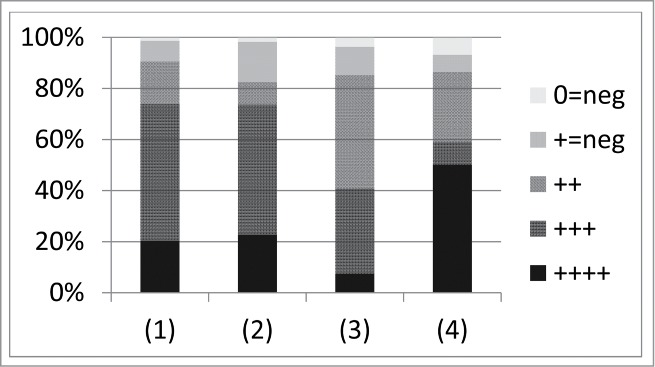
Summary of lung-treatment results provided by Ślopek et al.42 Numbers found along the x axis correspond to (1) “Inflammation of the upper and lower respiratory tract” (74 cases), (2) “Pneumonia, pneumonia abscedens, bronchopneumonia” (57 cases), (3) “Suppurative pneumonia, empyema with thoracic fistula” (27 cases), and (4) “Pleuritis with fistula” (22 cases). “++++” means “outstanding effect manifesting by a complete recovery,” “+++” means “elimination of suppurative process and healing of the local wounds,” “++” means “marked improvement with a tendency to healing of the local lesions with negative results of bacteriological control,” “+=neg” means only “transient improvement,” and “0=neg” means “no effect.” The authors suggest that (p. 570), “While evaluating the final results, it should be taken into consideration that in 518 cases, i.e., in 94.2% the treatment preceding phage therapy failed, among others, due to resistance of bacteria to antibiotics and chemotherapeutics used.”
Descriptions of individual case studies can be found in earlier publications by the same group. Note in particular the extensive use of oral phage administration43 as well as a combinations of oral along with local treatments.11 Durations of phage treatments typically were for multiple weeks. Ślopek et al.43 reports (p. 291) that “Bacteriophages are safe, side effects are rather rare and present no danger for a patient…” Ślopek et al.44 describe treatments of staphylococcal infections. In terms of “Diseases of the respiratory tract” they summarize (p. 270): “Ninety-three cases which belong to this category included 43 cases of suppurative inflammation of the upper respiratory tract (rhinitis, pansinusitis) and bronchitis, 26 cases of bronchogenic pneumonia, 11 cases of abscesses of lung and thoracic empyema with suppurative fistulas and 13 cases of thoracic empyema with fistulas. In 83 cases (89.2%) bacteriophages exerted the desired therapeutic effect, in 8 cases only a transitory improvement was confirmed and in 2 no therapeutic effect was observed. No differences dependent on the type of infection (monoinfections, polyinfections) were noted.” Phages were applied orally and locally including via inhalation (the later to treat a bilateral bronchogenic pneumonia from a patient suffering from multiple infections).
Phage treatment of children is the focus of Ślopek et al.,45 as published in 1985, with description of “Bronchopneumonia, pneumonia abscedens” as follows (p. 248): “To this category 11 cases of bronchogenic pneumonia were classified. All of them were resistant to antibiotics. Six cases were monoinfections and 5 polyinfections. The treatment with phages appeared effective in 10 cases and resulted only in a transitory improvement in 1 case of bilateral intraparenchymatous pneumonia (polyinfection) caused by pyogenic Staphylococci, Klebsiella and Proteus bacilli.” They also report on inhalation phage therapy for 5 weeks of a 2-year-old suffering from a variety of conditions including second and third degree burns, though ultimately this patient died. See also 46.
Weber-Dabrowska et al.47 in their 2000 publication provide an update to the report of Ślopek et al.42 with an additional 1307 cases. From p. 548: “The majority of cases were long, persisting infections in which antibiotic therapy had failed.” In their Table 1 they provide a summary of results. Under the heading of “Mucopurulent chronic bronchitis, laryngitis, rhinitis” (p. 549) they list “S. aureus, E. coli, Klebsiella, Proteus, Pseudomonas” and indicate a rate of “Full recovery and complete elimination of bacteria” in 82.6% (224 cases) and rate of “Improvement, bacteria still detectable” in 16.9% (46). Another 0.3% (1 case) they indicate as “no effect”. Under the heading of “Bronchopneumonia, empyema” they list the same etiologies. 82% of cases say “full recovery” (47 cases) vs. 18% with no effect (10 cases). For “Pleuritis with fistula”, again with the same etiologies, the rates are 86% (full recovery), 10% (“marked improvement”), and 4% (no effect), or 42, 5, and 2 cases, respectively. See Figure 5 for summary.
Figure 5.
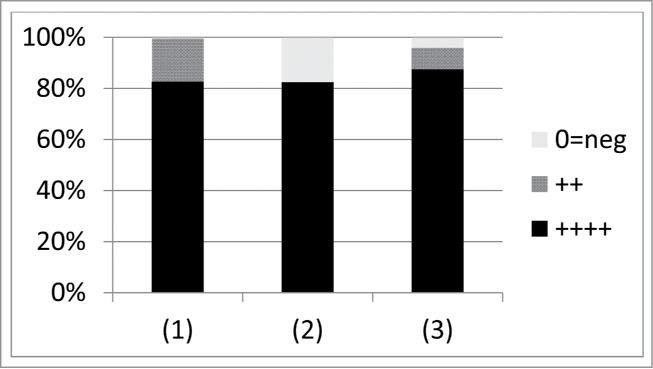
Summary of lung-treatment results provided by Weber-Dabrowska et al.47 Numbers found along the x axis correspond to (1) “Mucopurulent chronic bronchitis, laryngitis, rhinitis” (271 cases), (2) “Bronchopneumonia, Empyema” (57 cases), (3) “Pleuritis with fistula” (49 cases). “++++” means “Full recovery and complete elimination of bacteria”, “++” means “marked improvement” or “Improvement, bacteria still detectable”, and “0=neg” means “no effect.” These results cover years post those considered by Ślopek et al.42
Like the Hirszfeld Institute in Poland, the Eliava Institute of Bacteriophages, Microbiology and Virology in Tbilisi, Georgia (as it is currently named) has been involved in phage therapy for many decades, dating back in this case to the 1930s.3,48 Unlike the Polish approach, which involves the use of phage banks and monophage therapy, in Georgia as well as the former Soviet Union more generally it has predominately been phage cocktails that have been employed; for discussion of the distinctions between the two approaches, see 31, 49, and 50. From that institute, Kutateladze and Adamia48 describe the treatment of pulmonary infections (p. 428): “In the case of lung disease, the preparation was introduced intravenously, as well as by direct injection into the center of the infection. ¶ The staphylococcal phage preparation was used against various… acute and chronic lung abscesses; chronic pneumonia and bronchitis; bronchoectasis, purulent cysts…” Kvachadze et al.32 further elaborate on, in 2011, the use of anti-Staphylococcus phage therapy by the Eliava institute. They then provide a case study of phage treatment of a 7-year-old cystic fibrosis patient. They employed a combination of anti-staphylococcal and anti-pseudomonads phages via nebulization a total of 9 times once every 4 to 6 weeks, and this had been preceded by long-term antibiotic treatment. The result was substantial reductions in the presence of these bacteria, improvement in the patient's “general condition”, and a 50% reduction in required ongoing antibiotic treatment.
Saussereau et al.51 employed a cocktail of 10 phages to reduce P. aeruginosa loads within sputum samples derived from cystic fibrosis patients. The justification for this approach as a model for phage therapy is the complexity of the sputum environment, which presumably mimics the complexity of the bacteria-containing environment within lungs to at least some degree. The authors note in their abstract that “the addition of bacteriophages led to a significant decrease in the levels of P. aeruginosa strains, as shown by comparison with controls, taking two variables (time and bacteriophages) into account (p = 0.024). In 45.8% of these samples, this decrease was accompanied by an increase in the number of bacteriophages.” On p. O986 they indicate that following a 6 h incubation at 37° C bacterial counts within sputum samples “ranging from 33% to 6090% increase in the absence and from 18 to 98% reduction in the presence of bacteriophages.” These results were seen despite only 35% of isolated colonies from untreated sputum samples displaying sensitivity to the phage cocktail in terms of phage-induced lysis or inhibition of bacterial growth.
Though not a phage therapy study, James et al.52 monitored via real-time Q-PCR the densities of free phages within sputum samples derived from cystic fibrosis patients. They found that free-phage densities exceeded P. aeruginosa densities in all samples with mean ratios of phages to P. aeruginosa ranging from approximately 1 to 2 orders of magnitude. They speculate that temperate phage-induced lysis could play a role in regulating P. aeruginosa lung-densities in these patients. At a minimum, this work is suggestive that free phages can represent a normal constituent of bacteria-diseased lungs. See also the work of Friman et al.53 who suggest that P. aeruginosa isolated from the lungs of cystic fibrosis patients may become more susceptible, over the course chronic infections, to unassociated phages.
Pulmonary Phage Therapy, Animal Models
Relatively few studies have been published in which animal models have been used to test the potential of phage therapy for pulmonary bacterial infections of humans. Most of these studies have been performed as models for the treatment of bacterial-pathogen infections of cystic fibrosis patients, particularly those targeting Pseudomonas or Burkholderia species. As is often the case with animal models of disease, the infections treated are imperfect representations of disease in humans. In particular, it can be difficult with animal models to mimic chronic bacterial infections because of issues of too-rapid animal clearance of infecting bacteria or, at the other extreme, rapid fatality. Thus, experimental phage treatment in animals tends to be initiated relatively soon after bacterial challenge. For example, a full 24 h between bacterial challenge and the start of treatment can be viewed as a relatively long delay. Alternatively, and especially without substantial delays, e.g., <<24 h, the successful treatment of an experimental animal infection might be viewed simply as a phage-induced reduction in what otherwise would have been a lethal bacterial dose instead to a non-lethal bacterial dose. That is, without substantial delay between bacterial challenge and phage dosing, perhaps the initial bacterial dose is reduced early on via phage treatment to below the bacterium's lethal dose within the animal, rather than phage treatment instead representing phage therapy of previously existing bacterial disease. In the case of treatment of pulmonary infections, one can add as well difficulties in mimicking inhalation therapy.25 Thus, for example, one typically will see intranasal instillation of phages,54-59 or intraperitoneal application,54,60,61 both of which might less efficiently deliver phages into the lungs versus, for example, the use of nebulizers.12,59,62 Below I provide overviews of these studies. Note that most of these treatments consist of only a single phage dose vs., e.g., multiple dosing over the course of weeks for typical human treatments of pulmonary infections (above).
Chhibber et al.60 employed a mouse model of Klebsiella pneumoniae-associated pneumonia. Bacterial challenge was intranasal while phage administration was intraperitoneal with what appears to be 109 phages per animal. Protection from disease symptoms was observed only if no delay between challenge and treatment was allowed or instead with a 3-h prior phage treatment (with partial protection given a 6-h prior treatment). Microbiologically, it appears that either no-delay or 3-h prior phage treatment had the effect of reducing lung bacterial densities to below their infectious dose, as measured 5-days post challenge, whereas a 6-h prior phage treatment resulted in reductions in bacterial densities to zero, but not until day 7. Note that non-phage treated controls too displayed reduced bacterial densities over time, with roughly a 2-log reduction from starting bacterial densities and 6-log reduction from indicated peak bacterial densities by day 7.
Carmody et al.54 employed a mouse Burkholderia cenocepacia lung infection model. Bacterial challenge was intratracheal and treatment appeared to consist of 109 or 1010 phages per animal that were intranasally instilled or instead intraperitoneally (IP) delivered. There was a 24-h delay between challenge and treatment. An average of approximately a 2-log reduction in bacterial densities in the lungs was observed, but only with IP delivery.
Debarbieux et al.55 employed a mouse Pseudomonas aeruginosa lung infection model. Both bacterial challenge and phage treatment were via intranasal instillation. Phage doses appear to have been 108 per animal and 100-fold lower phage doses were found to be ineffective in preventing death. Bacterial densities were measured via bioluminescence. Treatment effectiveness in preventing lethality was found to decline from 100% survival at 72 h given a 2-h delay in phage treatment to 75% survival given 4-h delays and then to 25% survival given 6-h delays in phage instillations. Pretreatment with phages 24-h prior to bacterial challenge resulted in 100% survival.
From the same group as Debarbieux et al.55, Morello et al.56 also employed a mouse P. aeruginosa lung infection model. Essentially the same approach was taken except that delay before treatment was limited to 2 h, a multi-drug resistant mucoid strain isolated from a cystic fibrosis patient was employed as the target, and fewer phages were applied (3 × 107 versus 1 × 108 per animal). In addition, a preventative treatment 4 d prior to bacterial challenge of 3 × 108 phages/animal was used. Substantial survival (>90 % or 100%, respectively) was seen with these higher doses vs. 50% or 20% survival using 10-fold fewer phages. Higher-dose phage treatment, versus pretreatment, also had the effect of reducing bacterial densities more than 2 logs after 20 h as measured via broncho-alveolar lavage.
Though also employing a mouse P. aeruginosa lung-infection model, Alemayehu et al.57 are a different group from that of Debarbieux et al.55 and Morello et al.56 They used an intranasal bacterial challenge and phage dosing with a 2-h delay. Peak bacterial densities were reached after 6 h, as measured via bioluminescence, while phage treatment resulted in substantial declines in bacterial densities 2 h after phage application. This suggests that infections had matured little prior to bacterial eradication. No lethality with or without phage treatment is mentioned.
Henry et al.58 extended the P. aeruginosa mouse model of Debarbieux et al.55, comparing in vitro with in vivo characteristics for 9 phages. The in vivo assays consisted of mouse survival following a 2-h delay between bacterial challenge and phage application in combination with the mouse-infection bioluminescence assay of Debarbieux et al.55 The in vitro assay, by contrast, consisted of efficiency of plating in combination with lysis profiles, that is, where cultures are followed with and without phage addition in terms of optical density. This latter approach contrasts with the seemingly more rigorous “In vitro phage virulence tests” described by Smith and Huggins63, which I quote in full from their Materials and Methods (p. 2660): “Ten ml amounts of nutrient broth were inoculated with 3 × 108 viable E. coli organisms and three-fold falling numbers of phage particles and incubated for 5 h. The lowest inoculum of phage particles that produced complete clearing of the bacterial cultures was then recorded.” In addition, no effort appears to have been made by Henry et al. to determine phage growth parameters of adsorption rate constant, latent period, or burst size64 nor whether extended latent periods were a consequence of, for instance, lysis inhibition exhibited by these phages.65,66 Nevertheless, the simple characterization used by Henry et al. was predictive of the phage performance in a claimed 7 of 9 cases, though with a perhaps less stringent, qualitative assessment I would suggest that the 4 less effective phages in vitro as determined via lysis profile assay were also the 4 less effective phages in vivo. The particularly poor in vivo performance of 2 phages (PhiKZ and CHA_P1) in terms of rescuing mice, however, was not obvious from the lysis profile where instead these phages displayed only intermediately poor performance. Phage PhiKZ performance was easily improved via the application of greater phage numbers while that of phage CHA_P1 was not, though phage CHA_P1 nonetheless was effective at rescuing mice infected with the CHA strain of P. aeruginosa. A third phage, LBL3, by contrast displayed intermediately poor performance in both lysis profile and mouse survival assays while a fourth phage (LUZ19) displayed particularly poor performance via lysis profile but only intermediately poor performance in terms of rescuing mice, performance that possibly (I speculate) could be improved also via the application of greater phage numbers. A reasonable general conclusion from the study in terms of treatment efficacy nevertheless is that most of the phages tested were relatively effective in experimental treatment of mouse infections explored. The results are consistent, however, with not all phages necessarily being effective against all bacterial strains even given evidence of in vitro effectiveness; comparisons between phage in vitro and in vivo, i.e., phage therapy properties can be found as well in references 67-69. Additional characterization of the biology of the phages employed by Henry et al. has recently been published where they question the need for “complete molecular characterization of new bacteriophages” prior to their medical use.70
Semler et al.62 also employed a mouse B. cenocepacia lung infection model but are a different group from that of Carmody et al.54 Mice were prepared for infection by being chemically immunocompromised via cyclophosphamide application and both bacterial challenge and phage treatment were accomplished via nebulization. There was a 24-h delay between bacterial challenge and phage treatment, which as a delay in the initiation of therapy in animals can be viewed as fairly substantial. Treatment involved phage densities of potentially 1011/ml prior to nebulization, which can be interpreted as a possibly substantial phage dose. Somewhat lower phage densities as well as IP treatments were, by contrast, not effective in reducing bacterial densities. Approximately 4-log lower bacterial densities were seen with higher density phage treatment in comparison with control bacterial densities at 2 and 3 d. For an only single phage dosage given with a 1-d delay before phage addition, this level of bacterial reduction could be viewed as substantial. For technical reasons a second phage dose was not employed. It would be of interest to see, using the same protocol, to what extent phage effectiveness might be affected by longer delays between challenge and treatment, though for the same technical issues (requirements for additional cyclophosphamide application over time), that too might not be easily implemented.
Takemura-Uchiyama et al.23 describe the phage treatment of a lung-derived septicemia following intranasal inoculation of cyclophosphamide-treated mice with S. aureus. Phage application of 1010 plaque-forming units was intraperitoneal, 6-h post bacterial challenge, and resulted in nearly 70% mouse survival vs. 10% with mock treatment. Microbiologically, 48-h post bacterial challenge at least a one-log reduction in S. aureus densities were observed in the blood, liver, and spleen, relative to the phage-less control, and with the latter two the difference was statistically significant. Unexpectedly, however, no reduction in bacterial density was observed in the lungs despite observed phage densities being highest there (a reported 1010/g tissue versus, e.g., 108/ml blood) with phage densities also high 24-h post bacterial challenge as determined via bronchoalveolar lavage fluid (109/ml). Given phage application without bacterial challenge, by contrast, phage densities associated with lung tissue were much lower (~104/g tissue vs. 102/ml of blood) and essentially not present within bronchoalveolar lavage fluid. From these observations the authors note (p. 516) that the results likely are indicative of “diffusion from the bloodstream into the alveoli via the pores created by S. aureus invasion”, that phages were replicating within the lungs, and that phage distribution within the lungs nevertheless may have been heterogeneous. The intraperitoneally administered phages however were apparently unsuccessful in sufficiently controlling S. aureus population size within the lungs.
Cao et al.59 isolated a phage from hospital sewage against a P. aeruginosa isolate associated with hemorrhagic pneumonia in minks. A toxicity trial was performed involving the application intranasally of 60 μl of “crude phage preparation” containing 1012 plaque-forming units (PFU) per ml once a day for 3 d to mice. In comparison to physiological saline controls there were no deaths or differences in body weight, food consumption, or behavior though in one of the phage-treated mice there was (p. 11), “Minor hemorrhage in spleen and slight inflammatory cells infiltration in liver”. Phage treatment experiments involved intranasal challenge of minks with 108 CFU/ml of P. aeruginosa which was followed 2 h later with a 3-minute aerosol application to mink-containing chambers of phage with 3 ml of fluid atomized per min, a process which the authors describe as ultrasonically atomizing “phages at MOI 1, 10, 100”, though it is not obvious how these numbers translate into either phage density within aerosols or phage dose per animal. Application at the highest and second-highest number of phages reduced lung bacterial loads approximately 1 log per g tissue at 24 h. Survival or instead a lack of euthanization for humanitarian reasons, at or by 12 d for 5 animals per treatment group, was 100% with the highest phage dose, 80% for the second highest phage dose, 20% for the third, and zero for the control.
Singla et al.61 packaged phages within liposomes for intraperitoneal delivery in the treatment of experimental mouse K. pneumonia-associated lobar pneumonia. Substantial bacterial clearance was observed without treatment by 72 h post intranasal challenge with 104 CFU. Intranasal phage treatment resulted in no additional reductions in bacterial densities. IP application of phages alone, at a reported MOI of 1.0, completely eliminated bacteria by 24 h given a 6 h delay in phage treatment following bacterial challenge and an approximately 3-log reduction in bacteria at 48 h post challenge given a 24 h delay. IP liposome treatment alone had no discernable impact on bacterial densities. Phages packaged within liposomes given IP delivery, at a reported MOI of 0.1, reduced bacterial densities equivalently to MOI 1.0 unpackaged phages given for both a 6-h delay (i.e., bacteria were eliminated), reduced bacterial counts to zero by 72 h given a 24-h delay, reduced bacterial counts to zero at 5 d given a 48 h delay (a 5-log reduction relative to the control), and reduced bacterial counts to zero at 7 d given a 72-h delay (a 2-log reduction relative to the control). The impact of IP treatment using phages alone, 3 or 6 h prior to bacterial challenge, was to prevent infection but no effect was seen given 24 h prior treatment. Liposome entrapped phages provided complete protection given 6, 24, or 48 h IP delivery prior to bacterial challenge but no protection was seen given 72 h prior delivery.
Conclusion
The history of phage treatment of bacterial infections is such that substantial experimentation has taken place clinically prior to the development of robust experimental animal disease models and also outside of what today would be considered to be desirable practices for clinical trials (particularly, e.g., double-blind analysis). The result of this history, and not just regarding pulmonary treatment, is multifold: (1) There appears to exist or at least has existed more experience in using phages as antibacterial agents in clinical practice than there is in the laboratory despite the relative lack of formal approval for phage therapy use in clinical practice for Western medicine. (2) Reported phage therapy successes appear to be more numerous than reported pre-clinical treatment successes, and there otherwise is little evidence of this clinical use of phage therapy having been preceded by substantial pre-clinical study, as would have been expected following a typical modern pharmaceutical development pathway. (3) Clinical use of phages seems to have been more aggressive, e.g., more invasive as well as over longer times spans with multiple dosing, than appears to be the case with pre-clinical phage therapy studies.
For instance, with regard to the latter point: All but one of the animal studies involved only a single phage dosing and most23,54-59,62 involved topical phage application into the lungs. Exceptional were the mouse studies by Chhibber et al.,60 Semler et al.,62 and Singla et al.61 which also tested intraperitoneal phage application. Clinical treatments, by contrast, involved a diversity of approaches to phage application including more or less direct injection,28,38 puncture,28,40,41 or catheterization40 into or into the vicinity of infections as well as parenteral application including intravenous.27,40,40 Phage treatment over multiple days or weeks is also seen with clinical application, representing more or less the published pulmonary phage therapy norm.
As a result of these issues, pre-clinical characterization of phage therapy tends to find itself in a position where it is attempting to replicate or even re-derive previous clinical results, with the intention of eventual return to the clinic based on a more modern though not necessarily more robust model of pharmaceutical development. If nothing else, here I provide historical documentation of this seemingly “backward” process of antibacterial development. Whether current strategies of phage therapy research and development should be altered in light of the presented observations ultimately will be a function of the extent to which case-study results are believed to be legitimate, but also given a potential for existing regulatory frameworks for pharmaceutical development to allow for the commencement of clinical study other than strictly following highly robust pre-clinical development.71 This latter statement comes, though, with the caveat that a key component of earlier clinical efforts was presumably some degree of phage therapy experience and expertise by practitioners, which certainly must to a degree be re-acquired by any new-to-the-field clinical phage therapists prior to continuation of these past clinical approaches. If nothing else, however, it behooves pre-clinical researchers to pay attention to documentation of what may have worked in the past, clinically, in the course of their design of phage therapy animal experiments.
Footnotes
“pratiquement tous les antibiotiques ordinaires et spécifiques, thoracoplastie gauche, ponctions, drainages, lavages, etc.”
“le malade reprenait progressivement du poids et pouvait se lever”
“En conclusion nous pensons que, dans ces cas d'infection pyogène résistant aux antibiotiques, il vaut la peine d'envisager d'emblée un traitement combiné d'antibiotiques et de stock-phage que l'on remplacera par la suite par un auto-phage dont l'action est plus efficace.”
“une toux plus ou moins réfractaire aux moyens thérapeutiques habituels”
“Les résultats, en ce qui concerne la toux, peuvent être qualifiés de très favorables. Sur une série de 19 cas, je n'ai observé aucun échec total. Quinze guérisons furent complètes; l'une, suivie d'une réinfection bronchique, fut guérie à nouveau par le même moyen (cas 17); dans trois cas enfin, l'amélioration fut notable. Par guérison, j'entends la disparition complète de la toux et la suppression du caractère purulent des expectorations quand elles existaient. Dans tous les cas guéris, les symptômes concomitants ont également disparu…”
“Une fois, le produit a déclenché des réactions fébriles avec urticaire (cas 15). Cette réaction est de nature probablement allergique à l'un des composants du médicament, peut-être au milieu albumineux… mais a pu être empêchée par l'absorption prophylactique d'un antihistaminique. Je n'ai pas remarqué d'autres intolérances.”
“Wir verwendeten das Präparat Diriphagen [Diriphagene®: Chemische Fabrik Dr. Heinz Haury, München.], weil wir glaubten, daß die erwähnten Forderungen in diesem Präparat erfüllt sind. Das Präparat enthält nach Angaben 180–200 verschiedene Phagenstämme und besitzt somit ein breites Wirkungsspektrum.”
“…sogenannte gezielte Antimikrobika beigegeben zur Bekämpfung derjenigen Bakterien, die eine primäre Phagenresistenz aufweisen.”
“Wir haben anfangs Diriphagen unverdünnt aerosoliert und dabei nach der ersten Sitzung gelegentlich subfebrile Temperaturen beobachtet. Wir nehmen an, daß es sich um eine Reaktion auf Zerfallsprodukte der Bakterien handelt… Später verwendeten wir eine Verdünnung mit physiologischer NaCl bis 1 : 5 und sahen keine Nebenerscheinungen mehr.”
“Полученные дaнные позволяют зaключить, что бaктериофaг является эффективным препaрaтом, облaдaющим aктивностью в отношении подaвляющего большинствa штaммов стрептококкa, стaфилококкa и протея и его можно широко использовaть в медицинской прaктике при смешaнных зaболевaниях.”
“он не окaзывaет побочного действия, нaблюдaемого при использовaнии aнтибиотикa (дисбaктериоз, aллергические явления, обрaзовние резистентных культур и др.).”
“28 (62,2 %) больных удaлось добиться стойкой ремиссии с положительными клинико-рентгенологическими сдвигaми и снятия гнойной интоксикaции. У 14 (31,1 %) больных знaчительно уменьшилось количество гнойной мокроты и появилaсь зaметнaя положительнaя рентгенологическaя динaмикa. У 3 (6,6 %) пaциентов выделение мокроты хотя и уменьшилось, однaко онa былa зловонной…”
Стa филококковый бaктериофaг применяли кaк отдельно, тaк и в комбинaции с aнтибиотикaми… Бaктериофaг нaзнaчaли кaк местно — в виде эндобронхиaльных сaнaций (ингaляции, кaтетеризaция трaхеи, бронхоскопия) непосредственно в гнойный очaг в легком или плеврaльной полости (трaнсторaкaльнaя пункция и кaтетеризaция гнойных очaгов), тaк и пaрентерaльно — внутримышечно, внутривенно, путем длительной перфузии в бронхиaльную или легочную aртерию. Количество бaктериофaгa лимитировaлось местом введения: для эндобронхиaльных сaнaций — 10—30 мл, непосредственно в гнойный очaг в пaренхиме легкого — 10—50 мл, в полость эмпиемы — 20—100 мл, пaрентерaльно — 0,5—1,0 мл/кг. Длительность фaготерaпии зaвиселa от клинического и рентгенологического эффектa и состaвлялa 2—4 нед.”
“Стойкaя ремиссия, a в некоторых случaях и выздоровление…”
“Относительнaя ремиссия…”
“…летaльный исход или обострение гнойного процессa …”
“Местное и пaрентерaльное применение стaфилококкового бaктериофaгa с aнтибиотикaми не вызывaло отрицaтельных побочных явлений. При внутривенных инфузиях стaфилококкового бaктериофaгa в дозе 0,5—1 мл/кг aллергических или пирогенных реaкций не нaблюдaлось, лишь в единичных случaях темперaтурa повышaлaсь нa 0,3—0,6 °С.”
Disclosure of Potential Conflicts of Interest
The author has consulted for various companies with regard to phage therapy issues and is the founder of the website, phage.org, as well as the related phage-therapy.org.
Acknowledgments
Thank you to Andrey Letarov and Sarah Kuhl for reading the manuscript, providing comments, and correcting both Russian and French translation errors.
References
- 1.Harper DR. Biological control by microorganisms The Encyclopedia of Life Sciences. Chichester, England, UK: John Wiley & Sons, 2006:1–10. [Google Scholar]
- 2.Abedon ST. Kinetics of phage-mediated biocontrol of bacteria. Foodborne Pathog Dis 2009; 6:807–15; PMID:19459758; http://dx.doi.org/ 10.1089/fpd.2008.0242 [DOI] [PubMed] [Google Scholar]
- 3.Chanishvili N. Phage therapy—history from Twort and d'Herelle through Soviet experience to current approaches. Adv Virus Res 2012; 83:3–40; PMID:22748807; http://dx.doi.org/ 10.1016/B978-0-12-394438-2.00001-3 [DOI] [PubMed] [Google Scholar]
- 4.Summers WC. History of phage research and phage therapy In: Waldor M, Friedman D, Adhya S, eds. Phages: Their Role in Bacterial Pathogenesis and Biotechnology. Washington DC: ASM Press, 2005: [Google Scholar]
- 5.Kutter E, De Vos D, Gvaxalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST . Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 2010; 11:69–86; PMID:20214609; http://dx.doi.org/ 10.2174/138920110790725401 [DOI] [PubMed] [Google Scholar]
- 6.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage 2011; 1:66–85; PMID:22334863; http://dx.doi.org/ 10.4161/bact.1.2.15845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen HK, Trachsel J, Looft T, Casey TA. Finding alternatives to antibiotics. Ann N Y Acad Sci 2014; 1323:91–100; PMID:24953233; http://dx.doi.org/ 10.1111/nyas.12468 [DOI] [PubMed] [Google Scholar]
- 8.Baquero F, Coque TM, Canton R. Counteracting antibiotic resistance: breaking barriers among antibacterial strategies. Expert Opin Ther Targets 2014; 18:851–61; PMID:24881465; http://dx.doi.org/ 10.1517/14728222.2014.925881] [DOI] [PubMed] [Google Scholar]
- 9.Hoeflmayr J. Inhalationstherapie mit Bakteriophagen bei therapieresistenten Infektionen [Inhalation therapy with bacteriophages for treatment-resistant infections] In: Nückel H, ed. Fortschritte der biologischen Aerosol-Forschung in den Jahren 1957–1961 [Advances in Biological Aerosols Research in the Years 1957–1961]. Stuttgart, Germany: 1962:403–9. [Google Scholar]
- 10.Krestovikova VA. Phage therapy and phage prophylaxis and their development through the work of Soviet researchers. Zh Mikrobiol Epidemiol Immunobiol 1947; 3:56–65 [Google Scholar]
- 11.Slopek S, Durlakova I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. II. Detailed evalulation of the results. Arch Immunol Ther Exp 1983; 31:293–327; PMID:6651485 [PubMed] [Google Scholar]
- 12.Hoe S, Semler DD, Goudie AD, Lynch KH, Matinkhoo S, Finlay WH, Dennis JJ, Vehring R. Respirable bacteriophages for the treatment of bacterial lung infections. J Aerosol Med Pulm Drug Deliv 2013; 26:317–35; PMID:23597003; http://dx.doi.org/ 10.1089/jamp.2012.1001 [DOI] [PubMed] [Google Scholar]
- 13.Hoe S, Boraey MA, Ivey JW, Finlay WH, Vehring R. Manufacturing and device options for the delivery of biotherapeutics. J Aerosol Med Pulm Drug Deliv 2014; 27:315–28; PMID:24299502; http://dx.doi.org/ 10.1089/jamp.2013.1090 [DOI] [PubMed] [Google Scholar]
- 14.El-Gohary FA, Huff WE, Huff GR, Rath NC, Zhou ZY, Donoghue AM. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens. Poult Sci 2014; 93:2788–92; PMID:25214555; http://dx.doi.org/ 10.3382/ps.2014-04282 [DOI] [PubMed] [Google Scholar]
- 15.Tsonos J, Vandenheuvel D, Briers Y, De GH, Hernalsteens JP, Lavigne R. Hurdles in bacteriophage therapy: deconstructing the parameters. Vet Microbiol 2014; 171:460–9; PMID:24315040; http://dx.doi.org/ 10.1016/j.vetmic.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Abedon ST, Thomas-Abedon C. Phage therapy pharmacology. Curr Pharm Biotechnol 2010; 11:28–47; PMID:20214606; http://dx.doi.org/ 10.2174/138920-110790725410 [DOI] [PubMed] [Google Scholar]
- 17.Abedon S. Phage therapy pharmacology: calculating phage dosing. Adv Appl Microbiol 2011; 77:1–40; PMID:22050820; http://dx.doi.org/ 10.1016/B978-0-12-387044-5.00001-7 [DOI] [PubMed] [Google Scholar]
- 18.Ryan EM, Gorman SP, Donnelly RF, Gilmore BF. Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. Journal of Pharmacy and Pharamcology 2011; 63:1253–64; PMID:21899540; http://dx.doi.org/ 10.1111/j.2042-7158.2011.01324.x [DOI] [PubMed] [Google Scholar]
- 19.Abedon ST. Phage therapy best practices In: Hyman P, Abedon ST, eds. Bacteriophages in Health and Disease. Wallingford, UK: CABI Press, 2012:256–72. [Google Scholar]
- 20.Abedon ST. Bacteriophages as drugs: the pharmacology of phage therapy In: Borysowski J, Miedzybrodzki R, Górski A, eds. Phage Therapy: Current Research and Applications. Norfolk, UK: Caister Academic Press, 2014:69–100. [Google Scholar]
- 21.Abedon ST. Phage therapy: eco-physiological pharmacology. Scientifica 2014; 2014:581639; PMID:25031881; http://dx.doi.org/ 10.1155/2014/581639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, Bingen E, Bonacorsi S. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob Agents Chemother 2012; 56:3568–75; PMID:22491690; http://dx.doi.org/ 10.1128/AAC.06330-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takemura-Uchiyama I, Uchiyama J, Osanai M, Morimoto N, Asagiri T, Ujihara T, Daibata M, Sugiura T, Matsuzaki S. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes Infect 2014; 16:512–7; PMID:24631574; http://dx.doi.org/ 10.1016/j.micinf.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 24.Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, Fourie PB, Colombo P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb) 2011; 91:71–81; PMID:20875771; http://dx.doi.org/ 10.1016/j.tube.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Pandya SM, Mohammad I, Agrawal AK, Mohan M, Misra A. Particulate pulmonary delivery systems containing anti-tuberculosis agents. Crit Rev Ther Drug Carrier Syst 2013; 30:277–91; PMID:23662603; http://dx.doi.org/ 10.1615/CritRevTherDrugCarrierSyst.2013005684 [DOI] [PubMed] [Google Scholar]
- 26.Morrison S, Gardner RE. The treatment of a lung abscess due to Bacillus coli with a lytic filtrate. J Am Med Assoc 1936; 107:33–4;' http://dx.doi.org/ 10.1001/jama.1936.92770270005010c [DOI] [Google Scholar]
- 27.A Literature Review of the Practical Application of Bacteriophage Research Hauppauge, New York: Nova Publishers, 2012. [Google Scholar]
- 28.Shishenko SF. To the issue of application of D'Herelle's bacteriophage in children surgery. Select Art Azerbaijan Inst Micro Epidem 1938; 6:107–14 [Google Scholar]
- 29.Cevey M, Schwiez Z. Le bactériophage dans le traitement des empyèmes pleuraux à germes résistants aux antibiotiques [Bacteriophage in the treatment of pleural empyema antibiotic resistant bacteria]. Tuberk (Swiss Tuberculosis Journal or Schw Zt Tbc) 1958;; 15:34–9 [PubMed] [Google Scholar]
- 30.Kutter EM. Bacteriophage therapy: past and present In: Schaecter M, ed. Encyclopedia of Microbiology. Oxford: Elsevier, 2009:258–66. [Google Scholar]
- 31.Pirnay JP, De VD, Verbeken G, Merabishvili M, Chanishvili N, Vaneechoutte M, Zizi M, Laire G, Lavigne R, Huys I, et al.. The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharm Res 2011; 28:934–7; PMID:21063753; http://dx.doi.org/ 10.1007/s11095-010-0313-5 [DOI] [PubMed] [Google Scholar]
- 32.Kvachadze L, Balarjishvili N, Meskhi T, Tevdoradze E, Skhirtladze N, Pataridze T, Adamia R, Topuria T, Kutter E, Rohde C, et al.. Evaluation of lytic activity of staphylococcal bacteriophage Sb-1 against freshly isolated clinical pathogens. Microb Biotechnol 2011; 4:643–50; PMID:21481199; http://dx.doi.org/ 10.1111/j.1751-7915.2011.00259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delacoste P. Considérations sur le traitement des affections respiratoires banales au moyen de bactériophages [Considerations on the treatment of common respiratory diseases by means of bacteriophages]. Rev Med Suisse Romande 1959; 79:552–63; PMID:13815551 [PubMed] [Google Scholar]
- 34.Garsevanishvili TI. Некоторые методические aспекты применения ингaляции поливaлентного ьaктериофaгa при лечении пневмонии рaннего детского возрaстa [Certain methodological aspects of the use of inhalation of a polyvalent bacteriophage in the treatment of pneumonia of young children]. Pediat- Zhurnal G N Speranskogo 1974; 53;65–6; PMID:4276158 [PubMed] [Google Scholar]
- 35.Nikolaeva Y. Staphylococcal bacteriophage and its application in clinic. Selected Articles of the Jubilee Symposium Dedicated to the 50th Anniversary of the Tbilisi Institute of Vaccine and Sera. 1974;364–6 [Google Scholar]
- 36.Sakandelidze VM, Meipariani AN. Применение комбинировaнных фaгов при гнойно-воспaлительных зaьолевaниях [Use of combined phages in suppurative-inflammatory diseases]. Zh Mikrobiol Epidemiol Immunobiol 1974; 6:135–6; PMID:4411424 [PubMed] [Google Scholar]
- 37.Sulakvelidze A, Alavidze, Morris Z, Jr JG. Bacteriophage therapy. Antimicrob Agents Chemother 2001; 45:649–59; PMID:11181338; http://dx.doi.org/ 10.1128/AAC.45.3.649-659.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ioseliani GD, Meladze GD, Chkhetiia NS, Mebuke MG, Kiknadze NI. Применение бaктериофaгa с aнтибиотикaми для профилaктики острых пострезекционных эмпием плевры при хронических нaгноительных зaболевaниях легких [Use of bacteriophage with antibiotics for prevention of acute postresectional pleural empyemas in chronic pulmonary suppurations]. Grudnaia Khirurgiia 1980; 6:63–7; PMID:6450089 [PubMed] [Google Scholar]
- 39.Sulakvelidze A, Kutter E. Bacteriophage therapy in humans In: Kutter E, Sulakvelidze A, eds. Bacteriophages: Biology and Application. Boca Raton, Florida: CRC Press, 2005:381–436. [Google Scholar]
- 40.Meladze GD, Mebuke MG, Chkhetia NS, Kiknadze NI, Koguashvili GG, Timoshuk II, Larionova NG, Vasadze GK.. Эффектибность стaфилококкового бaктериофaгa при лечении гнойных зaьолевaний легких и плевры [Efficacy of staphylococcal bacteriophage in treatment of purulent diseases of lungs and pleura]. Grudnaia Khirurgiia 1982; 1:53–6 [PubMed] [Google Scholar]
- 41.Chkhetia NS. Treatment of lung disease 1984. Dissertation, Tbilisi, Georgia. [Google Scholar]
- 42.Ślopek S, Weber-Dąbrowska B, Dąbrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch Immunol Ther Exp 1987; 35:569–83; PMID:3455647 [PubMed] [Google Scholar]
- 43.Ślopek S, Durlakova I, Weber-Dąbrowska B, Kucharewicz-Krukowska A, Dąbrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of the results. Arch Immunol Ther Exp 1983; 31:267–91; PMID:6651484 [PubMed] [Google Scholar]
- 44.Ślopek S, Kucharewicz-Krukowska A, Weber-Dąbrowska B, Dąbrowski M. Results of bacteriophage treatment of suppurative bacterial infections. VI. Analysis of treatment of suppurative staphylococcal infections. Arch Immunol Ther Exp 1985; 33:261–73; PMID:2935117 [PubMed] [Google Scholar]
- 45.Ślopek S, Kucharewicz-Krukowska A, Weber-Dąbrowska B, Dąbrowski M. Results of bacteriophage treatment of suppurative bacterial infections. V. Evaluation of the results obtained in children. Archivum Immunologiae et Therapiae Experimentalis 1985; 33:241–59; PMID:2935116 [PubMed] [Google Scholar]
- 46.Ślopek S, Kucharewicz-Krukowska A, Weber-Dąbrowska B, Dąbrowski M. Results of bacteriophage treatment of suppurative bacterial infections. IV. Evaluation of results obtained in 370 cases. Arch Immunol Ther Exp 1985; 33:219–40; PMID:2935115 [PubMed] [Google Scholar]
- 47.Weber-Dąbrowska B, Mulczyk M, Górski A. Bacteriophage therapy of bacterial infections: An update of our institute's experience. Arch Immunol Ther Exp 2000; 48:547–51; PMID:11197610 [PubMed] [Google Scholar]
- 48.Kutateladze M, Adamia R. Phage therapy experience at the Eliava Institute. Med Mal Infect 2008; 38:426–30; PMID:18687542; http://dx.doi.org/ 10.1016/j.medmal.2008.06.023 [DOI] [PubMed] [Google Scholar]
- 49.Chan BK, Abedon ST. Phage therapy pharmacology: phage cocktails. Adv Appl Microbiol 2012; 78:1–23; PMID:22305091; http://dx.doi.org/ 10.1016/B978-0-12-394805-2.00001-4 [DOI] [PubMed] [Google Scholar]
- 50.Chan BK, Abedon ST, Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol 2013; 8:769–83; PMID:23701332; http://dx.doi.org/ 10.2217/fmb.13.47 [DOI] [PubMed] [Google Scholar]
- 51.Saussereau E, Vachier I, Chiron R, Godbert B, Sermet I, Dufour N, Pirnay JP, De Vos D, Carrié F, Molinari N, et al.. Effectiveness of bacteriophages in the sputum of cystic fibrosis patients. Clin Microbiol Infect 2014; 20:O983–O990; PMID:24920209; http://dx.doi.org/ 10.1111/1469-0691.12712 [DOI] [PubMed] [Google Scholar]
- 52.James CE, Davies EV, Fothergill JL, Walshaw MJ, Beale CM, Brockhurst MA, Winstanley C. Lytic activity by temperate phages of Pseudomonas aeruginosa in long-term cystic fibrosis chronic lung infections. ISME J 2014; PMID:25461970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friman VP, Ghoul M, Molin S, Johansen HK, Buckling A. Pseudomonas aeruginosa adaptation to lungs of cystic fibrosis patients leads to lowered resistance to phage and protist enemies. PLoS One 2013; 8:e75380; PMID:24069407; http://dx.doi.org/ 10.1371/journal.pone.0075380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmody LA, Gill JJ, Summer EJ, Sajjan US, Gonzalez CF, Young RF, LiPuma JJ. Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. J Infect Dis 1-15-2010; 201:264–71; PMID:20001604; http://dx.doi.org/ 10.1086/649227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis 4-1-2010; 201:1096–104; PMID:20196657; http://dx.doi.org/ 10.1086/651135 [DOI] [PubMed] [Google Scholar]
- 56.Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One 2011; 6:e16963; PMID:21347240; http://dx.doi.org/ 10.1371/journal.pone.0016963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alemayehu D, Casey PG, McAuliffe O, Guinane CM, Martin JG, Shanahan F, Coffey A, Ross RP, Hill C. Bacteriophages fMR299-2 and fNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. MBio 2012; 3:e00029-12; PMID:22396480; http://dx.doi.org/ 10.1128/mBio.00029-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henry M, Lavigne R, Debarbieux L. Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections. Antimicrob Agents Chemother 2013; 57:5961–8; PMID:24041900; http://dx.doi.org/ 10.1128/AAC.01596-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao Z, Zhang J, Niu YD, Cui N, Ma Y, Cao F, Jin L, Li Z, Xu Y. Isolation and Characterization of a “phiKMV-Like” Bacteriophage and Its Therapeutic Effect on Mink Hemorrhagic Pneumonia. PLoS One 2015; 10:e0116571; PMID:25615639; http://dx.doi.org/ 10.1371/journal.pone.0116571 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Chhibber S, Kaur S, Kumari S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol 2008; 57:1508–13; PMID:19018021; http://dx.doi.org/ 10.1099/jmm.0.2008/002873-0 [DOI] [PubMed] [Google Scholar]
- 61.Singla S, Harjai K, Katare OP, Chhibber S. Bacteriophage-loaded nanostructured lipid carrier: Improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae induced lobar pneumonia. J Infect Dis 2015; PMID:25605867 [DOI] [PubMed] [Google Scholar]
- 62.Semler DD, Goudie AD, Finlay WH, Dennis JJ. Aerosol phage therapy efficacy in Burkholderia cepacia complex respiratory infections. Antimicrob Agents Chemother 2014; 58:4005–13; PMID:24798268; http://dx.doi.org/ 10.1128/AAC.02388-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith HW, Huggins MB. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol 1983; 129:2659–75; PMID:6355391 [DOI] [PubMed] [Google Scholar]
- 64.Hyman P, Abedon ST. Practical methods for determining phage growth parameters. Meth Mol Biol 2009; 501:175–202; PMID:19066822; http://dx.doi.org/ 10.1007/978-1-60327-164-6_18 [DOI] [PubMed] [Google Scholar]
- 65.Abedon ST. Lysis and the interaction between free phages and infected cells In: Karam JD, Kutter E, Carlson K, Guttman B, eds. The Molecular Biology of Bacteriophage T4. Washington, DC: ASM Press, 1994:397–405. [Google Scholar]
- 66.Abedon ST. Bacteriophage T4 resistance to lysis-inhibition collapse. Genet Res 1999; 74:1–11; PMID:10505404; http://dx.doi.org/ 10.1017/S00166-72399003833 [DOI] [PubMed] [Google Scholar]
- 67.Bull JJ, Otto G, Molineux IJ. In vivo growth rates are poorly correlated with phage therapy success in a mouse infection model. Antimicrob Agents Chemother 2012; 56:949–54; PMID:22106213; http://dx.doi.org/ 10.1128/AAC.05842-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bull JJ, Gill JJ. The habits of highly effective phages: population dynamics as a framework for identifying therapeutic phages. Front Microbiol 2014; 5:618; PMID:25477869; http://dx.doi.org/ 10.3389/fmicb.2014.00618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindberg HM, McKean KA, Wang I-N. Phage fitness may help predict phage therapy efficacy. Bacteriophage 2014; 4:e964081; http://dx.doi.org/ 10.4161/21597073.2014.964081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry M, Bobay LM, Chevallereau A, Saussereau E, Ceyssens PJ, Debarbieux L. The search for therapeutic bacteriophages uncovers one new subfamily and two new genera of pseudomonas-infecting myoviridae. PLoS One 2015; 10:e0117163; PMID:25629728; http://dx.doi.org/ 10.1371/journal.pone.0117163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kutter EM, Kuhl S, Abedon ST. Reestablishing a place for phage therapy in western medicine. Future Microbiology 2015; http://dx.doi.org/ 10.2217/FMB.15.28 [DOI] [PubMed] [Google Scholar]


