Abstract
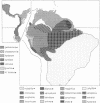
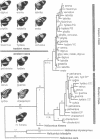
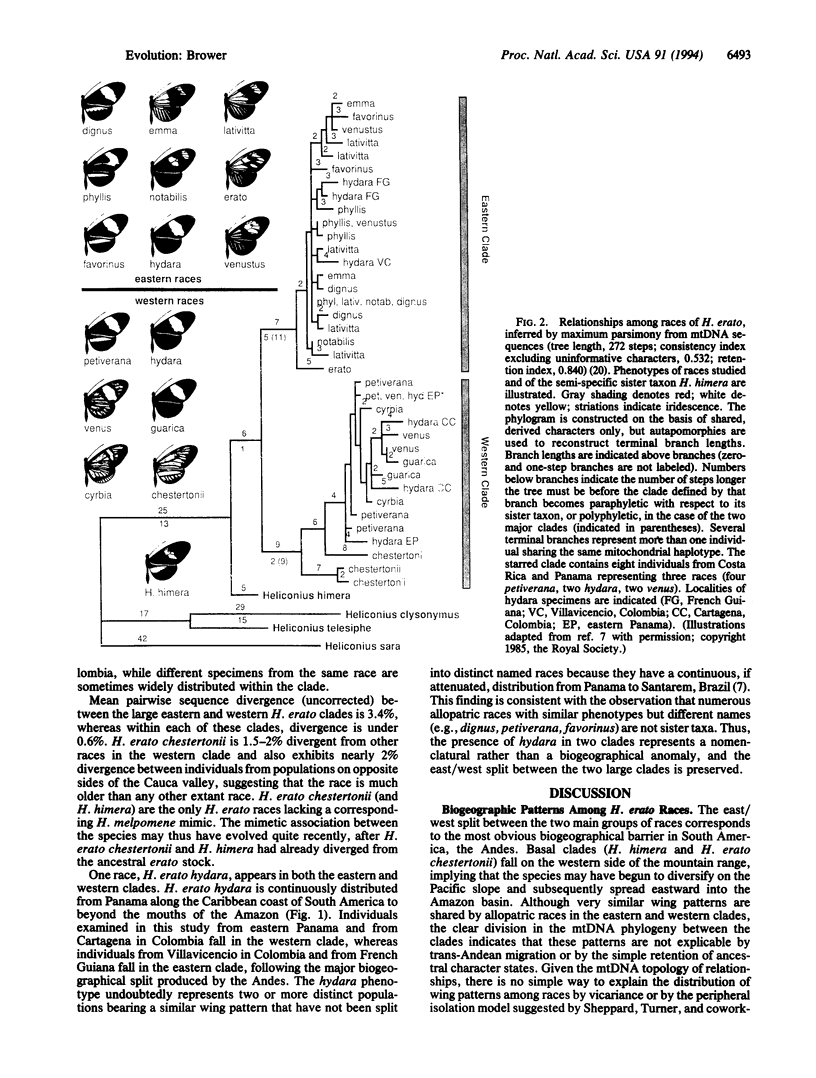
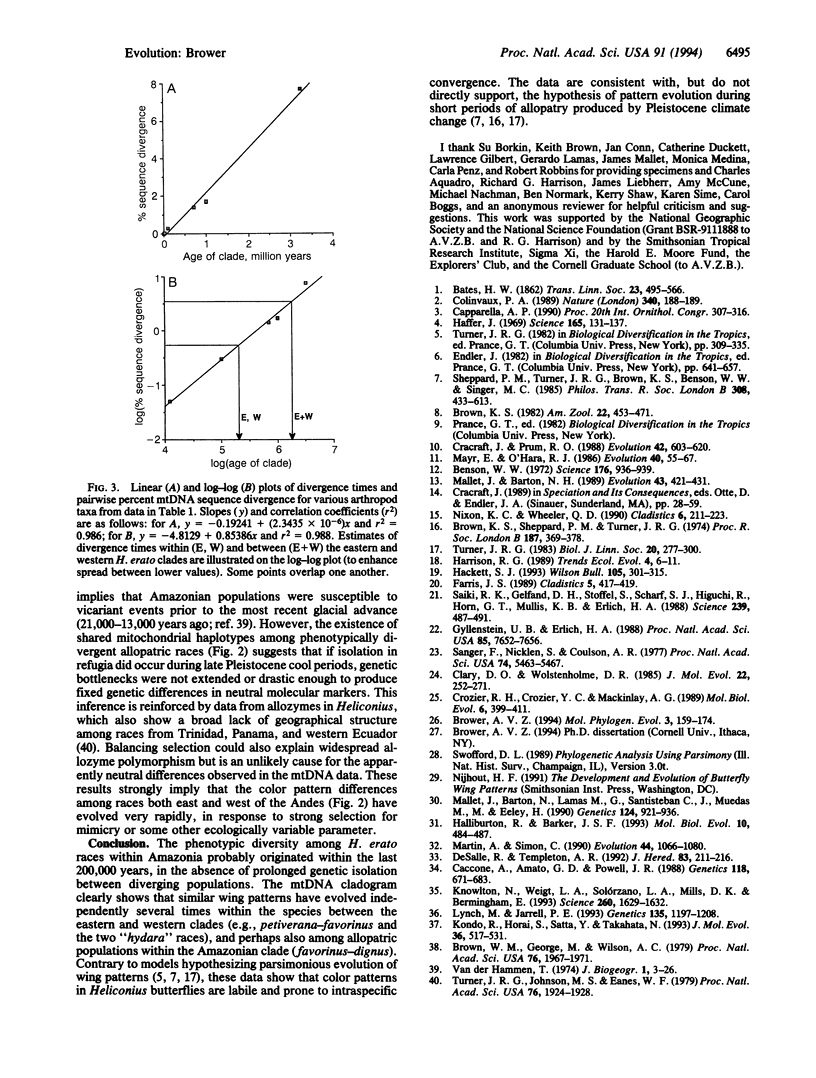
The neotropical Heliconius butterflies are famous examples of Müllerian mimicry, due to the diverse array of shared, brightly colored wing patterns that advertise the butterflies' unpalatability. The parallel geographical variation in these patterns within several widespread species has been invoked to support the controversial Pleistocene refugium hypothesis of tropical diversification. However, in no Heliconius species have either evolutionary rates or relationships among geographical races been explicitly examined. I present a phylogenetic hypothesis based on mitochondrial DNA sequences for 14 divergent races of Heliconius erato, which reveals that similar wing patterns have evolved rapidly and convergently within the species. There is a basal split between groups of races from east and west of the Andes, reflecting a vicariant separation at the base of the Pleistocene. Within each of these clades, sequence divergence is very low, and some haplotypes are shared between allopatric races with radically different wing patterns. The topology implies a simultaneous radiation of races in these two areas within the last 200,000 years. Ages for the clades are estimated by comparing sequence divergence to a plot of mitochondrial divergence in several arthropod taxa with independently dated divergence times. This plot is linear and suggests that mitochondrial DNA in arthropods evolves in a clocklike manner, at least initially, when sequence divergence is low.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson W. W. Natural Selection for Miillerian Mimicry in Heliconius erato in Costa Rica. Science. 1972 May 26;176(4037):936–939. doi: 10.1126/science.176.4037.936. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccone A., Amato G. D., Powell J. R. Rates and patterns of scnDNA and mtDNA divergence within the Drosophila melanogaster subgroup. Genetics. 1988 Apr;118(4):671–683. doi: 10.1093/genetics/118.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Crozier R. H., Crozier Y. C., Mackinlay A. G. The CO-I and CO-II region of honeybee mitochondrial DNA: evidence for variation in insect mitochondrial evolutionary rates. Mol Biol Evol. 1989 Jul;6(4):399–411. doi: 10.1093/oxfordjournals.molbev.a040553. [DOI] [PubMed] [Google Scholar]
- DeSalle R., Templeton A. R. The mtDNA genealogy of closely related Drosophila silvestris. J Hered. 1992 May-Jun;83(3):211–216. doi: 10.1093/oxfordjournals.jhered.a111195. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffer J. Speciation in amazonian forest birds. Science. 1969 Jul 11;165(3889):131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- Halliburton R., Barker J. S. Lack of mitochondrial DNA variation in Australian Drosophila buzzatii. Mol Biol Evol. 1993 Mar;10(2):484–487. doi: 10.1093/oxfordjournals.molbev.a040014. [DOI] [PubMed] [Google Scholar]
- Knowlton N., Weigt L. A., Solórzano L. A., Mills D. K., Bermingham E. Divergence in proteins, mitochondrial DNA, and reproductive compatibility across the isthmus of Panama. Science. 1993 Jun 11;260(5114):1629–1632. doi: 10.1126/science.8503007. [DOI] [PubMed] [Google Scholar]
- Kondo R., Horai S., Satta Y., Takahata N. Evolution of hominoid mitochondrial DNA with special reference to the silent substitution rate over the genome. J Mol Evol. 1993 Jun;36(6):517–531. doi: 10.1007/BF00556356. [DOI] [PubMed] [Google Scholar]
- Lynch M., Jarrell P. E. A method for calibrating molecular clocks and its application to animal mitochondrial DNA. Genetics. 1993 Dec;135(4):1197–1208. doi: 10.1093/genetics/135.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J., Barton N., Lamas G., Santisteban J., Muedas M., Eeley H. Estimates of selection and gene flow from measures of cline width and linkage disequilibrium in heliconius hybrid zones. Genetics. 1990 Apr;124(4):921–936. doi: 10.1093/genetics/124.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Johnson M. S., Eanes W. F. Contrasted modes of evolution in the same genome: allozymes and adaptive change in Heliconius. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1924–1928. doi: 10.1073/pnas.76.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zandt Brower A. Phylogeny of Heliconius butterflies inferred from mitochondrial DNA sequences (Lepidoptera: Nymphalidae). Mol Phylogenet Evol. 1994 Jun;3(2):159–174. doi: 10.1006/mpev.1994.1018. [DOI] [PubMed] [Google Scholar]