Abstract
The differential expression of Ia antigens was studied in freshly isolated rheumatoid nonlymphoid synovial lining cells (SLC) and rheumatoid synovial fibroblast cell lines cultured in the presence of Interferon-gamma, using a large panel of anti-Ia reagents with monomorphic or polymorphic specificities. All the HLA-DR or -DQ specificities detectable on the corresponding peripheral blood B cells were also expressed in freshly isolated SLC. However, in all instances, the number of DR-positive SLC exceeded the percentage of cells expressing DQ antigens. In addition, the epitope expression of Ia antigens varied within the DR or DQ populations of Ia molecules as revealed by polymorphic reagents. Double-label experiments or using the ingestion of Latex particles as a marker demonstrated that the synovial macrophages (type I SLC) primarily bear the DR+DQ+ phenotype, while there is an additional population of nonphagocytic SLC (previously termed type II SLC) that has a DR+ and monocyte marker negative phenotype but did not have detectable levels of DQ antigens as analyzed by both fluorescence microscopy and cell sorter analysis. This latter population frequently had a morphology showing dendritic processes and rapidly lost the expression of Ia antigens upon culture. Cells with a similar, primarily DR+ phenotype were readily obtained in synovial fibroblast cultures after treatment with Interferon-gamma. These data suggest that there are two populations of Ia+ synovial lining cells: the synovial macrophages (type I cells) with the DR+DQ+ phenotype, and cells probably related to fibroblasts with a DR+ phenotype without detectable DQ antigens (type II cells). The fact that the latter phenotype could be induced by Interferon-gamma treatment of cultured synovial fibroblasts suggests that this mediator may have a similar role in vivo in the activation of certain synovial cell populations.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Balaghi S., Ström H., Möller E. High incidence of spontaneous Ig-producing lymphocytes in peripheral blood and synovial fluid of patients with active seropositive rheumatoid arthritis. Scand J Immunol. 1982 Jul;16(1):69–76. doi: 10.1111/j.1365-3083.1982.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Amento E. P., Bhan A. K., McCullagh K. G., Krane S. M. Influences of gamma interferon on synovial fibroblast-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest. 1985 Aug;76(2):837–848. doi: 10.1172/JCI112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballardini G., Mirakian R., Bianchi F. B., Pisi E., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984 Nov 3;2(8410):1009–1013. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. A matrix approach to human class II histocompatibility antigens: reactions of four monoclonal antibodies with the products of nine haplotypes. Immunogenetics. 1984;19(3):179–194. doi: 10.1007/BF00364762. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Bodmer W. F. Monoclonal antibodies to HLA--DRw determinants. Tissue Antigens. 1980 Jul;16(1):30–48. doi: 10.1111/j.1399-0039.1980.tb00285.x. [DOI] [PubMed] [Google Scholar]
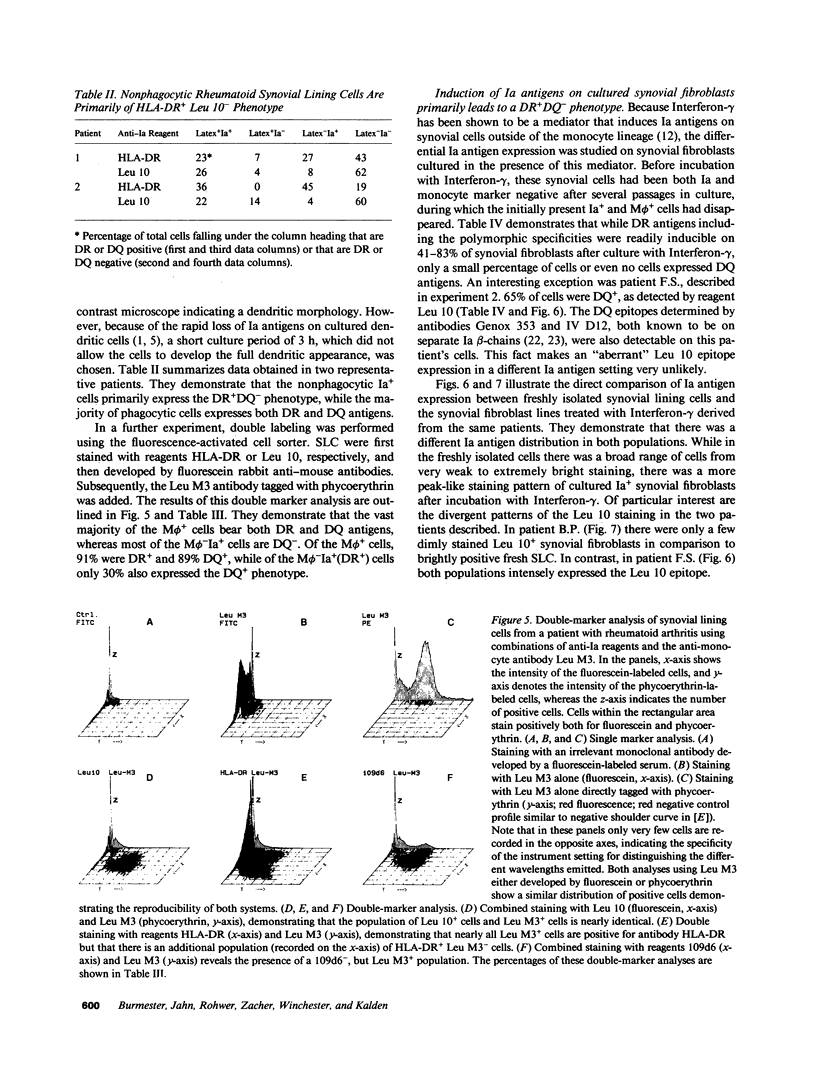
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Kalden J. R., Peter H. H., Schedel I., Beck P., Wittenborg A. Immunological and functional characteristics of peripheral blood and synovial fluid lymphocytes from patients with rheumatoid arthritis. Scand J Immunol. 1978;7(5):405–417. doi: 10.1111/j.1365-3083.1978.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Locher P., Koch B., Winchester R. J., Dimitriu-Bona A., Kalden J. R., Mohr W. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol Int. 1983;3(4):173–181. doi: 10.1007/BF00541597. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Menche D., Merryman P., Klein M., Winchester R. Application of monoclonal antibodies to the characterization of cells eluted from human articular cartilage. Expression of Ia antigens in certain diseases and identification of an 85-kD cell surface molecule accumulated in the pericellular matrix. Arthritis Rheum. 1983 Oct;26(10):1187–1195. doi: 10.1002/art.1780261003. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Schneeberger J., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Rheumatoid non-lymphoid synovial cells and the induction of mixed leukocyte reactions. Low-density preparations containing Ia+ macrophages and dendritic cells are less stimulatory than peripheral blood non-T cells. Rheumatol Int. 1984;4 (Suppl):31–37. doi: 10.1007/BF00541276. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Winchester R. J., Dimitriu-Bona A., Klein M., Steiner G., Sissons H. A. Delineation of four cell types comprising the giant cell tumor of bone. Expression of Ia and monocyte-macrophage lineage antigens. J Clin Invest. 1983 Jun;71(6):1633–1648. doi: 10.1172/JCI110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Cesario T. C., Andrews B. S., Martin D. A., Jason M., Treadwell T., Friou G., Tilles J. G. Interferon in synovial fluid and serum of patients with rheumatic disease. J Rheumatol. 1983 Aug;10(4):647–650. [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Passwell J. H., Schneeberger E. E., Krane S. M. Interactions among rheumatoid synovial cells and monocyte-macrophages: production of collagenase-stimulating factor by human monocytes exposed to concanavalin A or immunoglobulin Fc fragments. J Immunol. 1980 Apr;124(4):1712–1720. [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Giles R. C., Nunez G., Hurley C. K., Nunez-Roldan A., Winchester R., Stastny P., Capra J. D. Structural analysis of a human I-A homologue using a monoclonal antibody that recognizes an MB3-like specificity. J Exp Med. 1983 May 1;157(5):1461–1470. doi: 10.1084/jem.157.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Immunohistochemical studies of interleukin-2 and gamma-interferon in rheumatoid arthritis. Arthritis Rheum. 1985 Feb;28(2):174–181. doi: 10.1002/art.1780280212. [DOI] [PubMed] [Google Scholar]
- Jahn B., Burmester G. R., Schmid H., Weseloh G., Rohwer P., Kalden J. R. Changes in cell surface antigen expression on human articular chondrocytes induced by gamma-interferon. Induction of Ia antigens. Arthritis Rheum. 1987 Jan;30(1):64–74. doi: 10.1002/art.1780300109. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Meo T., Riethmüller G., Schendel D. J., Wank R. Direct demonstration of an HLA-DR allotypic determinant on the low molecular weight (beta) subunit using a mouse monoclonal antibody specific for DR3. J Exp Med. 1982 Jul 1;156(1):104–111. doi: 10.1084/jem.156.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Johnell O., Hulth A. Expression of HLA-DR and HLA-DQ antigens on cells within the cartilage-pannus junction in rheumatoid arthritis. Rheumatol Int. 1984;4 (Suppl):11–15. doi: 10.1007/BF00541273. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lee S. H., Gregersen P. K., Shen H. H., Nunez-Roldan A., Silver J., Winchester R. J. Strong association of rheumatoid arthritis with the presence of a polymorphic Ia epitope defined by a monoclonal antibody: comparison with the allodeterminant DR4. Rheumatol Int. 1984;4 (Suppl):17–23. doi: 10.1007/BF00541274. [DOI] [PubMed] [Google Scholar]
- Müller C., Ziegler A., Muller C., Hadam M., Waller H. D., Wernet P., Müller G. Divergent expression of HLA-DC/MB, -DR, and -SB region products on normal and pathological tissues as detected by monoclonal antibodies. Immunobiology. 1985 Apr;169(3):228–249. doi: 10.1016/s0171-2985(85)80036-x. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Navarrete C., Jaraquemada D., Hui K., Awad J., Okoye R., Festenstein H. Different functions and associations of HLA-DR and HLA-DQ(DC) antigens shown by serological, cellular and DNA assays. Tissue Antigens. 1985 Mar;25(3):130–141. doi: 10.1111/j.1399-0039.1985.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Oliveira D. B., Blackwell N., Virchis A. E., Axelrod R. A. T helper and T suppressor cells are restricted by the A and E molecules, respectively, in the F antigen system. Immunogenetics. 1985;22(2):169–175. doi: 10.1007/BF00563514. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel D. J., Johnson J. P. T cells specific for different antigens express different HLA-D region products. Eur J Immunol. 1985 Dec;15(12):1239–1243. doi: 10.1002/eji.1830151217. [DOI] [PubMed] [Google Scholar]
- Schober I., Braun R., Reiser H., Munk K., Leroux M., Kirchner H. la-positive T lymphocytes are the producer cells of interferon gamma. Exp Cell Res. 1984 Jun;152(2):348–356. doi: 10.1016/0014-4827(84)90636-0. [DOI] [PubMed] [Google Scholar]
- Sogn J. A., Robinson M. A., Kulaga H. Functional distinctions among the products of different class II subregions. Scand J Immunol. 1984 Dec;20(6):478–483. doi: 10.1111/j.1365-3083.1984.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Tjernlund U. M. Ia-like antigens in lichen planus. Acta Derm Venereol. 1980;60(4):309–314. doi: 10.2340/0001555560309314. [DOI] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Toguchi T., Burmester G., Nunez-Roldan A., Gregersen P., Seremetis S., Lee S., Szer I., Winchester R. Evidence for the separate molecular expression of four distinct polymorphic Ia epitopes on cells of DR4 homozygous individuals. Hum Immunol. 1984 Jun;10(2):69–81. doi: 10.1016/0198-8859(84)90074-0. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Burmester G. R. Demonstration of Ia antigens on certain dendritic cells and on a novel elongate cell found in human synovial tissue. Scand J Immunol. 1981 Oct;14(4):439–444. doi: 10.1111/j.1365-3083.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Brinckerhoff C. E., Mainardi C. L., Vater C. A., Evanson J. M., Harris E. D., Jr Collagenase production by rheumatoid synovial cells: morphological and immunohistochemical studies of the dendritic cell. Ann Rheum Dis. 1979 Jun;38(3):262–270. doi: 10.1136/ard.38.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Elferink B. G., Hermans J., de Vries R. R., van Rood J. J. Role of HLA class II products in proliferative T-lymphocyte responses to PPD. Evidence of a regulatory influence associated with MB1. Scand J Immunol. 1984 Dec;20(6):503–510. doi: 10.1111/j.1365-3083.1984.tb01032.x. [DOI] [PubMed] [Google Scholar]