Abstract
Extracellular matrix proteins of the tenascin family resemble each other in their domain structure, and also share functions in modulating cell adhesion and cellular responses to growth factors. Despite these common features, the 4 vertebrate tenascins exhibit vastly different expression patterns. Tenascin-R is specific to the central nervous system. Tenascin-C is an “oncofetal” protein controlled by many stimuli (growth factors, cytokines, mechanical stress), but with restricted occurrence in space and time. In contrast, tenascin-X is a constituitive component of connective tissues, and its level is barely affected by external factors. Finally, the expression of tenascin-W is similar to that of tenascin-C but even more limited. In accordance with their highly regulated expression, the promoters of the tenascin-C and -W genes contain TATA boxes, whereas those of the other 2 tenascins do not. This article summarizes what is currently known about the complex transcriptional regulation of the 4 tenascin genes in development and disease.
Keywords: cytokine, cancer, development, extracellular matrix, glucocorticoid, growth factor, gene regulation, gene promoter, homeobox gene, matricellular, mechanical stress, tenascin, transcription factor
Abbreviations
- AKT
v-akt murine thymoma viral oncogene homolog
- ALK
anaplastic lymphoma kinase
- ATF
activating transcription factor
- AP-1
activator protein-1
- BMP
bone morphogenetic protein
- CBP
CREB binding protein
- ChIP
chromatin immunoprecipitation
- CREB
cAMP response element-binding protein
- CREB-RP
CREB-related protein
- CYP21A2
cytochrome P450 family 21 subfamily A polypeptide 2
- EBS
Ets binding site
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ERK1/2
extracellular signal-regulated kinase 1/2
- ETS
E26 transformation-specific
- Evx1
even skipped homeobox 1
- EWS-ETS
Ewing sarcoma-Ets fusion protein
- FGF
fibroblast growth factor
- HBS
homeodomain binding sequence
- IL
interleukin
- ILK
integrin-linked kinase
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinase
- MHCIII
major histocompatibility complex class III
- miR
micro RNA
- MKL1
megakaryoblastic leukemia-1
- NGF
nerve growth factor; NFAT, nuclear factor of activated T-cells
- NFκB
nuclear factor kappa B
- OTX2
orthodenticle homolog 2
- p38 MAPK
p38 mitogen activated protein kinase
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol 3-kinase
- POU3F2
POU domain class 3 transcription factor 2
- PRRX1
paired-related homeobox 1
- RBPJk
recombining binding protein suppressor of hairless
- RhoA
ras homolog gene family member A
- ROCK
Rho-associated, coiled-coil-containing protein kinase
- SAP
SAF-A/B, Acinus, and PIAS
- SCX
scleraxix
- SEAP
secreted alkaline phosphatase
- SMAD
small body size - mothers against decapentaplegic
- SOX4
sex determining region Y-box 4
- SRE
serum response element
- SRF
serum response factor
- STAT
signal transducer and activator of transcription
- TGF-β
transforming growth factor-β
- TNC
tenascin-C
- TNF-α
tumor necrosis factor-α
- TNR
tenascin-R
- TNW
tenascin-W
- TNX
tenascin-X
- TSS
transcription start site
- UTR
untranslated region
- WNT
wingless-related integration site
Introduction: The Tenascin Gene Family
Tenascins are a family of large, oligomeric, multi-domain extracellular matrix (ECM) proteins.1 Four genes encoding tenascin-C (TNC), tenascin-R (TNR), tenascin-X (TNX), and tenascin-W (TNW) proteins exist in higher vertebrates, and a single tenascin gene is found in cephalochordates whereas similar genes and proteins do not seem to exist in other animal phyla.2-4 Tenascins are characterized by their unique domain structure. Each monomeric unit comprises an N-terminus with heptad repeats flanked by cysteine residues. This N-terminal oligomerization region is followed by EGF-like repeats, and a variable number of fibronectin type III repeats as a result of alternative mRNA splicing. At the C-terminus, each subunit ends with a large C-terminal fibrinogen related domain.1 Via their N-terminal oligomerization domain, tenascin subunits form disulfide-linked homo-trimers (TNR and TNX) or -hexamers (“hexabrachions;” TNC and TNW). Rather than representing bona fide structural components of the extracellular matrix, tenascins are “matricellular” proteins4 involved in modifying the interaction of cells with extracellular matrix and growth factors, and hence regulating cell adhesion, migration, growth and differentiation in a context-dependent manner5 (see other articles in this issue).
A number of earlier reviews have summarized the discovery,6-9 protein structure,1,8 splice variants,10,11 binding partners and cellular receptors,12 expression patterns13 and functions in vitro and in vivo9,14-17 of the 4 tenascins, and more information on these topics is to be found in other contributions to this special issue. The present article has a different and narrow focus, namely to summarize what is currently known about the regulation of expression of tenascins. We briefly review the expression patterns of the 4 tenascins in development, regeneration and disease, and focus on the transcriptional regulation of their respective genes by growth factors and mechanical stimuli. Except for TNX, which has a widespread distribution like many ECM proteins, the other 3 tenascins show a very restricted occurrence during embryogenesis, tissue remodeling and tumor formation.8,9,18,19 Their patterns of localization, which are specific for each of the 4 family members, point to tightly controlled spatial and temporal expression, and are likely to reflect complex gene regulation. To date, the promoter of the TNC gene has been studied most extensively in various species, whereas information on the gene promoters of the other 3 family members is still comparatively sparse. Thus, the apparent imbalance between chapters in this article reflects the current status of the literature.
Tenascin-C: Expression in Organogenesis, Inflammation, Tissue Repair and Cancer
Structure of the tenascin-C (TNC) gene
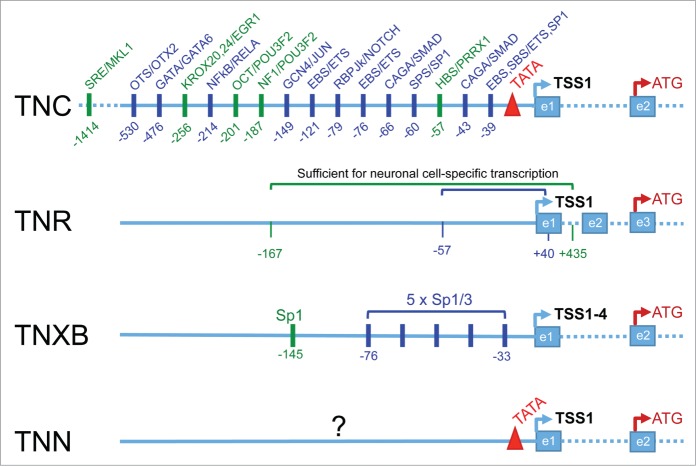
Tenascin-C (gene name TNC) is the founding member of the respective family of ECM proteins.5 The human TNC gene (gene ID: 3371) is on chromosome 9q33; it contains 29 exons of which 9 (each coding for a fibronectin type III domain) can be alternatively spliced.20-22 The transcript starts with a non-coding exon, separated by an intron >20 kb long, and followed by exon 2, which contains the ATG start codon for translation initiation. TNC mRNA from human fibroblasts and human melanoma cells analyzed by primer extension and S1 nuclease showed a single transcription start site (TSS) localized to the first exon (Fig. 1). Sequencing of approximatively 2300 bp of the TNC gene 5′-flanking region has revealed several potential binding sites for transcription factors (see below).20 The sequence of 220 bp upstream to the TSS was identified as region with high promoter activity; it contains a classical TATA box at −20 to −26 bp. A putative silencer sequence was localized to the fragment between −220 and −2300 bp.20 Similarly, primer extension analysis of mRNA isolated from brain tissue of mouse embryos showed a single TSS that lays 27 bp downstream of the TATA box.23 Moreover, the 230 bp proximal promoter sequence, which is conserved between species, was found to be highly active in driving reporter gene expression when transfected into both mouse and human fibroblasts.23 The chicken TNC (cytotactin) gene features a TATA box at a similar position as the mammalian counterparts.24 A comparison between the human, mouse and chicken TNC promoters has been presented by Jones and Jones (2000).25
Figure 1.
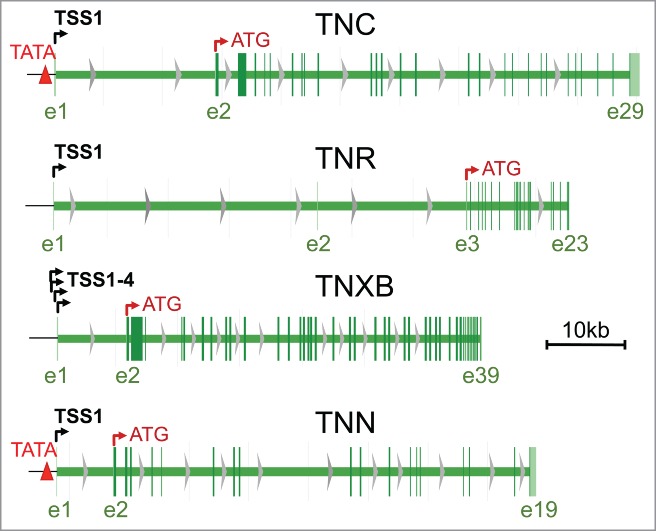
Schematic representation of all tenascin genes. Gene models of TNC, tenascin-C; TNN, tenascin-W; TNR, tenascin-R; TNXB, tenascin-X were captured from the NCBI database (http://www.ncbi.nlm.nih.gov/gene/). TNC, TNN and TNR have a single transcription start site (TSS1) whereas the TNXB gene has 4 closely clustered TSSs(TSS1–4) in its principle promoter shown here. Non-coding exons up to the first coding exons (indicated by the translation start codon ATG) as well as the last exons are numbered with e1, 2, … below the models. Note that the TNC and TNN genes possess TATA boxes (red triangles) whereas the TNR and the TNXB genes do not.
Tenascin-C gene regulation by patterning genes during development
TNC received much attention after its discovery because of its highly specific and restricted expression patterns during vertebrate embryogenesis.26,27 In contrast to many other ECM proteins, TNC often appears in an all-or-none fashion both in space and time. Specifically, the protein is an early marker of tendon, ligament and bone formation.26 Other prominent sources are neural crest cells in early embryos,28 Schwann cell precursors in developing peripheral nerves,29,30 and vascular smooth muscle cells around arteries.31 In addition, TNC expression is often associated with specific morphogenetic events during organogenesis, e.g. with the formation of somites, segmental nerves,30 mammary glands,27,32 teeth,33 kidneys34 and lungs.35 It was therefore an obvious possibility that the TNC gene could be controlled by segmentation and patterning genes. Indeed, some of the early publications on TNC promoters from different species investigated their regulation by homeobox transcription factors (for detailed information about the location and sequence of cis-acting elements in the TNC promoter, see Table 1 and Fig. 2). For example, the chicken promoter was found to be strongly activated by co-transfection of fibroblasts with even-skipped homeobox 1 (EVX1), and by mutational analysis, an AP1 element was identified that was essential for this response.36 The same AP1 site was found to mediate activation of the promoter by serum growth factors, and EVX1 overexpression potentiated the effect of serum. Thus, EVX1 appears to activate the TNC gene indirectly by synergizing with JUN/FOS transcription factors, which target the AP1 site.
Table 1.
Summary of transcriptional regulation of the tenascin gene promoters. For each publication cited, the species of the promoter analyzed, the transcription factor(s) over-expressed or growth factors added, the response elements and their sequences and relative position to the TSS are listed together with a short description of the main experimental evidence provided
| Gene | Promoter species | Transcription factor overexpressed or factors added | Response element | Sequence | Position | Functional assays |
|---|---|---|---|---|---|---|
| TNC | Chicken 24 | −3986 to + 121 | 3986bp more active than 721bp promoter in chicken embryo fibroblasts. 3986bp promoter active in human U251MG cells, but not in HT1080 cells. | |||
| Chicken 36 | EVX1 (mouse) | TRE/AP1 | CTGAGTCAT | −281 to −273 | Reporter activation in NIH3T3 cells and inactivation by mutation of response elements. | |
| Chicken 36 | HBS 1 | TAATGATGAT | −1354 to −1345 | In vitro binding assay with ftz homeodomain. | ||
| HBS 2 | TAATGATTCT | −1369 to −1360 | ||||
| Chicken 63 | AP1 | CTGAGTCAT | −281 to −273 | Reporter activation in chicken embryo fibroblasts; deletion of response element. | ||
| ECM | −570 to −469 | Reporter activation on attached but not floating collagen gels; transfer of response to SV40 promoter | ||||
| Mouse 23 | −247 to + 147 | 247bp promoter construct was more active than longer constructs in NIH3T3 and chicken fibroblasts | ||||
| Mouse 39 | POU3F2 (BRN2) | octamer | ATGCAATG | −201 to −193 | Reporter activation in mouse mouse N2A cells, but not in rat C6 cells. Binding assays (EMSA, footprint). | |
| NF1 | TGGCGGCGCGCCT | −187 to −165 | Inactivation of reporter activity by mutation of response element in N2A, C6 and NIH3T3 cells. | |||
| EGR1 (KROX-24) | KROX20/24 | GCGGGGGCG | −256 to −248 | In vitro binding assays. Presence of element represses reporter in N2A cells, but not in NIH3T3 or C6 cells. | ||
| Mouse 42,43 | PRRX1 (PRX1) | HBS | CATTAC | −57 to −52 | Reporter activation in rat A10 vascular SMCs and RFL-6 lung fibroblasts, EMSA and supershift. | |
| Mouse 58 | MKL1 | SRE (CArG) | CTATTTATGG | −1414 to −1423 | SRF-dependent reporter activation in NIH3T3 cells and inactivation by mutation of response elements. | |
| MKL1mutB1 | −247 to + 147 | SRF-independent SAP domain dependent promoter activation by cyclic strain; ChIP | ||||
| Mouse 59 | MKL1 MKL1mutB1 |
−247 to + 147 | SRF-independent SAP domain dependent promoter activation in HC11 mammary epithelial cells Transcipts induced in SOX4-transfected LNCaP cells. |
|||
| Human 86 | SOX4 | |||||
| Human 20 | −220 to +79 | 220bp promoter more active than longer constructs in SK-Mel-28 and InR1-G9 cells. Positive effect of first 20bp of first exon; inclusion of 1338bp of the first intron in the long promoter construct activates the reporter in SK-Mel-28, but not InR1-G9 cells. | ||||
| Human 37 | OTX2 | OTS | TAATCC | −530 to −525 | In vitro binding assays. | |
| Human 38 | OTX2 | OTS | TCTAATCCC | −531 to −523 | Reporter repression in U87-MG human glioblastoma cells; EMSA and binding studies. | |
| Human 78 | TGF-β and SMAD3 and 4ETS1 | CAGA1CAGA2EBS1 | TCTGGCAGAGTTCC | −66 to −62−43 to −39−121 to −118 | Reporter activation in human dermal fibroblasts in a complex with CBP-300; mutation of response elements; DNA affinity precipitation; Co-IP`s of complexes. | |
| EBS4 | GGAA | −39 to −36 | ||||
| SP1 | SBS2 | GGC | −60 to −58 | |||
| SBS3 | GGA | −39 to −37 | ||||
| TNC | Human 79 | PDGF and ETS1,2 and SP1 | EBS1EBS3EBS4SBS2 | TTCCGGAAAGGATGGAAGGC | −121 to −118−76 to −68−39 to −36−60 to −58 | Reporter activation in human dermal fibroblasts after PDGF stimulation; mutation of response elements; overexpression of dominant negative TFs; Co-IP`s of complexes |
| SBS3 | GGA | −39 to −37 | ||||
| PDGF and FLI1 | SBS2SBS3 | GGCGGA | −60 to −58−39 to −37 | Abrogation of PDGF-induced reporter expression in human dermal fibroblasts | ||
| Human 84 | EWS-FLI; EWS-ERG (EWS-ETS) | EBS1–4 | GGAA | −130 to −30 | Reporter activation in H1299 cells. Endogenous transcripts induced by EWS-ETS, but not FLI or ERG | |
| Human 85 | JUNRELA (p65) | GCN4NFkB | TGAGTGGGGAATTCCT | −149 to −144−214 to −207 | Synergistic reporter activation; mutation of response elements EMSA; transcripts induced by Jun overexpression in rat embryo fibroblasts. | |
| Human 92 | NOTCH2 | RBPJk | GTGGGAA | −79 to −73 | Reporter activation in Hs683 cells and inactivation by mutation of response element, or mutation of NOTCH2. | |
| Human 83 | GATA6 | TTATCC | −467 to −462 | Reporter repression in human dermal fibroblasts (both basal and IL-4 or TGF-β induced); mutation of binding element; ChIP | ||
| Human 62 | NFKBIA (IkBα); IKBKB (IKK-β) | NFkB | GGGAATTCCT | −214 to −207 | Deletion of binding element inhibits promoter activation; EMSA; Overexpression of dominant negative regulators of NFkB inhibit strain-induced promoter activation in neonatal rat cardiac myocytes. | |
| TNR | Mouse 101 | −167 to +435 | 167bp promoter was sufficient for full activity of reporter expression in cell lines of neural or glial origin but not in NIH3T3 and P19 teratocarcinoma cells. | |||
| Human 99 | −57 to +40 | 57bp promoter was sufficient for full activity of reporter expression in cell lines of neural or glial origin but not in SK-MEL28 and HeLa cells. | ||||
| TNXB | Mouse 124 | −150 to +333 | More active than longer or shorter constructs in mouse L and human 293Tcells | |||
| SP1 | GGGAGG | −145 to −150 | Mutation of binding element reduces reporter activity; EMSA and supershifts. | |||
| Human 121 | −181 to +88 | Higher activity in HT1080 cells than longer or shorter constructs; TSS in HT1080 cells and fibroblasts at +46bp. | ||||
| SP1, SP3 | SP1/3 | GGG…GGG…GGG…GGG…CCC | −33 to −76 | Activation of 311bp and 181bp reporter constructs in Drosophila S9 cells. Mutation of binding sites inhibits promoter activity; EMSA and supershifts. |
Figure 2.
Scheme of the gene promoters of the 4 tenascins. The transcription start sites (TSS) are indicated with blue arrows in front of the first exons (e1; blue boxes). The start codons (ATG) of the translation start sites are marked with red arrows in the second (e2) or third exon (e3; for TNR), respectively. The upstream promoter sequences are represented by horizontal light blue lines, on which the experimentally confirmed transcription factor binding sites are marked by vertical bars. Dark blue color refers to those sites reported for the human promoters; sites described so far in the mouse promoters only are labeled in green. For nomenclature of binding sites and transcription factors, see the list of abbreviations. For additional information and publications on individual binding sites, refer to Table 1. Note that the promoter sequences are not drawn to scale. However, the exact location of each binding site is indicated below the bars; numbers refer to the distance in base pairs from the transcription start site.
On the other hand, a homeobox transcription factor involved in anterior head formation, orthodenticle homolog 2 (OTX2), was shown to bind directly and with high affinity to the human TNC promoter and to suppress its transcriptional activity;37,38 the OTX2 target sequence is conserved in the mouse (but not chicken) gene. Similarly, the POU-homeodomain transcription factor POU3F2 (also called BRN2 or Oct-7) was demonstrated to interact directly with a reverse octamer sequence in the mouse Tnc promoter, which is conserved in the human and also the chicken gene.39
In addition, the proximal promoter of the chicken, mouse and human TNC gene contains another conserved homeodomain binding sequence (HBS).25 Paired-related homeobox 1 (PRRX1; formerly called PRX1 or Mhox), a transcription factor involved in limb and craniofacial morphogenesis,40 is a marker for periosteal cells41 and often co-expressed with TNC. Overexpression of PRRX1 strongly induced a full-length mouse Tnc promoter construct in a vascular smooth muscle cell line.42 Later, PRRX1 was demonstrated to trans-activate Tnc gene transcription in mouse pulmonary endothelial cells through direct interaction with the HBS located within the proximal promoter.43 Furthermore, increased deposition of TNC along the arterial wall in pulmonary vascular lesions of patients with mutated BMP type II receptors was highly associated with the expression of PRRX1.44
TNC is a prominent early marker for developing tendons.26 The basic helix-loop-helix transcription factor scleraxis (SCX) is essential for development of load-bearing tendons.45 The Tnc gene was therefore assumed to be a target gene of this tendon-specific transcription factor.46 In Scx null mouse embryos, however, TNC still accumulates in condensing mesenchyme where tendons normally develop.45 Therefore, the Tnc gene appears to be controlled by factors that act upstream of SCX during early tendon morphogenesis, such as PRRX1 (see above).
Tenascin-C gene regulation by mechanical stress
Whereas TNC is expressed transiently in many developing organs, it persists in the adult mainly in a few structures bearing high tensile stress, such as tendons, ligaments, and the smooth muscle walls of arteries.7,25 It was therefore speculated early on that its gene might be regulated by mechanical forces. Indeed, TNC expression was found to be induced in vivo e.g., by hypertension in the arterial walls of rats,47 or upon supra-physiological loading in skeletal muscle connective tissue of chicken,48 rat49 and human.50 Transduction of external mechanical stimuli requires integrins as bridges between ECM and the cytoskeleton.51 Depending on the precise nature of the stimulus, various integrin-dependent signaling pathways can then be triggered, such as Ca influx, activation of ERK1/2, NFκB, and RhoA/ROCK.52 An extensively studied mechanotransduction pathway concerns the rapid activation of the TNC gene by cyclic strain (10%, 0.3 Hz for 1–6 h) in chicken and mouse fibroblasts attached to elastic substrates, which depends on Rho/ROCKsignaling.53 Pericellular fibronectin, integrin α5β154 and integrin-linked kinase (ILK)55 were shown to be required for RhoA activation and TNC induction in response to cyclic stretch in mouse fibroblasts. Strain-mediated RhoA activation triggered an increase in cellular actin assembly,56 which in turn lead to translocation of megakaryoblastic leukemia-1 (MKL1; also called MAL or MRTF-A) from the cytoplasm to the nucleus,55 where it is known to act as a trancriptional regulator.57 Accordingly, TNC induction by cyclic strain was abolished by MKL1 knockdown in NIH3T3 fibroblasts.58 Furthermore, overexpression of MKL1 induced TNC expression in both fibroblasts58 and mammary epithelial cells.59 MKL1 regulates the transcriptional activity of serum response factor (SRF).60 Indeed, the mouse Tnc promoter contains a serum response element (SRE; CArG-box) located 1.4 kb upstream from the transcription start site, which is in part involved in its activation. However, Tnc induction by cyclic strain was found to be independent of SRF but strictly dependent on the interaction of the SAP domain of MKL1, a putative DNA-binding domain, with the proximal Tnc promoter.58
Among vascular diseases, hypertension is correlated with elevated TNC abundance around vessels, concomittently with an increase in wall stress. In human arterial smooth muscle cells, cyclic strain (13%, 0.5 Hz for 24 h) was found to control the expression and activity of nuclear factor of activated T cells 5 (NFAT5) in a JNK-dependent manner.61 Once translocated into the nucleus in response to strain, NFAT5 was able to induce TNC gene expression. Five NFAT consensus sequences were found in the first 3512 bp of the human TNC promoter sequence upstream of the transcription start site, and for the first of them (at -820 bp), cyclic strain-induced binding was demonstrated by ChIP analysis.61 Note that the Rho/MKL1 pathway described above directly activates the mouse Tnc gene within 1–3 hours in response to strain, whereas the JNK/NFAT5 pathway requires prior synthesis of a transcription factor and takes 24 h for human TNC induction.
Yet a different mechanotransduction pathway was found to be responsible for TNC induction by cyclic strain in rat cardiomyocytes. It is noteworthy that a similar strain amplitude (9–14%) but a higher frequency (1 Hz) was used.62 In this case, the response depended on release of reactive oxygen species and activation of NFκB. A consensus sequence for this transcription factor at -210 bp was required for mechanical activation of the rat Tnc promoter, and shown to bind the p50 subunit of NFκB in response to strain.62 Moreover, TNC expression is not only regulated by dynamic (cyclic) strain, but also by static tensile stress. For example, TNC expression by chicken fibroblasts was found to be high when they were embedded in an attached (stressed) collagen matrix, but diminished when the matrix was released from its anchors (relaxed). A conserved region in the the chicken TNC promoter was required for this response63 (Table 1). Interestingly, a GAGAC/TC motif was identified in this region.64 This motif is present in the control regions of other mechanoresponsive genes where it is recognized by NFκB,65 but the factors binding to it in the chicken TNC promoter have not been identified. In any case, these examples show that depending on the cell type and on the exact mode and dosis of mechanical stress, the TNC gene appears to be regulated via distinct mechanotransduction pathways (Table 1).
Tenascin-C gene regulation by growth factors during tissue repair
In the adult, TNC protein is restricted to few tissues.7,25,66 However, the protein becomes prominently expressed de novo in practically every tissue upon inflammation in response to chemical or mechanical injury, as well as due to other pathological processes.1 A number of cytokines has been shown to induce TNC expression in different cell types, among them pro-inflammatory (IL-1α67 and IL-1β68) as well as anti-inflammatory (IL-469 and IL-1370). Cytokines signal via various intracellular pathways (JAK/STAT, MAPK, NF-κB71,72). However, there are no direct functional studies so far on the control of TNC promoter activity by cytokines.
More is known about TNC gene regulation during wound repair. Transforming growth factor-β (TGF-β) plays an important role as inducer of extracellular matrix protein expression during development and in tissue regeneration.73,74 Stimulation of TNC synthesis has been detected after treatment of chicken embryo fibroblasts with TGF-β1.75 A direct role of TGF-β in promoting TNC expression was observed in mammary epithelial cells (HC11) and in mouse embryo fibroblasts.76 For astrocytes, it was shown that the expression of TNC is controlled by the canonical SMAD-mediated TGF-β signaling pathway and by fibroblast growth factor (FGF).77 Series of 5′-deletions of the human TNC promoter revealed the presence of 2 potential SMAD2/3 binding sites in the proximal promoter region78 (Table 1 and Fig. 2). In addition, it was shown that SMADs interact with co-factors such as SP1 or ETS1 in a complex with CBP/p300, which possess binding sites located within the same promoter region, in order to achieve proper TNC gene transcription induced by TGF-β in human dermal fibroblasts78 (Table 1 and Fig. 2).
In the same cells, platelet-derived growth factor (PDGF) regulates TNC gene expression via PI3K/AKT signaling, which triggers the interaction of transcription factors SP1 and ETS1/ETS2 in an active complex that recognizes ETS binding sites (EBS) in the promoter79 (Table 1 and Fig. 2).
In chicken embryo fibroblasts, PDGF and TGF-β growth factors were shown to act in an additive manner with tensile strain in promoting TNC mRNA expression,53 and thus an increase in these factors might indirectly stimulate TNC production in response to mechanical load. For example, TNC accumulates in angiotensin II- induced perivascular fibrotic lesions in hypertensive mice. Angiotensin II was shown to trigger aldosterone-induced inflammation, which indirectly stimulated TNC expression by upregulating PDGF-A/B, PDGF receptor-α, and TGF-β1 in this model.80
Glucocorticoids are potent anti-inflammatory steroid hormones. They function by binding to nuclear receptors that act as transcription factors, but can also negatively regulate gene expression by inhibiting the activity of other factors like AP1 and NFκB.81 Glucocorticoids have also been described as important hormones involved in myelopoiesis, and they can directly act at the progenitor cell level or by modifying the expression of ECM components. Down-regulation of TNC expression by glucocorticoids was shown in bone marrow stromal cells.82 These authors suggested that the different TNC distribution between bone marrow of newborn and adult mice controlled by glucocorticoids might in part influence the hematopoiesis process. Putative binding sites for glucocorticoid receptors have been identified in the chicken TNC promoter sequence,63 but their function in the hormone response has not been explored. A further repressor motif was mapped in the human TNC promoter and was demonstrated to bind GATA6, a zinc finger transcription factor known to regulate the synthetic phenotype of vascular smooth muscle cells. Exogenous expression of GATA6 in dermal fibroblasts negatively modulated the level of TNC protein, and inhibited its induction by IL-4 and TGF-β83 (Table 1).
Tenascin-C gene regulation in cancer
Ets binding sites within the TNC promoter are not only important for its activation by the PDGF/PI3K/Akt pathway (see above), but were also shown to be the targets of EWS-ETS transcription factor. EWS-ETS is a chimeric gene found in several tumor types such as Ewing sarcoma and peripheral primitive neuroectodermal tumors.84 In a similar setting, oncogenic transformation of primary rat embryonic fibroblasts can be the consequence of the activity of transcription factor c-Jun in cooperation with an activated Ras gene.85 The transient expression of TNC induced by c-JUN could facilitate the de-adhesion of fibroblasts from the extracellular matrix, thus promoting their transformation. The c-JUN transcription factor contains a bipartite DNA binding domain which recognizes GCN4/AP1 and NFκB binding sequences, located in the human TNC promoter region85 (see Table 1 and Fig. 2).
SOX4 is a transcription factor overexpressed in many human tumors, and TNC was identified as a direct SOX4 target gene.86 TGF-β is also associated with the enrichment of TNC protein in the stroma of malignant breast tumors.87,88 Through gene set enrichment analysis it was found that direct target genes of TGF-β–activated SMAD3 were also enriched in the list of the SOX4 target genes. In the context of malignancies, this would suggest a cooperation between SOX4 and TGF-β1 in controlling TNC expression.
In addition to SOX4, other patterning genes might be involved in TNC accumulation during malignancy in a cell- and tissue-specific manner. For example, overexpression of POU3F2 (Brn2; see 2.2.) stimulated transcription from the Tnc promoter in a neuroblastoma cell line, but had no effect in glioma cells.39 Interestingly, PRRX1, shown to induce TNC in vascular smooth muscle cells (see 2.2) was identified as an important inducer of mesenchymal-epithelial transition both in the embryo as well as in cancer cells.89 Thus, PRRX1 might also play a role in TNC induction in tumors to facilitate local invasion.
Notch is a large transmembrane protein that acts as receptor for cell-bound ligands Delta and Jagged; upon activation, its intracellular domain is cleaved and translocates to the nucleus where it acts as transcriptional regulator through binding to RBPJk/CSL.90 High levels of Delta-like-1 and Jagged-1 ligands expressed in many glioma cell lines and primary human gliomas were shown to be important for the induction of the Notch signaling pathway.91 The proximal promoter of the human TNC gene includes an RBPJk/CSL binding sequence responsible for Notch2-mediated trans-activation in glioblastomas, and is likely to mediate strong TNC induction in these tumors.92 Conversely, in lung metastases of breast cancer, TNC accumulation has been implicated in supporting the Notch signaling pathway.93 Taking the 2 findings together, there may be a positive feedback between Notch and TNC expression, which in turn will further amplify the Notch signaling pathway.
Posttranscriptional regulation of tenascin-C gene by microRNAs
The role of microRNAs as regulators of post-transcriptional gene silencing is well documented,94,95 and recent studies have shown that the repression of certain microRNAs corresponds to a more pronounced tumorigenesis.96 For example, downregulation of SOX4 and TNC is controlled by miR-335, and loss of this microRNA in breast cancer was shown to induce metastasis in part by increased TNC levels.97 Other findings show how TNC promotes oncosphere formation by a metastasis-initiating breast cancer cell population for lung colonization, and in this context, GATA3 and miR-335 were downregulated.93
Tenascin-R: An ECM Protein Mainly Restricted to the Central Nervous System
Structure of the tenascin-R (TNR) gene
The human TNR gene (gene ID: 7143) is located on chromosome 1q24 and contains 23 exons. The transcript starts with 2 non-coding exons, separated by large introns from exon 3 which contains the ATG start codon98,99 (Fig. 1). The non-coding part of the tenascin-R gene spans a much larger region than the region of the protein encoding exons (Fig. 1). As demonstrated by S1 nuclease analysis, TNR transcripts of fetal, adult, and neoplastic human brain all contained both exons 1 and 2, indicating the presence of a single TSS at exon 1.99 The proximal promoter region is not recognizable as such and lacks a TATA box, CAAT box, GC-rich regions, or an initiator element, but potential binding sites for GATA1/2, MyoD, glucocorticoid receptor and homeobox binding sites were present in the region of −111 to -974bp. A non-typical TATA box, multiple GAGA boxes and an initiator-like element were identified within exon 1.99 However, inclusion of exon 1 did not play any role in the transcriptional activation directed by a 230 bp promoter construct and a short 57bp promoter construct was sufficient for full and cell type-specific activity of the human TNR promoter in cell culture99 (Table 1 and Fig. 2). The gene and promoter structure is highly conserved also in the rat100 and the mouse101. The TATA-less mouse TNR promoter displays canonical binding sites for potential regulators such as GATA-1/2, AP1 and p53 transcription factors as well as glucocorticoid receptors.101 However, all these binding sites are outside the 167bp short promoter region and exon 1 shown to be sufficient for the induction of transcription in cells of neuronal origin101 (Table 1 and Fig. 2).
Expression of tenascin-R in neural development, injury and cancer
TNR, originally designated as janusin in rodents and restrictin in chicken, is almost exclusively located to the central nervous system,102-105 but transiently appears also in Schwann cells during peripheral nerve development.106 Previous work has shown that 2 TNR splice variants of 160 and 180 kDa are expressed in the central nervous system by oligodendrocytes and a few neuronal cell types, but not by astrocytes or fibroblasts.107 In the developing human cortex, the spatiotemporal distribution of TNR parallels neuronal migration.108 In vitro experiments have demonstrated that TNR promotes adhesion and differentiation of oligodendrocytes and astrocytes by binding to sulfatides on cell surfaces.105,109 Conversely, TNR can inhibit neurite outgrowth either by interacting with adhesion molecule contactin 1 (F3/F11) or by interfering with integrin-dependent adhesion to fibronectin (reviewed in Pesheva et al., 2000110 ).
In a pathological context, activation of microglial cells after facial nerve axotomy in rats has been shown to downregulate TNR protein expression with the subsequent loss of its anti-adhesive properties.111 On the other hand, TNR is up-regulated in the injured visual pathway of the lizard that has the capacity to regenerate.112 In brain cancer, TNR was reported to be overexpressed in pilocytic astrocytoma, oligodendroglioma and ganglioglioma, but not glioblastoma.113
Tenascin-R gene regulation by growth factors
In mice, oligodendrocyte precursor cells synthesize most tenascin-R, whereas expression decreases with differentiation. In more mature oligodendrocytes, expression of TNR was stimulated by coculture with astrocytes or neurons, and was also induced by adding platelet-derived growth factor (PDGF) but not basic fibroblast growth factor.114 Rat pheochromocytoma cells (PC12) express high levels of Tnr mRNA after nerve growth factor (NGF) treatment.102,114 In contrast, rat oligodendrocytes treated with conditioned medium from activated microglia show a reduced Tnr mRNA expression due to the release of injury factors such as TNF-α.111 Unfortunately, there are no studies yet how these growth factors and cytokines regulate the TNR gene on the promoter level.
Tenascin-X: A Regulatory Component of Collagen Fibers
Structure of the tenascin-X (TNXB) gene
The human TNX gene was discovered as unknown “gene X” present in the major histocompatibility locus III (MHCIII).115 There, it is found on the opposite strand of the CYP21A2 gene and partially overlaps with it.116,117 CYP21A2 encodes steroid 21-hydroxylase, and mutations in this gene cause congenital adrenal hyperplasia. However, a fraction of cases with deletions in this genomic region are also deficient for TNX; these patients suffer in addition from hyperextensible skin and joint laxity typical of Ehlers-Danlos syndrome.116,118,119 The MHCIII gene locus is very complex and has been partly duplicated resulting in 2 TNX gene copies TNXA and TNXB, of which TNXA is a truncated version.120,121 In the human genome TNXB (gene ID: 7148), the gene coding for the intact TNX protein, is located on chromosome 6p21.3; it counts 38 exons and transcription can take place from 3 different widely separated promoters.120 However, only one of the 3 promoters was shown to be the main control region for TNXB transcription in all tissues tested and has thus been analyzed in more detail120,121 (Table 1 and Fig. 2). This main TNXB promoter is depicted in Figure 2. It lacks TATA or CAAT boxes and drives transcription form 4 closely clustered TSSs distributed over 194bp in the region of the first non-translated exon.120,121 More recently, yet another promoter and TSS within the TNXB gene was described.122 This promoter was shown to be activated by hypoxia resulting in a transcript encoding a truncated short TNXB protein with cytoplasmic localization. The transcripts were mainly found in the adrenal gland.122 Little is known about a possible intracellular function of this truncated TNXB, except that it was found to interact with the mitotic motor kinesin Eg5.123 Analysis of the main promoter of the full length TNXB gene revealed several putative binding sites for Sp1/Sp3 transcription factors, of which a cluster of 5 sites close to the TSSs were proven to be functional and required for driving TNXB expression in fibroblasts.121 The same gene organization was found for mouse Tnxb, with a non-coding first exon, a TATA-less promoter and an Sp1 site 145 bp upstream of the major transcription start site with a critical role in transcription of this gene124 (Table 1 and Fig. 2).
Expression of tenascin-X in collagen-rich tissues
As mentioned above, mutations or deletions in the human TNXB gene cause Ehlers Danlos syndrome,118 and certain TNXB mutations are reported to cause another connective tissue disorder, vesicouretral reflux.125 Deletion of the Tnxb gene in mice was subsequently shown to phenocopy the connective tissue defects observed in affected human patients.126 Thus, in apparent contrast to the other tenascins, TNX protein has a clear structural role in connective tissue integrity. It is reported to (indirectly) bind to and bridge collagen fibrils127,128 and regulates collagen deposition in vivo.127-129 During rat embryonic development, Tnxb mRNA is especially prominent in the epicardium, skeletal muscle connective tissue, and tendon primordia.130 In the adult pig, TNXB mRNA becomes widely and constituitively expressed in most connective tissues, but is present at higher levels in tendons, ligaments, and perineural sheaths.131 Despite of considerable overlap on the tissue level especially in the embryo, on a smaller scale the distribution of mouse Tnxb mRNA and TNX protein was found to be distinct and often reciprocal to that of TNC.18 Also strikingly different from tenascin-C, there is so far no report indicating an induction of tenascin-X expression in inflammation or wound healing.
Tenascin-X gene regulation by growth factors and hormones
Unlike for the other proteins belonging to the tenascin family, there are so far no reports indicating that TNX is regulated by growth factors or cytokines. Thus, the signaling pathways that act on the Sp1/Sp3 sites described above in the TNX promoter are at present unknown. Like TNC and TNW, however, TNX is subjected to negative regulation by glucocorticoids,132 but again the mechanism has not been elucidated yet on the gene promoter level.
Tenascin-X gene regulation in cancer
Not only during embryogenesis, but also in the context of malignancy TNX appears to be regulated in an opposite way compared to TNC. For example, TNX expression is prominent in normal pig skin but strongly suppressed in cutaneous melanoma, where TNC is highly upregulated.133 In contrast to TNC, TNX is not induced in breast and ovarian carcinomas, but has been reported to be a marker for malignant mesothelioma.134
Tenascin-W: Expression in Osteogenesis and Tumorigenesis
Structure of the tenascin-W (TNN) gene
The first tenascin-W gene to be discovered and cloned was from zebrafish and named tnw.135 Unfortunately, its mouse ortholog136 was subsequently called tenascin-N;137 Tnn for the mouse and TNN for the human are now the official gene names in the NCBI data base. The complete characterization of the human TNN gene (ID: 63923; chromosome 1q23-q24) was carried out in 2007.138 The human TNN gene consists of a total of 19 exons spanning 80 kb of genomic DNA and the transcript starts with a non-coding exon (Fig. 1). The same gene organization is found for mouse Tnn, except for presence of 3 additional exons encoding 3 additional fibronectin type III repeats.139 Thus the mouse TNW protein is about 30kDa larger than the human counterpart. The first non-coding exons as well as about 600bp 5` of the transcription start are highly conserved between human and mouse orthologs indicating the presence of conserved promoters, as revealed by the conservation tracks of the UCSC genome browser (http://genome.ucsc.edu/). Using ConSite (http://consite.genereg.net/) to explore transcription factor binding sites shared by the putative human and mouse promoter sequences revealed a conserved TATA box as well as conserved SMAD binding elements. However, the relevance of these sites and the functionality of the TNN promoter still need to be tested experimentally.
Expression of tenascin-W in bone formation and cancer
TNC and TNW proteins show partially overlapping expression patterns in the developing and adult skeleton.140 Most of the research based on the regulation of TNW expression in a physiological context indicates its significant role in osteogenesis.9 For instance, addition of TNW to explant cultures of frontal bones increased their growth, suggesting that TNW can accelerate bone formation in a complex multicellular environment.141 Furthermore, in a transgenic mouse model, overexpression of GFP under a Prrx1 promoter was used to isolate osteochondro-progenitor cells from the periosteum.41 These cells expressed high levels of TNW protein, proving its association with PRRX1-positive bone progenitor cells. Thus, it is tempting to speculate that similarly to TNC, TNW might be regulated by PRRX1. In the adult organisms, TNW is predominantly expressed in the periosteum as well as in other stem cell niches similar to TNC.142
In tumors, TNW is again similarly distributed as TNC and high amounts are present in cancer stroma of most solid tumors.76,138,143 However, in comparison to TNC, TNW represents an even more specific tumor marker in several malignancies such as in kidney, lung and colon cancers.19,143 In brain cancers TNW was found to be specifically expressed in blood vessels, and in vitro studies showed pro-angiogenic activity of TNW added to endothelial cell cultures.144 Also in kidney and lung cancer, a strong association of TNW expression was found with tumor blood vessels.19 These studies support the potential of TNW as a tumor biomarker. Its strict association with blood vessels suggests a good accessibility from the blood stream for antibody-drug-conjugate based therapeutic strategies.
Tenascin-W gene regulation by growth factors
Bone morphogenetic protein 2 (BMP2) is able to induce TNW expression in periosteum during endochondral bone formation in mice.145 An in vitro osteogenesis model using the mouse bone marrow-derived Kusa-A1 cell line shows an increase of Tnn transcript starting with differentiation into osteoblasts.146 Similarly, mouse C2C12 myoblasts differentiate into osteoblastic cells upon treatment with BMP2 and concomitantly express TNW.136 The induction of TNW synthesis by BMP2 in mouse E14.5 primary embryo fibroblasts as well as in mouse HC11 mammary epithelial cells occurs via the non-canonical p38 MAPK signaling pathway.76 TNW was also strongly induced by BMP7 in embryonic cranial fibroblasts in vitro.147 Among other regulators of bone formation, WNT5a signaling is indirectly involved in promoting TNW expression through p38 activation of an unknown TNW inducer, thus controlling bone density.148 Many factors involved in the regulation of TNW expression in bone formation are also present in cancer tissues and may be responsible for the cancer-specific expression of TNW.
Conclusions and Outlook
The four members of the vertebrate tenascin family are quite similar not only in their overall domain organization, but as typical “matricellular” proteins also appear to fulfill similar functions: There is increasing evidence that they all modulate cell adhesion and cellular responses to growth factors and cytokines in a context-dependent manner.1 In view of these structural and functional similarities, it is surprising that the 4 tenascins exhibit vastly different expression patterns in space and time. TNR is almost exclusively found in the central nervous system and its expression level is affected by just a few known growth factors. In contrast, TNC is an “oncofetal” or “stress” protein that is controlled by many stimuli and can appear in almost any tissue and cell type in the embryo and the adult, however just at certain times and in specific locations. TNX is a constitutive component of most connective tissues and its level is barely influenced by growth factors, whereas the expression of TNW is again more similar to that of TNC, although it is even more restricted to developing/remodeling bone, certain stem cell niches,66 and to a subset of tumors. These observations point to very distinct mechanisms of regulation for the various family members. Fitting with a highly regulated versus a more constitutive expression, respectively, the gene promoters of TNC and TNN have classical TATA boxes ca. 20–40 bp upstream of their transcription start sites, whereas the promoters of TNR and TNXB are TATA-less. TNC and TNR genes have a first untranslated exon separated from the second ATG-containing exon by a very large intron, which is likely to be involved in gene regulation. TNR has 2 untranslated exons and the ATG translation start site is found in the third, whereas TNXB even possesses 3 alternative promoters and non-coding first exons that are subjected to alternative splicing (see above).
Because the TNC gene was the first of the family to be characterized, most is known about its regulation, although it turns out to be overwhelmingly complex. The responsiveness of the TNC gene to segmentation genes, growth factors/cytokines and mechanical stress appears to be very similar in different vertebrate species, which is reflected in the high sequence similarity in parts of the promoter regions. Nevertheless, although many of the same cis-acting regulatory elements have been identified in the chicken, mouse and human TNC promoter, there appear to be differences in their arrangement and activity.25 Although TNW is quite distantly related to TNC within this protein family, its expression pattern and the regulation of its gene TNN appears to be most similar to that of TNC, especially also in stem cell niches142 and in cancer. Future research is likely to reveal more about similarities and differences in the control of these 2 genes e.g. by TGF-βs vs. BMPs, or by various cytokines. Conversely, TNR is the closest family member of TNC on the protein level, but it is regulated completely differently. It is remarkable that only about 200 bp of the proximal promoter and sequences in the first exon of the TNR gene are necessary and sufficient for its expression exclusively in neuronal cells.101 Thus, this gene appears to represent a relatively simple case of tissue-specific regulation, and it will be interesting to work out the exact mechanism. As for TNX, it exhibits a constitutive expression more like TNR, but in a reciprocal fashion since it is found in most tissues except the CNS. Nothing is known yet about the mechanism for tissue-specific expression of the TNXB gene, and the lack of regulation by growth factors and the relative scarcity of putative cis-acting elements in its promoter are noteworthy.121 For more meaningful comparisons between the genes of this family, it would be important to learn more about the regulation of TNR, TNXB and TNWN genes in the future. In the case of TNC, systems biology and computational approaches will probably be required to fully understand how a dozen or more signaling pathways converge to control its very complex gene promoter.
Why is it relevant to study the regulation of tenascins in even more detail? TNR and TNX, because of their largely constitutive expression, might perhaps be less interesting in this respect. Of course, TNX will remain in focus because of its important function in tissue integrity and its association with human disease, and TNR might be further investigated as a prime example for highly tissue and cell type-specific gene regulation. In case of the highly regulated TNC and TNW, however, more and more evidence suggests that these 2 proteins are important modulators of cell division, migration and differentiation in adult stem cell niches66 as well as in cancer.149 Moreover, because of their very localized and high expression in the extracellular matrix, both TNC and TNR are very well suited for targeting antibodies and drugs to certain types of tumors.150 For this therapeutic approach to work effectively, it is important to know how TNC or TNW gene expression is affected e.g., by cytotoxic drugs in combined therapy, and what signaling pathways are involved. From a basic research point of view, the tenascins provide an intriguing example for a vertebrate protein family of paralogs with similar structure and function, but with distinct expression patterns in space and time that are generated by different mechanisms of regulation of the respective genes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work by the authors was funded by grants of the Swiss National Science Foundation to M.C. and R.C.-E. and by the Swiss Cancer League to R.C.-E.
References
- 1. Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol 2003; 200:488-99; PMID:12845616; http://dx.doi.org/ 10.1002/path.1415 [DOI] [PubMed] [Google Scholar]
- 2. Tucker RP, Chiquet-Ehrismann R. Evidence for the evolution of tenascin and fibronectin early in the chordate lineage. Int J Biochem Cell Biol 2009; 41:424-34; PMID:18761101; http://dx.doi.org/ 10.1016/j.biocel.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 3. Adams JC, Chiquet-Ehrismann R, Tucker RP. The evolution of tenascins and fibronectins. Cell Adh Migr 2014 Oct; 30:1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol 2014; 37:1-14; PMID:25064829; http://dx.doi.org/ 10.1016/j.matbio.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol 2011; 3:a004960; PMID:21441591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erickson HP. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr Opin Cell Biol 1993; 5:869-76; PMID:7694605; http://dx.doi.org/ 10.1016/0955-0674(93)90037-Q [DOI] [PubMed] [Google Scholar]
- 7. Chiquet-Ehrismann R. Tenascins, a growing family of extracellular matrix proteins. Experientia 1995; 51:853-62; PMID:7556567; http://dx.doi.org/ 10.1007/BF01921736 [DOI] [PubMed] [Google Scholar]
- 8. Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 2000; 218:235-59; PMID:10842355; http://dx.doi.org/ 10.1002/(SICI)1097-0177(200006)218:2%3c235::AID-DVDY2%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 9. Martina E, Chiquet-Ehrismann R, Brellier F. Tenascin-W: an extracellular matrix protein associated with osteogenesis and cancer. Int J Biochem Cell Biol 2010; 42:1412-5; PMID:20541035; http://dx.doi.org/ 10.1016/j.biocel.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 10. Joester A, Faissner A. The structure and function of tenascins in the nervous system. Matrix Biol 2001; 20:13-22; PMID:11246000; http://dx.doi.org/ 10.1016/S0945-053X(00)00136-0 [DOI] [PubMed] [Google Scholar]
- 11. Guttery DS, Shaw JA, Lloyd K, Pringle JH, Walker RA. Expression of tenascin-C and its isoforms in the breast. Cancer Metastasis Rev 2010; 29:595-606; PMID:20814719; http://dx.doi.org/ 10.1007/s10555-010-9249-9 [DOI] [PubMed] [Google Scholar]
- 12. Orend G. Potential oncogenic action of tenascin-C in tumorigenesis. Int J Biochem Cell Biol 2005; 37:1066-83; PMID:15743679; http://dx.doi.org/ 10.1016/j.biocel.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 13. Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta 2009; 1793:888-92; PMID:19162090; http://dx.doi.org/ 10.1016/j.bbamcr.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 14. Mackie EJ, Tucker RP. The tenascin-C knockout revisited. J Cell Sci 1999; 112 ( Pt 22):3847-53; PMID:10547346 [DOI] [PubMed] [Google Scholar]
- 15. Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci 2011; 68:3175-99; PMID:21818551; http://dx.doi.org/ 10.1007/s00018-011-0783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anlar B, Gunel-Ozcan A. Tenascin-R: role in the central nervous system. Int J Biochem Cell Biol 2012; 44:1385-9; PMID:22634605; http://dx.doi.org/ 10.1016/j.biocel.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 17. O'Connell M, Burrows NP, van Vlijmen-Willems MJ, Clark SM, Schalkwijk J. Tenascin-X deficiency and Ehlers-Danlos syndrome: a case report and review of the literature. Br J Dermatol 2010; 163:1340-5; PMID:20649799; http://dx.doi.org/ 10.1111/j.1365-2133.2010.09949.x [DOI] [PubMed] [Google Scholar]
- 18. Matsumoto K, Saga Y, Ikemura T, Sakakura T, Chiquet-Ehrismann R. The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C. J Cell Biol 1994; 125:483-93; PMID:7512972; http://dx.doi.org/ 10.1083/jcb.125.2.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brellier F, Martina E, Degen M, Heuze-Vourc'h N, Petit A, Kryza T, Courty Y, Terracciano L, Ruiz C, Chiquet-Ehrismann R. Tenascin-W is a better cancer biomarker than tenascin-C for most human solid tumors. BMC Clin Pathol 2012; 12:14; PMID:22947174; http://dx.doi.org/ 10.1186/1472-6890-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gherzi R, Carnemolla B, Siri A, Ponassi M, Balza E, Zardi L. Human tenascin gene. Structure of the 5′-region, identification, and characterization of the transcription regulatory sequences. J Biol Chem 1995; 270:3429-34; PMID:7531707; http://dx.doi.org/ 10.1074/jbc.270.7.3429 [DOI] [PubMed] [Google Scholar]
- 21. Sriramarao P, Bourdon MA. A novel tenascin type III repeat is part of a complex of tenascin mRNA alternative splices. Nucleic Acids Res 1993; 21:163-8; PMID:7680113; http://dx.doi.org/ 10.1093/nar/21.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mighell AJ, Thompson J, Hume WJ, Markham AF, Robinson PA. Human tenascin-C: identification of a novel type III repeat in oral cancer and of novel splice variants in normal, malignant and reactive oral mucosae. Int J Cancer 1997; 72:236-40; PMID:9219826; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970717)72:2%3c236::AID-IJC6%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 23. Copertino DW, Jenkinson S, Jones FS, Edelman GM. Structural and functional similarities between the promoters for mouse tenascin and chicken cytotactin. Proc Natl Acad Sci U S A 1995; 92:2131-5; PMID:7534412; http://dx.doi.org/ 10.1073/pnas.92.6.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones FS, Crossin KL, Cunningham BA, Edelman GM. Identification and characterization of the promoter for the cytotactin gene. Proc Natl Acad Sci U S A 1990; 87:6497-501; PMID:1697683; http://dx.doi.org/ 10.1073/pnas.87.17.6497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones PL, Jones FS. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol 2000; 19:581-96; PMID:11102748; http://dx.doi.org/ 10.1016/S0945-053X(00)00106-2 [DOI] [PubMed] [Google Scholar]
- 26. Chiquet M, Fambrough DM. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol 1984; 98:1926-36; PMID:6725406; http://dx.doi.org/ 10.1083/jcb.98.6.1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 1986; 47:131-9; PMID:2428505; http://dx.doi.org/ 10.1016/0092-8674(86)90374-0 [DOI] [PubMed] [Google Scholar]
- 28. Mackie EJ, Tucker RP, Halfter W, Chiquet-Ehrismann R, Epperlein HH. The distribution of tenascin coincides with pathways of neural crest cell migration. Development 1988; 102:237-50; PMID:2458221 [DOI] [PubMed] [Google Scholar]
- 29. Tucker RP, McKay SE. The expression of tenascin by neural crest cells and glia. Development 1991; 112:1031-9; PMID:1718677 [DOI] [PubMed] [Google Scholar]
- 30. Wehrle B, Chiquet M. Tenascin is accumulated along developing peripheral nerves and allows neurite outgrowth in vitro. Development 1990; 110:401-15; PMID:1723942 [DOI] [PubMed] [Google Scholar]
- 31. Imanaka-Yoshida K, Aoki H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol 2014; 5:283; PMID:25120494; http://dx.doi.org/ 10.3389/fphys.2014.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inaguma Y, Kusakabe M, Mackie EJ, Pearson CA, Chiquet-Ehrismann R, Sakakura T. Epithelial induction of stromal tenascin in the mouse mammary gland: from embryogenesis to carcinogenesis. Dev Biol 1988; 128:245-55; PMID:2456233; http://dx.doi.org/ 10.1016/0012-1606(88)90288-6 [DOI] [PubMed] [Google Scholar]
- 33. Thesleff I, Mackie E, Vainio S, Chiquet-Ehrismann R. Changes in the distribution of tenascin during tooth development. Development 1987; 101:289-96; PMID:2451586 [DOI] [PubMed] [Google Scholar]
- 34. Aufderheide E, Chiquet-Ehrismann R, Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol 1987; 105:599-608; PMID:2440899; http://dx.doi.org/ 10.1083/jcb.105.1.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koch M, Wehrle-Haller B, Baumgartner S, Spring J, Brubacher D, Chiquet M. Epithelial synthesis of tenascin at tips of growing bronchi and graded accumulation in basement membrane and mesenchyme. Exp Cell Res 1991; 194:297-300; PMID:1709104; http://dx.doi.org/ 10.1016/0014-4827(91)90368-5 [DOI] [PubMed] [Google Scholar]
- 36. Jones FS, Chalepakis G, Gruss P, Edelman GM. Activation of the cytotactin promoter by the homeobox-containing gene Evx-1. Proc Natl Acad Sci U S A 1992; 89:2091-5; PMID:1372434; http://dx.doi.org/ 10.1073/pnas.89.6.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Briata P, Ilengo C, Bobola N, Corte G. Binding properties of the human homeodomain protein OTX2 to a DNA target sequence. FEBS Lett 1999; 445:160-4; PMID:10069392; http://dx.doi.org/ 10.1016/S0014-5793(99)00113-1 [DOI] [PubMed] [Google Scholar]
- 38. Gherzi R, Briata P, Boncinelli E, Ponassi M, Querze G, Viti F, Corte G, Zardi L. The human homeodomain protein OTX2 binds to the human tenascin-C promoter and trans-represses its activity in transfected cells. DNA Cell Biol 1997; 16:559-67; PMID:9174161; http://dx.doi.org/ 10.1089/dna.1997.16.559 [DOI] [PubMed] [Google Scholar]
- 39. Copertino DW, Edelman GM, Jones FS. Multiple promoter elements differentially regulate the expression of the mouse tenascin gene. Proc Natl Acad Sci U S A 1997; 94:1846-51; PMID:9050867; http://dx.doi.org/ 10.1073/pnas.94.5.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin JF, Bradley A, Olson EN. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev 1995; 9:1237-49; PMID:7758948; http://dx.doi.org/ 10.1101/gad.9.10.1237 [DOI] [PubMed] [Google Scholar]
- 41. Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun 2009; 386:477-82; PMID:19538944; http://dx.doi.org/ 10.1016/j.bbrc.2009.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones FS, Meech R, Edelman DB, Oakey RJ, Jones PL. Prx1 controls vascular smooth muscle cell proliferation and tenascin-C expression and is upregulated with Prx2 in pulmonary vascular disease. Circ Res 2001; 89:131-8; PMID:11463719; http://dx.doi.org/ 10.1161/hh1401.093582 [DOI] [PubMed] [Google Scholar]
- 43. Ihida-Stansbury K, McKean DM, Gebb SA, Martin JF, Stevens T, Nemenoff R, Akeson A, Vaughn J, Jones PL. Paired-related homeobox gene Prx1 is required for pulmonary vascular development. Circ Res 2004; 94:1507-14; PMID:15117820; http://dx.doi.org/ 10.1161/01.RES.0000130656.72424.20 [DOI] [PubMed] [Google Scholar]
- 44. Ihida-Stansbury K, McKean DM, Lane KB, Loyd JE, Wheeler LA, Morrell NW, Jones PL. Tenascin-C is induced by mutated BMP type II receptors in familial forms of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2006; 291:L694-702; PMID:16782755; http://dx.doi.org/ 10.1152/ajplung.00119.2006 [DOI] [PubMed] [Google Scholar]
- 45. Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007; 134:2697-708; PMID:17567668; http://dx.doi.org/ 10.1242/dev.001933 [DOI] [PubMed] [Google Scholar]
- 46. della Gaspera B, Armand AS, Sequeira I, Lecolle S, Gallien CL, Charbonnier F, Chanoine C. The Xenopus MEF2 gene family: evidence of a role for XMEF2C in larval tendon development. Dev Biol 2009; 328:392-402; PMID:19389348; http://dx.doi.org/ 10.1016/j.ydbio.2009.01.039 [DOI] [PubMed] [Google Scholar]
- 47. Mackie EJ, Scott-Burden T, Hahn AW, Kern F, Bernhardt J, Regenass S, Weller A, Bühler FR. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol 1992; 141:377-88; PMID:1379781 [PMC free article] [PubMed] [Google Scholar]
- 48. Fluck M, Tunc-Civelek V, Chiquet M. Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by loading of skeletal muscle. J Cell Sci 2000; 113 ( Pt 20):3583-91; PMID:11017874 [DOI] [PubMed] [Google Scholar]
- 49. Fluck M, Chiquet M, Schmutz S, Mayet-Sornay MH, Desplanches D. Reloading of atrophied rat soleus muscle induces tenascin-C expression around damaged muscle fibers. Am J Physiol Regul Integr Comp Physiol 2003; 284:R792-801; PMID:12571079 [DOI] [PubMed] [Google Scholar]
- 50. Crameri RM, Langberg H, Teisner B, Magnusson P, Schroder HD, Olesen JL, Jensen CH, Koskinen S, Suetta C, Kjaer M. Enhanced procollagen processing in skeletal muscle after a single bout of eccentric loading in humans. Matrix Biol 2004; 23:259-64; PMID:15296940; http://dx.doi.org/ 10.1016/j.matbio.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 51. Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol 1997; 9:707-13; PMID:9330875; http://dx.doi.org/ 10.1016/S0955-0674(97)80125-1 [DOI] [PubMed] [Google Scholar]
- 52. Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports 2005; 15:223-30; PMID:15998339; http://dx.doi.org/ 10.1111/j.1600-0838.2005.00461.x [DOI] [PubMed] [Google Scholar]
- 53. Chiquet M, Sarasa-Renedo A, Tunc-Civelek V. Induction of tenascin-C by cyclic tensile strain versus growth factors: distinct contributions by Rho/ROCK and MAPK signaling pathways. Biochim Biophys Acta 2004; 1693:193-204; PMID:15363633; http://dx.doi.org/ 10.1016/j.bbamcr.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 54. Lutz R, Sakai T, Chiquet M. Pericellular fibronectin is required for RhoA-dependent responses to cyclic strain in fibroblasts. J Cell Sci 2010; 123:1511-21; PMID:20375066; http://dx.doi.org/ 10.1242/jcs.060905 [DOI] [PubMed] [Google Scholar]
- 55. Maier S, Lutz R, Gelman L, Sarasa-Renedo A, Schenk S, Grashoff C, Chiquet M. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim Biophys Acta 2008; 1783:1150-62; PMID:18269918; http://dx.doi.org/ 10.1016/j.bbamcr.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 56. Sarasa-Renedo A, Tunc-Civelek V, Chiquet M. Role of RhoA/ROCK-dependent actin contractility in the induction of tenascin-C by cyclic tensile strain. Exp Cell Res 2006; 312:1361-70; PMID:16448650; http://dx.doi.org/ 10.1016/j.yexcr.2005.12.025 [DOI] [PubMed] [Google Scholar]
- 57. Asparuhova MB, Gelman L, Chiquet M. Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand J Med Sci Sports 2009; 19:490-9; PMID:19422655; http://dx.doi.org/ 10.1111/j.1600-0838.2009.00928.x [DOI] [PubMed] [Google Scholar]
- 58. Asparuhova MB, Ferralli J, Chiquet M, Chiquet-Ehrismann R. The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. FASEB J 2011; 25:3477-88; PMID:21705668; http://dx.doi.org/ 10.1096/fj.11-187310 [DOI] [PubMed] [Google Scholar]
- 59. Gurbuz I, Ferralli J, Roloff T, Chiquet-Ehrismann R, Asparuhova MB. SAP domain-dependent Mkl1 signaling stimulates proliferation and cell migration by induction of a distinct gene set indicative of poor prognosis in breast cancer patients. Mol Cancer 2014; 13:22; PMID:24495796; http://dx.doi.org/ 10.1186/1476-4598-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113:329-42; PMID:12732141; http://dx.doi.org/ 10.1016/S0092-8674(03)00278-2 [DOI] [PubMed] [Google Scholar]
- 61. Scherer C, Pfisterer L, Wagner AH, Hodebeck M, Cattaruzza M, Hecker M, Korff T. Arterial wall stress controls NFAT5 activity in vascular smooth muscle cells. J Am Heart Assoc 2014; 3:e000626; PMID:24614757; http://dx.doi.org/ 10.1161/JAHA.113.000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamamoto K, Dang QN, Kennedy SP, Osathanondh R, Kelly RA, Lee RT. Induction of tenascin-C in cardiac myocytes by mechanical deformation. Role of reactive oxygen species. J Biol Chem 1999; 274:21840-6; PMID:10419501; http://dx.doi.org/ 10.1074/jbc.274.31.21840 [DOI] [PubMed] [Google Scholar]
- 63. Chiquet-Ehrismann R, Tannheimer M, Koch M, Brunner A, Spring J, Martin D, Baumgartner S, Chiquet M. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J Cell Biol 1994; 127:2093-101; PMID:7528751; http://dx.doi.org/ 10.1083/jcb.127.6.2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol 1996; 74:737-44; PMID:9164643; http://dx.doi.org/ 10.1139/o96-080 [DOI] [PubMed] [Google Scholar]
- 65. Khachigian LM, Resnick N, Gimbrone MA, Jr., Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest 1995; 96:1169-75; PMID:7635955; http://dx.doi.org/ 10.1172/JCI118106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biol 2014. [DOI] [PubMed] [Google Scholar]
- 67. Maqbool A, Hemmings KE, O'Regan DJ, Ball SG, Porter KE, Turner NA. Interleukin-1 has opposing effects on connective tissue growth factor and tenascin-C expression in human cardiac fibroblasts. Matrix Biol 2013; 32:208-14; PMID:23454256; http://dx.doi.org/ 10.1016/j.matbio.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 68. Chevillard G, Derjuga A, Devost D, Zingg HH, Blank V. Identification of interleukin-1beta regulated genes in uterine smooth muscle cells. Reproduction 2007; 134:811-22; PMID:18042638; http://dx.doi.org/ 10.1530/REP-07-0289 [DOI] [PubMed] [Google Scholar]
- 69. Gratchev A, Kzhyshkowska J, Utikal J, Goerdt S. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scand J Immunol 2005; 61:10-7; PMID:15644118; http://dx.doi.org/ 10.1111/j.0300-9475.2005.01524.x [DOI] [PubMed] [Google Scholar]
- 70. Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Upregulation of tenascin-C expression by IL-13 in human dermal fibroblasts via the phosphoinositide 3-kinase/Akt and the protein kinase C signaling pathways. J Invest Dermatol 2006; 126:551-60; PMID:16374482; http://dx.doi.org/ 10.1038/sj.jid.5700090 [DOI] [PubMed] [Google Scholar]
- 71. Kasza A. IL-1 and EGF regulate expression of genes important in inflammation and cancer. Cytokine 2013; 62:22-33; PMID:23481102; http://dx.doi.org/ 10.1016/j.cyto.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 72. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014; 2014:561459; PMID:24876674; http://dx.doi.org/ 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schiller M, Javelaud D, Mauviel A. TGF-β-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 2004; 35:83-92; PMID:15265520; http://dx.doi.org/ 10.1016/j.jdermsci.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 74. Gordon KJ, Blobe GC. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim Biophys Acta 2008; 1782:197-228; PMID:18313409; http://dx.doi.org/ 10.1016/j.bbadis.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 75. Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-β. EMBO J 1988; 7:2977-82; PMID:2460335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres AC, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-α induced expression in vitro. Oncogene 2005; 24:1525-32; PMID:15592496; http://dx.doi.org/ 10.1038/sj.onc.1208342 [DOI] [PubMed] [Google Scholar]
- 77. Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol 2012; 3:120; PMID:22740833; http://dx.doi.org/ 10.3389/fphar.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Tenascin-C upregulation by transforming growth factor-β in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene 2004; 23:1656-67; PMID:15001984; http://dx.doi.org/ 10.1038/sj.onc.1207064 [DOI] [PubMed] [Google Scholar]
- 79. Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Platelet derived growth factor induced tenascin-C transcription is phosphoinositide 3-kinase/Akt-dependent and mediated by Ets family transcription factors. J Cell Physiol 2006; 206:718-27; PMID:16245312; http://dx.doi.org/ 10.1002/jcp.20527 [DOI] [PubMed] [Google Scholar]
- 80. Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, Hiroe M, Imanaka-Yoshida K. Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol 2007; 49:261-8; PMID:17513943; http://dx.doi.org/ 10.1097/FJC.0b013e318033dfd4 [DOI] [PubMed] [Google Scholar]
- 81. Oppong E, Flink N, Cato AC. Molecular mechanisms of glucocorticoid action in mast cells. Mol Cell Endocrinol 2013; 380:119-26; PMID:23707629; http://dx.doi.org/ 10.1016/j.mce.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 82. Ekblom M, Fassler R, Tomasini-Johansson B, Nilsson K, Ekblom P. Downregulation of tenascin expression by glucocorticoids in bone marrow stromal cells and in fibroblasts. J Cell Biol 1993; 123:1037-45; PMID:7693719; http://dx.doi.org/ 10.1083/jcb.123.4.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ghatnekar A, Trojanowska M. GATA-6 is a novel transcriptional repressor of the human Tenascin-C gene expression in fibroblasts. Biochim Biophys Acta 2008; 1779:145-51; PMID:18177748; http://dx.doi.org/ 10.1016/j.bbagrm.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Watanabe G, Nishimori H, Irifune H, Sasaki Y, Ishida S, Zembutsu H, Tanaka T, Kawaguchi S, Wada T, Hata J, et al. Induction of tenascin-C by tumor-specific EWS-ETS fusion genes. Genes Chromosomes Cancer 2003; 36:224-32; PMID:12557222; http://dx.doi.org/ 10.1002/gcc.10153 [DOI] [PubMed] [Google Scholar]
- 85. Mettouchi A, Cabon F, Montreau N, Dejong V, Vernier P, Gherzi R, Mercier G, Binétruy B. The c-Jun-induced transformation process involves complex regulation of tenascin-C expression. Mol Cell Biol 1997; 17:3202-9; PMID:9154819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scharer CD, McCabe CD, Ali-Seyed M, Berger MF, Bulyk ML, Moreno CS. Genome-wide promoter analysis of the SOX4 transcriptional network in prostate cancer cells. Cancer Res 2009; 69:709-17; PMID:19147588; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mackie EJ, Chiquet-Ehrismann R, Pearson CA, Inaguma Y, Taya K, Kawarada Y, Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A 1987; 84:4621-5; PMID:2440026; http://dx.doi.org/ 10.1073/pnas.84.13.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Walker RA, Dearing SJ, Gallacher B. Relationship of transforming growth factor β 1 to extracellular matrix and stromal infiltrates in invasive breast carcinoma. Br J Cancer 1994; 69:1160-5; PMID:7515264; http://dx.doi.org/ 10.1038/bjc.1994.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell; 22:709-24; PMID:23201163; http://dx.doi.org/ 10.1016/j.ccr.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 90. Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol 2011; 43:1550-62; PMID:21854867; http://dx.doi.org/ 10.1016/j.biocel.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res 2005; 65:2353-63; PMID:15781650; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1890 [DOI] [PubMed] [Google Scholar]
- 92. Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M, Mergenthaler S, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res 2009; 69:458-65; PMID:19147558; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2610 [DOI] [PubMed] [Google Scholar]
- 93. Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 2011; 17:867-74; PMID:21706029; http://dx.doi.org/ 10.1038/nm.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005; 433:769-73; PMID:15685193; http://dx.doi.org/ 10.1038/nature03315 [DOI] [PubMed] [Google Scholar]
- 95. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281-97; PMID:14744438; http://dx.doi.org/ 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 96. Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 2007; 39:673-7; PMID:17401365; http://dx.doi.org/ 10.1038/ng2003 [DOI] [PubMed] [Google Scholar]
- 97. Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massagué J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008; 451:147-52; PMID:18185580; http://dx.doi.org/ 10.1038/nature06487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Leprini A, Gherzi R, Siri A, Querze G, Viti F, Zardi L. The human tenascin-R gene. J Biol Chem 1996; 271:31251-4; PMID:8940128; http://dx.doi.org/ 10.1074/jbc.271.49.31251 [DOI] [PubMed] [Google Scholar]
- 99. Gherzi R, Leprini A, Siri A, Zardi L. Structure of 5' region of human tenascin-R gene and characterization of its promoter. DNA Cell Biol 1998; 17:275-82; PMID:9539107; http://dx.doi.org/ 10.1089/dna.1998.17.275 [DOI] [PubMed] [Google Scholar]
- 100. Leprini A, Gherzi R, Vecchi E, Borsi L, Zardi L, Siri A. Rat tenascin-R gene: structure, chromosome location and transcriptional activity of promoter and exon 1. Cytogenet Cell Genet 1998; 83:115-23; PMID:9925948; http://dx.doi.org/ 10.1159/000015146 [DOI] [PubMed] [Google Scholar]
- 101. Putthoff P, Akyuz N, Kutsche M, Zardi L, Borgmeyer U, Schachner M. Structure of the murine tenascin-R gene and functional characterisation of the promoter. Biochem Biophys Res Commun 2003; 308:940-9; PMID:12927810; http://dx.doi.org/ 10.1016/S0006-291X(03)01506-7 [DOI] [PubMed] [Google Scholar]
- 102. Derr LB, McKae LA, Tucker RP. The distribution of tenascin-R in the developing avian nervous system. J Exp Zool 1998; 280:152-64; PMID:9433801; http://dx.doi.org/ 10.1002/(SICI)1097-010X(19980201)280:2%3c152::AID-JEZ6%3e3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 103. Fuss B, Wintergerst ES, Bartsch U, Schachner M. Molecular characterization and in situ mRNA localization of the neural recognition molecule J1-160/180: a modular structure similar to tenascin. J Cell Biol 1993; 120:1237-49; PMID:7679676; http://dx.doi.org/ 10.1083/jcb.120.5.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Norenberg U, Wille H, Wolff JM, Frank R, Rathjen FG. The chicken neural extracellular matrix molecule restrictin: similarity with EGF-, fibronectin type III-, and fibrinogen-like motifs. Neuron 1992; 8:849-63; PMID:1375037; http://dx.doi.org/ 10.1016/0896-6273(92)90199-N [DOI] [PubMed] [Google Scholar]
- 105. Pesheva P, Gloor S, Schachner M, Probstmeier R. Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J Neurosci 1997; 17:4642-51; PMID:9169525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Probstmeier R, Nellen J, Gloor S, Wernig A, Pesheva P. Tenascin-R is expressed by Schwann cells in the peripheral nervous system. J Neurosci Res 2001; 64:70-8; PMID:11276053; http://dx.doi.org/ 10.1002/jnr.1055 [DOI] [PubMed] [Google Scholar]
- 107. Pesheva P, Spiess E, Schachner M. J1-160 and J1-180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol 1989; 109:1765-78; PMID:2477380; http://dx.doi.org/ 10.1083/jcb.109.4.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. El Ayachi I, Fernandez C, Baeza N, De Paula AM, Pesheva P, Figarella-Branger D. Spatiotemporal distribution of tenascin-R in the developing human cerebral cortex parallels neuronal migration. J Comp Neurol 2011; 519:2379-89; PMID:21456020; http://dx.doi.org/ 10.1002/cne.22632 [DOI] [PubMed] [Google Scholar]
- 109. Morganti MC, Taylor J, Pesheva P, Schachner M. Oligodendrocyte-derived J1-160/180 extracellular matrix glycoproteins are adhesive or repulsive depending on the partner cell type and time of interaction. Exp Neurol 1990; 109:98-110; PMID:2192910; http://dx.doi.org/ 10.1016/S0014-4886(05)80012-3 [DOI] [PubMed] [Google Scholar]
- 110. Pesheva P, Probstmeier R. The yin and yang of tenascin-R in CNS development and pathology. Prog Neurobiol 2000; 61:465-93; PMID:10748320; http://dx.doi.org/ 10.1016/S0301-0082(99)00061-1 [DOI] [PubMed] [Google Scholar]
- 111. Angelov DN, Walther M, Streppel M, Guntinas-Lichius O, Neiss WF, Probstmeier R, Pesheva P. Tenascin-R is antiadhesive for activated microglia that induce downregulation of the protein after peripheral nerve injury: a new role in neuronal protection. J Neurosci 1998; 18:6218-29; PMID:9698315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lang DM, Monzon-Mayor M, Del Mar Romero-Aleman M, Yanes C, Santos E, Pesheva P. Tenascin-R and axon growth-promoting molecules are up-regulated in the regenerating visual pathway of the lizard (Gallotia galloti). Dev Neurobiol 2008; 68:899-916; PMID:18361401; http://dx.doi.org/ 10.1002/dneu.20624 [DOI] [PubMed] [Google Scholar]
- 113. El Ayachi I, Baeza N, Fernandez C, Colin C, Scavarda D, Pesheva P, Figarella-Branger D. KIAA0510, the 3'-untranslated region of the tenascin-R gene, and tenascin-R are overexpressed in pilocytic astrocytomas. Neuropathol Appl Neurobiol 2010; 36:399-410; PMID:20202125; http://dx.doi.org/ 10.1111/j.1365-2990.2010.01074.x [DOI] [PubMed] [Google Scholar]
- 114. Jung M, Pesheva P, Schachner M, Trotter J. Astrocytes and neurons regulate the expression of the neural recognition molecule janusin by cultured oligodendrocytes. Glia 1993; 9:163-75; PMID:8294147; http://dx.doi.org/ 10.1002/glia.440090302 [DOI] [PubMed] [Google Scholar]
- 115. Morel Y, Bristow J, Gitelman SE, Miller WL. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci U S A 1989; 86:6582-6; PMID:2475872; http://dx.doi.org/ 10.1073/pnas.86.17.6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bristow J, Carey W, Egging D, Schalkwijk J. Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet 2005; 139C:24-30; PMID:16278880; http://dx.doi.org/ 10.1002/ajmg.c.30071 [DOI] [PubMed] [Google Scholar]
- 117. Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol 1993; 122:265-78; PMID:7686164; http://dx.doi.org/ 10.1083/jcb.122.1.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schalkwijk J, Zweers MC, Steijlen PM, Dean WB, Taylor G, van Vlijmen IM, van Haren B, Miller WL, Bristow J. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N Engl J Med 2001; 345:1167-75; PMID:11642233; http://dx.doi.org/ 10.1056/NEJMoa002939 [DOI] [PubMed] [Google Scholar]
- 119. Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet 1997; 17:104-8; PMID:9288108; http://dx.doi.org/ 10.1038/ng0997-104 [DOI] [PubMed] [Google Scholar]
- 120. Speek M, Barry F, Miller WL. Alternate promoters and alternate splicing of human tenascin-X, a gene with 5' and 3' ends buried in other genes. Hum Mol Genet 1996; 5:1749-58; PMID:8923003; http://dx.doi.org/ 10.1093/hmg/5.11.1749 [DOI] [PubMed] [Google Scholar]
- 121. Wijesuriya SD, Bristow J, Miller WL. Localization and analysis of the principal promoter for human tenascin-X. Genomics 2002; 80:443-52; PMID:12376099; http://dx.doi.org/ 10.1006/geno.2002.6852 [DOI] [PubMed] [Google Scholar]
- 122. Kato A, Endo T, Abiko S, Ariga H, Matsumoto K. Induction of truncated form of tenascin-X (XB-S) through dissociation of HDAC1 from SP-1/HDAC1 complex in response to hypoxic conditions. Exp Cell Res 2008; 314:2661-73; PMID:18588874; http://dx.doi.org/ 10.1016/j.yexcr.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 123. Endo T, Ariga H, Matsumoto K. Truncated form of tenascin-X, XB-S, interacts with mitotic motor kinesin Eg5. Mol Cell Biochem 2009; 320:53-66; PMID:18679583; http://dx.doi.org/ 10.1007/s11010-008-9898-y [DOI] [PubMed] [Google Scholar]
- 124. Minamitani T, Ariga H, Matsumoto K. Transcription factor Sp1 activates the expression of the mouse tenascin-X gene. Biochem Biophys Res Commun 2000; 267:626-31; PMID:10631113; http://dx.doi.org/ 10.1006/bbrc.1999.2006 [DOI] [PubMed] [Google Scholar]
- 125. Gbadegesin RA, Brophy PD, Adeyemo A, Hall G, Gupta IR, Hains D, Bartkowiak B, Rabinovich CE, Chandrasekharappa S, Homstad A, et al. TNXB mutations can cause vesicoureteral reflux. J Am Soc Nephrol 2013; 24:1313-22; PMID:23620400; http://dx.doi.org/ 10.1681/ASN.2012121148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, Rubin EM, Bristow J. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet 2002; 30:421-5; PMID:11925569; http://dx.doi.org/ 10.1038/ng850 [DOI] [PubMed] [Google Scholar]
- 127. Lethias C, Carisey A, Comte J, Cluzel C, Exposito JY. A model of tenascin-X integration within the collagenous network. FEBS Lett 2006; 580:6281-5; PMID:17078949; http://dx.doi.org/ 10.1016/j.febslet.2006.10.037 [DOI] [PubMed] [Google Scholar]
- 128. Veit G, Hansen U, Keene DR, Bruckner P, Chiquet-Ehrismann R, Chiquet M, Koch M. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem 2006; 281:27461-70; PMID:16861231; http://dx.doi.org/ 10.1074/jbc.M603147200 [DOI] [PubMed] [Google Scholar]
- 129. Minamitani T, Ikuta T, Saito Y, Takebe G, Sato M, Sawa H, Nishimura T, Nakamura F, Takahashi K, Ariga H, et al. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp Cell Res 2004; 298:305-15; PMID:15242785; http://dx.doi.org/ 10.1016/j.yexcr.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 130. Burch GH, Bedolli MA, McDonough S, Rosenthal SM, Bristow J. Embryonic expression of tenascin-X suggests a role in limb, muscle, and heart development. Dev Dyn 1995; 203:491-504; PMID:7496040; http://dx.doi.org/ 10.1002/aja.1002030411 [DOI] [PubMed] [Google Scholar]
- 131. Geffrotin C, Garrido JJ, Tremet L, Vaiman M. Distinct tissue distribution in pigs of tenascin-X and tenascin-C transcripts. Eur J Biochem 1995; 231:83-92; PMID:7543048; http://dx.doi.org/ 10.1111/j.1432-1033.1995.0083f.x [DOI] [PubMed] [Google Scholar]
- 132. Sakai T, Furukawa Y, Chiquet-Ehrismann R, Nakamura M, Kitagawa S, Ikemura T, Matsumoto K. Tenascin-X expression in tumor cells and fibroblasts: glucocorticoids as negative regulators in fibroblasts. J Cell Sci 1996; 109 ( Pt 8):2069-77; PMID:8856503 [DOI] [PubMed] [Google Scholar]
- 133. Geffrotin C, Horak V, Crechet F, Tricaud Y, Lethias C, Vincent-Naulleau S, Vielh P. Opposite regulation of tenascin-C and tenascin-X in MeLiM swine heritable cutaneous malignant melanoma. Biochim Biophys Acta 2000; 1524:196-202; PMID:11113568; http://dx.doi.org/ 10.1016/S0304-4165(00)00158-6 [DOI] [PubMed] [Google Scholar]
- 134. Yuan Y, Nymoen DA, Stavnes HT, Rosnes AK, Bjorang O, Wu C, Nesland JM, Davidson B. Tenascin-X is a novel diagnostic marker of malignant mesothelioma. Am J Surg Pathol 2009; 33:1673-82; PMID:19738457; http://dx.doi.org/ 10.1097/PAS.0b013e3181b6bde3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Weber P, Montag D, Schachner M, Bernhardt RR. Zebrafish tenascin-W, a new member of the tenascin family. J Neurobiol 1998; 35:1-16; PMID:9552162; http://dx.doi.org/ 10.1002/(SICI)1097-4695(199804)35:1%3c1::AID-NEU1%3e3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- 136. Scherberich A, Tucker RP, Samandari E, Brown-Luedi M, Martin D, Chiquet-Ehrismann R. Murine tenascin-W: a novel mammalian tenascin expressed in kidney and at sites of bone and smooth muscle development. J Cell Sci 2004; 117:571-81; PMID:14709716; http://dx.doi.org/ 10.1242/jcs.00867 [DOI] [PubMed] [Google Scholar]
- 137. Neidhardt J, Fehr S, Kutsche M, Lohler J, Schachner M. Tenascin-N: characterization of a novel member of the tenascin family that mediates neurite repulsion from hippocampal explants. Mol Cell Neurosci 2003; 23:193-209; PMID:12812753; http://dx.doi.org/ 10.1016/S1044-7431(03)00012-5 [DOI] [PubMed] [Google Scholar]
- 138. Degen M, Brellier F, Kain R, Ruiz C, Terracciano L, Orend G, Chiquet-Ehrismann R. Tenascin-W is a novel marker for activated tumor stroma in low-grade human breast cancer and influences cell behavior. Cancer Res 2007; 67:9169-79; PMID:17909022; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0666 [DOI] [PubMed] [Google Scholar]
- 139. Tucker RP, Drabikowski K, Hess JF, Ferralli J, Chiquet-Ehrismann R, Adams JC. Phylogenetic analysis of the tenascin gene family: evidence of origin early in the chordate lineage. BMC Evol Biol 2006; 6:60; PMID:16893461; http://dx.doi.org/ 10.1186/1471-2148-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Meloty-Kapella CV, Degen M, Chiquet-Ehrismann R, Tucker RP. Avian tenascin-W: expression in smooth muscle and bone, and effects on calvarial cell spreading and adhesion in vitro. Dev Dyn 2006; 235:1532-42; PMID:16534782; http://dx.doi.org/ 10.1002/dvdy.20731 [DOI] [PubMed] [Google Scholar]
- 141. Meloty-Kapella CV, Degen M, Chiquet-Ehrismann R, Tucker RP. Effects of tenascin-W on osteoblasts in vitro. Cell Tissue Res 2008; 334:445-55; PMID:18985388; http://dx.doi.org/ 10.1007/s00441-008-0715-4 [DOI] [PubMed] [Google Scholar]
- 142. Tucker RP, Ferralli J, Schittny JC, Chiquet-Ehrismann R. Tenascin-C and tenascin-W in whisker follicle stem cell niches: possible roles in regulating stem cell proliferation and migration. J Cell Sci 2013; 126:5111-5; PMID:24101721; http://dx.doi.org/ 10.1242/jcs.134650 [DOI] [PubMed] [Google Scholar]
- 143. Degen M, Brellier F, Schenk S, Driscoll R, Zaman K, Stupp R, Tornillo L, Terracciano L, Chiquet-Ehrismann R, Rüegg C, et al. Tenascin-W, a new marker of cancer stroma, is elevated in sera of colon and breast cancer patients. Int J Cancer 2008; 122:2454-61; PMID:18306355; http://dx.doi.org/ 10.1002/ijc.23417 [DOI] [PubMed] [Google Scholar]
- 144. Martina E, Degen M, Ruegg C, Merlo A, Lino MM, Chiquet-Ehrismann R, Brellier F. Tenascin-W is a specific marker of glioma-associated blood vessels and stimulates angiogenesis in vitro. FASEB J 2010; 24:778-87; PMID:19884327; http://dx.doi.org/ 10.1096/fj.09-140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kimura H, Akiyama H, Nakamura T, de Crombrugghe B. Tenascin-W inhibits proliferation and differentiation of preosteoblasts during endochondral bone formation. Biochem Biophys Res Commun 2007; 356:935-41; PMID:17395156; http://dx.doi.org/ 10.1016/j.bbrc.2007.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mikura A, Okuhara S, Saito M, Ota M, Ueda K, Iseki S. Association of tenascin-W expression with mineralization in mouse calvarial development. Congenit Anom (Kyoto) 2009; 49:77-84; PMID:19489959; http://dx.doi.org/ 10.1111/j.1741-4520.2009.00227.x [DOI] [PubMed] [Google Scholar]
- 147. d'Amaro Scheidegger R. R, Blumer S, Pazera P, Katsaros C, Graf D, Chiquet M. Putative functions of extracellular matrix glycoproteins in secondary palate morphogenesis. Front Physiol 2012; 3:377; PMID:23055981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Morgan JM, Wong A, Yellowley CE, Genetos DC. Regulation of tenascin expression in bone. J Cell Biochem 2011; 112:3354-63; PMID:21751239; http://dx.doi.org/ 10.1002/jcb.23265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Oskarsson T, Massague J. Extracellular matrix players in metastatic niches. EMBO J 2012; 31:254-6; PMID:22179697; http://dx.doi.org/ 10.1038/emboj.2011.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Aloj L, D'Ambrosio L, Aurilio M, Morisco A, Frigeri F, Caraco C, Di Gennaro F, Capobianco G, Giovannoni L, Menssen HD, et al. Radioimmunotherapy with Tenarad, a 131I-labelled antibody fragment targeting the extra-domain A1 of tenascin-C, in patients with refractory Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging 2014; 41:867-77; PMID:24435772; http://dx.doi.org/ 10.1007/s00259-013-2658-6 [DOI] [PubMed] [Google Scholar]