Abstract
Atherosclerosis and valvular heart disease often require treatment with corrective surgery to prevent future myocardial infarction, ischemic heart disease, and heart failure. Mechanisms underlying the development of the associated complications of surgery are multifactorial and have been linked to inflammation and oxidative stress, classically as measured in the blood or plasma of patients. Post-operative pericardial fluid (PO-PCF) has not been investigated in depth with respect to the potential to induce oxidative stress. This is important since cardiac surgery disrupts the integrity of the pericardial membrane surrounding the heart, and causes significant alterations in the composition of the pericardial fluid (PCF). This includes contamination with hemolyzed blood and high concentrations of oxidized hemoglobin, which suggests that cardiac surgery results in oxidative stress within the pericardial space. Accordingly, we tested the hypothesis that PO-PCF is highly pro-oxidant and that the potential interaction between inflammatory cell-derived hydrogen peroxide with hemoglobin is associated with oxidative stress. Blood and PCF were collected from 31 patients at the time of surgery and postoperatively from 4 to 48 hours after coronary artery bypass grafting, valve replacement, or valve repair (mitral or aortic). PO-PCF contained high concentrations of neutrophils and monocytes which are capable of generating elevated amounts of superoxide and hydrogen peroxide through the oxidative burst. In addition, PO-PCF primed naïve neutrophils resulting in an enhanced oxidative burst upon stimulation. The PO-PCF also contained increased concentrations of cell-free oxidized hemoglobin which was associated with elevated levels of F2α-isoprostanes and prostaglandins, consistent with both oxidative stress and activation of cyclooxygenase. Lastly, protein analysis of the PO-PCF revealed evidence of protein thiol oxidation and protein carbonylation. We conclude that PO-PCF is highly pro-oxidant and speculate that it may contribute to the risk of post-operative complications.
Introduction
In the United States there are approximately 750,000 cardiac surgeries performed each year (1). Depending on the procedure, the incidence of postoperative complications such as atrial fibrillation is 15-50%, which results in a prolonged hospital stay and an estimated $10,000 increase in hospital cost per occurrence (2). Therapeutic strategies are only partially effective, and a lack of understanding of the complication has resulted in minimal improvement in treatment over the last several years (3, 4). It has been documented that inflammatory markers are elevated in the serum of postoperative cardiac surgery patients and this correlates with increased occurrences of cardiac dysfunction and atrial fibrillation.(5-7). Some investigators have reported partial improvement in cardiac complications with systemic anti-inflammatory treatments (8). However, these studies have focused on serum concentrations of inflammatory molecules and have not evaluated the composition of the pericardial fluid, which may be more reflective of local environment around the heart, especially for highly labile oxidation products and pro-inflammatory cytokines. Oxidative stress is known to exacerbate heart injury and can occur secondary to inflammation, surgical trauma, and cardiac ischemia (7, 9-11). Oxidative stress has been implicated in the development of atrial fibrillation immediately following surgery, but the extent of its contribution and mechanisms responsible have yet to be elucidated (12-15).
The pericardium is a specialized membrane surrounding the heart that performs specific physiological roles needed for cardiac function (16, 17). In addition to acting as a lubricant, the pericardial fluid (PCF) contains several paracrine modulators, such as prostanoids, natriuretic peptides, and endothelins, which may regulate sympathetic tone, coronary vasomotor tone, heart rate, blood pressure and complement responses (16, 18). The pericardium is breached at the time of surgery, causing significant alterations in the PCF. In pericardial diseases, it has been shown that the composition and volume of postoperative PCF (PO-PCF) is altered and associated with cardiac dysfunction (19-23). Specifically, pericarditis, inflammation of the pericardium, has been shown to cause increased fluid volume and accumulation of inflammatory cells in the PCF and contributes to decreased cardiac function. These findings provide evidence that components of PCF can act as modulators of cardiomyocyte function despite the presence of the epicardium (24-27). In this study we tested the hypothesis that cardiac surgery results in the generation of highly pro-oxidant and pro-inflammatory molecules in the PCF and that these lead to oxidative damage in the pericardial environment.
Materials and Methods
Surgery, Blood Collection and Cell Isolations
All study protocols for the collection and handling of human samples were reviewed and approved by the Institutional Review Board, University of Alabama at Birmingham. Consent was obtained from adult (>19 years old) patients undergoing cardiac surgery for ischemic heart disease or valvular heart disease: coronary artery bypass graft +/- aortic valve replacement/mitral valve repair/replacement, tricuspid valve repair or replacement, or a valve procedure alone. Patients with ventricular assist devices, atrial fibrillation surgery, thoracic aorta surgery, non-cardiac surgery, and patients with atrial fibrillation within six months were excluded from the study.
Blood samples (1-2 tubes, 8.5 ml/tube) were collected from 31 patients (22 males and 9 females, 62 (± 26) years of age (Table 1) in vacutainers (BD Biosciences) containing 1.5 ml ACD solution (trisodium citrate, 22.0 g/L; citric acid, 8.0 g/L; and dextrose 24.5 g/L) as anticoagulant and processed within 2 hours of collection. Procedures were designed to prevent activation of the cells during isolation and were performed at room temperature unless otherwise specified. PO-PCF was obtained and assayed in parallel with peripheral blood drawn from the same patient at the time of the start of surgery, and 4, 12, 24, and 48 h after the patient left the operating room. PO-PCF was allowed to accumulate no more than 1 h in the mediastinal drainage tube prior to collection. Cells were prepared from blood or PCF as described previously (28, 29). Monocytes and neutrophils were pelleted, counted, and plated on an extracellular flux analysis plate for measurement of oxidative burst. Prepared cells were ≥95% viable as determined by Trypan Blue exclusion (data not shown).
Table 1. Patient demographics.
Table of the patient demographics involved in this study. The age, weight, height, Body mass index (BMI), and presentation of Atrial fibrillation after surgery were obtained from a total of 31 patients. Data presented as mean ± SD.
|
Patient Characteristics
| ||||||
|---|---|---|---|---|---|---|
| Age (years) | Weight (kg) | Height (cm) | BMI | Gender | Afib | Procedure |
| 62.3 (± 25.6) | 83.4 (± 27.8) | 166.3 (± 24.4) | 30.9 (± 16.2) | M 71% F 29% | 32% | CABG (Pump) 19% |
| CABG (No Pump) 52% | ||||||
| Valve 16% | ||||||
| Valve + CABG 13% | ||||||
Measurement of Myoglobin, Troponin and Creatine Kinase
Cardiac injury markers were assessed by a Luminex bead-based multiplex assay using fluorescent bead technology (Milliplex kit, Millipore Corp.) in a 96-well format. Measurements were conducted in compliance with the manufacturer’s instructions. Standard curves correlating mean fluorescence intensity with concentration were generated for each analyte using the standards supplied by the manufacturer, and unknown values were calculated within the linear portion of the generated standard curves.
Hemoglobin concentration
The postoperative plasma and PCF were centrifuged at 1500 × g for ten min to remove cells and then at 5000 × g for 10 min to remove cellular debris. Absorbance spectra from 500-700nm was measured using a DU 800 spectrophotometer (Beckman-Coulter). Total hemoglobin was determined after subtracting the background absorbance at 700nm from the isosbestic point for Methemoglobin (MetHb) and Oxyhemoglobin (OxyHb) at 523nm with an extinction coefficient of 7.12 as reported by Snell and Marini (30). MetHb concentration was calculated using the extinction coefficient of 4.4 mM-1 cm-1 at 631nm (31). Assuming the two major species of hemoglobin, MetHb and OxyHb, were present in the PCF, OxyHb was measured as the difference between MetHb and total hemoglobin.
Flow Cytometry analysis and complete blood count in PCF
Pericardial fluid collected at the time of surgery and for the first 48 hours after surgery was centrifuged and the buffy coat isolated as described above. Isolated leukocytes were separated by forward and side scatter and analyzed by flow cytometry using CD14-PE and CD16-APC to differentiate monocyte and neutrophil populations.
Determination of lipid peroxidation products by mass spectrometry
Isoprostane and prostaglandin extractions of human serum and pericardial fluid were performed as described previously (32). Briefly, aliquots of patient PCF were stored frozen (-80°C) and contained 1 mM BHT and 50 μM EDTA and DTPA. Samples (1 ml) were thawed and diluted to 3 ml with 0.02 M Bis-Tris, pH 6.0 containing 8-iso-PGF2α-d4 as an internal standard and 1 mM BHT and 50 μM EDTA prior to being loaded on to prepared Strata X-AW 33u polymeric weak anion exchange cartridges (Phenomenex, Torrance, CA). Eluted samples were dried under a constant stream of nitrogen and reconstituted in methanol containing 1 mM BHT and 50 μM EDTA. F2α-Isoprostanes and prostaglandins were measured by isotope dilution, reverse-phase liquid chromatography-multiple reaction ion monitoring mass spectrometry as previously described (32)
Protein Thiol Oxidation
To detect protein thiols, Bodipy-IAM (Bodipy-iodoacetamide; Invitrogen, Eugene, OR), a fluorescently labeled alkylating agent capable of forming covalent adducts with cysteine thiol groups that are not involved in disulfide linkages was utilized. In brief, PCF samples from individual patients (0 and 4 h post-surgery) were diluted 1:10 with de-ionized water prior to determining protein concentration using the Lowry DC protein assay (Bio-Rad). Subsequently, 5 μg of PCF protein was treated with 500 μM Bodipy-IAM for 30 min to assess protein thiol modifications. The reaction was stopped with 5X SDS-PAGE sample buffer (1M Tris-HCl, pH 6.8, 10% SDS, 30% glycerol, 0.05% bromophenol blue) containing 5% β-mercaptoethanol.
Samples were resolved using 12.5% SDS-PAGE gels and imaged in-gel using a Typhoon imager (GE Healthcare Biosciences, Pittsburgh, PA). Following imaging, gels were immediately stained with Coomassie Brilliant Blue G-250 (coomassie blue; Bio-Rad Laboratories, Hercules, CA) for 1 hour, destained overnight, and imaged using the AlphaView SA imager (Protein Simple, Santa Clara, CA) to evaluate protein loading. The Bodipy-IAM fluorescent signal intensity for albumin was quantified using ImageQuantTL analysis software (GE Healthcare Biosciences, Pittsburgh, PA) and the coomassie blue protein stain for albumin was quantified using the AlphaView SA software.
Protein Carbonylation
To determine the extent of protein carbonylation due to aldehyde/ketone moieties on proteins in PO-PCF (0 and 4 h), samples were first depleted of albumin. PCF samples (100 μl) were loaded onto rehydrated SwellGel blue Resin (Thermo Scientific) and eluted as per the manufacturer’s instructions. The protein content was measured in the flow-through material. Next, 100 μg protein was incubated in the presence of 1 mM biotin hydrazide (Pierce) for 30 min at 37°C. The reaction was stopped with the addition of 1M Tris-HCl, pH 7.4 after which proteins were resolved by SDS-PAGE and transferred to nitrocellulose.
Measurement of the Oxidative Burst
Monocytes and neutrophils were isolated from peripheral blood and PCF at 4 hours post-surgery and compared with healthy donors (29). Where indicated, healthy donor neutrophils were pretreated for 1.5 h with the post centrifugation (1500 × g) supernatant from the 4 h PO-PCF sample (0-10% supernatant). After incubation, the oxidative burst was stimulated with phorbol 12-myristate 13-acetate (PMA, 100 ng/ml), after inhibition of mitochondrial respiration using antimycin A (10 μM). The oxygen consumption rate was measured using an extracellular flux analyzer (Seahorse Bioscience) as previously described (33). The area under the curve (AUC) after PMA stimulation was subtracted by the rate of oxygen consumption after antimycin A treatment to give the total generation of ROS over a period of 24 or 48 min. The oxidant production has previously been shown to be dependent on NADPH oxidase activity (33).
Statistics
Each blood sample was analyzed with the number of replicates indicated and data are presented as mean ± SEM. Statistical significance was determined using either student’s T-test or One-way Anova, as indicated, followed by Tukey’s Post hoc analysis, with p≤0.05 designated as statistically significant.
Results
Cell-free OxyHb and MetHb are present in the pericardial fluid surrounding the heart after cardiac surgery and followed by increased markers of myocardial damage
Following cardiac surgery, the pericardial membrane is left partially open leading to the mixing of blood from lacerations, lipids from the myocardial, perivascular, and epicardial fat surrounding the heart and from the traumatized tissue and exuded fluids from the surgical wounds with the PCF in the pericardial space. Figure 1 illustrates a chest CT scan of a representative patient 12 h after surgery and depicts the location of the mediastinal drains and the effusion surrounding the pericardial space. These drains typically capture 100-300 ml in the first few hours following surgery and then taper to about <150 ml/24 h before the drains are removed. A major constituent of the post-operative pericardial fluid in the first few hours after surgery is blood from surgical trauma to the heart and vessels as well as non-cardiac sources. The proportion of blood gradually diminishes and is largely absent by 24 h post-surgery in a normal post-operative course.
Figure 1. Post-operative pericardial fluid accumulates around the heart.
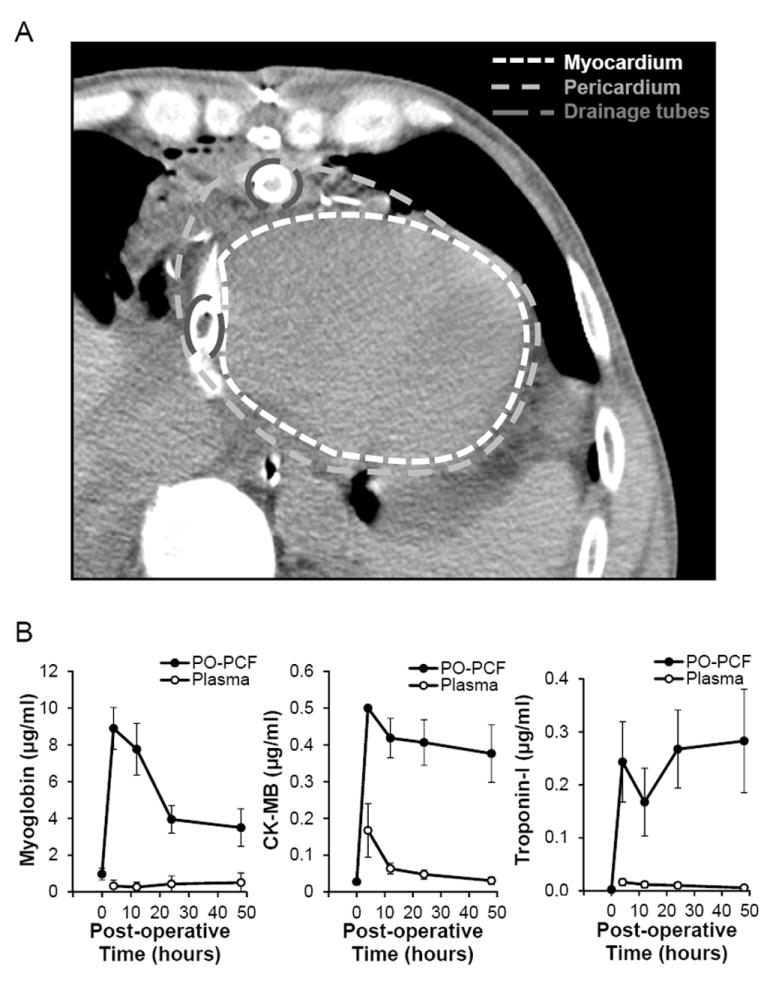
A) Post-operative axial CT scan image of a cardiac surgery patient demonstrates accumulation of pericardial fluid around the heart. B) The cardiac injury markers, myoglobin, CK-MB and troponin-I are significantly elevated in the postoperative pericardial fluid compared to their plasma controls (p<0.05).
Continuous drainage of the PO-PCF is maintained up to 72 h post-surgery. As shown in (Figure 1A), PCF accumulates around the heart even in the presence of mediastinal drainage tubes in place. Indicators of cardiac muscle injury following surgery, including myoglobin, troponin-I, and CK-MB (Figure 1B), were present in the PO-PCF at significantly greater levels when compared to patient serum and increased in the PCF subsequent to elevated markers of oxidative stress. Following surgery, blood was present in the PCF and significant hemolysis was also detected. Figure 2A and B show the oxyhemoglobin (OxyHb) and methemoglobin (MetHb) concentrations in PCF as determined by the visible absorbance spectra at 0-48 h post-surgery, indicating significant hemolysis and oxidation of the cell-free hemoglobin. Shown in Figure 2C is the quantification of the (MetHb)/ (OxyHb) ratio in the intraoperative and postoperative samples. The ratio is significantly increased at 12 h and consistent with a pro-oxidant environment.
Figure 2. Hemoglobin and methemoglobin in postoperative blood and PCF.
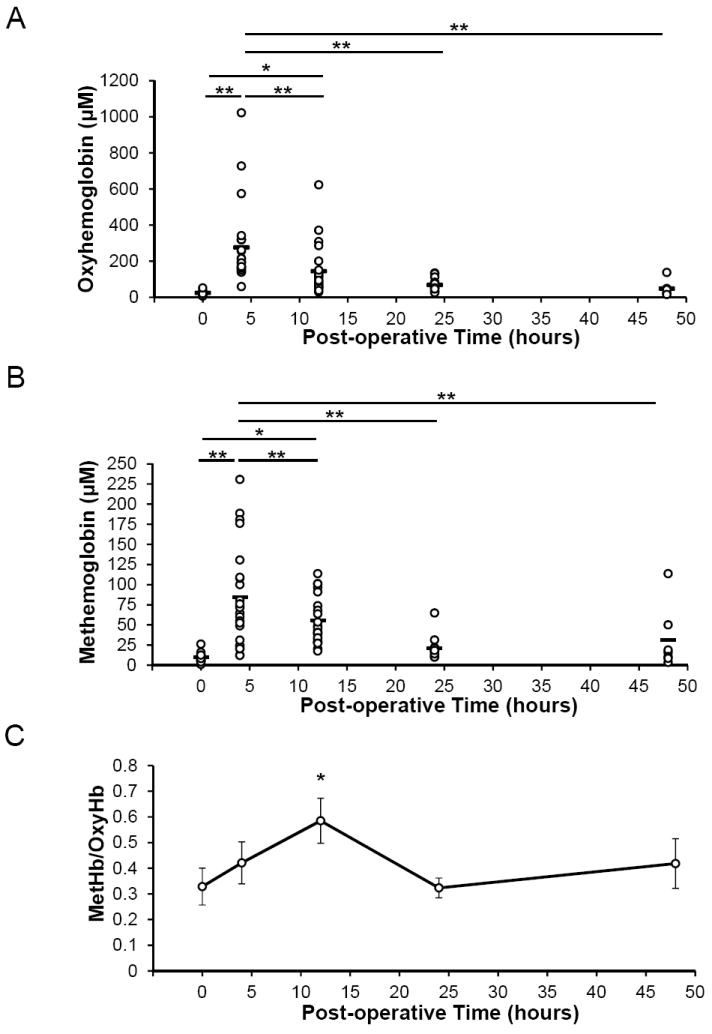
Samples collected from the same patient during surgery (0 hour) and post-surgery (4, 12, 24, 48 hours) were immediately centrifuged at 500g for 15 minutes, supernatants collected, and centrifuged again to remove cells and large debris at 1,500g for 10 minutes. A) The oxyhemoglobin and B) methemoglobin levels are visualized for each patient at the corresponding time after surgery as measured by spectrophotometry. C) The methemoglobin/oxyhemoglobin ratio (MetHb/OxyHb) is shown to determine time points of relatively greater oxidative stress compared to the 0 hour control. The mean values are represented by a horizontal bar. Data from 31 patients. *= p<0.05, **= p<0.005.
The pro-inflammatory environment in the postoperative PCF promotes monocyte and neutrophil recruitment
Flow cytometry analysis of the PCF and blood at 4-48 h showed two major populations of leukocytes. The predominant population, visible by four hours, was CD14-CD16+ neutrophils which comprised 64.0 ± 19.7% of cells found in the PCF by 12 h (Figure 3A). The next most abundant lineage was CD14+CD16+ monocytes which were 1.01 ± 0.25% of cells at 12 h and increased to 6.02 ± 2.53% of total leukocytes by 24 h (Figure 3A). The lineages of these cells were also confirmed by CD66b and MHCII expression (data not shown). Monocytes were detectable in the 0 hour PCF as confirmed by a complete blood count performed on unprocessed PCF, but counts did not significantly increase after surgery (Figure 3B). Neutrophil counts were similar to monocytes at the commencement of surgery but were significantly elevated after 4 hours and for the duration of recovery (Figure 3C).
Figure 3. Flow cytometric analysis of the PCF.
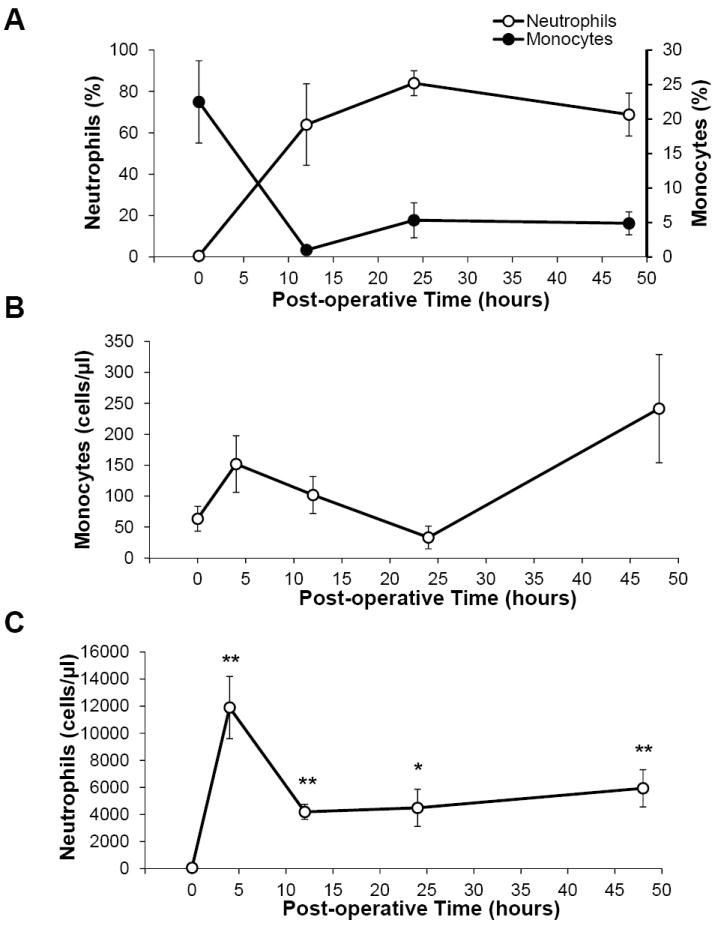
A) The proportions of gated cells expressing CD14 and CD16 characteristic of neutrophils and monocytes respectively were enumerated at each time point. Note the differences in the Y-axis scales for macrophages and neutrophils. B) Absolute monocyte C) and neutrophil counts were performed on unaltered PCF drawn during surgery (0 hour) and post-surgery (4, 12, 24, 48 hours). Data reported as mean ± SEM. * = p ≤ 0.05, **= p<0.005.
The postoperative pericardial environment primes the oxidative burst in monocytes and neutrophils
A major source of hydrogen peroxide production during inflammation is NADPH oxidase 2 (NOX2) in monocytes and neutrophils. Using the monocytes and neutrophils isolated from both PCF and blood, the potential maximal activity of the NADPH oxidase system was determined. We found that monocytes isolated from PCF exhibited an increased oxidative burst when stimulated by PMA (100 ng/ml) as compared to monocytes isolated from the peripheral blood of healthy age-matched controls (Figure 4A). No significant difference was seen between the oxidative burst of monocytes (Figure 4A and 4C, p = 0.15) or neutrophils (Figure 4B and 4C, p = 0.33) isolated from postoperative patient blood and PCF at 4 h. However, to test the acute effect of PO-PCF on the priming of neutrophils to generate high levels of ROS, neutrophils from a healthy donor were incubated for 1.5 h with increasing concentrations of PO-PCF obtained from a patient at 4 h post-surgery and then stimulated with PMA as described above. Quantitation of oxygen consumption demonstrated that PO-PCF exposure primes the neutrophils to increase the production of superoxide and hydrogen peroxide compared to the untreated controls, and the response is dose dependent (Figure 5A&B).
Figure 4. Priming of the oxidative burst of monocytes and neutrophils isolated from the blood and pericardial fluid of postoperative cardiac surgery patients.
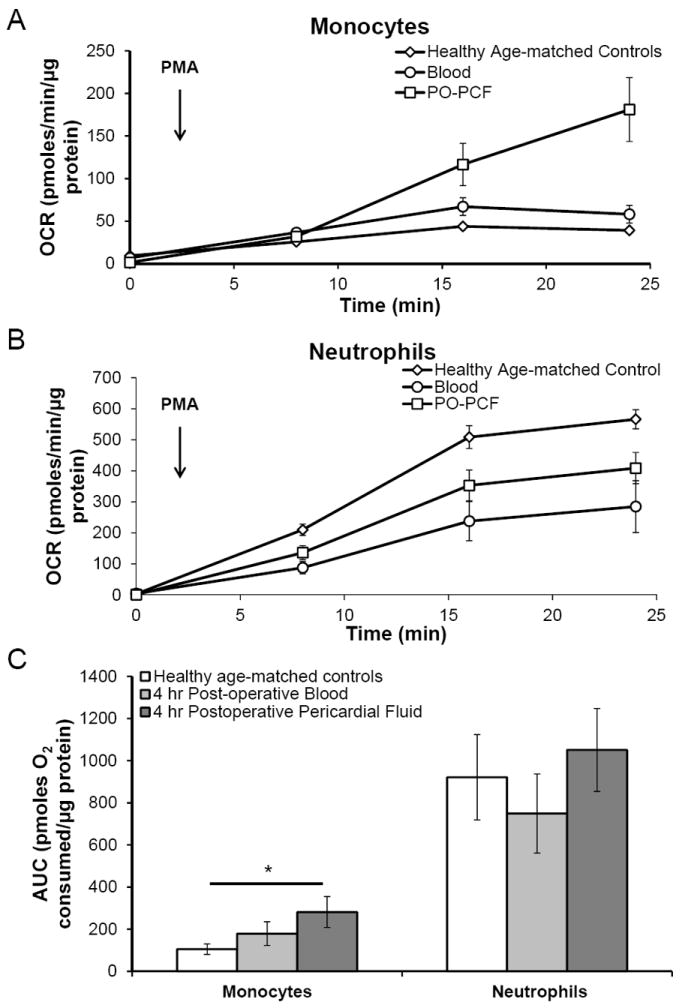
A) Healthy age matched control (n=8) monocytes and B) neutrophils isolated from freshly drawn blood were stimulated with PMA (100 ng/ml). Oxygen consumption was monitored over time on a Seahorse Extracellular Flux analyzer and compared with cells collected from blood and pericardial fluid obtained from at 4 hours post-surgery (n=7). C) The oxidative burst was measured as the area under the curve (AUC) of oxygen consumption over 24 minutes following PMA injection, and normalized to the oxygen consumption rate after antimycin A (10 μM) injection to inhibit mitochondrial respiration. 250k cells/well (monocytes) and 125k cells/well (neutrophils), 3-5 replicates per sample. Data reported as mean ± SEM. * = p ≤ 0.05
Figure 5. Post-operative pericardial fluid primes neutrophils for increased oxidative burst.
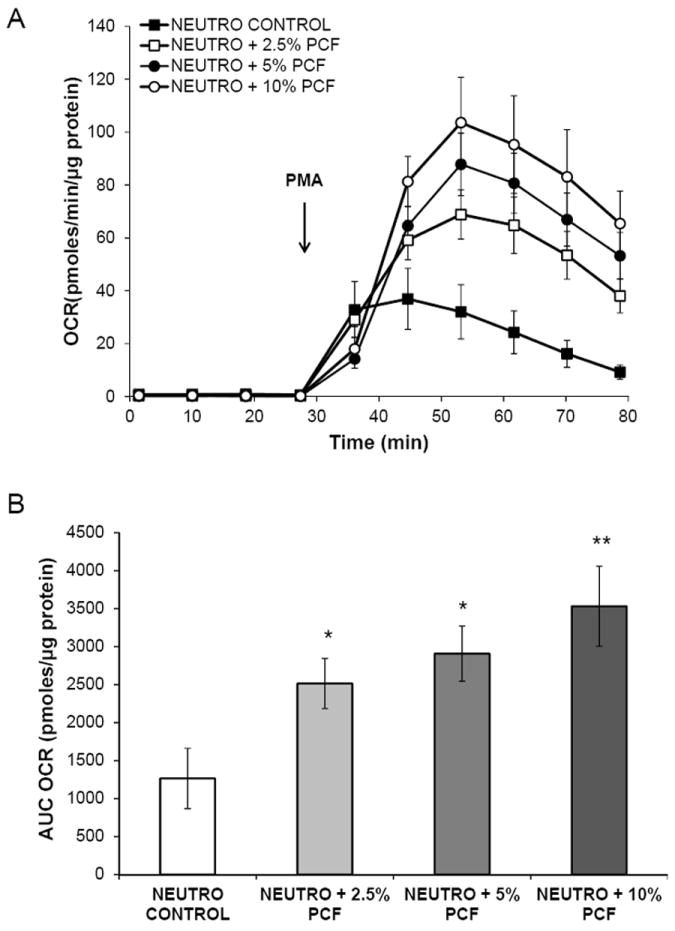
Priming of the oxidative burst in normal, healthy donor neutrophils collected from pericardial fluid at 4 hours post-surgery. Pericardial fluid was centrifuged at 1,500xg and then diluted to 2.5%, 5%, and 10% in XF DMEM. It was then added to freshly isolated and plated neutrophils 1.5 hours prior to the assay. A) Baseline OCR was measured in neutrophils prior to inhibition of mitochondrial respiration with antimycin A (10 μM) and induction of the oxidative burst with PMA (100 ng/mL). B) The area under the curve (AUC) during the 48 minute oxidative burst response was calculated and plotted by PCF dose. *= p<0.05, **= p<0.005. 3-5 replicated wells per group.
Lipid peroxidation products indicate a pro-oxidant environment in the postoperative pericardial fluid
MetHb and myoglobin promote lipid peroxidation particularly in the presence of hydrogen peroxide (31, 34). Mass spectrometry analysis revealed high and variable concentrations of the non-specific lipid peroxidation products of arachidonic acid derived isoprostane 8-iso-PGF2α and its stereoisomer 8-iso-15-PGF2α (F2 isoprostanes) in the PCF at 4 and 12 h following surgery (Figure 6A). These concentrations returned to near basal levels by 24-48 h. The prostaglandin F2α (PGF2α) and its stereoisomer 15(R)-PGF2α (Prostaglandins) showed a similar pattern although levels were approximately 10-fold higher than the isoprostanes consistent with the activation of cyclooxygenase (Figure 6B). Interestingly, the levels of isoprostanes were well correlated with prostaglandins (Figure 6C) suggesting that the available arachidonic acid, which is the substrate for both these lipid oxidation products, may also be increasing in PO-PCF.
Figure 6. Quantitation of F2α Isoprostanes and Prostaglandins in postoperative PCF by LCMS/MS.
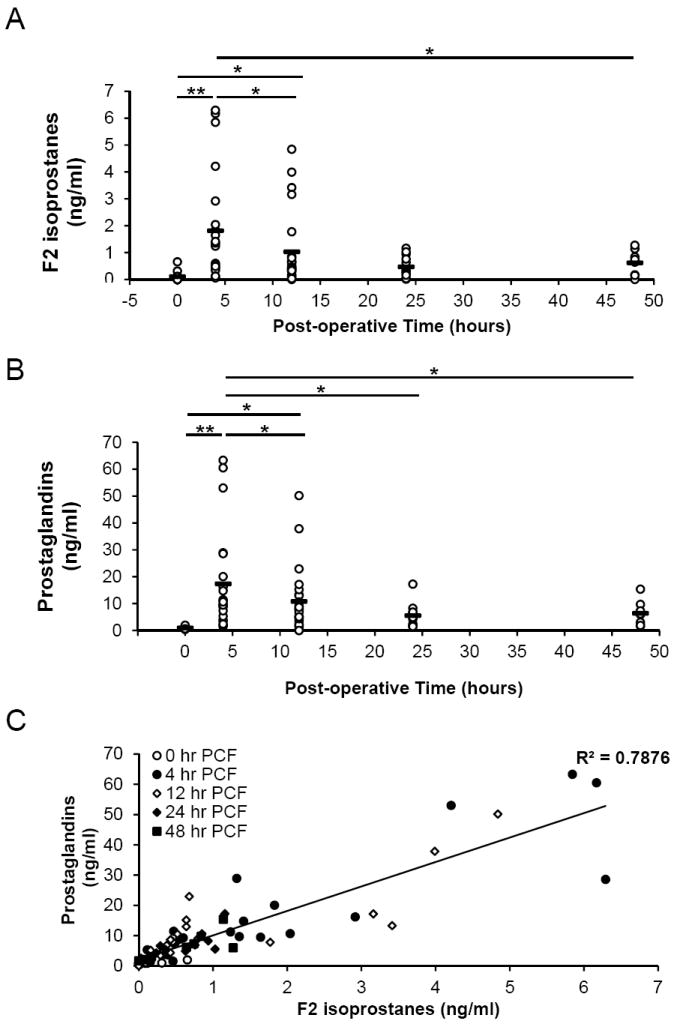
Lipids were extracted from post-operative pericardial fluid using solid phase extraction columns as described in the methods and analyzed by mass spectrometry. A) 8-iso-prostaglandin F2α concentrations and B) prostaglandin F2α concentrations were assessed in 0, 4, 12, 24, and 48 hour postoperative PCF patient samples. C) Regression analysis of F2α Isoprostanes plotted against prostaglandins over all time points. Data represented as individual patient values plus with the mean represented by a horizontal bar at 0 hours (n=13), 4 hours (n=22), 12 hours (n=23), 24 hours (n=12), and 48 hours (n=8). Statistical significance was determined using One-way Anova followed by Tukey’s Post hoc analysis, with p<0.05 indicating statistical significance. *= p<0.05, **= p<0.005.
Protein thiol oxidation and carbonyl formation are increased in postoperative pericardial fluid
Oxidants such as hydrogen peroxide and lipid peroxidation products are capable of modifying cysteine residues on proteins. To determine the extent of these reactions in the PCF, free thiol and carbonyl formation detection techniques were utilized. As shown in Figure 7A, Bodipy-IAM labeling of albumin, which has a single available thiol and is the predominant protein in both plasma and PO-PCF, was significantly decreased in the 4 h PO-PCF post-surgery samples compared to the 0 h PCF samples. Carbonylation of PCF proteins as determined by biotin hydrazide labeling of albumin, detected significant carbonyl formation of proteins at 4 h post-surgery compared to the intraoperative sample (0h PO-PCF). These findings indicate that significant oxidative modification and adduct formation of proteins occurs following cardiac surgery (Figure 7B). Furthermore, these data are consistent with the presence of hemoglobin and isoprostanes observed at the same time points (Figures 2 and 6).
Figure 7. Oxidative modifications to proteins in Postoperative PCF.
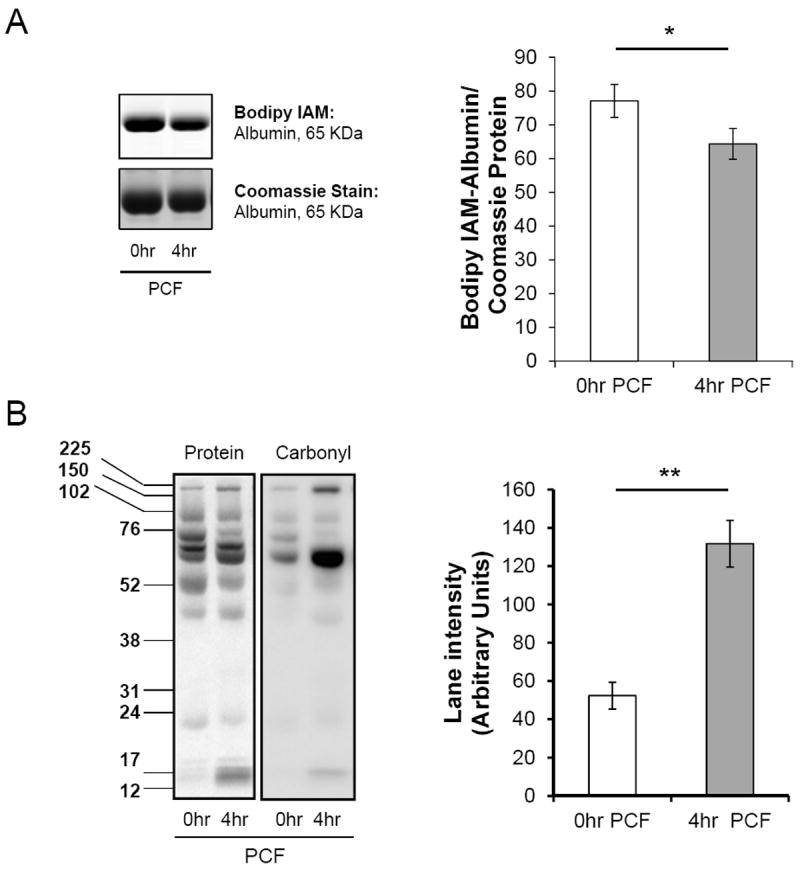
A) Representative in-gel fluorescence image of individual patient PCF (0 and 4 hr) treated with 500 μM Bodipy-iodoacetamide (Bodipy-IAM) for 30 min to assess protein thiol modifications, where lower fluorescence intensity represents increased oxidized protein thiols. Following alkylation, 5 μg of protein was resolved by 12.5% SDS–PAGE and imaged in-gel using a Typhoon imager. The bottom panel shows coomassie blue stain to confirm equal protein loading. Quantitation of the Bodipy-IAM fluorescence signal for albumin normalized to coomassie blue protein staining. n=7 patients; Data expressed as mean ± SEM.*= p<0.05 compared to 0 hr PCF. B) Carbonylated proteins (100 μg) from 0 and 4 hr albumin depleted PCF were derivatized with biotin hydrazide (1 mM) for 30 min at 37°C. Reactions were stopped with 1M Tris-HCl, pH 7.4 and proteins (10 μg) separated using SDS-PAGE. The extent of carbonylation was determined by western blotting using Streptavidin. The quantification of lane intensities is shown to the right. n = 4 patient matched sets; Statistical significance was measured using Student’s T-Test. Data expressed as mean ± SEM. *= p<0.05, **= p<0.005.
Discussion
Alterations in the composition and volume of the pericardial fluid occur after cardiac surgery. These alterations include the accumulation of inflammatory cells and oxidative molecules which can potentially lead to postoperative complications and alter cardiac function. As shown these changes include elevated cardiac injury markers, increased levels of cell free hemoglobin and methemoglobin, inflammatory cell infiltration, and the accumulation of lipid peroxidation products. Taken together, surgical trauma initiates red blood cell hemolysis, pro-inflammatory cell recruitment, and the active generation of pro-oxidative and pro-inflammatory molecules. It is likely that a major source of pro-oxidative molecules in the pericardial space post-surgery is the neutrophil population that immigrates into the area in large numbers.
An established link between oxidant production and inflammation is the oxidative burst, a process by which inflammatory stimuli and antigens activate NADPH oxidase to produce superoxide and hydrogen peroxide as an innate host defense. Inflammatory cells are a source of superoxide and hydrogen peroxide as well as nitric oxide and hypochlorous acid (35-37). The oxidative burst is capable of generating these oxidative species in the micromolar range, which will oxidize MetHb to the ferryl form, an efficient initiator of lipid peroxidation (34, 38-42). Inflammation has been found to be associated with atrial fibrillation, although most investigators have monitored the systemic inflammatory markers rather than the cardiac compartment in which inflammation directly occurs (43, 44). Recently, Pinho-Gomes et al. demonstrated an association between ROS in atrial tissue and the onset of postoperative atrial fibrillation after cardiac surgery (45) providing evidence that ongoing myocardial injury from oxidative stress may contribute to post-operative complications such as atrial fibrillation.
The post-operative pericardial environment is lipid-rich, given the proximity of epicardial and pericardial fat (46, 47). The enzymatic (cyclooxygenase mediated) and non-enzymatic (heme-protein mediated) oxidation of polyunsaturated fatty acids generates biologically significant oxidation products which play a role in inflammation, signaling, and cell damage. Lipid peroxidation products have also been linked to the oxidative stress observed in ischemic heart disease (48-52) and might also contribute to postoperative cardiac dysfunction.
In this study, the PO-PCF showed high concentrations of oxidized hemoglobin and evidence of lipid peroxidation, which has been shown to be a quantitative marker of oxidant stress and has previously been associated with increased incidence of heart failure (53). The formation of lipid radicals ultimately results in bioactive and/or electrophilic lipids capable of modifying nucleophilic protein residues such as cysteine, tyrosine, arginine, and histidine. Consistent with oxidative protein modification, the available thiol pool on albumin was significantly decreased at 4 h post-surgery in PCF. Isoprostanes, a common product of non-enzymatic arachidonic acid peroxidation, are commonly used markers of oxidative stress in vivo and were present at elevated levels in the PCF at post-operative time points 4 and 12 h (Figure 6). Although the pericardial drains serve the purpose of limiting postoperative tamponade, the cardiac tissues are still exposed to an effusion containing high levels of oxidative molecules which can contribute to postoperative cardiac dysfunction, possibly including electromechanical disturbances which are characteristic of atrial fibrillation. This concept is further supported by data from animal models demonstrating that oxidant production in the heart can lead to disruption of gap junctions and arrhythmic events, the blockade of reactive oxygen species (ROS) resulted in dramatic decreases in arrhythmias (54).
Oxidative stress has been shown to contribute to worse patient outcomes following cardiac surgery (55, 56). Traditionally, these markers were measured by the presence of reactive lipids such as malondialdehyde and HNE as well as the presence of antioxidant enzymes such as catalase (57). Using the thiol alkylating reagent iodoacetamide linked to the fluorophore Bodipy, we showed that albumin in the PO-PCF contained significantly fewer available thiols when compared to pre-operative PCF. Additionally, we examined protein carbonylation, a marker of oxidative protein modification in intra-operative and post-operative PCF samples. Consistent with other indices of oxidative stress, the amount of protein carbonylation in post-operative PCF was significantly increased compared to intra-operative PCF (Figure 7). These data support increased production of reactive lipids and increased oxidant production in the PCF following surgery.
Superoxide production plays an important role in ROS mediated inflammatory signaling (58-62). Under basal conditions, NADPH oxidase activity remains low; however under stimuli such as increased pro-inflammatory cytokines, enzymatic activity can be increased by a process known as priming (63-67). Under chronic levels of low stimuli, phagocytic cells such as neutrophils can be primed, resulting in a heightened response to exogenous stimuli such as PMA. We determined whether monocytes and neutrophils isolated from the post-operative pericardial space or treated with pericardial fluid were primed for oxidant production by stimulation with PMA. When stimulated, monocytes from the pericardial space showed a much higher capacity for oxidative burst when compared to blood monocytes isolated from the same patient and age-matched controls. Using neutrophils isolated from healthy donors, we showed a dose-dependent oxidative burst capacity in response to priming with increasing amounts of 4 h PO-PCF (Figure 5). These data clearly demonstrate that monocytes and neutrophils, in response to the post-operative PCF milieu, are primed for oxidant production, supporting the concept that oxidative stress in the pericardium following surgery is in part due to the infiltrating inflammatory cells.
In summary, these data show that post-operative PCF contains high levels of cell free hemoglobin, isoprostanes and prostaglandins, which were not found in concurrent sampling of blood serum from the same patients or in the intra-operative PCF. We also demonstrate that a highly pro-oxidant environment is generated in the post-operative pericardial fluid of cardiac surgery patients. Evidence supports that this pro-oxidant milieu surrounding the heart may trigger post-operative atrial fibrillation. The incomplete drainage of this effusion after surgery may not be sufficient to prevent oxidative injury to myocytes. Additional studies are required to investigate if therapies to inhibit oxidative stress in the pericardium may improve outcomes by preventing the onset or severity of atrial fibrillation. These data suggest an interesting relationship between oxidant production by infiltrating leukocytes, MetHb, and lipid peroxidation products and encourage larger scale trials to determine the nature and mechanism of this relationship and its potential role in the development of post-operative complications.
Acknowledgments
The authors thank Gloria A Benavides for her valuable technical assistance. This work was supported by T32HL07918 (PAK), P30DK056336 (BC), DK079337 (VDU,SB), HL103859 (SWB), T32HL007457 (TM), UAB CCTS pilot grant award (SJM), Birmingham Veterans Administration Merit Review grants (LJD) and the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research (DK079337). The mass spectrometer used in these studies was purchased with an award (SB) from the UAB Health Services Foundation General Endowment Fund.
Abbreviations
- AF
Atrial fibrillation
- PO-PCF
Postoperative Pericardial Fluid
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- RLS
Reactive lipid species
- PCF
Pericardial fluid
- PB
Peripheral blood
- WBC
White blood cell
- CABG
Coronary Artery By-pass Graft surgery
- AVR
Aortic valve replacement/repair
- MVR
Mitral valve replacement/repair
- FACS
Fluorescence–activated cell sorting
- BHT
butylated hydroxyl toluene
- COX-2
Cyclooxygenase 2
- NOX2
NADPH oxidase 2
- iNOS
inducible nitric-oxide synthase
- OCR
Oxygen consumption rate
- PMA
phorbol 12-myristate 13-acetate
- PKC
Protein Kinase C
- DPI
diphenylene iodonium
- Bodipy IAM
Bodipy-iodoacetamide
- MetHb
methemoglobin
- OxyHb
oxyhemoglobin
Footnotes
Author contributions: SJM, VDU, PAK, MSJ and BKC designed the experiments; PAK, BKC, SR, MSJ, TM and JFG performed the experiments and analyzed the data; VDU, SJM, PAK, TM and BC wrote and edited the paper; SB, LJD, MSJ and JFG reviewed and edited the paper.
References and Notes
- 1.Popovic JR. National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. Vital and health statistics. 1999;2001(151):i–v. 1–206. doi: 10.1037/e309042005-001. [DOI] [PubMed] [Google Scholar]
- 2.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94(3):390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 3.Parkash R, Verma A, Tang AS. Persistent atrial fibrillation: current approach and controversies. Current opinion in cardiology. 25(1):1–7. doi: 10.1097/HCO.0b013e3283336d52. [DOI] [PubMed] [Google Scholar]
- 4.Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. European journal of internal medicine. 2009;20(7):672–681. doi: 10.1016/j.ejim.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Gedikli O, Dogan A, Altuntas I, et al. Inflammatory markers according to types of atrial fibrillation. International journal of cardiology. 2007;120(2):193–197. doi: 10.1016/j.ijcard.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Chelazzi C, Villa G, De Gaudio AR. Postoperative atrial fibrillation. ISRN cardiology. 2011 doi: 10.5402/2011/203179. 203179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldhoon B, Melenovsky V, Peichl P, Kautzner J. New insights into mechanisms of atrial fibrillation. Physiological research / Academia Scientiarum Bohemoslovaca. 59(1):1–12. doi: 10.33549/physiolres.931651. [DOI] [PubMed] [Google Scholar]
- 8.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 124(21):2290–2295. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 9.Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil. 2008;15(6):735–741. doi: 10.1097/HJR.0b013e328317f38a. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigo R, Castillo R, Cereceda M, Asenjo R, Zamorano J, Araya J. Non-hypoxic preconditioning of myocardium against postoperative atrial fibrillation: mechanism based on enhancement of the antioxidant defense system. Medical hypotheses. 2007;69(6):1242–1248. doi: 10.1016/j.mehy.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Aronson D, Boulos M, Suleiman A, et al. Relation of C-reactive protein and new-onset atrial fibrillation in patients with acute myocardial infarction. The American journal of cardiology. 2007;100(5):753–757. doi: 10.1016/j.amjcard.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Lappegard KT, Hovland A, Pop GA, Mollnes TE. Atrial fibrillation: inflammation in disguise? Scand J Immunol. 2013;78(2):112–119. doi: 10.1111/sji.12061. [DOI] [PubMed] [Google Scholar]
- 13.Fontes ML, Mathew JP, Rinder HM, et al. Atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesth Analg. 2005;101(1):17–23. doi: 10.1213/01.ANE.0000155260.93406.29. [DOI] [PubMed] [Google Scholar]
- 14.Chen MC, Chang JP, Liu WH, et al. Increased inflammatory cell infiltration in the atrial myocardium of patients with atrial fibrillation. Am J Cardiol. 2008;102(7):861–865. doi: 10.1016/j.amjcard.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111(22):2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 16.Dudzinski DM, Mak GS, Hung JW. Pericardial diseases. Current problems in cardiology. 37(3):75–118. doi: 10.1016/j.cpcardiol.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Zipes DP. Atrial fibrillation: a review of pathophysiology. Semin Interv Cardiol. 1997;2(4):203–213. [PubMed] [Google Scholar]
- 18.Bodson L, Bouferrache K, Vieillard-Baron A. Cardiac tamponade. Current opinion in critical care. 17(5):416–424. doi: 10.1097/MCC.0b013e3283491f27. [DOI] [PubMed] [Google Scholar]
- 19.Karatolios K, Pankuweit S, Maisch B. Diagnostic value of biochemical biomarkers in malignant and non-malignant pericardial effusion. Heart failure reviews. 2013;18(3):337–44. doi: 10.1007/s10741-012-9327-x. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Horin S, Shinfeld A, Kachel E, Chetrit A, Livneh A. The composition of normal pericardial fluid and its implications for diagnosing pericardial effusions. Am J Med. 2005;118(6):636–640. doi: 10.1016/j.amjmed.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 21.Selmeci L, Antal M, Horkay F, et al. Enhanced accumulation of pericardial fluid ferritin in patients with coronary artery disease. Coronary artery disease. 2000;11(1):53–56. doi: 10.1097/00019501-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Gibson AT, Segal MB. A study of the composition of pericardial fluid, with special reference to the probable mechanism of fluid formation. The Journal of physiology. 1978;277:367–377. doi: 10.1113/jphysiol.1978.sp012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisalo A, Konttinen A. Composition of pericardial fluid in cholesterol pericarditis. Acta medica Scandinavica. 1972;191(1-2):125–128. [PubMed] [Google Scholar]
- 24.Megged O, Argaman Z, Kleid D. Purulent pericarditis in children: is pericardiotomy needed? Pediatric emergency care. 27(12):1185–1187. doi: 10.1097/PEC.0b013e31823b44af. [DOI] [PubMed] [Google Scholar]
- 25.Sparano DM, Ward RP. Pericarditis and pericardial effusion: management update. Current treatment options in cardiovascular medicine. 13(6):543–555. doi: 10.1007/s11936-011-0151-8. [DOI] [PubMed] [Google Scholar]
- 26.Katz LH, Pitlik S, Porat E, Biderman P, Bishara J. Pericarditis as a presenting sign of infective endocarditis: two case reports and review of the literature. Scandinavian journal of infectious diseases. 2008;40(10):785–791. doi: 10.1080/00365540802169106. [DOI] [PubMed] [Google Scholar]
- 27.Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363(9410):717–727. doi: 10.1016/S0140-6736(04)15648-1. [DOI] [PubMed] [Google Scholar]
- 28.Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. Journal of visualized experiments : JoVE. 2014(85) doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chacko BK, Kramer PA, Ravi S, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Laboratory investigation; a journal of technical methods and pathology. 2013;93(6):690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snell SM, Marini MA. A convenient spectroscopic method for the estimation of hemoglobin concentrations in cell-free solutions. Journal of biochemical and biophysical methods. 1988;17(1):25–33. doi: 10.1016/0165-022x(88)90075-9. [DOI] [PubMed] [Google Scholar]
- 31.Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. Amsterdam: North-Holland Pub. Co.; 1971. p. 436. [Google Scholar]
- 32.Prasain JK, Arabshahi A, Taub PR, et al. Simultaneous quantification of F2-isoprostanes and prostaglandins in human urine by liquid chromatography tandem-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;913-914:161–168. doi: 10.1016/j.jchromb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chacko BK, Kramer PA, Ravi S, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013;93(6):690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore KP, Holt SG, Patel RP, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273(48):31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 35.Chedid P, Hurtado-Nedelec M, Marion-Gaber B, et al. Adiponectin and its globular fragment differentially modulate the oxidative burst of primary human phagocytes. The American journal of pathology. 2011;180(2):682–692. doi: 10.1016/j.ajpath.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Santos SS, Brunialti MK, Rigato O, Machado FR, Silva E, Salomao R. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock. 2012;38(1):18–23. doi: 10.1097/SHK.0b013e318257114e. [DOI] [PubMed] [Google Scholar]
- 37.Jukic T, Ihan A, Jukic D. Tetrahydrophthalazine derivative “sodium nucleinate” exert its anti-inflammatory effects through inhibition of oxidative burst in human monocytes. Collegium antropologicum. 2012;36(2):409–412. [PubMed] [Google Scholar]
- 38.Heyn J, Beiras-Fernandez A, Luchting B, Briegel J, Weis F. Inflammatory reactions and hydrocortisone in the setting of cardiac surgery: an overview. Cardiovascular & hematological agents in medicinal chemistry. 2011;9(1):56–61. doi: 10.2174/187152511794182800. [DOI] [PubMed] [Google Scholar]
- 39.Fink R, Al-Obaidi M, Grewal S, Winter M, Pepper J. Monocyte activation markers during cardiopulmonary bypass. Perfusion. 2003;18(2):83–86. doi: 10.1191/0267659103pf645oa. [DOI] [PubMed] [Google Scholar]
- 40.Franke A, Lante W, Fackeldey V, et al. Proinflammatory and antiinflammatory cytokines after cardiac operation: different cellular sources at different times. The Annals of thoracic surgery. 2002;74(2):363–370. doi: 10.1016/s0003-4975(02)03658-5. discussion 370-361. [DOI] [PubMed] [Google Scholar]
- 41.Edmunds LH., Jr Inflammatory response to cardiopulmonary bypass. The Annals of thoracic surgery. 1998;66(5 Suppl):S12–16. doi: 10.1016/s0003-4975(98)00967-9. discussion S25-18. [DOI] [PubMed] [Google Scholar]
- 42.Khare S, Bhutani LK, Rao DN. Modulation of peripheral blood derived monocytes/macrophages from leprosy patients using ‘tuftsin’ for production of reactive oxygen intermediates. Leprosy review. 1993;64(3):208–218. doi: 10.5935/0305-7518.19930023. [DOI] [PubMed] [Google Scholar]
- 43.Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. The American journal of cardiology. 2004;93(9):1176–1178. doi: 10.1016/j.amjcard.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. The Annals of thoracic surgery. 2005;79(5):1530–1535. doi: 10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Pinho-Gomes AC, Reilly S, Brandes RP, Casadei B. Targeting Inflammation and Oxidative Stress in Atrial Fibrillation: Role of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibition with Statins. Antioxidants & redox signaling. 2014;20(8):1268–85. doi: 10.1089/ars.2013.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzzardi MA, Iozzo P. Fatty heart, cardiac damage, and inflammation. Rev Diabet Stud. 8(3):403–417. doi: 10.1900/RDS.2011.8.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacks HS, Fain JN, Cheema P, et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metabolic syndrome and related disorders. 2011;9(6):433–439. doi: 10.1089/met.2011.0024. [DOI] [PubMed] [Google Scholar]
- 48.Dincic D, Jovic P, Obradovic S, Popovic P, Prcovic M. Lipid peroxidation intensity and lipid status parameters in the estimation of the severity of ischemic heart disease. Vojnosanitetski pregled. 1998;55(4):359–367. [PubMed] [Google Scholar]
- 49.Chandra M, Chandra N, Agrawal R, Kumar A, Ghatak A, Pandey VC. The free radical system in ischemic heart disease. International journal of cardiology. 1994;43(2):121–125. doi: 10.1016/0167-5273(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 50.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. The Biochemical journal. 2012;442(3):453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higdon AN, Landar A, Barnes S, Darley-Usmar VM. The Electrophile Responsive Proteome: Integrating Proteomics and Lipidomics with Cellular Function. Antioxidants & redox signaling. 2012;17(11):1580–9. doi: 10.1089/ars.2012.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eaton P, Hearse DJ, Shattock MJ. Lipid hydroperoxide modification of proteins during myocardial ischaemia. Cardiovascular research. 2001;51(2):294–303. doi: 10.1016/s0008-6363(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 53.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97(16):1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 54.Sovari AA, Rutledge CA, Jeong EM, et al. Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circulation Arrhythmia and electrophysiology. 2013;6(3):623–631. doi: 10.1161/CIRCEP.112.976787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karu I, Taal G, Zilmer K, Pruunsild C, Starkopf J, Zilmer M. Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scandinavian cardiovascular journal : SCJ. 2010;44(2):119–124. doi: 10.3109/14017430903490981. [DOI] [PubMed] [Google Scholar]
- 56.McDonald CI, Fraser JF, Coombes JS, Fung YL. Oxidative stress during extracorporeal circulation. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezt637. in press. [DOI] [PubMed] [Google Scholar]
- 57.Vukasovic JL, Moraga F, Diaz-Araya G, et al. Oxidative stress in pericardial fluid and plasma and its association with ventricular function. Int J Cardiol. 2005;101(2):197–201. doi: 10.1016/j.ijcard.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Maulik SK, Kumar S. Oxidative stress and cardiac hypertrophy: a review. Toxicology mechanisms and methods. 2012;22(5):359–366. doi: 10.3109/15376516.2012.666650. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19(10):1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, Perino A, Ghigo A, Hirsch E, Shah AM. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal. 2013;18(9):1024–1041. doi: 10.1089/ars.2012.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen GX. Mitochondrial dysfunction, oxidative stress and diabetic cardiovascular disorders. Cardiovascular & hematological disorders drug targets. 2012;12(2):106–112. doi: 10.2174/1871529x11202020106. [DOI] [PubMed] [Google Scholar]
- 62.Hafstad AD, Nabeebaccus AA, Shah AM. Novel aspects of ROS signalling in heart failure. Basic research in cardiology. 2013;108(4):359. doi: 10.1007/s00395-013-0359-8. [DOI] [PubMed] [Google Scholar]
- 63.Koval’chuk LV, Klebanov GI, Ribarov SR, et al. Priming of phagocytes by cytokines and water-soluble products of lipid peroxidation. Biomed Sci. 1991;2(3):221–231. [PubMed] [Google Scholar]
- 64.Kharazmi A, Nielsen H, Rechnitzer C, Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989;21(2):177–184. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- 65.Hurtado-Nedelec M, Makni-Maalej K, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Assessment of priming of the human neutrophil respiratory burst. Methods in molecular biology. 2014;1124:405–412. doi: 10.1007/978-1-62703-845-4_23. [DOI] [PubMed] [Google Scholar]
- 66.Finkel TH, Pabst MJ, Suzuki H, et al. Priming of neutrophils and macrophages for enhanced release of superoxide anion by the calcium ionophore ionomycin. Implications for regulation of the respiratory burst. J Biol Chem. 1987;262(26):12589–12596. [PubMed] [Google Scholar]
- 67.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30(3):279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]


