Abstract
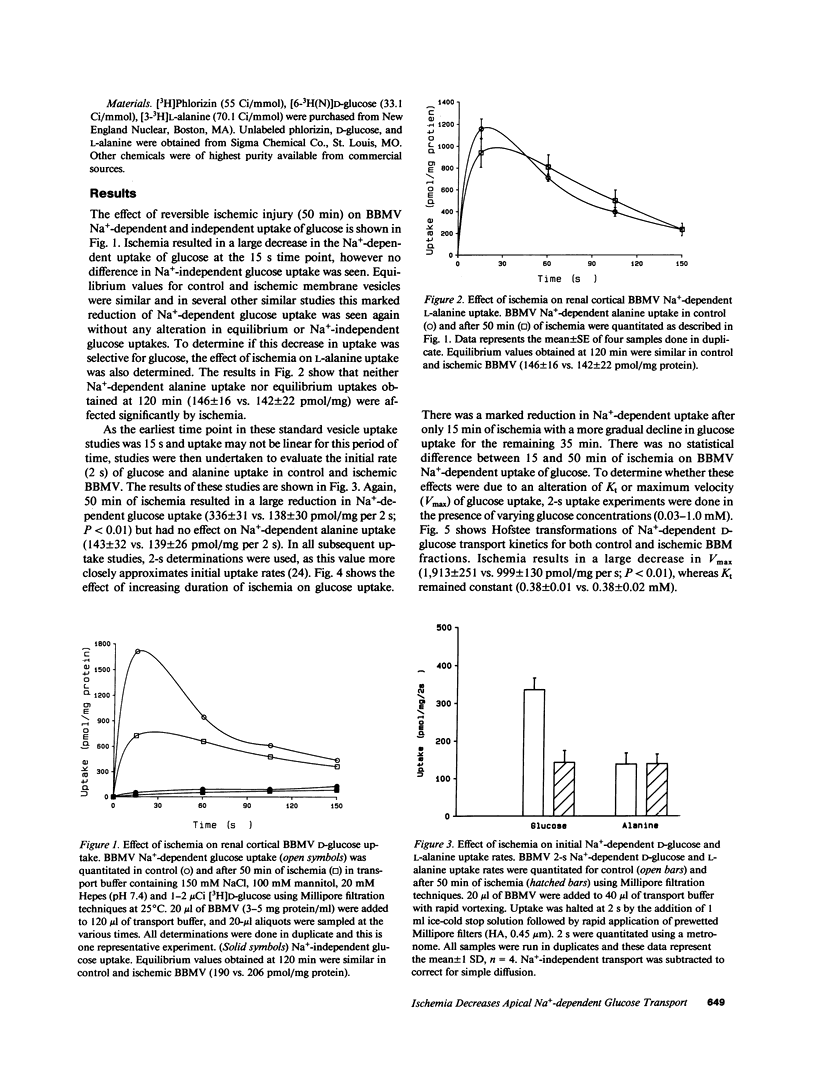
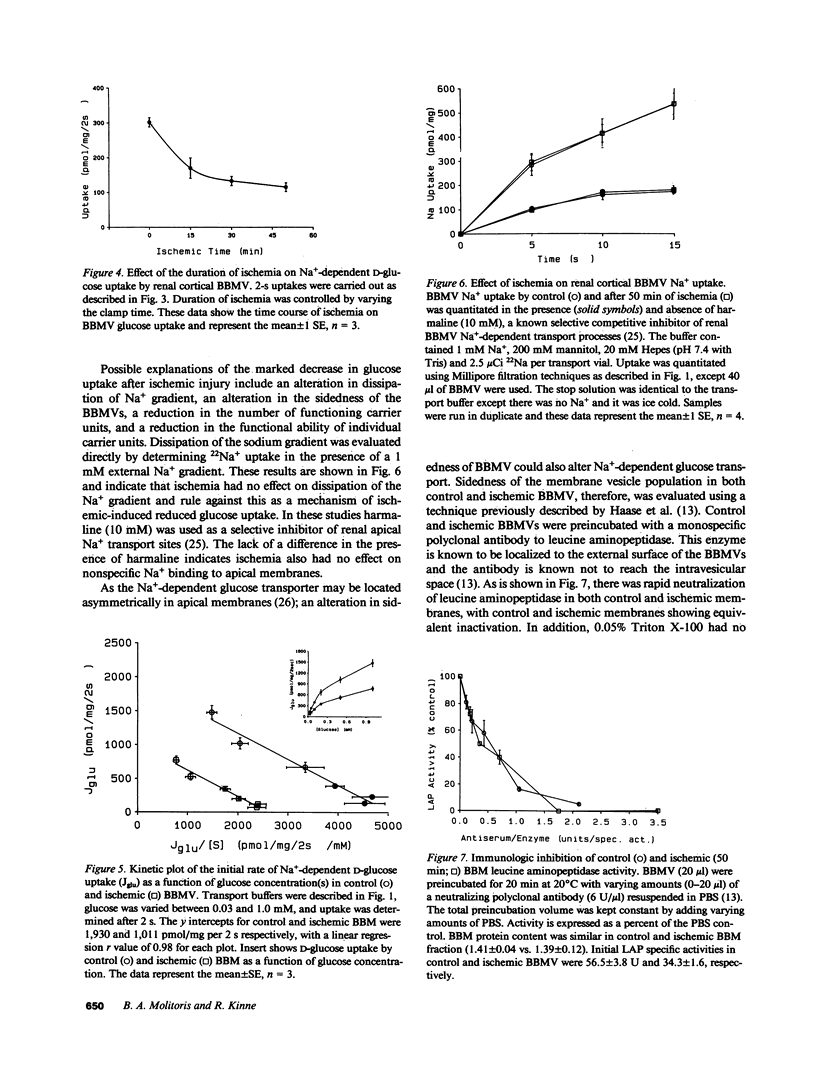
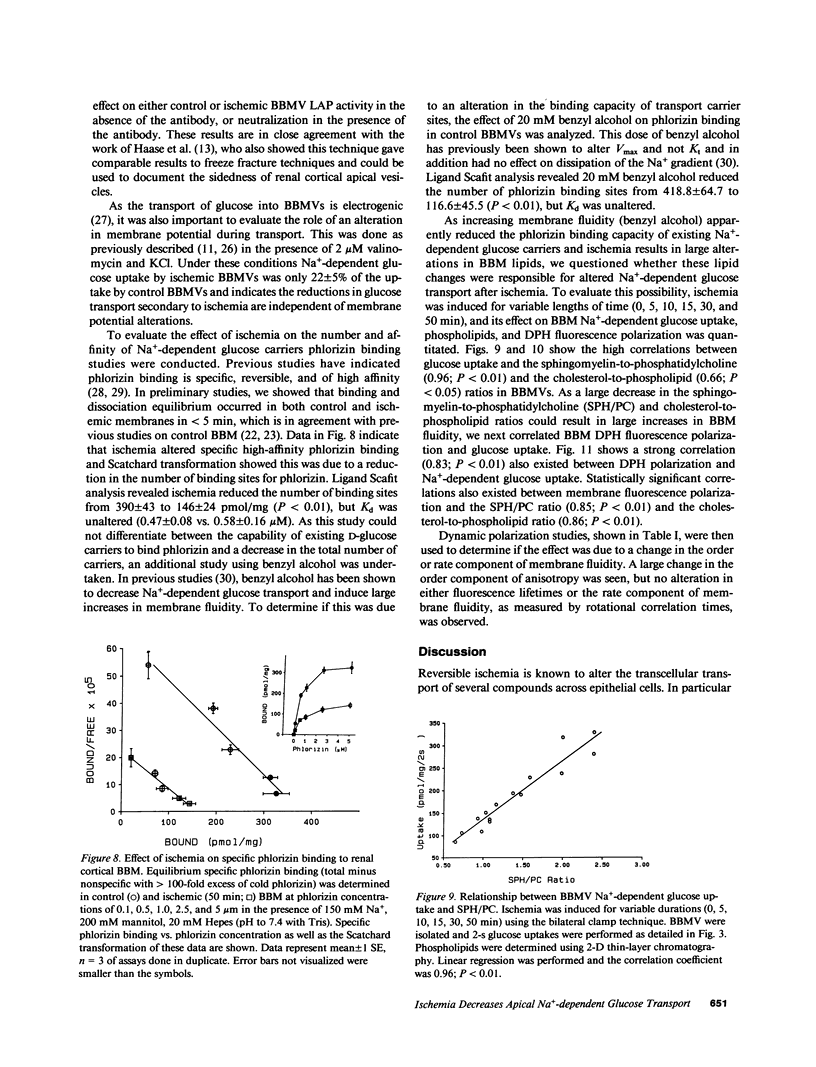
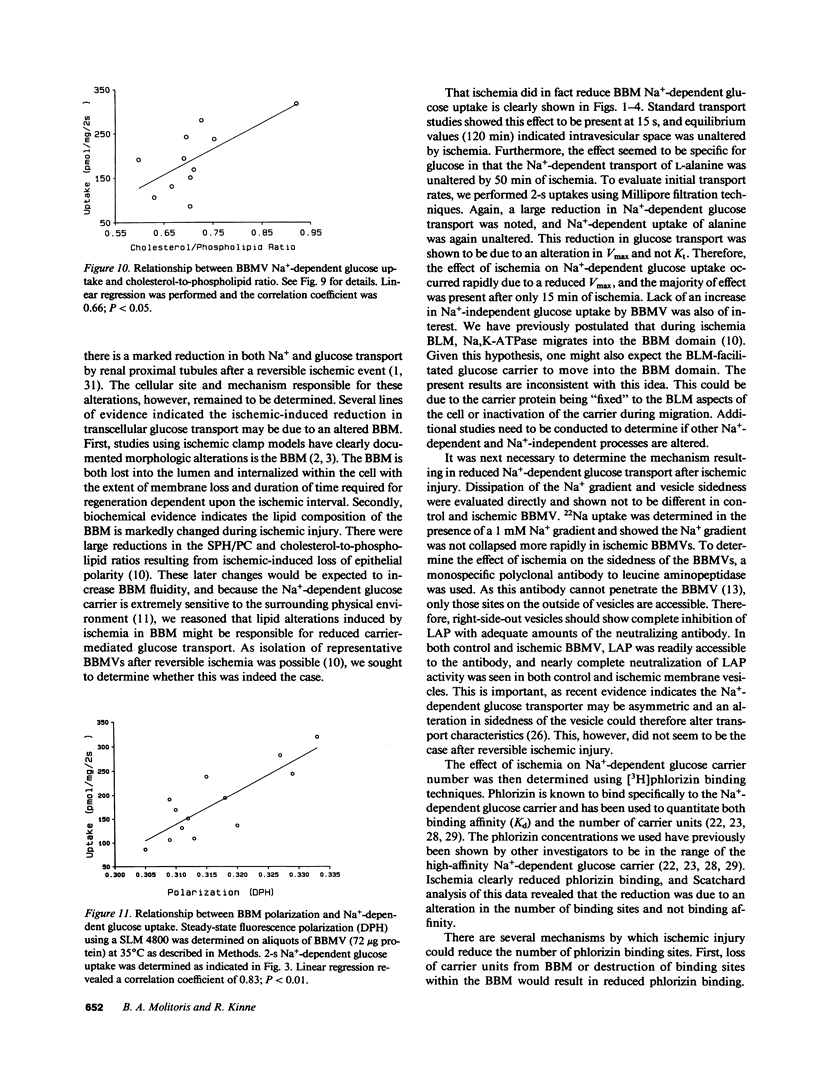
Reversible ischemia reduced renal cortical brush border membrane (BBM) Na+-dependent D-glucose uptake (336 +/- 31 vs. 138 +/- 30 pmol/mg per 2 s, P less than 0.01) but had no effect on Na+-independent glucose or Na+-dependent L-alanine uptake. The effect on D-glucose uptake was present after only 15 min of ischemia and was due to a reduction in maximum velocity (1913 +/- 251 vs. 999 +/- 130 pmol/mg per 2 s; P less than 0.01). This reduction was not due to more rapid dissipation of the Na+ gradient, altered sidedness of the vesicles, or an alteration in membrane potential. Ischemia did, however, reduce the BBM sphingomyelin-to-phosphatidylcholine (SPH/PC) and cholesterol-to-phospholipid ratios and the number of specific high-affinity Na+-dependent phlorizin binding sites (390 +/- 43 vs. 146 +/- 24 pmol/mg; P less than 0.01) without altering the binding dissociation constant (Kd). 20 mM benzyl alcohol also reduced the number of Na+-dependent phlorizin binding sites (418 +/- 65 vs. 117 +/- 46; P less than 0.01) without altering Kd. The reduction in Na+-dependent D-glucose transport correlated with ischemic-induced changes in the BBM SPH/PC and cholesterol-to-phospholipid ratios and membrane fluidity. Taken together these data indicate the cellular site responsible for ischemic-induced reduction in renal cortical transcellular glucose transport is the BBM. We propose the mechanism involves marked alterations in BBM lipids leading to large increases in BBM fluidity which reduces the binding capacity of Na+-dependent glucose carriers. These data indicate that reversible ischemia has profound effects on the surface membrane function of epithelial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Aronson P. S., Bounds S. E. Harmaline inhibition of Na-dependent transport in renal microvillus membrane vesicles. Am J Physiol. 1980 Mar;238(3):F210–F217. doi: 10.1152/ajprenal.1980.238.3.F210. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barrow D. A., Lentz B. R. Membrane structural domains. Resolution limits using diphenylhexatriene fluorescence decay. Biophys J. 1985 Aug;48(2):221–234. doi: 10.1016/S0006-3495(85)83775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Rodrigue F., Carrière S., Le Grimellec C. Composition and physical properties of lipids from plasma membranes of dog kidney. Biochim Biophys Acta. 1985 Aug 27;818(2):149–157. doi: 10.1016/0005-2736(85)90557-7. [DOI] [PubMed] [Google Scholar]
- Carrière B., Le Grimellec C. Effects of benzyl alcohol on enzyme activities and D-glucose transport in kidney brush-border membranes. Biochim Biophys Acta. 1986 May 28;857(2):131–138. doi: 10.1016/0005-2736(86)90340-8. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- De Smedt H., Kinne R. Temperature dependence of solute transport and enzyme activities in hog renal brush border membrane vesicles. Biochim Biophys Acta. 1981 Nov 6;648(2):247–253. doi: 10.1016/0005-2736(81)90040-7. [DOI] [PubMed] [Google Scholar]
- Donohoe J. F., Venkatachalam M. A., Bernard D. B., Levinsky N. G. Tubular leakage and obstruction after renal ischemia: structural-functional correlations. Kidney Int. 1978 Mar;13(3):208–222. doi: 10.1038/ki.1978.31. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Farber J. L., Martin J. T., Chien K. R. Irreversible ischemic cell injury. Prevention by chlorpromazine of the aggregation of the intramembranous particles of rat liver plasma membranes. Am J Pathol. 1978 Sep;92(3):713–732. [PMC free article] [PubMed] [Google Scholar]
- Fernandez Y. J., Boigegrain R. A., Cambon-Gros C. D., Mitjavila S. E. Sensitivity of Na+-coupled D-glucose uptake, Mg2+-ATPase and sucrase to perturbations of the fluidity of brush-border membrane vesicles induced by n-aliphatic alcohols. Biochim Biophys Acta. 1984 Mar 14;770(2):171–177. doi: 10.1016/0005-2736(84)90127-5. [DOI] [PubMed] [Google Scholar]
- Frasch W., Frohnert P. P., Bode F., Baumann K., Kinne R. Competitive inhibition of phlorizin binding by D-glucose and the influence of sodium: a study on isolated brush border membrane of rat kidney. Pflugers Arch. 1970;320(3):265–284. doi: 10.1007/BF00587458. [DOI] [PubMed] [Google Scholar]
- Frederiks W. M., Myagkaya G. L., van Veen H. A., Vogels I. M. Biochemical and ultrastructural changes in rat liver plasma membranes after temporary ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(4):269–282. doi: 10.1007/BF02890316. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Phlorizin receptors in isolated kidney brush border membranes. J Biol Chem. 1972 Dec 10;247(23):7779–7789. [PubMed] [Google Scholar]
- Gray G. M., Santiago N. A. Intestinal surface amino-oligopeptidases. I. Isolation of two weight isomers and their subunits from rat brush border. J Biol Chem. 1977 Jul 25;252(14):4922–4928. [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herminghuysen D., Welbourne C. J., Welbourne T. C. Renal sodium reabsorption, oxygen consumption, and gamma-glutamyltransferase excretion in the postischemic rat kidney. Am J Physiol. 1985 Jun;248(6 Pt 2):F804–F809. doi: 10.1152/ajprenal.1985.248.6.F804. [DOI] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Rennke H., Levinsky N. G. Recovery of proximal tubular function from ischemic injury. Am J Physiol. 1984 Feb;246(2 Pt 2):F159–F166. doi: 10.1152/ajprenal.1984.246.2.F159. [DOI] [PubMed] [Google Scholar]
- Kania R. K., Santiago N. A., Gray G. M. Intestinal surface amino-oligopeptidases. II. Substrate kinetics and topography of the active site. J Biol Chem. 1977 Jul 25;252(14):4929–4934. [PubMed] [Google Scholar]
- Kaunitz J. D., Wright E. M. Kinetics of sodium D-glucose cotransport in bovine intestinal brush border vesicles. J Membr Biol. 1984;79(1):41–51. doi: 10.1007/BF01868525. [DOI] [PubMed] [Google Scholar]
- Kessler M., Semenza G. The small-intestinal Na+, D-glucose cotransporter: an asymmetric gated channel (or pore) responsive to delta psi. J Membr Biol. 1983;76(1):27–56. doi: 10.1007/BF01871452. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin J. T., Szwarc K., Kinne R., Jung C. Y. Structural state of the Na+/D-glucose cotransporter in calf kidney brush-border membranes. Target size analysis of Na+-dependent phlorizin binding and Na+-dependent D-glucose transport. Biochim Biophys Acta. 1984 Nov 7;777(2):201–208. doi: 10.1016/0005-2736(84)90421-8. [DOI] [PubMed] [Google Scholar]
- Malathi P., Preiser H. Isolation of the sodium-dependent d-glucose transport protein from brush-border membranes. Biochim Biophys Acta. 1983 Nov 23;735(3):314–324. doi: 10.1016/0005-2736(83)90144-x. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Simon F. R. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turnover. J Membr Biol. 1985;83(3):207–215. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Wilson P. D., Schrier R. W., Simon F. R. Ischemia induces partial loss of surface membrane polarity and accumulation of putative calcium ionophores. J Clin Invest. 1985 Dec;76(6):2097–2105. doi: 10.1172/JCI112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Evidence for tyrosyl residues at the Na+ site on the intestinal Na+/glucose cotransporter. J Biol Chem. 1985 May 25;260(10):6026–6031. [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Sodium-induced conformational changes in the glucose transporter of intestinal brush borders. J Biol Chem. 1984 Nov 25;259(22):14105–14112. [PubMed] [Google Scholar]
- Petrovich D. R., Finkelstein S., Waring A. J., Farber J. L. Liver ischemia increases the molecular order of microsomal membranes by increasing the cholesterol-to-phospholipid ratio. J Biol Chem. 1984 Nov 10;259(21):13217–13223. [PubMed] [Google Scholar]
- Saito Y., Silbert D. F. Selective effects of membrane sterol depletion on surface function thymidine and 3-O-methyl-D-glucose transport in a sterol auxotroph. J Biol Chem. 1979 Feb 25;254(4):1102–1107. [PubMed] [Google Scholar]
- Silverman M., Black J. High affinity phlorizin receptor sites and their relation to the glucose transport mechanism in the proximal tubule of dog kidney. Biochim Biophys Acta. 1975 Jun 11;394(1):10–30. doi: 10.1016/0005-2736(75)90201-1. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Kempner E. S. Radiation inactivation studies of the renal brush-border membrane phlorizin-binding protein. J Biol Chem. 1982 Sep 25;257(18):10794–10797. [PubMed] [Google Scholar]
- Venkatachalam M. A., Jones D. B., Rennke H. G., Sandstrom D., Patel Y. Mechanism of proximal tubule brush border loss and regeneration following mild renal ischemia. Lab Invest. 1981 Oct;45(4):355–365. [PubMed] [Google Scholar]
- Wright S. H., Hirayama B., Kaunitz J. D., Kippen I., Wright E. M. Kinetics of sodium succinate cotransport across renal brush-border membranes. J Biol Chem. 1983 May 10;258(9):5456–5462. [PubMed] [Google Scholar]
- Yuli I., Wilbrandt W., Shinitzky M. Glucose transport through cell membranes of modified lipid fluidity. Biochemistry. 1981 Jul 21;20(15):4250–4256. doi: 10.1021/bi00518a003. [DOI] [PubMed] [Google Scholar]



