Abstract
Background
Tens of thousands of health-related smartphone applications (apps), including hundreds of weight loss apps, are available but little is known about the effectiveness of these programs.
Objective
To evaluate the impact of introducing patients to a popular, free smartphone app for weight loss, MyFitnessPal, in a primary care setting.
Design
Randomized, controlled trial.
Setting
Two primary care clinics in the University of California Los Angeles Health System.
Patients
212 primary care patients with body mass index greater than or equal to 25 kg/m2.
Intervention
6 months of usual care (n = 107) or usual care plus research assistant help in downloading the MyFitnessPal app onto the patient’s smartphone (n = 105).
Measurements
Weight loss at six months (primary outcome), change in systolic blood pressure (SBP), change in behavioral mediators, satisfaction with the app, and frequency of app use (secondary outcomes).
Results
There was no significant difference between intervention and control groups in weight change (mean between group difference, −0.67 lb [CI, −3.3 to 2.1lb]; p = 0.63) or in SBP (mean between group difference, −1.7 mmHg [CI, −7.1 to 3.8]; p = 0.55). The intervention group exhibited increased use of a personal calorie goal compared to the control group (mean between group difference, 2.0 days per week [CI, 1.1 to 2.9]; p < .001), though changes in other self-reported behaviors did not differ between the groups. Most users reported high satisfaction with MyFitnessPal but logins dropped sharply after the first month.
Limitation
Despite blinding to the name of the app, fourteen control group participants (16%) used MyFitnessPal. 32% of intervention group participants and 19% of control group participants were lost to follow-up at 6 months. The app was given to patients by research assistants, not by physicians.
Conclusion
Smartphone apps for weight loss may be useful for individuals who are ready to self-monitor calories. For the average overweight primary care patient, however, introducing a smartphone app is unlikely to produce significant weight change.
INTRODUCTION
It is well known that the United States is facing an obesity epidemic and the long-term sequela are costly (1,2). Researchers continue the search for effective weight loss interventions that can be applied in outpatient settings but these are often time consuming and resource intensive, requiring repeated counseling (3). It is no surprise that primary care providers often omit discussing weight loss with obese patients and rarely spend adequate time on counseling (4,5).
Smartphone applications (apps) may provide an alternative to resource intensive weight loss programs. In December 2013, a Pew survey found that 58% of Americans own smartphones and ownership is increasing among every demographic group including low-income populations (6). The nascent field of mobile health (mHealth) is rapidly expanding with experts estimating as many as 40,000 health related apps available in 2012, comprising a $718 million industry (7). Many of these apps aim to help individuals change behaviors to improve health, including weight loss, yet exceedingly few have been rigorously evaluated. An effective app for reducing weight could produce tremendous cost-savings by preventing long-term complications such as diabetes and cardiovascular events. To our knowledge, however, no studies have examined the effectiveness of delivering or “prescribing” an app for weight loss to patients in a clinical setting.
The present study evaluates one of the most popular, publically available apps for weight loss, MyFitnessPal (MFP). MFP receives the highest possible rating, five out of five stars, from thousands of reviewers on the Apple and Android app stores. MFP has nearly one million “likes” on Facebook and the company reports having over 50 million registered users. MFP incorporates elements of social cognitive theory, including self-monitoring, goal setting, and feedback. The objective of this study is to test the impact of providing this free, widely-used smartphone application for weight loss to patients in their primary care clinic.
METHODS
Design Overview
The Mobile Fitness Study (mFit) was a randomized controlled trial with participants randomized to either usual primary care (n = 107) or usual primary care plus MFP app (n = 105) (Figure 1). Assessments were completed at baseline, 3 months and 6 months between August 2012 and May 2013. The institutional review board of the University of California, Los Angeles approved the study and all participants provided written informed consent. Study data were collected on iPads® using REDCap™ electronic data capture tools hosted at UCLA. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing an intuitive interface, audit trails, and automated export (8).
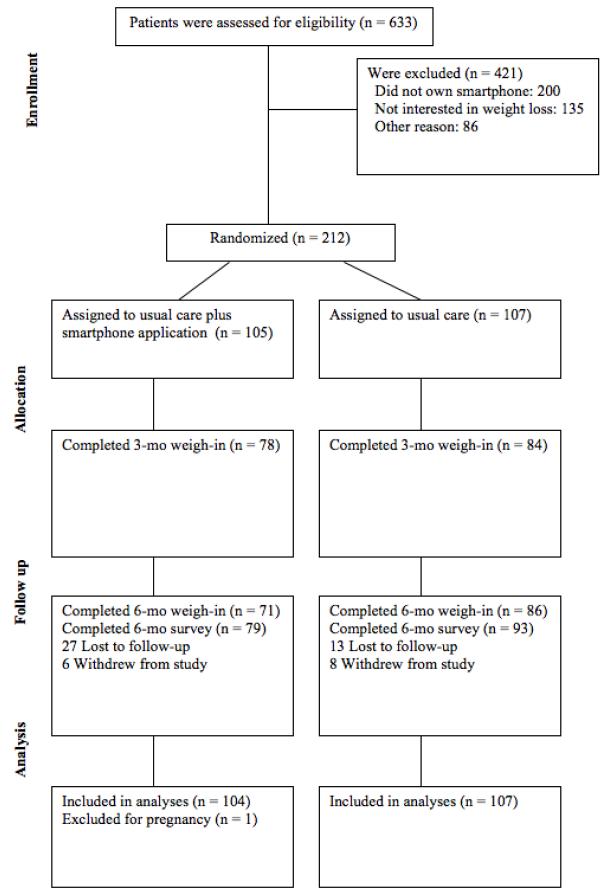
Figure 1.
Study Flow Diagram
Among the 6 intervention group participants who withdrew, 3 intervention group participants reported not having enough time to use the app, and 2 reported they did not have time to follow-up and 1 was no longer interested in participating in the study. Among the 8 control group participants, 6 reported not having time to return for follow-up and 2 reported they were no longer interested in participating in the study
Setting and Participants
Participants were recruited from two UCLA primary care clinics that serve ethnically and socioeconomically diverse patient populations. Eligibility criteria included an age of 18 years or older, body mass index (BMI) of 25 kg/m2 or greater, and smartphone ownership. Participants also had to answer “yes” when asked, “Are you interested in losing weight?” Exclusion criteria were current, planned, or previous pregnancy within six months, hemodialysis, life expectancy less than six months, lack of interest in weight loss, or current use of a smartphone app for weight loss.
Screening and Randomization
Patients were recruited during routine primary care visits at their respective clinics. The research team provided a script to medical assistants to use with any patients with BMI greater than 25. Patients interested in enrollment were referred to the on-site research assistant who screened, consented, and completed surveys with each patient. Participants were block randomized by BMI 25-30 and BMI >30 to ensure roughly equal distribution of “overweight” and “obese” patients between intervention and control groups. Our statistician used R to generate the permuted block sequence. We printed the sequence and placed it in opaque envelopes. Research assistants helped intervention group participants download the MFP app onto their phone and showed them an instructional video developed by MyFitnessPal (available at http://youtu.be/fu9RKqlmD1Q). These participants also received a phone call from the same research assistant one week after enrollment to assist with any technical problems with the app. Research assistants told control group patients to “choose any activities you’d like to lose weight” without specifying any particular interventions. Control group participants were aware that they were participating in a study of a weight loss app but were blinded to the name of the app. To minimize contamination of the control group, providers and clinic staff were also blinded to the name of the app and to group assignment. At the 3-month follow-up visit, all participants received an educational one-page handout on healthy eating from www.myplate.gov. Participants received a $20 gift card for attending each follow-up visit. Each participant’s primary care provider was notified of their enrollment in the study. Blood pressure was measured once at baseline, three months, and six months by trained research assistants using an automated Dynamap ® monitor.
Intervention
We selected MyFitnessPal as our intervention based on two focus groups held with overweight primary care patients. Patients were asked about their impressions of a variety of short message service (SMS) text-based programs and smartphone apps. Overall, there was much more interest in smartphone apps compared to text-based programs. A few participants stated they enjoyed using MyFitnessPal and a majority of participants expressed great interest in trying this app. Although we selected MFP as our intervention, there are many similar, publically available apps which may be just as popular as MFP. Some of these apps have been assessed in prior studies but to our knowledge none have been evaluated in a randomized trial (9).
MFP was designed by software engineers in collaboration with dieticians to create an app for calorie counting. The app provides a database of over 3 million foods and an easy-to-use interface for logging food and exercise. Users enter their current weight, goal weight, and goal rate of weight loss (limited to 0.5 to 2 lb per week). The MFP app then shows the user their daily, individualized calorie goal. Each day, the app displays the user’s calorie goal relative to their recorded caloric intake. MFP also generates real-time reports showing users their weight trend, caloric intake in the past week and nutritional summaries of their diet (e.g. grams of fat, carbohydrates, protein, milligrams of sodium, etc.). The app also includes a barcode scanner for store-bought foods and a social networking feature that enables users to find friends and share their progress. Study participants were encouraged to use the social networking feature with friends and to set reminders to log their food.
MFP incorporates an evidence-based and theory-based approach to weight loss. Setting a realistic weight loss goal of 0.5 to 2 lbs per week is supported in self-regulation theory and is a standard setting of the MFP app (10). The social networking feature of MFP may be important given prior studies demonstrating the benefits of social support on weight loss (11). Self-monitoring, consisting of recording dietary intake, physical activity and weight, is also strongly associated with weight loss (12). One pilot trial recently demonstrated that adherence to diet self-monitoring is higher among patients using a smartphone app compared to patients using a paper diary (13).
Outcomes and Follow-up
The primary outcome was change in weight at six months in the intervention group compared to the usual primary care group. Weight was measured at baseline, 3 and 6 months. Data for secondary outcomes were also collected at baseline, 3 and 6 months, including systolic blood pressure (SBP) as well as three self-reported, behavioral mediators of weight loss: exercise, dieting, and self-efficacy in weight loss (Appendix A). The behavioral survey items were adapted from the TRIAD (Translating Research into Action for Diabetes) study (14) and the Diabetes Empowerment Scale (15).
The MyFitnessPal company also shared user data with the research team to investigate frequency of app logins over time. Each time a participant opened the app counted as a “login.” We assessed for contamination at the end of the trial by asking control group participants if they happened to use the MyFitnessPal app in the past 6 months.
At 6 months, participants in the intervention group completed a survey on their experience using MFP (Appendix B). In addition, we interviewed six participants who lost more than 10 pounds to ask if they thought MFP helped them lose weight, and if so, how.
Statistical Analysis
We determined that a total sample size of 82 patients (41 per arm) would allow us 80% power to detect a 5.5 lb difference in weight change at six months between the two groups, assuming a standard deviation of 8.8 lbs. We set a goal enrollment of 180 to account for rates of attrition as high as 55%.
We used a linear mixed effects model (PROC MIXED) to compare weight change, SBP change, and change in behavioral survey items between groups from baseline to 3 and 6 months, while controlling for clinic site. Month, including baseline, was modeled as a categorical term in the mixed effects model. This model included fixed effects for clinic, intervention, month, and an intervention by month interaction, and used an unstructured variance-covariance matrix to model the covariance structure among the repeated measures by participant. All participants were included in this primary analysis based upon their randomized intervention assignment, except for one patient in the intervention group who became pregnant and no longer met inclusion criteria.
The portion of participants in each group who lost at least 6 pounds at 6 months was also calculated. The analysis of this dichotomous outcome had not been pre-specified in the protocol but was added to assess subgroups of patients who achieved significant weight loss.
Bivariate correlation and linear regression analyses were run to assess the relationship between background characteristics and extent of application use. Linear regression was also used to determine if baseline self-efficacy was a significant predictor of weight change while controlling for interaction between baseline self-efficacy and group assignment. All analyses were performed using SAS, version 9.3.
We conducted two sensitivity analyses to further evaluate our primary outcome results. The first analysis explored the impact of possible informative drop-out based on a selection model using PROC QLIM (16). This model assumes the existence of unobserved factors related to both outcomes and missingness, considered as a “missing not at random” (MNAR) assumption. We included income, education, diet experience, treatment group, and baseline value as covariates in the binary model for missingness. The second sensitivity analysis gauged the effect of excluding one outlier participant in the control group who made extensive use of MyFitnessPal and lost the most weight of any participant.
Role of Funding Source
This research was supported by The Robert Wood Johnson Foundation Clinical Scholars Program grant 69003. The use of REDCap, and a portion of Drs. Mangione and Bell’s efforts are supported by the NIH/National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute Grant UL1TR000124. Dr. Mangione received support from the University of California at Los Angeles (UCLA), Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly under National Institutes of Health (NIH)/NIA Grant P30-AG021684. Dr. Mangione holds the Barbara A. Levey and Gerald S. Levey Endowed Chair in Medicine, which partially supported her work. None of the funding sources had a role in the design, conduct, or analysis of the study.
RESULTS
Baseline Characteristics of Participants
The participants were mostly women (73%) with a mean (SD) age of 43.3 (14.3),and mean BMI of 33.4 (7.09) kg/m2. 33% of participants self-identified as Hispanic/Latino, 48% identified as white, 19% as black, 8% as Asian, and 2% as Native American or Pacific Islander (participants were allowed to choose more than one race/ethnicity option). Additional sample characteristics are described in Table 1. Characteristics by clinic site are reported in Appendix G.
Table 1.
Characteristics of Participants
| Characteristic | Control (N = 107) |
Intervention (N = 105) |
|---|---|---|
| Female - n(%) | 81 (75.7) | 73 (69.5) |
|
Self-reported Race/Ethnicity
- n(%) |
||
| Hispanic | 34 (31.8) | 34 (33.3) |
| White | 43 (42.2) | 55 (52.9) |
| Black | 20 (19.6) | 19 (18.3) |
| Asian | 10 (9.8) | 7 (6.7) |
| Native American or Pacific Islander | 1 (0.49) | 3 (1.46) |
| Education | ||
| High school grad or less | 26 (24.3) | 15 (14.3) |
| Some college or college grad | 59 (55.1) | 66 (62.9) |
| More than 4 years college | 22 (20.6) | 24 (22.9) |
| Annual Income | ||
| Less than $30,000 | 28 (27.5) | 23 (24.7) |
| $30,000 - $49,000 | 22 (21.6) | 12 (12.9) |
| $50,000-74,999 | 18 (17.6) | 20 (21.5) |
| $75,000 or more | 34 (33.3) | 38 (40.9) |
| Mean Age – yr (SD) | 43.2 (14.5) | 43.1 (14.0) |
| BMI - kg/m2 (SD) | 33.3 (7.17) | 33.3 (6.8) |
| No. of pts BMI 25-30 | 41 | 40 |
| No. of pts BMI > 30 | 66 | 65 |
| Systolic blood pressure | 123 (18.1) | 126 (15.8) |
| mm Hg (SD) | ||
| Baseline self-reported behaviors | ||
| mean number of days per week (SD) | ||
| Healthy diet* | 3.18 (2.45) | 2.89 (2.39) |
| Used calorie goal * | 1.31 (2.44) | 1.31 (2.28) |
| Physical activity * | 3.81 (2.21) | 3.55 (2.22) |
| Exercise sessions * | 1.83 (2.04) | 1.92 (2.10) |
| Self-efficacy in achieving weight | 7.77 (2.39) | 8.12 (1.98) |
| loss goal (0-10 scale) | ||
| Self-efficacy in making healthy | 7.94 (2.62) | 7.84 (2.29) |
| food/exercise choices (0-10 scale) | ||
| Like using smartphone (0-10 scale) | 8.35 (2.30) | 8.00 (2.33) |
| Type of smartphone (%) | ||
| iPhone® | 50 (50.5) | 46 (44.7) |
| Android® | 43 (43.4) | 45 (43.7) |
| Blackberry® | 6 (6.0) | 12 (11.7) |
BMI = body mass index
Number of days in the last 7 days in which the behavior was followed or practiced.
At 3 months, 26% of intervention group participants and 21% of control group participants were lost to follow-up or had withdrawn from the study (p = .69). At 6 months, 32% of intervention group participants and 19% of control group participants were lost to follow-up or had withdrawn from the study (p = 0.063).
Weight Loss, and Systolic Blood Pressure
There was minimal weight change in both groups and no statistically significant difference between groups. At 3 months, the control group gained an average of .54 pounds whereas the intervention group lost 0.06 pounds for a between group difference of −0.60 lbs [CI, −2.5 to 1.3 lb]; (p = 0.53). At 6 months, the control group gained an average of 0.60 pounds and the intervention group lost .07 pounds for a between group difference of −0.67 lbs [CI, −3.3 to 2.1], (p = 0.63) (Table 2). These confidence intervals exclude our pre-determined clinically significant difference in weight change between groups of 5.5 lbs. Difference in systolic blood pressure change between groups was also minimal. The sensitivity analysis based on possible informative dropout provided consistent results (between group difference at 6 months, 0.08 lb [CI −3.04 to 3.20]; p = 0.96) (Appendix C).
Table 2.
Mean changes in weight, blood pressure, and behavioral mediators of weight loss
| Within Group Change From Baseline |
Predicted
Between-group Difference in Change† (intervention – control) |
||||
|---|---|---|---|---|---|
| Measure | Control | Intervention |
95% CI |
P Value |
|
|
Weight Change
(lb) |
|||||
| At month 3 | 0.54 | −.06 | −.60 | −2.5 to 1.3 | 0.53 |
| At month 6 | 0.60 | −0.07 | −.67 | −3.3 to 2.1 | 0.63 |
|
Systolic Blood
Pressure Change (mmHg) |
|||||
| At month 3 | 4.9 | .92 | −4.3 | −9.4 to 0.73 | .093 |
| At month 6 | 1.5 | −.34 | −1.7 | −7.1 to 3.8 | .55 |
| Healthy diet in last 7 days |
|||||
| At month 3 | 0.34 | 0.3 | 0.03 | −0.74 to 0.80 |
0.94 |
| At month 6 | 0.67 | 0.9 | 0.29 | −0.51 to 1.1 | 0.48 |
| Used calorie goal in the last 7 days |
|||||
| At month 3 | −0.15 | 1.8 | 1.9 | 1.0 to 2.8 | <.0001 |
| At month 6 | 0.27 | 2.3 | 2.0 | 1.1 to 2.9 | <.0001 |
| Physical activity in last 7 days |
|||||
| At month 3 | 0.24 | 0.87 | 0.62 | −0.12 to 1.4 | 0.10 |
| At month 6 | 0.66 | 0.87 | 0.20 | −0.49 to 0.90 |
0.56 |
| Exercise sessions in last 7 days |
|||||
| At month 3 | 0.17 | 0.19 | 0.016 | −0.63 to 0.66 |
0.96 |
| At month 6 | 0.62 | 1.02 | 0.40 | −0.35 to 1.2 | 0.29 |
| Self-efficacy
in achieving weight loss goal (0 - 10 scale) |
|||||
| At month 3 | 0.50 | −0.44 | −0.85 | −1.6 to - 0.10 |
0.026 |
| At month 6 | 0.49 | −.03 | −0.44 | −1.1 to 0.21 | 0.19 |
| Self-efficacy in making healthy food/exercise choices |
|||||
| (0 – 10 scale) At month 3 |
0.14 | 0.14 | −0.0 | −0.81 to 0.81 |
1.0 |
| At month 6 | 0.44 | 0.41 | −0.03 | −0.74 to 0.69 |
0.94 |
using mixed effects model
Among participants with 6 month measurements, 14 of 87 participants (16%) in the control group lost 6 lbs or more whereas 13 of 71 in the intervention group (18%) lost 6 lbs or more.
Self-reported Behavioral Mediators
At three and six months, intervention group participants reported using a “personal calorie goal” more often compared to control group participants (mean between group difference at three months = 1.9 days per week [CI, 1.0 to 2.8]; p<.001 and mean between group difference at six months = 2.0 days per week [CI, 1.1 to 2.9]; p < .001). At month three, intervention group participants reported decreased self-efficacy in achieving a weight loss goal compared to control group participants (−0.85 on a 10-point scale [CI, −1.6 to −0.10]; p = 0.026). At month six, however, this effect was not significant. We found no statistically significant difference in other self-reported behaviors around diet, exercise, and self-efficacy in weight loss (Table 2). Baseline self-efficacy was not associated with weight loss either alone or when interacted with group assignment, indicating those with greater baseline self-efficacy did not lose more weight and did not differentially lose more with the intervention.
App Usage
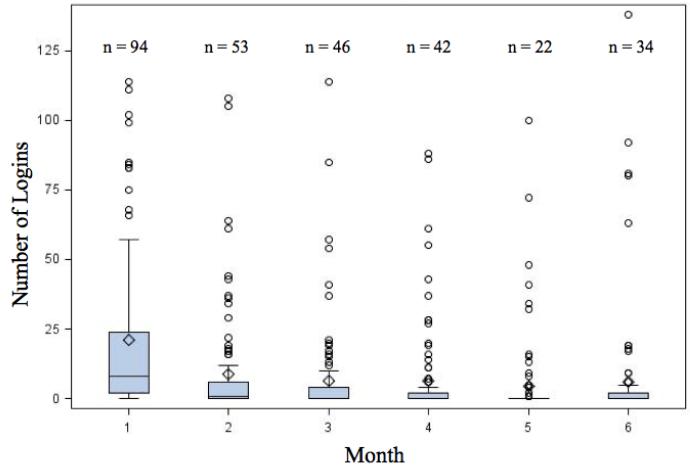
Over the six month study period, the mean total logins was 61 and median total logins was 19. Frequency of logins among most users declined rapidly after enrollment (Table 3). The median (IQR) number of logins was 8 (2,24) in the first month and 0 (0, 2) in the sixth month). The number of participants who actually used the app in the first month was 94 compared to 34 in the sixth month. There were a few individuals who continued to use the app at least 30 times in month 6 (Figure 2). Among the 105 intervention group participants, three never logged in and eight did not have data available from MyFitnessPal.
Table 3.
Summary of Logins by Month in Participants Randomized to MyFitnessPal
| Month | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Number of participants who logged in |
94 (97%) | 53 (55%) | 46 (47%) | 42 (43%) | 22 (23%) | 34 (35%) |
| Mean number of logins | 20.9 | 8.6 | 65 | 6.3 | 43 | 6.2 |
| Median number of logins | 8 | 1 | 0 | 0 | 0 | 0 |
| IQR | (2,24) | (0,6) | (0,4) | (0,2) | (0,0) | (0,2) |
| Range | (0,114) | (0,108) | (0,114) | (0,88) | (0,100) | (0,138) |
Figure 2.
Number of Logins by Month Among MyFitnessPal Users
Boxes represent median number of logins and interquartile range.
n = number of participants who logged in that month
○ = outliers more than 1.5x interquartile range
◇ = mean logins
Although clinicians, clinic staff, and control group patients were blinded to the name of the app, 14 of the 107 control group participants ended up using MyFitnessPal during the trial. The individual who used the app the most (782 logins) and lost the most weight (29 lbs) was from the control group. A sensitivity analysis excluding this outlier individual did not change our main findings (between group difference at 6 months, −1.0 lb [CI, −3.36 to 1.62]; p = 0.46) (Appendix D). None of the participants reported using a weight loss app other than MFP. There was no statistically significant association between baseline characteristics and extent of app usage or weight change.
Reviews of MyFitnessPal
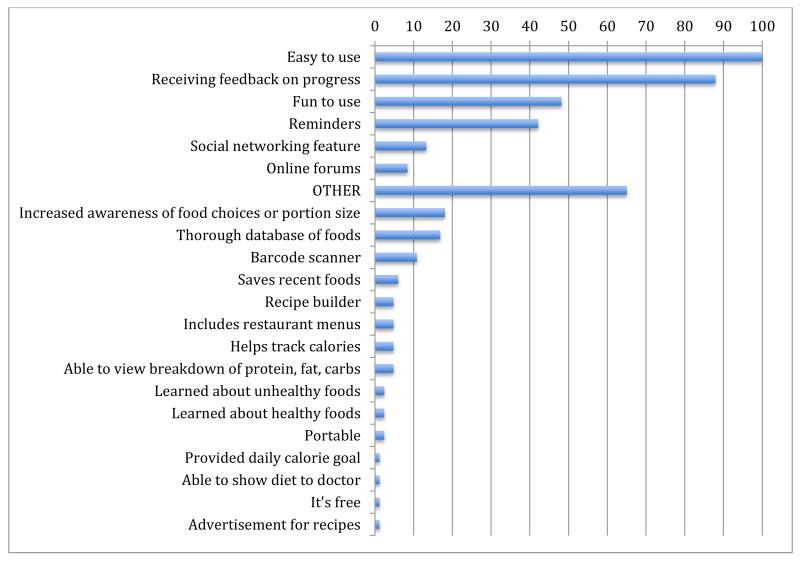
Although app usage dropped during the study, participants who completed the survey reported high satisfaction at 6 months, with 79% of participants stating they were somewhat or completely satisfied with the app and 92% reporting they would recommend it to a friend. 80% indicated they plan to continue using MFP. Using a checklist we asked participants “What do you like about MFP?”. Of the 83 participants who responded, 100% reported it was easy to use, 88% reported they enjoyed receiving feedback on their progress, 48% reported it was fun to use, 42% enjoyed the reminder feature, 13% liked the social networking feature, and 83% reported “Other.” The most common “Other” reasons were that MFP increased awareness of food choices or portion size (18%), provided a thorough database of foods (17%), and included a barcode scanner (10%) (Appendix E). Some participants commented that they were able to maintain an improved diet but stopped using the app.
Responses from interviewees who had lost more than 10 pounds included the following: “I realized I was consuming 5,000 to 6,000 (calories) per day and afterward I never ate that much again!”; “the app showed me where my problems are - so I reduced portion sizes and cut back on alcohol, carbs, and sweets.”; “It really makes you look at what you’re eating. It helped me select healthier foods and stay on track.”; “Thanks so very much for introducing me to this excellent weight loss program. It has been a life saver.” Most participants, however, did not use the app regularly and they reported the most common reasons for ceasing use of the app were that it was tedious or that they were too stressed or busy. Overall, usage of the social networking feature was minimal with 80% of participants reporting having “no friends” in the MFP app.
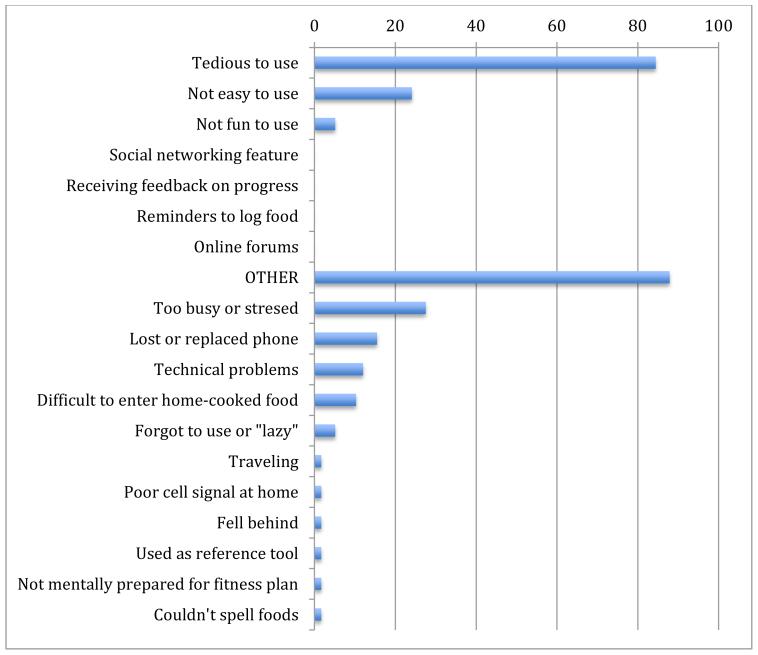
Not all reviews were positive. Using a checklist, we asked intervention group participants, “If you stopped using MFP, why did you stop using it?”. Of the 58 individuals who responded, 84% reported it was tedious, 24% reported it was not easy to use, and 88% reported “Other.” The most common “Other” reasons included being too busy or stressed (28%), losing or replacing a phone (16%), technical issues (7%), and difficulty logging home-cooked foods (6%) (Appendix F).
DISCUSSION
The principal finding of this six-month trial was that the MyFitnessPal app delivered to overweight patients in primary care did not result in increased weight loss compared to usual primary care. Most participants rarely used the app after the first month of the study and a few individuals continued to login regularly in the sixth month. Given these results, it may not be worth a clinician’s time to “prescribe” MyFitnessPal to every overweight patient with a smartphone. If a patient seems particularly motivated to lose weight and track calories, however, this app may serve as a helpful tool. Our analysis did not show any demographic covariates to be important predictors of app use.
One possible explanation for our negative results is that our participants may have wanted to lose weight but were not ready to put in the necessary work to self-monitor their diet. Although all participants responded that they were “interested in losing weight” during the screening process, we did not explicitly measure readiness for change or motivation. The relative lack of changes in behavioral mediators may suggest that most participants were not ready to invest the time in self-monitoring calories. Our results are also consistent with prior research demonstrating that frequent recording of food intake is key to treating obesity(17).
An alternative explanation of our results is that MFP and similar weight-loss apps may need to be substantially more engaging or less time-consuming to produce weight reduction in the average overweight patient. Most participants’ use of the app plummeted after the first month because they found it tedious or felt it took too much time. We also found that MFP may actually decrease a user’s confidence in their ability to achieve a weight loss goal. This may be because the app makes users set an explicit weight loss goal and subsequently increases awareness of whether or not they achieved it.
There are myriad opportunities to improve app content and delivery. Based on patient feedback, a faster, streamlined interface for entering foods may be a priority. Alternatively, weight-loss apps could assess an individual’s readiness for self-monitoring before using the app and could prepare new users for the potential time commitment. Delivering engaging messages to educate users about the importance of self-monitoring and to incentivize patients to use the app may increase adherence (18). Brief, daily or weekly feedback and encouragement could also boost usage and self-efficacy in dieting (13). “Gamification” of the app, financial incentives, or delivering the app in a setting of group competition could also be important adjuncts to increase motivation to use the app and lose weight (19). Combining a weight loss app with a proven weight-loss counseling program could also be a powerful combination of tools (3). Of note, smartphone apps are constantly undergoing updates so the features of MyFitnessPal have changed over time, although the core features in the version tested here have remained constant. .
If an enhanced version of MyFitnessPal or similar app prove to be effective at reducing weight in the future, it could easily be distributed to patients at minimal cost. MFP is free and could be introduced to patients by a medical assistant in less than five minutes. In contrast, the long term consequences of obesity, such as diabetes and cardiovascular disease, are immensely expensive for the U.S. healthcare system.
Strengths of this study include the randomized design, implementation in real-world primary care settings, and use of a commercially available, free smartphone application. We searched PubMed and could not find any other randomized controlled trials of a weight loss app delivered in primary care.
There were also several limitations. Contamination of the control group may have impacted our results. Another limitation was the relatively high attrition rate. It is possible that some intervention group participants did not follow-up because they failed to lose weight or did not find the app helpful. If we assume that the non-completers lost less weight than participants who completed the study, our estimate of treatment effect is on the conservative end. In other words, it is unlikely that the missing data would have changed our main findings from negative to positive. Patients were followed up for only 6 months, but we suspect a trial longer than six months would also be unlikely to change our main findings. Finally, we did not have clinicians recommending the app to patients nor did we ask clinicians to follow-up with patients regarding use of the app or the patients’ progress with weight loss. A clinician’s recommendation could motivate a patient to use the app more frequently.
In summary, we did not find that introducing a weight loss app to overweight patients in primary care resulted in increased weight loss. In the hands of a patient who is truly ready to self-monitor calories, however, it may be a useful tool for losing weight. For now, readiness and adherence to self-monitoring must be addressed in order for apps such as MyFitnessPal to impact obesity and its costly, long-term consequences in primary care settings.
Acknowledgements
The authors would like to thank the leadership at MyFitnessPal, and all the staff of the UCLA Family Health Center and UCLA 16th Street Internal Medicine clinic for making this study possible.
Source of Funding: The Robert Wood Johnson Foundation Clinical Scholars Program grant 69003, CTSI award UL1TR000124, and Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly under National Institutes of Health (NIH)/NIA Grant P30-AG021684.
Appendix A. Behavioral Survey Questions
How many of the last seven days have you followed a diet that was about half vegetables & fruit, a quarter whole grains, a quarter lean protein, and limited in fat, salt & added sugar?
How many of the last seven days have you tried to stay within a personal calorie goal? If you don’t have a personal calorie goal, select “0.”
These questions are about exercise. If you were sick during the past 7 days, please think back to the last seven days that you were not sick. On how many of the last seven days did you participate in at least 30 minutes of physical activity? DEFINITION: Physical activity includes exercise sessions, as well as things you do around the house or as a part of your work or daily life, including walking, gardening (anything that gets you perspiring or gets your heart rate up). When deciding if you participated in 30 minutes of physical activity, add up the total minutes that you did physical activity for that day.
-
On how many of the last seven days did you participate in EXERCISE sessions for at least 30 minutes, such as swimming or walking, OTHER THAN what you do around the house or as part of your work or daily life?
For the next two questions, indicate if you disagree or agree with the following statements using a 0-10 scale. (0 = strongly disagree, 5 = neutral, 10 = strongly agree)
In general, I believe that I am able to turn a weight loss goal into a workable plan.
In general, I believe I know enough to make the right choices about food and exercise to lose weight.
Appendix B. MyFitnessPal Survey Questions
What do you like about MyFitnessPal?
-
□
Easy to use
-
□
Fun to use
-
□
Social networking feature
-
□
Receiving feedback on progress
-
□
Reminders
-
□
Online forums
-
□
Other
If you stopped using MyFitnessPal, why did you stop using it?
□ Not easy to use
-
□
Not fun to use
-
□
It’s tedious
-
□
Social networking feature
-
□
Getting feedback on progress
-
□
Reminders
-
□
Online forums
-
□
Other: please specify
Appendix C. Predicted mean change in weight accounting for possible informative dropout
| Weight Change (lb) |
Intervention vs control |
95% CI | p-value |
|---|---|---|---|
| 3m | −0.62 | −2.90 to 1.65 | 0.59 |
| 6m | 0.08 | −3.04 to 3.20 | 0.96 |
Appendix D. Predicted mean change in weight excluding outlier participants in control group who used the app 782 times and lost 29 pounds
| Weight Change (lb) |
Intervention vs control |
95% CI | p-value |
|---|---|---|---|
| 3m | −0.73 | −2.57 to 1.11 | 0.44 |
| 6m | −1.0 | −3.36 to 1.62 | 0.46 |
Appendix E. Participant responses to “What do you like about MyFitnessPal?” n = 83
*Reasons listed below “OTHER” represent free-text responses, categorized by the investigators.
Appendix F. Participant responses to “If you stopped using MyFitnessPal, why did you stop? n= 58
*Reasons listed below “OTHER” represent free-text responses, categorized by the investigators.
Appendix G. Baseline Characteristics by Clinic Site
| Characteristic | Clinic 1 (N = 120) |
Clinic 2 (N = 92) |
|---|---|---|
| Female - n(%) | 80 (66.7) | 74 (80.4) |
|
Self-reported
Race/Ethnicity - n(%) |
||
| Hispanic | 41 (34.2) | 27 (29.3) |
| White | 56 (46.7) | 42 (45.7) |
| Black | 23 (19.8) | 16 (17.8) |
| Asian | 7 (6.0) | 10 (11.1) |
| Native American or Pacific Islander | 3 (0.03) | 1 (0.01) |
| Education | ||
| High school grad or less | 32 (26.7) | 9 (9.8) |
| Some college or college grad | 68 (56.7) | 57 (62.0) |
| More than 4 years college | 20 (16.8) | 26 (28.3) |
| Annual Income | ||
| Less than $50,000 | 56 (48.3) | 29 (36.7) |
| Greater than $50,000 | 60 (51.7) | 50 (63.3) |
| No. of pts BMI 25-30 | 43 | 38 |
| No. of pts BMI > 30 | 77 | 54 |
| Type of smartphone (%) | ||
| iPhone® | 51 (43.2) | 45 (52.9) |
| Android® | 57 (48.3) | 32 (37.7) |
| Blackberry® | 10 (8.5) | 8 (9.4) |
Footnotes
ClinicalTrials.gov: NCT01650337
AUTHOR ADDRESSES
Brian Yoshio Laing, 12246 Montana Ave, #206, Los Angeles, CA 90049
Carol Mangione, UCLA Med-GIM & HSR, BOX 957394, 10940 Wilshire Blvd Ste 700, Los Angeles, CA 90095-7394
Douglas Bell, UCLA Med-GIM & HSR, BOX 951736, 911 Broxton Plz, Los Angeles, CA 90095-1736
Mei Leng, UCLA Med-GIM & HSR, BOX 957394, 10940 Wilshire Blvd. Ste 700, Los Angeles, CA 90095-7394
Chi-hong Tseng, UCLA Medicine/GIM, BOX 951736, 911 Broxton Ave., Los Angeles, CA 90095-1736
Megha Mahida, 10235 Whitetail Drive, Oakdale, CA 95361
Katia Vaisberg, 964 Vasco Da Gama Ln., Foster City, CA 94404
Donald Morisky, UCLA Pub Hlth-Cmnty Hlth Sci, BOX 951772, 46-071A CHS, Los Angeles, CA 90095-1772
Michelle Bholat, UCLA Fam Med, BOX 957197, 1920 Colorado Blvd, Santa Monica CA, Los Angeles, CA 90095-7197
Eve Glazier, UCLA Med-GIM & HSR, BOX 951736, 1245 16th St. #125, Santa Monica, CA 90404, Los Angeles, CA 90095-1736
Reproducible Research Statement: Study protocol and statistical code available from Dr. Laing (blaing@stanfordalumni.org). Data set: not available.
References
- 1.Stein C, Colditz G. The Epidemic of Obesity. The Journal of Endocrinology & Metabolism. 2004:2522–5. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999-2010. JAMA. 2012 Feb 1;307(5):491. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A Two-Year Randomized Trial of Obesity Treatment in Primary Care Practice. The New England Journal of Medicine. 2011 Nov 24;365(21):1969–79. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galuska D, Will J, Serdula M. Are health care professionals advising obese patients to lose weight? JAMA. 1999;16:1576–8. doi: 10.1001/jama.282.16.1576. [DOI] [PubMed] [Google Scholar]
- 5.Heath C, Grant W, Marcheni P. Do family physicians treat obese patients? Family Medicine. 1993;6:401–2. [PubMed] [Google Scholar]
- 6.Smith A. Smartphone Ownership: 2013 Update. pewinternetorg. Jun 3, 2013. pp. 1–12. [Google Scholar]
- 7.Cohn M. Tens of thousands of health apps available, but which ones work? [Internet]. Baltimore Sun. 2012 [cited 2012 May 30]. Available from: http://articles.baltimoresun.com/2012-03-14/health/bs-hs-mobile-health-apps-20120314_1_health-apps-mhealth-mobile-health.
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azar KMJ, Lesser LI, Laing BY, Stephens J, Aurora MS, Burke LE, et al. AMEPRE. 5. Vol. 45. Elsevier; Nov 1, 2013. Mobile Applications for Weight Management; pp. 583–9. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A, Simon KM. The role of proximal intentions in self-regulation of refractory behavior. Cogn Ther Res. 1977 Sep;1(3):177–93. [Google Scholar]
- 11.McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight-loss interventions: a systematic review of randomised trials. International Journal of Obesity. 2003:987–1005. doi: 10.1038/sj.ijo.0802383. [DOI] [PubMed] [Google Scholar]
- 12.Burke LE, Wang J, Sevick MA. J Am Diet Assoc. 1. Vol. 111. Elsevier Inc. Elsevier Inc; Jan 1, 2011. Self-Monitoring in Weight Loss: A Systematic Review of the Literature; pp. 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter MC, Burley VJ, Nykjaer C, Cade JE. J Med Internet Res. 4. Vol. 15. JMIR Publications Inc.; Toronto, Canada: 2013. Adherence to a Smartphone Application for Weight Loss Compared to Website and Paper Diary: Pilot Randomized Controlled Trial; p. e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.TRIAD Study Group The Translating Research Into Action for Diabetes (TRIAD) study: a multicenter study of diabetes in managed care. Diabetes Care. 2002 Feb;25(2):386–9. doi: 10.2337/diacare.25.2.386. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RM, Funnell MM, Fitzgerald JT, Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self-efficacy. Diabetes Care. 2000 Jun;23(6):739–43. doi: 10.2337/diacare.23.6.739. [DOI] [PubMed] [Google Scholar]
- 16.Allison PD. Missing data -- Quantitative applications in the social sciences. SAGE; 2002. pp. 79–82. [Google Scholar]
- 17.Wadden TA, Berkowitz RI, Womble LG. Randomized Trial of Lifestyle Modification and Pharmacotherapy for Obesity. The New England journal of medicine. 2005 Nov 4;353(20):2111–20. doi: 10.1056/NEJMoa050156. al E. [DOI] [PubMed] [Google Scholar]
- 18.Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11(1):e1–9. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullgren JT, Troxel AB, Loewenstein G, Asch DA, Norton LA, Wesby L, et al. Individual-versus group-based financial incentives for weight loss: a randomized, controlled trial. Annals of Internal Medicine. 2013 Apr 2;158(7):505–14. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]