Abstract
Context:
Antibodies against thyroid peroxidase (TPOAbs) are detected in 90% of all patients with Hashimoto thyroiditis, the most common cause of hypothyroidism. Hypothyroidism is associated with a range of adverse outcomes. The current knowledge of its genetic underpinnings is limited.
Objective:
The purpose of this study was to identify novel genetic variants associated with TPOAb concentrations and positivity using genome-wide association data and to characterize their association with thyroid function and disease.
Design, Setting, and Participants:
We studied European ancestry participants of 3 independent prospective population-based studies: Atherosclerosis Risk In Communities study (n = 7524), Study of Health in Pomerania (n = 3803), and Study of Health in Pomerania-TREND (n = 887).
Exposure:
Single nucleotide polymorphisms (SNPs), individually and combined into a genetic risk score (GRS), were examined.
Main Outcomes:
The main outcomes were TPOAb concentrations and positivity, thyroid hormone concentrations (TSH, free T4), and clinical thyroid diseases (subclinical and overt hypothyroidism and goiter).
Results:
Significantly associated single nucleotide polymorphisms (P < 5 · 10−8) mapped into 4 genomic regions not previously implicated for TPOAbs (RERE, extended HLA region) and into 5 previously described loci. A higher Genetic Risk Score (GRS) based on these 9 SNPs showed strong and graded associations with higher TPOAb, TSH, and lower free T4 concentrations (P < .001). Compared with individuals in the lowest GRS quartile, those in the highest quartile had 1.80-fold higher odds of subclinical hypothyroidism (95% confidence interval, 1.27–2.55) and 1.89-fold higher odds of overt hypothyroidism (95% confidence interval, 1.24–2.87).
Conclusion:
The identification of 4 novel genetic loci associated with TPOAb concentrations and positivity gives further insight into the genetic underpinnings of hypothyroidism. A GRS showed strong and graded associations with markers of thyroid function and disease in independent population–based studies.
Hypothyroidism has been related to fatigue, depression, heart failure, metabolic syndrome, and mortality (1–5). Thyroid dysfunction is seen in up to 10% of the adult population, and its prevalence increases with age (6). The most common cause of hypothyroidism in iodine-sufficient areas of the world is Hashimoto thyroiditis, which is characterized by gradual autoimmune-mediated destruction of the thyroid gland. High autoantibody titers against thyroid peroxidase (TPOAbs) are a sensitive clinical marker of Hashimoto thyroiditis and are detected in 90% of all patients with Hashimoto thyroiditis as opposed to 5% to 24% in the general population (6, 7). Despite the prevalence and adverse outcomes of autoimmune-mediated thyroid disease, its etiology remains incompletely understood (6, 8–11), complicating the identification of individuals at risk.
Autoimmune thyroid disease (AITD) is thought to arise from a combination of genetic susceptibility and environmental factors. Substantial progress has been made recently in the identification of such genetic susceptibility factors to AITD and other autoimmune diseases (12). Among the reported genetic risk loci are associations with HLA class I and II genes, CTLA-4, PTPN22, IL2RA, FOXP3, CD40, and FCRL3 (7, 9, 12). Estimates from twin studies indicate that the heritability to develop TPOAbs is around 70% (13), but the identified risk loci for AITD have been reported to account for only a minor proportion of the heritability (14, 15).
Genome-wide association studies (GWASs) are one way to gain novel insights into the pathophysiology of complex diseases. We undertook this study for several reasons: first, with use of previous GWAS findings as a basis, to identify additional novel genetic variants via meta-analysis with additional data from an independent study; second, to characterize associations of novel and known genetic variants in 3 large, community-based populations (the Atherosclerosis Risk in Communities [ARIC] study and the 2 Study of Health in Pomerania [SHIP] cohorts) with different levels of iodine supply; and third, to gain insight into the combined effect of the genetic variants by constructing a genetic risk score (GRS) and evaluating the GRS associations with measures of thyroid dysfunction and disease.
Subjects and Methods
Study populations
The ARIC study is a population-based prospective observational cohort of 15 792 adults in 4 US communities aged 45 to 64 years at the baseline visit in 1987 to 1989. Details of the study design were reported previously (16). In brief, 4 visits, each 3 years apart, were conducted between 1987 and 1998; a fifth visit was conducted from 2011 to 2013. Similar to previous large genetic studies of TPOAb concentrations (14), analyses in this report were limited to 7524 ARIC study participants of European ancestry with nonmissing information on thyroid hormone measurements, genotype information, and covariates.
The SHIP is a population-based project in northeastern Germany consisting of 2 independent longitudinal cohorts, SHIP (SHIP-0) and SHIP-TREND (SHIP-T) (17–19). For SHIP-0, 4308 participants aged 20 to 81 years were recruited from 1997 to 2001. For SHIP-T, 4420 Caucasian adults aged 20 to 81 years were recruited from 2008 to 2012. Written informed consent was obtained from all study participants for the ARIC and SHIP studies, and the protocol was approved by all ethic boards of the participating studies.
Measurement of thyroid hormones and ultrasound examination
Measures of thyroid function in the ARIC study were assayed in 2012 to 2013 from all participants with stored serum samples available from the second (1990–1992) and fifth (2011–2013) ARIC visits. Assays from Roche Diagnostics were implemented on an Elecsys 2010 analyzer (visit 2) and a Roche e411 analyzer (visit 5). All interassay coefficients of variation were ≤10% (Supplemental Table 1). Thyroid hormone intake was ascertained from information on the current intake of medications collected by trained interviewers.
For SHIP-0, serum TSH concentrations were analyzed on a Byk-Sangtec Diagnostica analyzer. The functional sensitivity of the assay was 0.03 mIU/L (17). In SHIP-T, serum TSH concentrations measured on a Siemens Dimension Vista analyzer. The functional sensitivity of the TSH assay was 0.014 mIU/L. Serum TPOAbs were measured on an Elias Medizintechnik GmbH VARELISA analyzer. The functional sensitivity of this assay was 1 IU/mL (Supplemental Table 1).
Thyroid ultrasonography was performed in SHIP-0 using an ultrasound VST-Gateway with a 5-MHz linear array transducer (Diasonic). In SHIP-T, ultrasonography was performed with a portable device using a 13-MHz linear array transducer (Vivid I; General Electric). In both cohorts, intra- and interobserver reliabilities were assessed before the baseline of the studies and semiannually during the studies. All measurements of the thyroid volume for interobserver and interdevice variability showed mean differences (2 SD) of the mean bias of <5% (<25%) (18). As described in the pertinent references, thyroid volume was calculated as length × width × depth × 0.479 (mL) for each lobe (20). Goiter was defined as a thyroid volume of >18 mL in women and >25 mL in men (21, 22). The normal thyroid echo pattern was classified as homogeneous. A homogeneous echo pattern with reduced echogenicity was defined as hypoechogenic.
Thyroid marker reference ranges and definition of thyroid disease
In clinical practice, the TPOAb status (positive or negative) is important in a first categorization of patients (6, 23), but both the presence and the concentrations of TPOAbs are relevant for disease onset (15, 24). Furthermore, different pathophysiological processes may be involved in the initiation and severity of the autoimmune response (15, 25). Besides TPOAb positivity, we therefore also tested the associations with continuous TPOAb concentrations. TPOAb positivity in the ARIC study was defined as >34 kIU/L as recommended by the assay manufacturer. Reference values for TSH, free T4 (FT4), and T3 in the ARIC study were derived from their distributions in a healthy subset of ARIC participants (6). Participants were excluded from this healthy subset for the following reasons: ethnicity other than European ancestry or African American, self-reported goiter and/or thyroid disease before visit 2, intake of thyroid medication, estrogen, androgen, and/or lithium use, laboratory evidence of overt hyperthyroidism (TSH of <0.27 mIU/L; FT4 of >21.88 pmol/L), overt hypothyroidism (TSH of >4.2 mIU/L; FT4 of <11.97 pmol/L), elevated TPOAb concentrations (>34 kIU/L), or missing information on TSH, FT4, T3, or TPOAb measurements. Among the resulting 7394 participants, reference intervals were established using the 2.5th and 97.5th percentiles for thyroid hormone measurements. For European American (EA) individuals, reference intervals were as follows: TSH, 0.61 to 5.4 mIU/L; and FT4, 11.2 to 19.3 pmol/L (Supplemental Table 2). Subclinical hypothyroidism was defined as elevated serum TSH concentrations but normal FT4 concentrations and overt hypothyroidism as elevated TSH concentrations and FT4 below the established reference range (3).
For SHIP-0 and SHIP-T, TPOAb positivity as well as the TSH and FT4 reference values were defined (TPOAb positivity as >60 kIU/L for men and >100 kIU/L for women; SHIP-0: TSH, 0.3–3.0 mIU/L; and FT4, 7.7–23.2 pmol/L; SHIP-T: TSH, 0.3–3.74 mIU/L; and FT4, 8.4–18.9 pmol/L) as provided by the manufacturer and implemented previously (26). In SHIP-T, FT4 was only measured for samples with TSH values outside of the reference range. Subclinical and overt hypothyroidism were defined as in the ARIC study using the SHIP-specific reference ranges.
In the ARIC study, 2 measures of thyroid function markers were available a median of 20.8 years (20.1, 21.5 years) apart, allowing us to evaluate prospective associations. Because of the long observational time gap between study visits 4 and 5 with limited information on potential informative censoring, incident thyroid disease associations were only evaluated in secondary analyses. Incident TPOAb positivity at ARIC visit 5 was defined as above among those who were TPOAb negative at the second visit. Incident subclinical and overt hypothyroidism at visit 5 were defined as above among participants with TSH and FT4 concentrations within the reference range at visit 2.
Identification of associated genetic variants and genotyping information
To identify TPOAb-associated single nucleotide polymorphisms (SNPs) that could be combined into a GRS, we used information from a recently published large GWAS meta-analysis of TPOAb concentrations and positivity, to which the SHIP studies contributed but the ARIC study did not (14). The study reported 20 index SNPs associated with measures of AITD at P < 10−6 (7 associated with TPOAb concentrations, 10 with TPOAb positivity, and 3 with both traits). In the ARIC study, genotypes at these SNPs were available from the Affymetrix Genome-Wide Human SNP Array 6.0. Quality control procedures and genotype imputation were described previously (27). The published effect estimates for the 20 index SNPs were meta-analyzed with the corresponding SNP estimates and traits from the ARIC study using a sample-size weighted meta-analysis as implemented in METAL (28). Subsequently, genotypes of all previously identified replicated loci and newly identified genome-wide significant (P < 5 · 10−8) index SNPs were used for the calculation of the GRS. Genotyping and imputation quality of the SNPs studied in this report are listed in Supplemental Table 3.
GRS calculation and statistical analysis
The GRS for each individual was calculated across the associated SNPs as the weighted sum of all TPOAb positivity risk alleles carried by a person, with weights proportional to the effect estimates from the previously published meta-analysis (14). GRS quartiles were generated from the global distribution of the score. Supplemental Table 4 shows handling of participants with thyroid hormone measurements above the upper limit of detection and below the lower limit of detection before association analyses (14). We then estimated the age-, sex- and study center–adjusted associations between each of the SNPs and the natural logarithm of TPOAb concentrations as well as inverse normal transformations of TSH and FT4 concentrations. Associations with thyroid volume, goiter, and low echogenicity were examined using multiple linear and logistic regression as appropriate. The proportion of TPOAb variance explained was estimated from a linear regression model.
The odds ratio (OR) of TPOAb positivity was estimated for each GRS quartile compared with the lowest GRS quartile (reference). The P value for trend was estimated by entering the GRS as a continuous variable into the regression model. The associations between the GRS quartiles and transformed thyroid hormone concentrations, as well as subclinical and overt hypothyroidism, thyroid volume, goiter, and low echogenicity were computed in an analogous fashion. Analyses on subclinical and overt hyperthyroidism were not conducted because of the very small case numbers. In a secondary analysis, associations of the GRS with incident TPOAb positivity and subclinical hypothyroidism were estimated using a multivariable adjusted Poisson regression model.
All GRS analyses were conducted using Stata/SE 13 or the R statistical framework (version 2.15.3; R Project for Statistical Computing; www.r-project.org).
Results
Study population and thyroid marker characteristics
Table 1 shows TPOAb concentrations and positivity in all cohorts. The prevalence of TPOAb positivity was 16% among 7524 ARIC participants, 7% among 3803 SHIP-0 participants, and 4% among 887 SHIP-T participants. Thyroid hormone replacement therapy ranged from 5.4% (ARIC) to 10% (SHIP-T), and the prevalence of goiter in both SHIP studies was around 35%.
Table 1.
Characteristics of the Study Populations
| ARIC | SHIP-0 | SHIP-T | |
|---|---|---|---|
| Sample size | 7524 | 3803 | 887 |
| Female sex, % (n) | 55 (4101) | 48 (1844) | 54 (475) |
| Age, y, mean ± SD | 57 ± 5.7 | 49 ± 16.3 | 50 ± 13.7 |
| TSH, mIU/L, median (p25, p75) | 1.9 (1.3, 2.9) | 0.66 (0.44, 0.97) | 1.2 (0.81, 1.6) |
| FT4, ng/dL, mean ± SD | 1.1 ± 0.19 | 1.0 ± 0.3 | NA |
| TPOAb, IU/mL, median (p25, p75) | 10.9 (7.9, 18.0) | 4.7 (1.9, 34.4) | 11.0 (10.0, 14.0) |
| TPOAb positivity, % (n) | 16 (1240) | 7 (265) | 4 (36) |
| Thyroid hormone replacement, % (n) | 5.4 (407) | 6.7 (272)a | 10.0 (99)a |
| Antithyroid drug use, % (n) | 0.03 (2) | NA | NA |
| Goiter, % (n) | NA | 38 (1428) | 34 (303) |
Abbreviation: NA, not available.
Genotyped samples before exclusion.
Nine genetic loci associated with TPOAbs
Table 2 shows a summary of the SNP with the lowest P value (index SNP) at 9 genetic loci that contained variants associated with TPOAb concentrations (n = 1), positivity (n = 3), and both (n = 5) at genome-wide significance. Four of these 9 common SNPs, rs301799 near RERE and 3 markers in the HLA region (rs3094228, rs1894407, and rs9277555), mapped into a region that showed genome-wide significant associations with TPOAb measures for the first time and thus represent novel risk loci for elevated TPOAb concentrations and positivity.
Table 2.
GWSAs With TPOAb Concentrations or TPOAb Positivity
| Variant | Chr | Position (hg 19) | Genesa | Risk Allele/Other Allele | Risk Allele Frequency | TPOAb Concentrationsb |
TPOAb Positivityb |
||
|---|---|---|---|---|---|---|---|---|---|
| Z Score | P Value | Z Score | P Value | ||||||
| rs301799 | 1 | 8489302 | RERE | C/T | 0.43 | 4.22 | 2.5E–05 | 5.68 | 1.4E–08 |
| rs1230666 | 1 | 114173410 | MAGI3, [PTPN22] | A/G | 0.14 | 6.65 | 2.9E–11 | 5.52 | 3.5E–08 |
| rs11675434 | 2 | 1407815 | TPO | T/C | 0.42 | 8.67 | 4.3E–18 | 9.57 | 1.1E–21 |
| rs2010099 | 3 | 124300257 | KALRN | C/T | 0.89 | 4.92 | 8.8E–07 | 3.73 | 1.9E–04 |
| rs3094228 | 6 | 31429927 | HCP5, MICA | C/T | 0.19 | 5.14 | 2.7E–07 | 5.69 | 1.3E–08 |
| rs1894407 | 6 | 32787036 | HLA-DOB, TAP2 | C/A | 0.61 | 5.49 | 4.0E–08 | 5.67 | 1.4E–08 |
| rs9277555 | 6 | 33055605 | HLA-DPB1 | G/A | 0.74 | 6.94 | 3.9E–12 | 6.30 | 3.0E–10 |
| rs10944479 | 6 | 90880393 | BACH2 | A/G | 0.19 | 4.89 | 1.0E–06 | 6.19 | 5.8E–10 |
| rs653178 | 12 | 112007756 | ATXN2, [SH2B3] | C/T | 0.50 | 6.28 | 3.5E–10 | 7.10 | 1.3E–12 |
Annotation is based on RefSeq genes, hg19: variant in gene if underlined, closest gene, or flanking genes if both are in same linkage disequilibrium block. Nearby genes that contain correlated functional variants are enclosed in brackets.
Number of individuals with available SNP information (n) ranges from 30501–35332. Sample size varies because of missing genotype information across studies and between markers because of handling of values outside the assay detection range.
Individual SNP association with serum thyroid function tests
Next, the 9 associated SNPs were characterized for their association with TPOAb concentrations, and additional markers of thyroid function and disease in the ARIC and SHIP studies. Supplemental Table 5 shows that all risk alleles showed positive associations with TPOAb concentrations in the combined ARIC and SHIP studies, all of which except rs2010099 were statistically significant after correction for multiple testing (P < 5.6 · 10−3). The SNPs rs11675434 (TPO) and rs9277555 (HLA region) showed nominally significant associations (P < .05) with all 3 thyroid function measures evaluated (TPOAbs, TSH, and FT4).
In the individual studies, the direction of effect for the risk allele at each SNP was almost all positive and of comparable magnitude across studies for TPOAb concentrations and TSH concentrations and mostly negative for FT4 concentrations, indicating that the risk alleles not only associate with higher TPOAb concentrations but also with higher TSH and lower FT4 concentrations.
The GRS associates with thyroid function markers and thyroid dysfunction
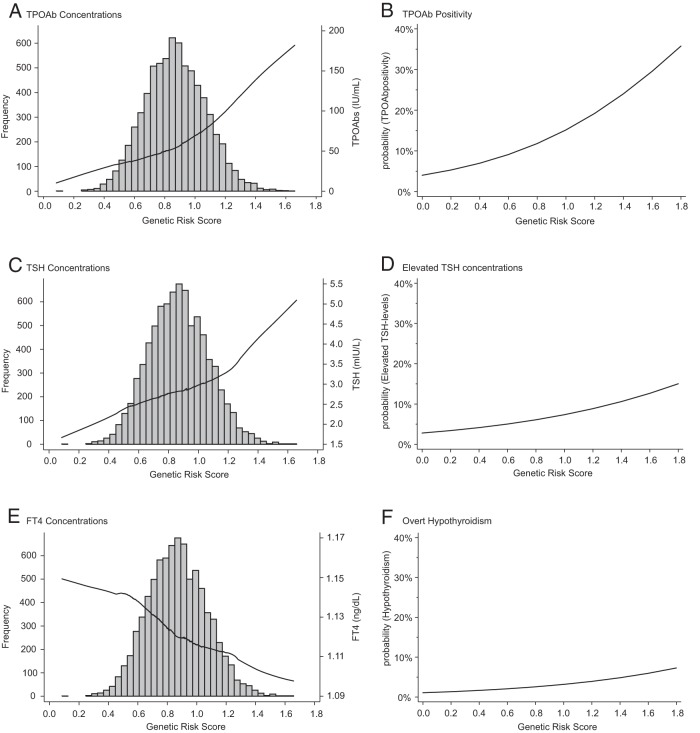
In the ARIC study, the proportion of variance explained in TPOAb concentrations increased from 3.2% using a GRS based on the 5 previously published loci to 4.1% by the addition of the novel 4 risk loci identified here. Using this improved GRS, we studied the effect of the continuous GRS on TPOAb, TSH, and FT4 concentrations, as well as on the odds of TPOAb positivity, elevated TSH, and overt hypothyroidism. Figure 1 shows a strong and graded positive association of the GRS with TPOAb concentrations (Figure 1A) and TSH concentrations (Figure 1C) and a strong, graded, inverse association with FT4 (Figure 1E). The predicted probability of TPOAb positivity ranged from 5% to 35% across the GRS range (Figure 1B). This effect was also present for elevated TSH concentrations (3%–15%, Figure 1D), subclinical hypothyroidism (2%–9%, graph not shown), and overt hypothyroidism (1%–7%, Figure 1F).
Figure 1.
Cross-sectional association of the GRS with TPOAbs, TH markers and clinical outcomes. A, Association of TPOAb concentrations and GRS (GRS distribution in the background). B, Association of TPOAb positivity and GRS. C, Association of TSH concentrations and GRS (GRS distribution in the background). D, Association of elevated TSH concentrations and GRS. E, Association of FT4 concentrations and GRS (GRS distribution in the background). F, Association of over hypothyroidism and GRS.
Subsequently, we evaluated the multivariable-adjusted association between quartiles of the GRS, TPOAb positivity, and hypothyroidism (Table 3). There was a strong, graded, and significant association across risk score quartiles with prevalent TPOAb positivity in all 3 studies. Compared with the lowest GRS quartile (reference), the ORs for TPOAb positivity for those in the highest GRS quartile were 2.10 (95% confidence interval [CI], 1.74–2.55) in the ARIC study and 3.84 (95% CI, 2.50–5.92) and 3.89 (95% CI, 1.73–8.75) for the SHIP-0 and SHIP-T studies. The P values for trend were highly significant in all 3 studies. Similarly, the GRS was significantly associated with 1.2-fold higher odds of elevated TSH per each higher quartile (P < .0001), with similar estimates for subclinical and overt hypothyroidism in the ARIC study, in which sufficient case numbers were available. The OR for overt hypothyroidism was 1.89 (95% CI, 1.24–2.87) for those in the highest GRS quartile compared with that for those in the lowest. Supplemental Table 6 shows associations for the continuous thyroid function measures TPOAbs, TSH, and FT4 with the GRS: there was a strong, graded, and highly significant association between higher GRS quartiles with TPOAb concentrations in the ARIC and SHIP-0 cohorts and a strong association with higher TSH and lower FT4 in the ARIC study (P for trend significant for all 3 markers in the large ARIC study). For the smaller SHIP-T cohort, there were no significant associations between the GRS and the evaluated measures, but the effect directions were consistent with those the other studies.
Table 3.
Prevalent Associations Between the GRS and Biomarker-Based Thyroid Outcomes in the ARIC and SHIP Studies
| Q1 | Q2 | Q3 | Q4 | Overall (trend) | |
|---|---|---|---|---|---|
| TPOAb positivity | |||||
| ARIC | |||||
| No. case patients/control subjects | 186/1826 | 243/1768 | 282/1730 | 353/1658 | 1064/6982 |
| OR (95% CI) | Ref | 1.36 (1.11–1.66) | 1.63 (1.33–1.98) | 2.10 (1.74–2.55) | 1.27 (1.20–1.35) |
| P value | 3.4E–03 | 1.6E–06 | 3.1E–14 | 2.9E–15 | |
| SHIP-0 | |||||
| No. case patients/control subjects | 28/924 | 62/887 | 77/874 | 98/853 | 265/3538 |
| OR (95% CI) | Ref | 2.35 (1.49–3.72) | 3.01 (1.93–4.70) | 3.84 (2.50–5.92) | 1.47 (1.30–1.65) |
| P value | 2.6E–04 | 1.2E–06 | 1.0E–09 | 2.1E–10 | |
| SHIP-T | |||||
| No. case patients/control subjects | 8/214 | 14/207 | 17/205 | 28/194 | 67/820 |
| OR (95% CI) | Ref | 1.79 (0.73–4.40) | 2.22 (0.94–5.27) | 3.89 (1.73–8.75) | 1.55 (1.22–1.97) |
| P value | 2.0E–01 | 7.0E–02 | 1.0E–03 | 3.6E–04 | |
| Elevated TSH: ARICa | |||||
| No. case patients/control subjects | 88/1881 | 127/1837 | 153/1826 | 153/1814 | 521/7358 |
| OR (95% CI) | Ref | 1.51 (1.14–2.00) | 1.82 (1.39–2.39) | 1.82 (1.39–2.39) | 1.20 (1.11–1.30) |
| P value | 3.9E–03 | 1.5E–05 | 1.6E–05 | 8.5E–06 | |
| Subclinical hypothyroidism: ARICa | |||||
| No. case patients/control subjects | 52/1881 | 72/1836 | 93/1825 | 89/1814 | 306/7356 |
| OR (95% CI) | Ref | 1.46 (1.01–2.09) | 1.87 (1.32–2.65) | 1.80 (1.27–2.55) | 1.21 (1.09–1.34) |
| P value | 4.3E–02 | 4.0E–04 | 1.0E–03 | 4.1E–04 | |
| Overt hypothyroidism: ARICa | |||||
| No. case patients/control subjects | 35/1881 | 55/1836 | 60/1825 | 63/1814 | 213/7356 |
| OR (95% CI) | Ref | 1.64 (1.07–2.53) | 1.80 (1.18–2.76) | 1.89 (1.24–2.87) | 1.20 (1.06–1.36) |
| P value | 2.4E–02 | 6.4E–03 | 3.1E–03 | 3.9E–03 |
The ARIC study only because of sample size.
In the secondary analysis, the association of the GRS with incident TPOAb positivity (OR, 1.21; 95% CI, 1.03–1.43) and subclinical hypothyroidism (OR, 1.14; 95% CI, 1.01–1.28) at visit 5 was of a magnitude comparable to the association with prevalent TPOAb positivity and prevalent subclinical hypothyroidism at visit 2.
Associations between the GRS and thyroid ultrasound findings
Supplemental Table 7 shows a graded and significant association between higher GRS and lower thyroid volume in SHIP-0, which was not observed in SHIP-T. There was a significant inverse association across quartiles of the GRS with lower odds of goiter in SHIP-0 (OR, 0.92; 95% CI, 0.86–0.98), which was also not observed in SHIP-T. Low echogenicity did not show an association with the GRS in either SHIP-0 or SHIP-T.
Discussion
There are 2 principal findings of our study. First, we identified 4 novel genomic regions (1 including the RERE gene and 3 in the extended HLA region) that contain variants associated with TPOAbs at genome-wide significance. We further confirmed associations at 5 known genetic loci. Second, a weighted GRS based on the independent index SNPs at these 9 genetic loci showed strong and graded associations with TPOAbs, TSH, and FT4 concentrations as well as with the risk of subclinical and overt hypothyroidism and goiter in the general population.
One of the major strengths of GWASs is the ability to map previously unknown risk genes in an unbiased fashion, which can serve as the basis for gaining pathophysiological insights (29). This is true even if the associated variants only explain a modest part of the trait variance as observed here and also if the variants themselves do not significantly improve already well-calibrated clinical prediction models. In our study, we identified 4 novel associated loci. The intronic index SNP rs301799 on chromosome 1p36.23 maps into the RERE gene, which encodes the arginine-glutamic acid dipeptide [RE] repeats protein. The variant identified here has been reported to associate with levels of the RERE transcript (30). A highly correlated genetic variant in this gene (r2 of 0.97 in 1000 Genomes CEU data) has previously been reported to be associated with the autoimmune disease vitiligo (31) and thus represents another example of a genetic variant associated with more than 1 autoimmune disease. Individuals with vitiligo show an increased prevalence of AITD (32), and our findings suggest that RERE may be a gene contributing to the shared genetic predisposition (33). The RERE protein is a transcriptional corepressor that is highly expressed in lymphoid cells and is thought to regulate apoptosis (34, 35), which seems to play an important role in the development of AITD, leading ultimately to the destruction of the thyroid gland (36–38).
The other 3 newly genome-wide significant SNPs map into the extended HLA region on chromosome 6, concordant with many already known susceptibility loci for autoimmune diseases including AITD by candidate gene and GWASs (11, 12). The marker rs9277555 maps into HLA-DPB1; genetic variants in HLA-DPB1 have previously been shown to associate with Graves disease (39).
The intergenic marker rs3094228 maps into a region of extended linkage disequilibrium. The closest genes are HCP5 and MICA; other genes include MICB, HLA-B, and HLA-C, all of which are plausible biological candidates. HLA-C was previously demonstrated to have a strong association with Graves disease in Caucasians (40). HLA-B was found to be associated independent of HLA-C with Graves disease and was a risk factor for Hashimoto disease in Han Chinese (40, 41). HCP5 (HLA complex P5) is also a HLA class I gene consistent with a potential role in autoimmunity (42). MICA has been reported to be associated with insulin-dependent diabetes mellitus, celiac disease, and Behçet disease (43–45). The third index SNP on chromosome 6, rs1894407, maps close to TAP2 and HLA-DOB. The membrane-associated protein encoded by TAP2 is a member of the superfamily of ATP-binding cassette transporters. This locus has been implicated in the susceptibility to other autoimmune disease, such as Graves disease, lupus erythematosus, psoriasis, rheumatoid arthritis, insulin-dependent diabetes mellitus, and celiac disease (46–50). HLA-DOB, the other gene mapping into the same linkage disequilibrium block, is thought to also have an important role in autoimmunity and has been associated with various other autoimmune diseases, such as diabetes mellitus type 1, systemic lupus erythematosus, rheumatoid arthritis, and celiac disease (52, 53).
Taken together, the four novel loci highlight the role of the general immune response in different autoimmune diseases. Functional data to illuminate the molecular mechanisms underlying the associations are needed to understand and target disease-specific mechanisms.
A GRS incorporating information from a previous GWASs showed strong associations in the large, independent ARIC study. This observation suggests that winner's curse is unlikely to have had a major impact on results of the published GWASs and that the GRS is likely to perform well in additional independent studies of EA participants. The GRS is based on common TPOAb-associated SNPs and facilitated the identification of a subgroup in the general population with a 2-fold higher prevalence of TPOAb positivity. So far, clinical algorithms, such as the THEA score, have been studied to predict the risk of development of clinical thyroid disease in individuals with first- or second-degree relatives with documented Hashimoto or Graves disease (24). Information on affected relatives was not available in our population-based studies, and we only had information about incident thyroid disease after a long gap between study visits. Future prospective studies are needed to evaluate whether addition of the GRS to clinically validated scores such as the THEA score can improve risk prediction.
The GRS also showed a significant strong and graded association with subclinical hypothyroidism. Subclinical hypothyroidism has increasingly been recognized as an important clinical entity. Besides the fact that it may lead to overt hypothyroidism, subclinical hypothyroidism itself has been associated with hypertension, dyslipidemia, cardiovascular abnormalities, and endothelial dysfunction with consecutive increased cardiovascular morbidity and mortality, reduced fertility, and adverse pregnancy outcomes (2, 51).
Our study has some limitations. Because the ARIC and SHIP studies are population-based studies, there were insufficient case numbers to evaluate subclinical and overt hyperthyroidism, but TPOAbs are clinically considered to be much more a marker of Hashimoto thyroiditis than Graves disease, for which TSH receptor antibodies are more important. Information on goiter, low echogenicity, and thyroid volume was only available for the SHIP cohorts, but outcomes that were available in both ARIC and SHIP showed consistent associations with the GRS despite their locations on different continents with different iodine status of populations. This finding suggests that genetic risk is evident even with variable environmental exposures. Although study heterogeneity and differences in the tests used to assess thyroid function represent limiting factors, the fact that our findings are generally consistent across studies increases confidence related to their validity and generalizability. Because our analyses were restricted to participants of EA descent, our results may not be generalizable to populations with other ancestries.
Because of the unavailability of more closely spaced information on thyroid disease, we could not reliably assess prospective associations of the GRS.
In summary, we identified 4 novel genetic loci and confirmed 5 known loci associated with TPOAbs. A GRS based on index SNPs in these 9 loci showed strong and graded associations not only with TPOAb concentrations and positivity but also with TSH and FT4 concentrations, as well as with the clinical entities subclinical and overt hypothyroidism and goiter. Our results support the generalizability of the GRS in populations of European ancestry even under different environmental exposures and warrant an evaluation of its predictive ability in combination with clinical patient characteristics in future studies of incident thyroid disorders.
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions. Reagents for the thyroid assays in the ARIC study were donated by Roche Diagnostics.
SHIP is part of the Community Medicine Research net of the University of Greifswald, Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (Grants 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania, and the network Greifswald Approach to Individualized Medicine (GANI_MED) funded by the Federal Ministry of Education and Research (Grant 03IS2061A). Genome-wide data were supported by the Federal Ministry of Education and Research (Grant 03ZIK012) and by a joint grant from Siemens Healthcare, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania. The University of Greifswald is a member of the Center of Knowledge Interchange program of Siemens AG and the Caché Campus program of InterSystems GmbH. The SHIP authors gratefully acknowledge the support from the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG] Vo 955/10-2 and the framework of the Priority Program (Schwerpunktprogramm) 1692 “Thyroid Trans Act” of the DFG WA 1328/5-1). U.T.S., Y.L., and A.K. were funded by the Emmy Noether Programme of the German Research Foundation (DFG KO-3598/2-1), and U.T.S. was funded by the Else Kröner-Fresenius-Stiftung (2013_Kolleg.03), Bad Homburg, Germany. E.S. was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK089174. M.M., L.C., and R.P.P. were funded by a ZonMW TOP grant to R.P.P. (92112044). The ARIC study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (Contracts HHSN268201100005C, HSN268201100006C, HHSN268201100007C, HHSN268201100008C, HSN268201100009C, HHSN268201100010C, HHSN 268201100011C, HHSN268201100012C, R01HL087641, R01HL59367, and R01HL086694), the National Human Genome Research Institute (Contract U01HG004402), and the National Institutes of Health (Contract HHSN268200625226C). Infrastructure was partly supported by Grant UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AITD
- autoimmune thyroid disease
- ARIC
- Atherosclerosis Risk In Communities
- CI
- confidence interval
- EA
- European American
- FT4
- free T4
- GRS
- genetic risk score
- GWAS
- genome-wide association studies
- OR
- odds ratio
- SHIP
- SHIP-0, Study of Health in Pomerania
- SHIP-T
- Study of Health in Pomerania-TREND
- SNP
- single nucleotide polymorphism
- TPOAb
- autoantibody titer against thyroid peroxidase.
References
- 1. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. [DOI] [PubMed] [Google Scholar]
- 2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. [DOI] [PubMed] [Google Scholar]
- 3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028. [DOI] [PubMed] [Google Scholar]
- 4. Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. [DOI] [PubMed] [Google Scholar]
- 5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. [DOI] [PubMed] [Google Scholar]
- 6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499. [DOI] [PubMed] [Google Scholar]
- 7. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646–2655. [DOI] [PubMed] [Google Scholar]
- 8. Brix TH, Hegedüs L, Gardas A, Banga JP, Nielsen CH. Monozygotic twin pairs discordant for Hashimoto's thyroiditis share a high proportion of thyroid peroxidase autoantibodies to the immunodominant region A. Further evidence for genetic transmission of epitopic “fingerprints.” Autoimmunity. 2011;44:188–194. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen CH, Brix TH, Leslie RG, Hegedüs L. A role for autoantibodies in enhancement of pro-inflammatory cytokine responses to a self-antigen, thyroid peroxidase. Clin Immunol. 2009;133:218–227. [DOI] [PubMed] [Google Scholar]
- 10. Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226. [DOI] [PubMed] [Google Scholar]
- 11. Weetman AP. Diseases associated with thyroid autoimmunity: explanations for the expanding spectrum. Clin Endocrinol (Oxf). 2011;74:411–418. [DOI] [PubMed] [Google Scholar]
- 12. Simmonds MJ. GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol. 2013;9:277–287. [DOI] [PubMed] [Google Scholar]
- 13. Hansen PS, Brix TH, Iachine I, Kyvik KO, Hegedüs L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: a study of healthy Danish twins. Eur J Endocrinol. 2006;154:29–38. [DOI] [PubMed] [Google Scholar]
- 14. Medici M, Porcu E, Pistis G, et al. Identification of novel genetic loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10:e1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014;170:R241–E252. [DOI] [PubMed] [Google Scholar]
- 16. ARIC Investigators. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17. Völzke H, Alte D, Kohlmann T, et al. Reference intervals of serum thyroid function tests in a previously iodine-deficient area. Thyroid. 2005;15:279–285. [DOI] [PubMed] [Google Scholar]
- 18. Völzke H, Werner A, Wallaschofski H, et al. Occupational exposure to ionizing radiation is associated with autoimmune thyroid disease. J Clin Endocrinol Metab. 2005;90:4587–4592. [DOI] [PubMed] [Google Scholar]
- 19. Völzke H, Alte D, Schmidt CO, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40:294–307. [DOI] [PubMed] [Google Scholar]
- 20. Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound (author's transl). Dtsch Med Wochenschr. 1981;106:1338–1340. [DOI] [PubMed] [Google Scholar]
- 21. Gutekunst R, Becker W, Hehrmann R, Olbricht T, Pfannenstiel P. Ultrasonic diagnosis of the thyroid gland [in German]. Dtsch Med Wochenschr. 1988;113:1109–1112. [DOI] [PubMed] [Google Scholar]
- 22. Teumer A, Rawal R, Homuth G, et al. Genome-wide association study identifies four genetic loci associated with thyroid volume and goiter risk. Am J Hum Genet. 2011;88:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong YH, Fu DG. Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharmacol Sci. 2014;18:3611–3618. [PubMed] [Google Scholar]
- 24. Strieder TG, Tijssen JG, Wenzel BE, Endert E, Wiersinga WM. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008;168:1657–1663. [DOI] [PubMed] [Google Scholar]
- 25. Utiger RD. The pathogenesis of autoimmune thyroid disease. N Engl J Med. 1991;325:278–279. [DOI] [PubMed] [Google Scholar]
- 26. Völzke H, Lüdemann J, Robinson DM, et al. The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid. 2003;13:803–810. [DOI] [PubMed] [Google Scholar]
- 27. Köttgen A, Pattaro C, Böger CA, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westra HJ, Peters MJ, Esko T, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin Y, Birlea SA, Fain PR, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vrijman C, Kroon MW, Limpens J, et al. The prevalence of thyroid disease in patients with vitiligo: a systematic review. Br J Dermatol. 2012;167:1224–1235. [DOI] [PubMed] [Google Scholar]
- 33. Spritz RA. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid. 2010;20:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L, Tsai CC. Atrophin proteins: an overview of a new class of nuclear receptor corepressors. Nucl Recept Signal. 2008;6:e009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waerner T, Gardellin P, Pfizenmaier K, Weith A, Kraut N. Human RERE is localized to nuclear promyelocytic leukemia oncogenic domains and enhances apoptosis. Cell Growth Differ. 2001;12:201–210. [PubMed] [Google Scholar]
- 36. Bossowski A, Czarnocka B, Bardadin K, et al. Identification of apoptotic proteins in thyroid gland from patients with Graves' disease and Hashimoto's thyroiditis. Autoimmunity. 2008;41:163–173. [DOI] [PubMed] [Google Scholar]
- 37. Wang SH, Baker JR. The role of apoptosis in thyroid autoimmunity. Thyroid. 2007;17:975–979. [DOI] [PubMed] [Google Scholar]
- 38. Mikos H, Mikos M, Obara-Moszynska M, Niedziela M. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (AITD). Endokrynol Pol. 2014;65:150–155. [DOI] [PubMed] [Google Scholar]
- 39. Chen PL, Fann CS, Chu CC, et al. Comprehensive genotyping in two homogeneous Graves' disease samples reveals major and novel HLA association alleles. PLoS One. 2011;6:e16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simmonds MJ, Howson JM, Heward JM, et al. A novel and major association of HLA-C in Graves' disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet. 2007;16:2149–2153. [DOI] [PubMed] [Google Scholar]
- 41. Huang CY, Chang TY, Chu CC, et al. The HLA-B gene and Hashimoto disease in Han Chinese children: a case-control and family-based study. Tissue Antigens. 2012;80:431–436. [DOI] [PubMed] [Google Scholar]
- 42. Liu Y, Helms C, Liao W, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4:e1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hüe S, Mention JJ, Monteiro RC, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. [DOI] [PubMed] [Google Scholar]
- 44. Gambelunghe G, Ghaderi M, Tortoioli C, et al. Two distinct MICA gene markers discriminate major autoimmune diabetes types. J Clin Endocrinol Metab. 2001;86:3754–3760. [DOI] [PubMed] [Google Scholar]
- 45. Wallace GR, Verity DH, Delamaine LJ, et al. MIC-A allele profiles and HLA class I associations in Behçet's disease. Immunogenetics. 1999;49:613–617. [DOI] [PubMed] [Google Scholar]
- 46. Penfornis A, Tuomilehto-Wolf E, Faustman DL, Hitman GA. Analysis of TAP2 polymorphisms in Finnish individuals with type I diabetes. Hum Immunol. 2002;63:61–70. [DOI] [PubMed] [Google Scholar]
- 47. Zhang SL, Chabod J, Penfornis A, et al. TAP1 and TAP2 gene polymorphism in rheumatoid arthritis in a population in eastern France. Eur J Immunogenet. 2002;29:241–249. [DOI] [PubMed] [Google Scholar]
- 48. Chen RH, Wang TY, Chen WC, Tsai CH, Tsai FJ. Association between the TAP2 gene codon 665 polymorphism and Graves' disease. J Clin Lab Anal. 2006;20:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Correa PA, Molina JF, Pinto LF, Arcos-Burgos M, Herrera M, Anaya JM. TAP1 and TAP2 polymorphisms analysis in northwestern Colombian patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krämer U, Illig T, Grune T, Krutmann J, Esser C. Strong associations of psoriasis with antigen processing LMP and transport genes TAP differ by gender and phenotype. Genes Immun. 2007;8:513–517. [DOI] [PubMed] [Google Scholar]
- 51. Biondi B. Natural history, diagnosis and management of subclinical thyroid dysfunction. Best Pract Res Clin Endocrinol Metab. 2012;26:431–446. [DOI] [PubMed] [Google Scholar]
- 52. Johansson S, Lie BA, Todd JA, Pociot F, Nerup J, Cambon-Thomsen A, et al. Evidence of at least two type 1 diabetes susceptibility genes in the HLA complex distinct from HLA-DQB1, -DQA1 and -DRB1. Genes Immun. 2003;4(1):46–53. [DOI] [PubMed] [Google Scholar]
- 53. van Lith M, van Ham M, Neefjes J. Novel polymorphisms in HLA-DOA and HLA-DOB in B-cell malignancies. Immunogenetics. 2002;54(8):591–5. [DOI] [PubMed] [Google Scholar]