Abstract
Context:
Currently there are no efficacious therapies for patients with anaplastic thyroid carcinoma (ATC) that result in long-term disease stabilization or regression.
Objective:
We sought to identify pathways critical for ATC cell progression and viability in an effort to develop new therapeutic strategies. We investigated the effects of targeted inhibition of stearoyl-CoA desaturase 1 (SCD1), a constituent of fatty acid metabolism overexpressed in ATC.
Design:
A gene array of ATC and normal thyroid tissue was performed to identify gene transcripts demonstrating altered expression in tumor samples. Effects of pharmacological and the genetic inhibition of SCD1 on tumor cell viability as well as cell signaling responses to therapy were evaluated in in vitro and in vivo models of this rare, lethal malignancy.
Results:
The gene array analysis revealed consistent distortion of fatty acid metabolism and overexpression of SCD1 in ATC and well-differentiated thyroid carcinomas. SCD1 is critical for ATC cell survival and proliferation, the inhibition of which induced endoplasmic reticulum stress, activation of the unfolded protein response, and apoptosis. Combined suppression of endoplasmic reticulum-associated degradation, a prosurvival component of the unfolded protein response, using proteasome inhibitors resulted in a synergistic decrease in tumor cell proliferation and increased cell death.
Conclusions:
SCD1 is a novel oncogenic factor specifically required for tumor cell viability in ATC. Furthermore, the expression of SCD1 appears to be correlated with thyroid tumor aggressiveness and may serve as a prognostic biomarker. These findings substantiate SCD1 as a novel tumor-specific target for therapy in patients with ATC and should be further investigated in a clinical setting.
Anaplastic thyroid carcinoma (ATC) is arguably the most lethal solid tumor known to man. The median overall survival for these patients is abysmal: 3–5 months (1–3). Although this subtype is rare, accounting for 1%–2% of all diagnosed cases of thyroid cancers, ATC disproportionately accounts for 14%–39% of all thyroid cancer related deaths (2). ATC is very aggressive, and patients often present with advanced localized invasion, limiting the opportunity for surgical resection of the bulk tumor. A reported 20%–50% of patients present with distant metastases, and 25% of patients develop new metastases after initial diagnosis (2). As such, the American Joint Committee on Cancer has classified ATC as stage IV disease, regardless of its tumor size, and local or distal involvement. While response rates are low, for those who want aggressive therapy (especially stages IVA and IVB), multimodal treatment with intensity modulated radiation therapy with chemotherapy, preferably after maximal surgical resection, is the current recommended therapy (4). In addition, many classes of targeted therapies have been investigated, including antiangiogenic therapy, vascular-disrupting agents, tyrosine kinase inhibitors, histone deacetylase inhibitors, and peroxisomal proliferator-activated receptor-γ agonists independently or in combination with chemotherapy (2, 3, 5, 6). Although some marginal success has been observed, the prognosis for these patients remains desolate. Therefore, a clear need for efficacious therapies that substantially improve patient overall survival remains.
Recent investigations have implicated the importance of fatty acid metabolism in cell transformation and cancer progression (7). Fatty acids are involved in many facets of cell growth and survival because they not only make up the major structural elements of cell membranes but they also contribute to hormone synthesis, triglyceride synthesis, and energy storage and serve as signaling molecules or second messengers. Modifications in fatty acid metabolism can result in altered membrane fluidics, affect availability of energy stores, influence drug resistance, mediate proliferation and survival, and instigate communications with the extracellular environment through secretion of lipid-based ligands (8, 9). Normally de novo biosynthesis of fatty acids and cholesterol is tightly regulated, restricted to specific tissues such as liver, adipose, and breast (9, 10). Other nontransformed mammalian cells typically obtain free fatty acids through extracellular sources, including those with high proliferative rates such as intestinal epithelia or hematopoietic cells (9, 10). Interestingly, transformed cells and malignant tissues overexpress constituents involved in de novo lipid metabolism (10–13). This tumor-specific need for increased lipid bioavailability presents as an attractive target for therapeutic intervention.
Gene array analysis of ATC patient tissue compared with matched and unmatched normal thyroid tissue was performed in an effort to identify pathways critical for ATC survival and progression. The results revealed dysregulation of several factors involved in fatty acid metabolism. Of these, expression levels of stearoyl-CoA desaturase (SCD1) were highly elevated in tumor samples. Upon further investigation we identified increased SCD1 protein not only in ATC patient tissues but also in papillary thyroid carcinoma (PTC) and advanced follicular thyroid carcinoma (FTC).
SCD1 is a key player in de novo lipid biosynthesis. A fatty acyl desaturase, its enzymatic activity catalyzes the insertion of a cis-double bond at the delta 9 position of the carbon chain primarily in the saturated fatty acids stearic and palimitic acid (14, 15). This rate-limiting reaction results in the production of the monounsaturated fatty acids oleoyl-CoA (oleic acid), and palmitoleoyl-CoA (palmitoleic acid), essential components of triglycerides, sphingolipids, glycolipids, phospholipids, and other lipoproteins (16). Currently five isoforms of this protein have been identified, although the expression of only two have been characterized in humans: SCD1 and SCD5 (17).
Our group has recently established an oncogenic role for SCD1 in clear cell renal cell carcinoma (18), another radiation and chemorefractory solid tumor that frequently manifests as metastatic disease. SCD1 has also been implicated as tumorigenic in other cancers including hepatocellular carcinoma (19), gastric cancer (20), and nonsmall cell lung cancer (20, 21). In our study, pharmacological and genetic inhibition of SCD1 attenuated tumor cell proliferation and induced cell death in both in vitro and in vivo models of ATC, providing mounting evidence for SCD1 as an oncogenic mediator in aggressive cancers.
Materials and Methods
Patient tissues and cell lines
All patient tissues used in this study are deidentified. This study has been approved by the Mayo Institutional Review Board. KTC1 cells were kindly provided by Dr Fagin (Memorial Sloan Kettering, New York, New York). KTC2 cells were kindly provided by Dr Kurebayashi (Kawasaki Medical School, Kurashiki, Japan). TT2609-CO2, FTC133, ML1, and WRO were kindly provided by Dr Schweppe (University of Colorado, Boulder, Colorado) with permission from each originator. K1 cells were kindly provided by Dr Wynford-Thomas (Cardiff University, Cardiff, United Kingdom). The TPC1 cells were kindly provided by Dr Jhiang (Ohio State University, Columbus, Ohio). The FRO cells were kindly provided by Dr Haugan (University of Colorado). The FF1 cells were kindly provided by Dr Frasca (Università di Catania, Catania, Italy). The ATC-derived THJ and LAM1 cells (22) were established in the laboratory of J.A.C. as described (23) along with PTC (LAM136) and FTC (SDAR1 and EAM306) cell lines. The remaining THJ cells were used as primary cultures representing normal thyrocytes and follicular adenomas. Cells were cultured in RPMI 1640 (Corning) containing 5% fetal bovine serum (Hyclone) and 1× penicillin-streptomycin (Invitrogen) at 37°C in humidified conditions with 5% CO2. All cell lines were short tandem repeat analysis validated as previously described (18).
DNA microarray
RNA was isolated from patient tissues using TRIzol reagent (Invitrogen) per the manufacturer's protocol. Sample purity and yield was assessed using a Nanodrop spectrophotometer (Thermo Scientific) prior to gene array expression analysis using Affymetrix Human Genome U133 Plus 2.0 Array chips, performed by the Mayo Clinic Advanced Genomic Technology Center Gene Expression Core. Expression data are deposited at the Gene Expression Omnibus Database (accession number GSE65144). The details of data processing and methodology are previously described (24). Genespring GX 7.3.1 (Agilent Technologies) was used to create heat maps using Affymetrix default analysis settings and standard Genespring normalizations. Ingenuity Systems software was used to model signaling pathways.
Compounds
A939572 was purchased from BioFine International. MF-438 [2-methyl-5-(6-[4-(2-(trifluoromethyl)phenoxy]piperidin-1-yl)pyridazin-3-yl-1,3,4-thiadiazole] was synthesized by the medicinal chemistry group at Sanford-Burnham according to the published procedure and was determined to be greater than 95% pure by liquid chromatography and mass spectrometry. Carfilzomib and bortezomib were purchased from Selleck Chemicals.
Growth assays
All drug stocks were prepared at 1000× in dimethyl sulfoxide (DMSO; Sigma) and added to media at a final concentration of 1× growth media before being added to cells. Cells were plated (1 × 105/well) in 24-well plates (Midwest Scientific) in triplicate. After 72 hours of treatment, cells were tryspinized (Corning), and cell count was established using a Coulter particle counter (Beckman). For drug combination studies, cells were seeded at 5000 cells/well in clear-bottom 96-well black plates in triplicate per group. After 48 hours, cells were washed one time with PBS (Corning), and analysis was performed using CyQuant proliferation analysis kit (Invitrogen) per the manufacturers' protocol. Where specified, oleic acid-albumin (Sigma-Aldrich) was added to media at 5 μM along with the drug.
RNA isolation and quantitative PCR (QPCR)
Total mRNA was isolated from tissue using Totally RNA (Ambion) per the manufacturer's protocol, followed by ethanol precipitation and Chromaspin column. Purity and yield were assessed using a Nanodrop (Thermo Scientific). The reverse transcription step was performed using the high-capacity reverse transcription kit as per the manufacturer's protocol (Applied Biosystems). Applied Biosystems' assays-on-demand assay mix of primers and TaqMan MGB probes (FAM dye labeled) were used for the following genes: GAPDH (Hs99999905_m1), SCD1 (Hs01682761_m1), HSPA5 (Hs99999174_m1), GADD45A (Hs00169255_m1), DDIT3 (Hs01090850_m1), and HERPUD1 (Hs01124269_m1) were used for QPCR. Data were normalized to GAPDH for each sample. Fold-change comparisons between normal vs tumor and DMSO vs drug-treated samples were calculated using the delta cycle threshold method (25).
Western blot analysis
Protein extraction and Western blot analysis were performed as previously described (26). Primary antibodies included the following: SCD1 (Sigma-Aldrich; number HPA012107), poly(ADP-ribose) polymerase (PARP; Cell Signaling; number 9542), C/EBP-Homologous Protein (CHOP; Cell Signaling; number 2895), Heat Shock 70kDa Protein 5 (BiP; GRP78; Abcam; number ab32618), spliced X-box binding protein (sXBP1; Santa Cruz Biotechnology; number sc-7160), and β-actin (Sigma-Aldrich; number A5441).
Immunohistochemistry (IHC) and immunofluorescence
Formalin-fixed, paraffin-embedded tissues were mounted on slides, blocked with Diluent (Dakocytomation) for 30 minutes, and probed for SCD (Sigma-Aldrich; number HPA012107), Ki67 (Dakocytomation; number M7240), caspase-3 (Cell Signaling; number 9662), CD31 (Santa Cruz Biotechnology; number sc-1506), homocysteine-inducible, endoplasmic reticulum stress inducible, ubiquitin-like-1 (HERPUD1; Lifespan; number ABIN466153), and survivin (R&D Systems; number AF886). H scores were calculated based on signal intensity (0–3+) using the following formula: [(1+% × 1) + (2+% × 2) + (3+% × 3)]. Cases with insufficient tumor tissue were excluded. The ×20 images were obtained using Scanscope XT and Imagescope software. Immunofluorescence was performed as previously described (26). The primary antibody was HERPUD1 (Lifespan; number ABIN466153). The negative sections were prepared by incubating slides in the absence of a primary antibody.
Lentiviral infection
MISSION shRNA pLKO.1 constructs (Sigma-Aldrich) were used to make self-inactivating shRNA lentiviruses for human SCD1 [clones: NM_005063.3–1200s1c1 (shSCD1200), NM_005063.3–780s1c1 (shSCD780)] and a nontarget (NT) random scrambled sequence control (SHC002) in 293FT viral progenitor cells (Invitrogen). Lipofectamine 2000 (Invitrogen) and ViraPower (Invitrogen) were used to transfect the 293FT cells. Seventy two hours after the transfection, lentiviral particles were collected and filtered through a 0.45-μm polyvinylidene fluoride syringe filter (Millipore) and stored at −80°C. Cells were incubated with lentivirus plus 5 μg/mL polybrene (American Bioanalytical) overnight. Puromycin (Fischer) was added 24 hours after the infection for 72 hours. All cell assays were plated 5 days after the infection.
Cell death flow cytometry
Cells were seeded at 1 × 106 in 60-mm, culture-treated dishes and allowed to adhere overnight. The drug was added to the culture media. After 48 hours, adhered and floating cells were collected using Accutase (Innovative Cell Technologies, Inc), washed two times with cold 1× PBS, and were suspended in 1× cold binding buffer [PBS (Corning; no Ca2+, Mg2+) + 1% BSA (Sigma) + 25 mM HEPES (Corning) + 1 mM EDTA (Promega); pH 7.2] at 1 × 106 cells/mL. Cells were stained with propidium iodide (BD Pharmingen) for 10 minutes. The analysis was performed using an Accuri C6 flow cytometer. Unstained DMSO-treated cells were used to set the population parameters. Lentivirus-targeted cells were seeded at 1 × 106 in 60-mm dishes 5 days after the infection. After an additional 72 hours, cells were collected and stained as described. Unstained NT cells were used to set population parameters.
In vivo analysis
1 × 106 THJ16T ATC cells were sc injected in female athymic nu/nu mice (Harlan Laboratories). Treatment was initiated once tumor volumes reached 50–100 mm3. MF-438 was suspended in 0.5% carboxymethyl-cellulose containing strawberry drink flavoring (0.1 g/mL) in sterilized H2O at 25 mg/kg in a 100-μL dose, and administered via oral gavage once daily. Carfilzomib was solubilized in DMSO (4% of final concentration), suspended in 100% corn oil at 4 mg/kg per 50-μL dose and administered via an ip injection twice weekly on consecutive days. Tumor volume [0.5236 (LWH)] and body weight were measured two time per week (n = 10 mice/group).
Statistical analysis
Data values are presented as fold change or percentage of control ± SD unless otherwise specified. Fold-change values greater than 1.5 are considered statistically significant. Treatment group comparisons were analyzed using a two-tailed paired Student's t test, with P < .05 considered statistically significant, indicated by an asterisk. Drug synergy as determined using CalcuSyn (27, 28) is described in text.
Results
SCD1 expression profile in thyroid cancer
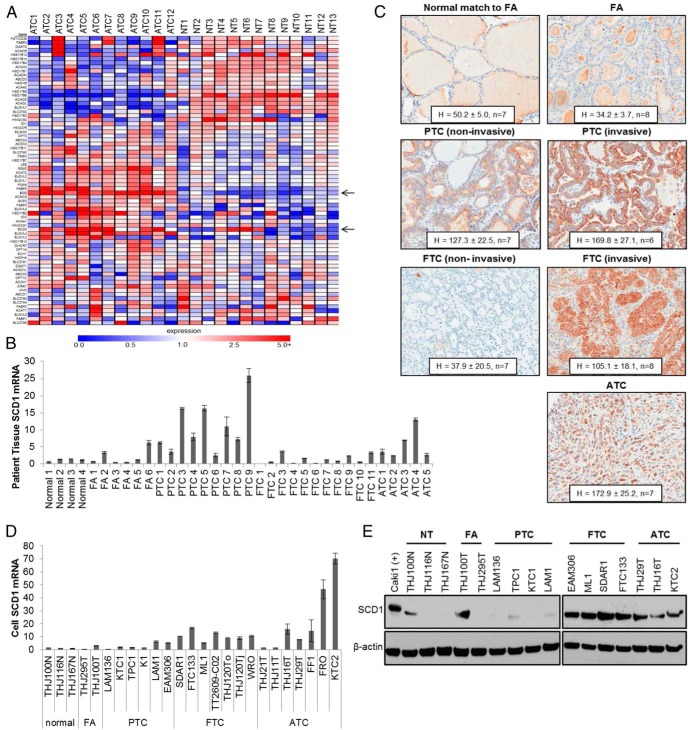
Gene array analysis was performed using 12 ATC tumor and 13 normal throid (NT) tissue specimens. Results revealed altered expression in fatty acid metabolism including fatty acid synthesis, cholesterol synthesis, mitochondrial and peroxisomal fatty acid oxidation, and cellular uptake of fatty acids. Findings are depicted in the heat map in Figure 1A, and mean fold change values compared with normal thyroid tissue is presented in Supplemental Table 1. SCD expression was consistently elevated in tumor samples, induced by 8.8 and 2.4-fold for SCD1 and SCD5, respectively (Figure 1A and Supplemental Table 1). To evaluate the expression profile of SCD1 in thyroid malignancy, QPCR as well as IHC of patient samples was performed. SCD1 was transcriptionally up-regulated in all ATC and PTC samples examined as well as several samples of follicular adenoma (FA) and FTC (Figure 1B). IHC analysis confirmed elevated SCD1 protein in ATC, noninvasive and invasive PTC, and invasive FTC lesions, in which low SCD1 expression was observed in noninvasive FTC lesions, FA, and NT tissue (Figure 1C). To evaluate whether cell line models recapitulate SCD1 expression patterns observed in patient tissue, representative patient-derived cells from normal, benign, well-differentiated, and ATC tissues were examined. SCD1 was found to be consistently overexpressed in ATC and FTC cells, both transcriptionally and at the protein level (Figure 1, D and E). Because most established FTC cell models are derived from advanced lesions, these results are consistent with those observed in tissue samples. Surprisingly, low levels of SCD1 were observed in PTC cell lines (Figure 1, D and E).
Figure 1.
SCD1 expression profile in thyroid malignancy. A, Heat map of fatty acid metabolism in ATC vs normal thyroid patient tissue samples is shown. Black arrows indicate SCD1 and SCD5 expression. B, Results of QPCR for SCD1 mRNA levels in normal, FA, PTC, FTC, and ATC patient tissue samples expressed as fold change compared with Normal 1 patient, whose level is set to 1. C, IHC expression of SCD1 in normal, FA, PTC, FTC, and ATC patient tissues were quantitated using the H-score method as described in Materials and Methods. PTC and FTC samples are further sorted into noninvasive and invasive groups. Mean H-score ± SD is given. D, QPCR for SCD1 mRNA levels in normal, FA, PTC, FTC, and ATC patient-derived cells. Levels are normalized to mean normal expression, which was set at 1. E, Western blot analysis of SCD1 protein expression in normal, FA, PTC, FTC, and ATC patient-derived cells. β-Actin is shown to demonstrate equal protein loading.
Effects of pharmacological and genetic SCD1 inhibition in thyroid cells
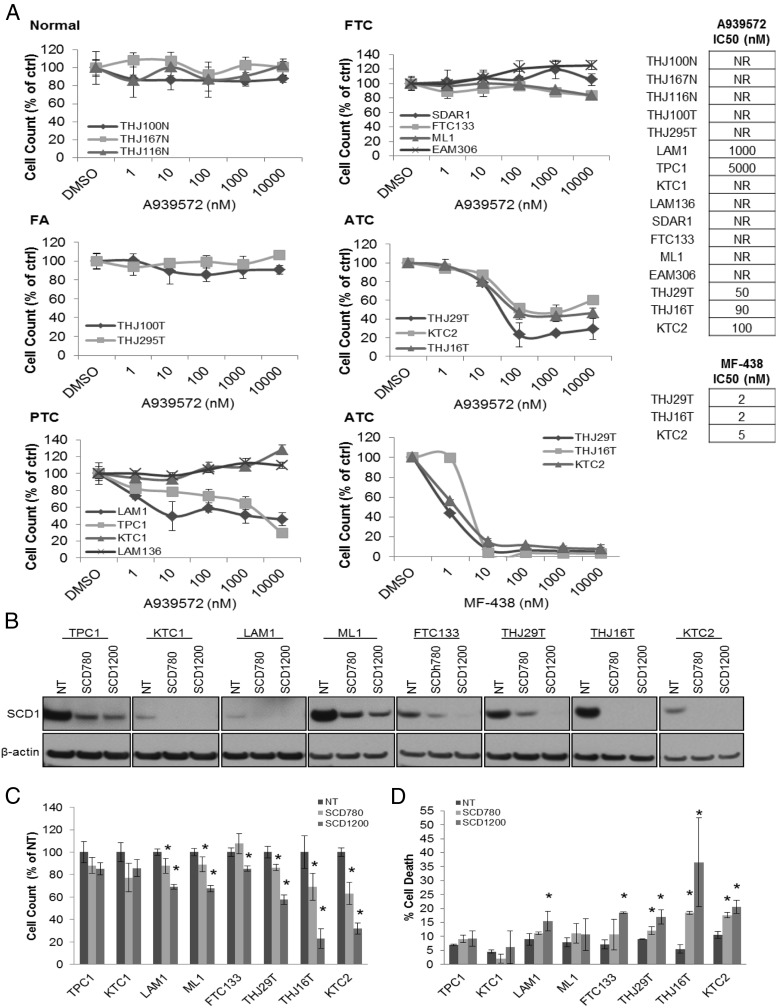
We next evaluated the efficacy of pharmacological inhibition of SCD on tumor cell proliferation after a 72-hour treatment using A939572, a commercially available small molecule SCD1 inhibitor (29). No response was observed in either NT or FA cells (Figure 2A). A minimal response was observed in two of four PTC cells at high doses (1000–5000 nM) of A939572 (Figure 2A). Despite conspicuous SCD1 expression in all four FTC cells tested, no response to A939572 was observed (Figure 2A). All ATC cells exhibited a dose-dependent decrease in proliferation (Figure 2A), with 50% inhibitory concentrations (IC50) in the low nanomolar dose range (50–100 nM). MF-438 (30), a potent and orally bioavailable SCD1 inhibitor that has been shown to be effective in rodent models for diabetes and obesity and is chemically distinct from A939572, similarly produced a dose-dependent decrease in ATC proliferation with IC50 values in the low nanomolar dose range (2–5 nM) (Figure 2A). To confirm a protumorigenic role of SCD1 in each tumor cell subtype, expression was knocked down using targeted lentiviruses in select cell lines representing PTC, FTC, and ATC. Excellent SCD1 protein knockdown was achieved in all cell lines tested using two separate lentiviral construct (SCD780, SCD1200) as compared with NT control infected cells (Figure 2B). Targeted knockdown of SCD1 resulted in an attenuated proliferation in one of the three PTC and both FTC cell lines tested. A more pronounced decrease was observed in each ATC cell line, particularly with the SCD1200 construct (Figure 2C). A significant increase in cell death as measured by flow cytometry of propidium iodide-stained cells was observed in all three ATC cells using both lentiviral constructs and in one PTC and FTC cell line using the SCD1200 lentivirus (Figure 2D).
Figure 2.
Effects of pharmacological and genetic SCD1 inhibition in thyroid cells. A, Proliferative dose response of thyroid cells to the SCD1 small molecule inhibitor A939572 in normal thyroid cells, FA cells, PTC cells, FTC cells, and ATC cells. Proliferative dose response of ATC cells to the small molecule SCD1 inhibitor MF-438. Results are presented as percent cell number relative to DMSO-treated control. B, Western blot for SCD1 protein expression in NT control and target SCD1 (SCD780, SCD1200) lentivirus shRNA-infected cells. β-Actin is shown to demonstrate equal protein loading. C, Proliferation of NT vs target SCD1 knockdown cells. Results are presented as percent cell number relative to NT control. D, Summary bar graph of cell death analysis of NT and target SCD1 knockdown cells. *, P < .05 as compared with NT control cells.
Evaluation of the endoplasmic reticulum (ER) stress response in SCD1 inhibitor-treated ATC cells
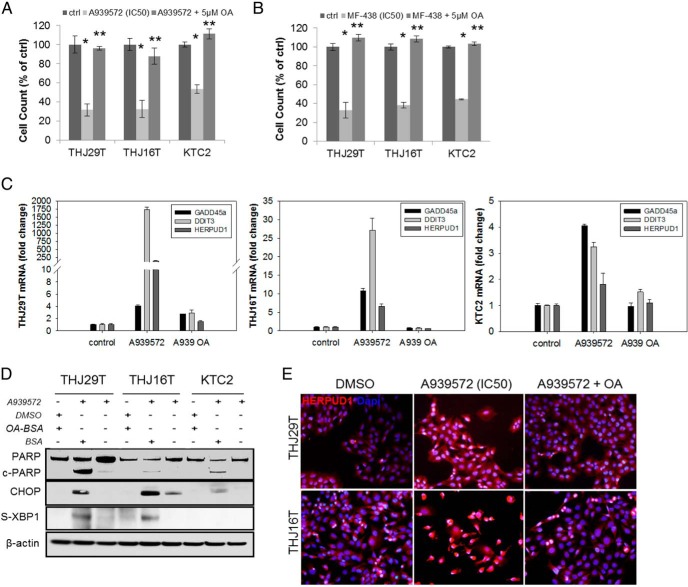
Previous studies have demonstrated that exogenous application of monounsaturated fatty acids, such as oleic or palmitoleic acid, can rescue the proliferative defects generated by SCD1 inhibition because these are the primary products of SCD1 enzymatic activity (20). THJ29T, THJ16T, and KTC2 cells were treated with the IC50 dose of A939572 or MF-438 for 72 hours with or without 5 μM of oleic acid (OA). Concomitant treatment of cells using either SCD1 inhibitor with OA resulted in a complete rescue of proliferation (Figure 3, A and B). In our previous work evaluating RCC cellular response to targeted inhibition of SCD1, we determined that the ER stress pathway, or UPR, is activated (18). We therefore examined SCD1 inhibitor treated ATC cells for UPR activation to evaluate whether these cells were responding similarly. THJ29T, THJ16T, and KTC2 cells were treated with a 10-μM dose of A939572 with or without 5 μM OA for 24 hours. The transcriptional up-regulation of UPR markers including growth arrest and DNA damage-inducible alpha (GADD45a), DNA damage-inducible transcript 3 (DDIT3, CHOP), and homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 (HERPUD1) were evaluated by QPCR. All three were transcriptionally up-regulated in response to A939572 in all cell lines, in which the simultaneous treatment with OA restored expression back to control levels (Figure 3C). Additionally, treatment of all three ATC cells with A939572 (IC50 dose) for 72 hours induced apoptosis as evaluated by PARP cleavage; this was abrogated with OA treatment (Figure 3D). Similarly, the protein expression of UPR factors, CHOP, s-XBP1 (Figure 3D), and HERPUD1 (Figure 3E), could be reversibly induced with SCD1 inhibitor treatment in ATC cells.
Figure 3.
SCD1 inhibitors induce ER stress in ATC cells. ATC cell proliferative rescue assay using exogenous OA in A939572- (A) and MF-438 (B)-treated cells. Control cells were treated with DMSO plus BSA. Results are presented as percent cell number relative to control. *, P < .05, monotherapy as compared with control; **, P < .05, monotherapy + OA as compared with monotherapy alone. C, QPCR for transcriptional up-regulation of UPR genes in ATC cells treated with A939572 ± 5 μM OA (A939 OA). Fold change was compared with DMSO control. D, Western blot for protein expression of PARP as well as the UPR genes CHOP and spliced XBP1 (sXBP1) in ATC cells treated with the IC50 values of A939572 ± 5 μM OA. β-Actin is shown to demonstrate equal protein loading. E, Immunofluorescence for expression of the ERAD marker HERPUD1 in ATC cells treated with the IC50 values of A939572 ± 5 μM OA. HERPUD1 demonstrates cytoplasmic localization. 4′,6′-Diamino-2-phenylindole was used as a nuclear marker.
Drug synergy: assessing the combination of SCD1 and proteasome inhibitors in ATC
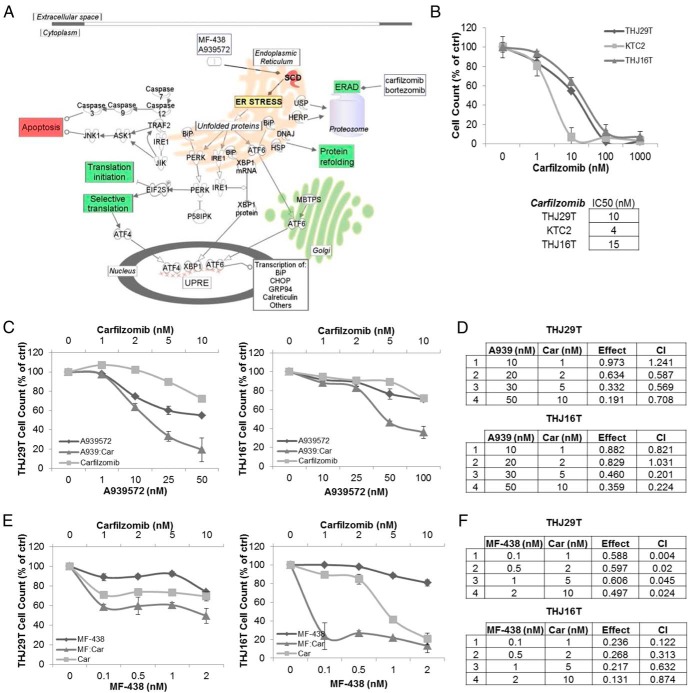
The UPR facilitates the attenuation of protein translation, molecular chaperone-mediated protein refolding, and endoplasmic reticulum associated degradation (ERAD) in response to a variety of stressors in an effort to restore cellular homeostasis. In extreme or prolonged cases of stress, it can also initiate cellular senescence or death (31–33). We hypothesized that the targeted inhibition of the ERAD component of the UPR using a proteosome inhibitor such as bortezomib or carfilzomib in conjunction with SCD1 inhibition would enhance cellular stress and drive tumor cells toward cell death in a synergistic manner (Figure 4A).
Figure 4.
SCD1 inhibitor induced ER stress and the UPR response in ATC cells. A, Proposed mechanism of combination therapy: A939572- or MF-438-mediated activation of the UPR through SCD1 inhibition prompts selective translation of ER stress response genes that drive prosurvival responses including decreased translation, ERAD, and chaperone-mediated protein refolding (highlighted in the green boxes). Targeted inhibition of ERAD using proteasome inhibitors such as bortezomib or carfilzomib is thought to further induce cellular stress, resulting in tumor cell apoptosis (red box). B, Monotherapeutic dose response of carfilzomib in ATC cells. IC50 values are listed. Combinatorial dose response of carfilzomib with A939572 (C) or MF-438 (E) in THJ29T and THJ16T cells are shown. D and F, Combination index values evaluating drug synergy generated using CalcuSyn software as described in the text in THJ29T and THJ16T cells. Car, carfilzomib; CI, combination index.
THJ29T, THJ16T, and KTC2 cells were treated with carfilzomib (Figure 4B) or bortezomib (Supplemental Figure 1A) for 72 hours to establish IC50 values. All three cell lines responded in a dose-dependent manner to either inhibitor, yielding IC50s in the low nanomolar dose range. Antiproliferative drug synergy was evaluated in THJ29T and THJ16T treated with either A939572 or MF-438 in combination with either bortezomib or carfilzomib for 48 hours. Synergy was assessed using CalcuSyn analytical software, which determines the combination index (CI) based on the Chou-Talalay method in which the CI values greater than 1 represent an antagonistic effect, values equaling 1 represent an additive effect, and values less than 1 represent synergy (in which the lower values signify enhanced synergy) (28). Strong synergy was observed in both cell lines using A939572 together with either carfilzomib (Figure 4, C and D) or bortezomib (Supplemental Figure 1, B and C) and similarly using MF-438 together with either carfilzomib (Figure 4, E and F) or bortezomib (Supplemental Figure 1, D and E).
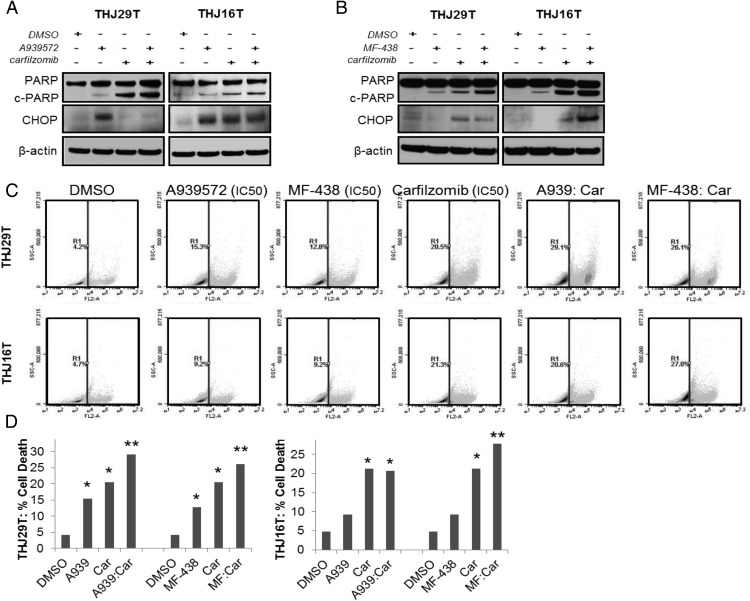
We next determined whether antiproliferative synergy was associated with increased cell death. THJ29T and THJ16T cells treated for 48 hours with IC50 doses of either A939572 or MF-438 in combination with either carfilzomib or bortezomib were probed for PARP cleavage and CHOP expression, both markers indicative of apoptosis (Figure 5, A and B, and Supplemental Figure 2, A and B). In THJ29T and THJ16T cells treated with either A939572 or MF-438 and carfilzomib, significant PARP cleavage was observed in both monotherapy and combinatorial groups as compared with DMSO control (Figure 5, A and B); however, a clear increase in PARP cleavage was observed only in the MF-438- and carfilzomib combinatorial-treated groups as compared with monotherapy (Figure 5B). Additionally, CHOP expression was significantly increased in THJ16T MF-438/carfilzomib (Figure 5B) as well as the A939572/bortezomib combinatorial group (Supplemental Figure 2B); however, no clear increase was observed in the other treatment combinations or in THJ29T as compared with monotherapy. Cell death was additionally examined via flow cytometry using propidium iodide staining. THJ29T and THJ16T cells were treated for 48 hours with IC50 doses of either A939572 or MF-438 in combination with either carfilzomib or bortezomib prior to staining and analysis. Increased cell death in the combination group vs the control and either monotherapy was observed in both cell lines treated with MF-438 and carfilzomib and in THJ29T treated with A939572 and carfilzomib (Figure 5D). No additive increases in cell death were observed with either SCD1 inhibitor with bortezomib (Supplemental Figure 2C).
Figure 5.
Evaluation of tumor cell viability in response to combinatorial therapy. Western blot for PARP cleavage and CHOP expression in THJ29T and THJ16T ATC cells treated with A939572 (A) or MF-438 (B) in combination with carfilzomib. β-Actin is shown to demonstrate equal protein loading. C, Cell death analysis using flow cytometry sorting of propidium iodide-stained cells treated with the IC50 dose of indicated drugs after a 48-hour treatment. Car, carfilzomib. Graphical results are shown in panel D. *, 5% or greater increase in cell death as compared with DMSO control; **, 5% or greater increase in cell death as compared with monotherapy.
In vivo evaluation of combinatorial therapy
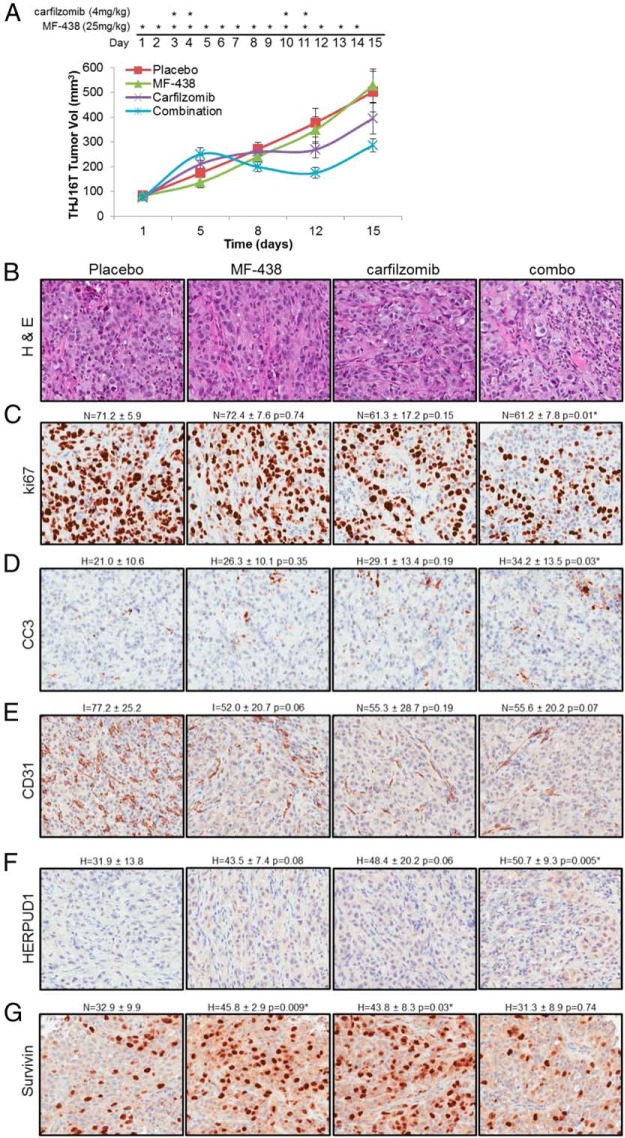
Based on these results, MF-438 was tested in combination with carfilzomib in vivo in a THJ16T xenograft model. This cell line harbors mutations in TP53, RB, and PI3KCA, and also demonstrates elevated expression of survivin, a negative regulator of apoptosis that inhibits caspase activation (23). A depiction of the treatment map is shown in Figure 6A. Dehydration and acute inflammation characterized by redness and mild swelling at the tumor site was observed at days 4–5 and 11–12 in both the carfilzomib and combination groups. Saline was administered as necessary. These adverse events resolved 24–48 hours after the second administration of carfilzomib. Discharge from the eye and squinting was seen in groups treated with MF-438 by day 12 of the protocol, as has been previously observed for SCD1 inhibitors (14); however, no other significant adverse events were observed. A regression in mean tumor volume was observed in the combination group beginning day 6 and continued until day 12 after which tumor volume began to increase. Tumor growth temporarily plateaued in the carfilzomib group by day 8, after which tumor growth continued (Figure 6A). A 21% (P = .32) and 43% (P = .03) reduction in mean tumor burden as compared with the placebo control was observed in the carfilzomib and combination groups, respectively (Figure 6A). At the dose tested, no tumor response with MF-438 monotherapy was observed. IHC analysis of tumors resected from each treatment group was performed. Hematoxylin and eosin stains are provided in Figure 6B. Results revealed a trend of decreased ki67 staining in both carfilzomib and combination groups (Figure 6C) and increased cleaved caspase-3 in all treatment groups as compared with placebo control (Figure 6D); however, only the combination group achieved statistical significance. A trend toward decreased CD31, indicative of decreased tumor vascularization, was observed in both monotherapeutic and combination groups (Figure 6E). Increased HERPUD1, a marker for ER stress, was observed in all treatment groups as compared with placebo control (Figure 6F), with statistically significant combinatorial values. Both MF-438 and carfilzomib induced survivin expression as single agents; however, combinatorial therapy did not result in any significant changes in expression (Figure 6G). These data support that combinatorial therapy induces ER stress in vivo, leading to decreased tumor cell proliferation and increased tumor cell death.
Figure 6.
Results of combinatorial therapy in an in vivo model of ATC. A, Mean THJ16T cell xenograft tumor volume of mice treated with MF-438 and carfilzomib independently or in combination. A treatment map depicting drug administration is included. B–G, Hematoxylin and eosin (H&E) staining as well as ki67 (proliferative index), cleaved caspase-3 (CC3; apoptosis marker), CD31 (endothelial cell marker), HERPUD1 (ER stress marker), and survivin (inhibitor of apoptosis, inhibitor of apoptosis family) was performed. Asterisks indicate statistically significant changes in IHC scores as compared with placebo control. P ≤ .05.
Discussion
ATC is thought to arise from preexisting thyroid malignancies through the acquisition of additional genetic deviations. Various genetic arrays have revealed a plethora of abnormalities in these lesions including chromosomal deletions, amplifications, and fusions; activating/inactivating point mutations in known oncogenes and tumor suppressor genes; and alterations in epigenetic regulators. Adaptations in molecular factors thought to contribute to ATC progression are numerous (1, 34, 35). Gene array analysis of ATC patient samples illuminated significant alterations in fatty acid metabolism in these tumors (Figure 1A and Supplemental Table 1), presenting an innovative therapeutic opportunity for this aggressive malignancy. Of the genes evaluated, SCD1 was consistently elevated in all tumor samples as compared with normal levels (Figure 1 and Supplemental Table 1).
Although other thyroid malignancies also demonstrate SCD1 up-regulation both transcriptionally and at the protein level (Figure 1), interestingly ATC cells are more dependent on SCD1 activity for tumor cell proliferation and viability (Figure 2). These findings highlight a tumor-specific role for SCD1 because normal and benign thyroid cells not only exhibit much lower SCD1 expression as compared with tumor, but they also remained unaffected by pharmacological inhibitors of the enzyme. Lentiviral mediated knockdown of SCD1 demonstrated some antitumor activity in PTC- and FTC-derived cell lines; however, ATC cells remained more sensitive to loss of this protein (Figure 2). Of note, patient-derived PTC cell lines did not recapitulate the high level of SCD1 overexpression observed in tumor tissue samples. One possible explanation may be that elevated SCD1 occurs within a specific population of tumor cells in PTC, and these were not immortalized in any of the monoclonal cell lines used in this study. Additionally, this may explain the limited responsiveness to SCD1 knockdown and inhibition observed in vitro in this particular subtype. Taken together, these results may suggest a more potent role for SCD1 in poorly differentiated lesions or alternatively that well-differentiated lesions demonstrate drug resistance against inhibition of SCD1. Further investigation into the specific mechanism of this discrepancy is needed.
Inhibition of SCD1 induces ER stress, triggering the activation of the UPR (Figures 3 and 4) (18, 20). The UPR pathway is activated by a variety of cellular stressors, which leads to an accumulation of misfolded proteins within the ER. There are three transmembrane ER resident master regulators of the UPR: eukaryotic translation initiation factor 2α kinase 3 (PERK; EIF2AK3), endoplasmic reticulum to nucleus signaling 1 (ERN1, IRE1), and activating transcription factor 6 (ATF6) (31, 33, 36). PERK activation leads to repression of protein translation. ERN1 (IRE1) activation triggers endoribonuclease activity that splices X-box binding protein, a molecular chaperone and transcriptional activator of other ER stress response proteins that facilitate growth arrest or apoptosis (33). ATF6 is a transcription factor that is proteolytically processed at the Golgi into its active state and targets promoter regions containing UPR elements (ER stress elements) in the nucleus (37). Altogether the UPR facilitates the attenuation of protein translation, protein refolding, and ERAD. The primary goal of this pathway is to restore cellular homeostasis; however, in extreme or prolonged cases of stress, it can also initiate cellular senescence or death (31–33).
Because ATC is a very aggressive, drug-resistant cancer, we sought to enhance cellular stress caused by SCD1 inhibition through the combined application of a proteasome inhibitor (Figure 4) to promote cell death. Proteosome inhibitors have also been investigated in the context of ATC as antitumor agents (38, 39). Our results demonstrate that both drugs when used in combination significantly decrease tumor growth and induce tumor cell death in vitro and in vivo (Figures 4–6 and Supplemental Figures 1 and 2). Surprisingly, SCD1 inhibition in vivo using MF-438 fails to demonstrate tumor response as a monotherapy yet effectively leads to decreased tumor growth when combined with carfilzomib (Figure 6). Elevated HERPUD1 expression was observed in all treatment groups, including MF-438, indicative of therapeutic activation of ER stress. Dose escalation of SCD1 inhibitors or modified methods of administration should be investigated in an effort to characterize the efficacy of SCD1 inhibitors as single agent therapeutics.
Notably, survivin expression was induced by each monotherapy yet remained suppressed by combination treatment (Figure 6). ER stress is known to promote proinflammatory regulators such as nuclear factor-κB of which survivin is a downstream target (40, 41). Survivin expression also has been shown to be regulated in a cell-cycle-dependent manner through the ubiquitin-proteosome pathway (42), and therefore, its expression may be stabilized through treatment with proteasome inhibitors. One may speculate that the decreased levels of survivin observed in the combination therapy may be due to the attenuated transcription or translation as a result of compounding ER stress; however, this requires additional examination.
In summary, our findings support that ATC cells are dependent on de novo lipogenesis for cell viability. Of the genes dysregulated in this metabolic pathway, SCD1 expression is consistently elevated in a tumor-specific manner. SCD1 may serve as a biomarker for disease aggressiveness in FTC, given its unique expression pattern in low-grade, localized tumors vs high-grade, invasive lesions (Figure 1). We further propose SCD1 as a novel therapeutic target in ATC, whose inhibition may provide clinical benefit to these patients. Additional investigations into other constituents of lipid metabolism that demonstrate altered expression patterns as compared with normal tissue may provide fresh insight into thyroid carcinogenesis as well as reveal other attractive therapeutic targets.
Acknowledgments
This work was supported in part by National Institutes of Health; National Cancer Institute Grant R01CA136665 (to J.A.C. and R.C.S.); Florida Department of Health Bankhead-Coley Cancer Research Program [Grant FL09B202 (to J.A.C. and R.C.S.) Grant FL3BF01 (to J.A.C.)]; Mr and Mrs Ompal Chauhan Research Fund (to J.A.C.); Scheidel Foundation (to J.A.C.); Fraternal Order of Eagles, Florida State Auxiliary (to J.A.C.); a grant for rare cancers from Dr Ellis and Dona Brunton (to J.A.C.); Mayo-Sanford-Burnham Medical Research Institute Collaborative Drug Discovery Program; a generous gift from Alfred D. and Audrey M. Petersen (to R.C.S.); the Francis and Miranda Childress Foundation Fund for Cancer Research (to J.A.C.); John A. and Bette B. Klacsmann Fund for Cancer Research at Mayo Clinic in Florida (to J.A.C.); and the Betty G. Castigliano Fund in Cancer Research Honoring S. Gordon Castigliano, MD cancer research at Mayo Clinic Florida (to J.A.C.).
Disclosure Summary: The authors have nothing disclose.
Footnotes
- ATC
- anaplastic thyroid carcinoma
- ATF6
- activating transcription factor 6
- CHOP
- CCAAT/enhancer-binding protein homologous protein
- DMSO
- dimethylsulfoxide
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- FA
- follicular adenoma
- FTC
- follicular thyroid carcinoma
- HERPUD1
- homocysteine-inducible, endoplasmic reticulum stress inducible, ubiquitin-like-1
- IHC
- immunohistochemistry
- NT
- nontarget
- OA
- oleic acid
- PARP
- poly(ADP-ribose) polymerase
- PERK
- eukaryotic translation initiation factor 2α kinase 3
- PTC
- papillary thyroid carcinoma
- QPCR
- quantitative PCR
- SCD
- stearoyl-CoA desaturase
- shRNA
- short hairpin RNA
- sXBP1
- spliced X-box binding protein.
References
- 1. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010;22(6):486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perri F, Lorenzo GD, Scarpati GD, Buonerba C. Anaplastic thyroid carcinoma: a comprehensive review of current and future therapeutic options. World J Clin Oncol. 2011;2(3):150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deshpande HA, Roman S, Sosa JA. New targeted therapies and other advances in the management of anaplastic thyroid cancer. Curr Opin Oncol. 2013;25(1):44–49. [DOI] [PubMed] [Google Scholar]
- 4. Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. [DOI] [PubMed] [Google Scholar]
- 5. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–1335. [DOI] [PubMed] [Google Scholar]
- 6. Smallridge RC, Copland JA, Brose MS, et al. Efatutazone, an oral PPAR-γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab. 2013;98(6):2392–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13(4):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279(15):2610–2623. [DOI] [PubMed] [Google Scholar]
- 9. Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6(6):1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abramson HN. The lipogenesis pathway as a cancer target. J Med Chem. 2011;54(16):5615–5638. [DOI] [PubMed] [Google Scholar]
- 11. Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13(1):27–29. [PubMed] [Google Scholar]
- 12. Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31(9):1509–1515. [DOI] [PubMed] [Google Scholar]
- 13. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–777. [DOI] [PubMed] [Google Scholar]
- 14. Kim YC, Ntambi JM. Regulation of stearoyl-CoA desaturase genes: role in cellular metabolism and preadipocyte differentiation. Biochem Biophys Res Commun. 1999;266(1):1–4. [DOI] [PubMed] [Google Scholar]
- 15. Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40(9):1549–1558. [PubMed] [Google Scholar]
- 16. Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49(2):186–199. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Yu L, Schmidt RE, et al. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332(3):735–742. [DOI] [PubMed] [Google Scholar]
- 18. von Roemeling CA, Marlow LA, Wei JJ, et al. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. 2013;19(9):2368–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yahagi N, Shimano H, Hasegawa K, et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41(9):1316–1322. [DOI] [PubMed] [Google Scholar]
- 20. Roongta UV, Pabalan JG, Wang X, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. 2011;9(11):1551–1561. [DOI] [PubMed] [Google Scholar]
- 21. Noto A, Raffa S, De Vitis C, et al. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. 2013;4:e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Copland JA, Marlow LA, Williams SF, et al. Molecular diagnosis of a BRAF papillary thyroid carcinoma with multiple chromosome abnormalities and rare adrenal and hypothalamic metastases. Thyroid. 2006;16(12):1293–1302. [DOI] [PubMed] [Google Scholar]
- 23. Marlow LA, D'Innocenzi J, Zhang Y, et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J Clin Endocrinol Metab. 2010;95(12):5338–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tun HW, Marlow LA, von Roemeling CA, et al. Pathway signature and cellular differentiation in clear cell renal cell carcinoma. PLoS One. 2010;5(5):e10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 26. Copland JA, Marlow LA, Kurakata S, et al. Novel high-affinity PPARγ agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25(16):2304–2317. [DOI] [PubMed] [Google Scholar]
- 27. Chou TC, Hayball MP. CalcuSyn for Windows: Multiple-Drug Dose Effect Analyzer and Manual. Cambridge, United Kingdom: Biosoft; 1997. [Google Scholar]
- 28. Chou TC, Talalay P. Analysis of combined drug effects—a new look at a very old problem. Trends Pharmacol Sci. 1983;4(11):450–454. [Google Scholar]
- 29. Xin Z, Zhao H, Serby MD, et al. Discovery of piperidine-aryl urea-based stearoyl-CoA desaturase 1 inhibitors. Bioorg Med Chem Lett. 2008;18(15):4298–4302. [DOI] [PubMed] [Google Scholar]
- 30. Leger S, Black WC, Deschenes D, et al. Synthesis and biological activity of a potent and orally bioavailable SCD inhibitor (MF-438). Bioorg Med Chem Lett. 2010;20(2):499–502. [DOI] [PubMed] [Google Scholar]
- 31. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115(10):2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7(12):1013–1030. [DOI] [PubMed] [Google Scholar]
- 33. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. [DOI] [PubMed] [Google Scholar]
- 34. Rusinek D, Szpak-Ulczok S, Jarzab B. Gene expression profile of human thyroid cancer in relation to its mutational status. J Mol Endocrinol. 2011;47(3):R91–R103. [DOI] [PubMed] [Google Scholar]
- 35. Guerra A, Di Crescenzo V, Garzi A, et al. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(suppl 2):S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–719. [DOI] [PubMed] [Google Scholar]
- 37. Shen J, Prywes R. ER stress signaling by regulated proteolysis of ATF6. Methods. 2005;35(4):382–389. [DOI] [PubMed] [Google Scholar]
- 38. Altmann A, Markert A, Askoxylakis V, et al. Antitumor effects of proteasome inhibition in anaplastic thyroid carcinoma. J Nucl Med. 2012;53(11):1764–1771. [DOI] [PubMed] [Google Scholar]
- 39. Mitsiades CS, McMillin D, Kotoula V, et al. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab. 2006;91(10):4013–4021. [DOI] [PubMed] [Google Scholar]
- 40. Tracey L, Perez-Rosado A, Artiga MJ, et al. Expression of the NF-κB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206(2):123–134. [DOI] [PubMed] [Google Scholar]
- 41. Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7(10):e45078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113 Pt 23:4363–4371. [DOI] [PubMed] [Google Scholar]