Abstract
Context:
Low levels of serum 25 Hydroxyvitamin D [25(OH)D] have been linked to greater fracture risk in older women.
Objective:
This study aimed to determine whether higher 25(OH)D is associated with slower loss of bone mineral density (BMD) and lower fracture risk during the menopausal transition.
Design, Setting, and Participants:
This was a prospective cohort study at five clinical centers in the United States. Mean age was 48.5 ± 2.7 years. The fracture analysis included 124 women with an incident traumatic fracture, 88 with incident nontraumatic fracture, and 1532 women without incident fractures; average followup was 9.5 years. BMD analysis included 922 women with a documented final menstrual period.
Main Outcome Measures:
Serum 25(OH)D was measured by liquid chromatography tandem mass spectrometry at the third annual clinic visit. BMD was measured and incident fractures ascertained at each annual visit.
Results:
The mean 25(OH)D was 21.8 ng/mL; seven-hundred two (43%) of the women had 25(OH)D values <20 ng/mL. There was no significant association between 25(OH)D and traumatic fractures. In multivariate adjusted hazards models, the hazard ratio (HR) for nontraumatic fractures (95% confidence interval [CI]) was 0.72 (0.54–0.96) for each 10-ng/mL increase in 25(OH)D. Comparing women whose 25(OH)D was ≥20 vs <20 ng/mL, the HR (95% CI) for fracture was 0.54 (0.32–0.89). Changes in lumbar spine and femoral neck bone mineral density across menopause were not significantly associated with serum 25(OH)D level.
Conclusion:
Serum 25(OH)D levels are inversely associated with nontraumatic fracture in mid-life women. Vitamin D supplementation is warranted in midlife women with 25(OH)D levels <20 ng/mL.
The menopausal transition (MT) is a time of dynamic changes in a variety of physiologic and psychosocial parameters. Accelerated bone loss is one of the classic changes associated with the MT. An early study by Heaney et al (1) showed in 1978 that perimenopausal women and estrogen-treated women reached neutral calcium balance with calcium intakes of 990 mg/d, whereas untreated postmenopausal women required more daily calcium (1504 mg/d) to achieve neutral calcium balance. Vitamin D plays an important role in calcium absorption and this early report suggested that menopausal status may influence calcium balance (1).
Low 25(OH)D levels have been linked to fracture in women in some studies (2–7) but not all (8–11). For the most part, an increased fracture risk was observed with hip fractures and results were less consistent for nonhip fractures. Finally, all of the previous reports in women have focused on postmenopausal women, most age 65 years or older. There is little known regarding 25(OH)D and fracture in women at midlife, who are transitioning through menopause. If an association exists, then identification of midlife women with low 25(OH)D and providing supplementation may reduce their fracture risk.
The 2010 Institute of Medicine (IOM) report concluded for individuals aged 19–50 and 51–70 years, that there is “fair” evidence to support an association between 25(OH)D and bone mineral density (BMD) or changes in BMD (12). However, none of the evidence characterized women by their menopausal status. It is currently unknown whether 25(OH)D modifies the rates of transmenopausal bone loss, whether there is a specific threshold of 25(OH)D below which rates of bone loss accelerate and fractures increase and whether known racial differences in 25(OH)D levels contributes to racial/ethnic variation in rates of bone loss across menopause.
We have shown in the Study of Women's Health Across the Nation (SWAN) that the cumulative amount of bone loss was greatest from 1 year before through 2 years after the final menstrual period (FMP), termed the transmenopause (13). Changes in estradiol and follicle stimulating hormone (FSH) paralleled these BMD changes in the lumbar spine (14). It is possible that other biomarkers, eg, serum 25(OH)D also contribute to these transmenopausal changes in BMD.
We used data from the SWAN study to test the hypothesis that circulating 25(OH)D level measured in women who were for the most part either premenopausal or perimenopausal predicted fractures and BMD changes over the MT.
Study sample
SWAN is a multisite, community-based, longitudinal cohort study of the MT (15). Eligibility criteria were: age between 42 and 52 years, intact uterus and at least one intact ovary, not using hormone therapy (HT) at SWAN baseline, at least one menstrual period in the 3 months before screening, and self identification as a member of one of five eligible race/ethnic groups. Participants were enrolled at seven sites in the United States: Boston, MA; Chicago, IL; Detroit, MI; Pittsburgh, PA; Los Angeles, CA; Newark, NJ; and Oakland, CA (N = 3302). All sites enrolled White participants; Boston, Chicago, Detroit, and Pittsburgh enrolled Black participants, and the remaining three sites enrolled Japanese, Hispanic, and Chinese women, respectively at baseline (visit 0). The Chicago and Newark sites did not measure BMD, leaving a potential of 2413 participants for the SWAN BMD cohort. Of these, 2067 were enrolled in the bone cohort at visit 2. The current analysis includes data from visit 2 (1998–2000) to follow-up visit 12 (2009–2011).
Blood collection
If women were cycling regularly, fasting blood samples were collected before 1000 h on days 2–5 of the menstrual cycle whenever possible. If 60 days passed without being able to collect the samples in this window, fasting blood samples were collected before 1000 h any time over the next 30 days. We noted whether the sample was collected in the window or not.
Serum 25 hydroxyvitamin D
25(OH)D was measured by liquid chromatography tandem mass spectrometry using visit 2 fasting serum in women enrolled in the BMD cohort (16). The limit of detection for D2 was 2 ng/mL and D3, 3 ng/mL. D2 and D3 were summed and total 25(OH)D was reported. The interassay coefficient of variation for a quality control serum containing a total vitamin D concentration of 23 ng/mL was 7.5%.
Covariates
Time-varying covariates were updated at each annual visit. Age (y), education, self-defined race/ethnicity (Black, white, Chinese, Japanese), menstrual bleeding patterns, menopausal HT use (yes/no, time varying), use of any medication that affects bone density (yes/no, time varying) were obtained using annual, standardized interviews. Menopause transition stages (time varying, based on reported annual bleeding patterns) were defined as: premenopausal (regular menses, no change from individual's pattern), early perimenopausal (menses within the last 3 months but less predictable than individual's pattern), late perimenopausal (≥3 but <12 consecutive mo amenorrhea), and postmenopausal ≥12 mo without menses). Weight (kg, time varying) and height (m) were assessed annually using calibrated scales and stadiometers. Body mass index (BMI; kg/m2 time varying) was calculated annually.
Questionnaires administered at visit 2 included information on smoking, self-reported health status, fracture history, prevalent conditions (diabetes, arthritis,osteoporosis) and the 36-item short form (SF-36) physical functioning score (17). Fracture history includes all fractures prior to SWAN and up to and including fracture history at SWAN visit 2. Physical activity was measured using the modified Baecke instrument (18). A food frequency questionnaire was administered at baseline (2 y before 25(OH)D was measured). Information on supplemental calcium and vitamin D (yes/no) was obtained at visit 2, concurrent with 25(OH)D measures.
Bone mineral density
Lumbar spine (LS) and femoral neck (FN) BMD (g/cm2) were measured annually using Hologic instruments (Hologic, Inc., Waltham, MA). Three sites used Hologic 4500A models throughout. Two sites upgraded from 2000 to 4500A models at follow-up visit 8 (2004–2006). These sites scanned 40 women on both their old and new machines to develop cross-calibration regression equations. A standard quality control program conducted in collaboration with Synarc, Inc. (Newark, CA) included daily phantom measurements, local site review of all scans, central review of scans that met problem-flagging criteria, and central review of a 5% random sample of scans. Short-term in vivo measurement variability was 0.014 g/cm2 (1.4%) for the LS and 0.016 g/cm2 (2.2%) for the FN. The BMD trajectories were divided into three linear segments in relations to the FMP (time 0): years −5 to −1, relative to the FMP, termed pretransmenopause; years −1 to +2 relative to the FMP, termed transmenopause and years +2 to +8 after the FMP termed postmenopause.
Fractures
Incident fractures that occurred after visit 2 (1998–2000) were ascertained at each annual visit by self report. Digit and facial fractures were excluded. The accuracy of self report of fractures was verified from visits 7 (2003–2005) to 12 (2009–2011) by review of radiology reports. The false-positive rate was 4.6%. If a fracture occurred, the participants were asked about the circumstances of the fracture. A traumatic fracture was defined as a fracture that resulted from a fall greater than a standing height, motor vehicle accident, occurred while playing sports, or occurred because something fell on them. Nontraumatic fractures were those that resulted from a standing height or less. The average followup for this analysis was 9.5 ± 2.62 years (range, 1–11 y).
Statistical analyses
The flow chart for the analytic sample is shown in Supplemental Figure 1. Of the 1756 women with 25(OH)D and BMD data at visit 2, 124 experienced a traumatic fracture and 88, a nontraumatic fracture. The analytic sample for BMD changes over the MT included 922 with FMP documentation. FMP was defined retrospectively using bleeding diaries.
We used χ2 tests and ANOVA to compare characteristics of women at visit 2 who did not fracture and those that experienced an incident traumatic or nontraumatic fracture. For continuous variables that were skewed, we used Wilcoxon Rank Sum tests. To assess confounding, we compared the characteristics of women by race/ethnicity. We assessed the association between serum 25(OH)D and fracture using discrete time survival analyses method. We calculated the hazard ratio (HR) (95% confidence interval) per 10 ng/mL increase in 25(OH)D and in women with 25(OH)D at least 20 ng/mL vs less than 20 ng/mL. The cutoff of 20 ng/mL was chosen because of the IOM recommendation (12). The base model was adjusted for age, site, and race/ethnicity. Multivariate models included variables in the base model as well as prior fracture history, SF-36 physical functioning score, current use of HT, BMI, physical activity, education, lumbar spine BMD, calcium and vitamin D supplements, corticosteroids, diabetes, and dietary calcium. We used Kaplan-Meier cumulative hazard curves with log-rank tests to assess the cumulative hazard of fracture using the IOM cutoff. To test for a threshold of 25(OH)D and fracture, we used cubic splines with automatic placement of knots at 5, 25, 50, and 75th percentile of 25(OH)D. We compared the average annual percent decline in BMD in the premenopause, transmenopausal, and postmenopause comparing women with 25(OH)D less than 20ng/mL vs at least 20 ng/mL using mixed models. All BMD models were adjusted for age, site, and BMI. Black women had lower 25(OH)D and slower BMD loss over the MT (13). Thus, because of confounding of 25(OH)D, race and annualized % change in BMD, we ran separate models for Black and Non-Black women.
Results
The mean 25(OH)D was 21.8 ng/mL but differed markedly by race/ethnicity with white participants having the highest 25(OH)D levels; Black women, the lowest; and Chinese and Japanese women having intermediate levels (Table 1). Overall, 752 (43%) had 25(OH)D less than 20.0 ng/mL and 380 (22%) had a value at least 30 ng/mL (Table 1). However, 80% of Black women had a 25(OH)D less than 20 ng/mL. Women were on average 48 years old at the time 25(OH)D was measured. The average BMI was 27.6 kg/m2 but ranged from 23.5 kg/m2 in the Asian women to 31.7 kg/m2 in the Black women. BMD was greatest in the Black women and approximately 1 SD higher than BMD in the Chinese and Japanese women. Most women had a high school education or greater. Overall, approximately 12% of women were current smokers and the rate of smoking was highest in the Black women (21%). The prevalence of preexisting conditions was low, although 12% of Black women had diabetes compared with an overall cohort prevalence of 5%. Physical activity scores were highest in Caucasian and Japanese women and lowest in Chinese and Black women. Few women reported poor/fair health status with a higher prevalence among Chinese, Black, and Japanese women compared with white women.
Table 1.
Characteristics of SWAN Women With 25(OH)D Measurements
| Variable | Blacks | Whites | Chinese | Japanese | Total | P Value |
|---|---|---|---|---|---|---|
| N | 405 | 894 | 217 | 240 | 1756 | |
| Age, ya,b | 48.4 (2.6) | 48.5 (2.8) | 48.6 (2.6) | 48.8 (2.6) | 48.5 (2.7) | .19 |
| BMI, kg/m2a,b | 31.7 (7.1) | 28.0 (6.5) | 23.5 (3.8) | 23.5 (3.6) | 27.6 (6.7) | .0001 |
| Height, cma,b | 163.6 (5.8) | 164.1 (6.1) | 158.0 (5.3) | 157.0 (4.8) | 162.1 (6.6) | .0001 |
| Weight, kga,b | 84.5 (19.2) | 75.3 (18.2) | 58.5 (10.1) | 57.7 (8.8) | 72.9 (19.2) | .0001 |
| Spine BMDa,b | 1.1 (0.15) | 1.06 (0.13) | 1.03 (0.12) | 1.01 (0.12) | 1.1 (0.14) | .0001 |
| Femoral nck BMDa,b | .94 (0.14) | 0.82 (0.11) | 0.77 (0.10) | 0.77 (0.09) | 0.83 (0.13) | .0001 |
| Serum 25(OH)D, ng/mLa,b | 14.1 (7.5) | 25.3 (9.7) | 20.1 (7.2) | 23.4 (7.8) | 21.8 (9.8) | .0001 |
| < 20 ng/dLc | 322 (79.5) | 256 (28.6) | 105 (48.4) | 69 (28.8) | 752 (42.8) | .0001 |
| Educationc | .0001 | |||||
| ≤ High school | 120 (30.2) | 123 (13.8) | 60 (27.7) | 42 (17.5) | 345 (19.7) | |
| > High school | 277 (69.8) | 768 (86.2) | 157 (72.3) | 198 (82.5) | 1400 (80.2) | |
| Tobacco Usea,c | .0001 | |||||
| Past | 4 (1.0) | 7 (0.8) | 0 (0.0) | 1 (0.1) | 12 (0.7) | |
| Current | 86 (21.2) | 91 (10.2) | 3 (1.3) | 24 (1.4) | 204 (11.6) | |
| Pre-existing conditionsa,c | ||||||
| Diabetes | 49 (12.1) | 36 (4) | 6 (2.8) | 4 (1.7) | 95 (5.4) | .0001 |
| Osteoporosis | 4 (1.0) | 2 (0.9) | 1 (0.4) | 1 (0.1) | 8 (0.5) | .31 |
| Arthritis | 65 (16.0) | 71 (7.9) | 13 (6.0) | 8 (3.3) | 157 (8.9) | .0001 |
| Physical activity scorea,b | 7.3 (1.8) | 8.1 (1.8) | 7.3 (1.7) | 7.9 (1.6) | 7.8 (1.8) | .0001 |
| Self-reported health status, poor/faira,c | 70 (17.3) | 53 (6.0) | 47 (21.7) | 34 (14.2) | 204 (11.7) | .0001 |
| Calcium (mg/d) dietaryd | 568 (460.7) | 742.7 (506.7) | 599.3 (486.9) | 585 (427.2) | 652.6 (490) | .0001 |
| Vitamin D (IU) dietaryd | 91.3 (96.8) | 119.7 (122.8) | 1.0.03 (122.1) | 89.9 (112.3) | 105.2 (115.2) | .0001 |
| History of fractures, prior or/at visit 2a,c | 63 (15.6) | 175 (19.6) | 18 (8.3) | 13 (5.4) | 269 (15.3) | .0001 |
| Menopausal status, visit 2a,c | .0001 | |||||
| Premenopausal | 59 (14.6) | 162 (18.1) | 39 (18.0) | 39 (16.3) | 299 (17.0) | |
| Early perimenopausal | 225 (55.6) | 485 (54.3) | 137 (63.1) | 155 (64.6) | 1002 (57.1) | |
| Late perimenopausal | 43 (10.6) | 55 (6.2) | 11 (5.1) | 18 (7.5) | 127 (7.2) | |
| Postmenopausal | 26 (6.4) | 36 (4.0) | 17 (7.8) | 9 (3.8) | 88 (5.0) | |
| Unknown | 52 (12.8) | 156 (17.4) | 13 (6.0) | 19 (7.9) | 240 (13.7) | |
| Menopausal hormone user, visit 0, 1, or 2c | 47 (11.6) | 166 (18.6) | 15 (6.9) | 21 (8.8) | 249 (14.2) | .0001 |
Abbreviation: IQR, interquartile range.
Information collected at visit 2; all other information at visit 0.
Data presented as mean (sd).
Data present as n (%).
Data presented as mean (IQR).
At visit 2, 1301 (74%) of the participants were pre or early perimenopausal; 127 (7.2%), late perimenopausal, and 88 (5%), postmenopausal. Menopausal status was unknown for 240 (13.7%) women primarily because of a prior hysterectomy or use of HT. Dietary calcium and vitamin D intake averaged approximately 650 mg and 105 IU/d, respectively. Use of calcium or vitamin D supplements was reported by approximately 40–45% of the women. Approximately 16% of women reported a positive history of fracture with higher prevalence in Whites and Blacks compared with Japanese and Chinese women.
Fracture and 25(OH)D
Over an average of 9.5 years 124 (7.0%) experienced an incident traumatic fracture and 88 (5.4%) an incident nontraumatic fracture. Seventy-nine (8.9%) White women experienced a traumatic fracture as well as 21 (5.2%) Black women, 16 (7.4%) Chinese women, and eight (3.3%) Japanese women. Nontraumatic fractures were experienced in 52 (5.9%) White women, 22 (5.45%) Black women, five (2.3%) Chinese women, and nine (3.75%) Japanese women. The most common traumatic fractures were leg (33), hand/wrist (23), and foot/ankle (19). The most common nontraumatic fractures were leg (28), foot/ankle (17), and hand/wrist (12). In comparison with women who did not fracture, women who experienced a traumatic fracture were slightly older, taller, had significantly lower BMD, and were more likely to report prevalent diabetes, a prior history of fractures, calcium supplement use, and corticosteroid use (Table 2). Of interest, mean 25(OH)D was higher in women with a traumatic fracture compared with women without a fracture.
Table 2.
Characteristics of Women at Visit 2 by Incident Fracture Status After Visit 2
| Variable | Fracture |
P Values |
|||
|---|---|---|---|---|---|
| None (n = 1532) | Traumatic (n = 124) | Non-Traumatic (n = 88) | Non-Traumatic Versus No Fracture | Traumatic Versus No Fracture | |
| Age, ya | 48.5 (2.7) | 49.0 (2.9) | 48.4 (8.6) | .74 | .04 |
| BMI, kg/m2a | 27.6 (6.7) | 27.1 (5.8) | 29.0 (7.5) | .07 | .39 |
| Height, cma | 162.0 (6.6) | 163.1 (5.9) | 162.5 (5.9) | .55 | .08 |
| Weight, kga | 72.9 (19.3) | 72.5 (16.7) | 76.8 (20.9) | .07 | .80 |
| Spine BMD, g/cm2a | 1.07 (0.14) | 1.04 (0.13) | 1.05 (0.12) | .17 | .01 |
| Femoral Neck BMD, g/cm2a | 0.84 (0.13) | 0.80 (0.11) | 0.83 (0.12) | .84 | .002 |
| Total Hip BMD, g/cm2a | 0.96 (0.14) | 0.92 (0.13) | 0.95 (0.14) | .85 | .012 |
| Serum 25(OH)D, ng/mLa | 21.8 (9.7) | 24.0 (10.7) | 19.7 (8.6) | .06 | .025 |
| Educational attainmentb | .40 | .11 | |||
| ≤ High school | 305 (19.9) | 15 (12.1) | 22 (25.0) | — | — |
| > High school | 1217 (79.4) | 108 (87.1) | 66 (75.0) | — | — |
| Tobacco useb | .04 | .95 | |||
| Past | 9 (0.6) | 1 (0.8) | 2 (2.3) | — | — |
| Current | 180 (11.7) | 15 (12.1) | 6 (6.8) | — | — |
| Pre-existing conditionsb | — | — | |||
| Diabetes | 74 (4.8) | 12 (9.7) | 8 (9.1) | .08 | .019 |
| Arthritis | 128 (8.4) | 17 (13.7) | 11 (12.5) | .38 | .12 |
| Physical activitya | 7.8 (1.8) | 7.8 (1.8) | 7.6 (1.8) | .49 | .85 |
| Self-reported health status, poor/fairb | 173 (11.3) | 16 (12.9) | 13 (14.8) | .72 | .88 |
| Menopausal statusb | .86 | .29 | |||
| Premenopausal | 270 (17.6) | 15 (12.1) | 15 (12.1) | — | — |
| Early perimenopausal | 875 (57.1) | 71 (57.3) | 71 (57.3) | — | — |
| Late perimenopausal | 109 (7.1) | 11 (8.9) | 11 (8.9) | — | — |
| Postmenopausal | 73 (4.8) | 10 (8.1) | 10 (8.1) | — | — |
| Unknown/hysterectomy | 205 (13.4) | 17 (13.7) | 17 (13.7) | — | — |
| SF-36 role–physical scores (higher value better function)b | .063 | .082 | |||
| 0–24 | 168 (11.0) | 21 (16.9) | 13 (14.9) | — | — |
| 25–49 | 81 (5.3) | 8 (6.5) | 9 (10.3) | — | — |
| 50–74 | 106 (6.9) | 7 (5.7) | 9 (10.3) | — | — |
| 75–99 | 137 (9.0) | 14 (11.3) | 10 (11.5) | — | — |
| 100 | 1036 (67.8) | 74 (59.7) | 46 (52.9) | — | — |
| Calcium, mg/d | |||||
| Dietaryc,d | 651.5 (487.6) | 680.2 (525.2) | 616.4 (435.1) | .32 | .68 |
| Supplements, yesb | 678 (44.3) | 74 (59.7) | 38 (43.2) | .82 | .003 |
| Vitamin D, IU | |||||
| Dietaryc,d | 106.7 (116.3) | 101.8 (112.5) | 78.4 (108.9) | .02 | .57 |
| Supplements, yesb | 578 (37.7) | 64 (51.6) | 29 (33.0) | .24 | .45 |
| History of fractures prior or at visit 2b | 205 (13.4) | 46 (32.3) | 22 (25.0) | .54 | <.0001 |
| Corticosteroid medicationsb | 114 (7.4) | 18 (14.5) | 11 (12.5) | .08 | .005 |
| Menopausal hormone use (visit 0, 1, or 2)b | 234 (15.3) | 24 (19.4) | 19 (21.6) | .11 | .23 |
Abbreviation: IQR, interquartile range.
Data presented as mean (sd).
Data present as n (%).
Data presented as mean (IQR).
Dietary data limited to food frequency questionnaire administered at baseline (visit 0).
Women who experienced a nontraumatic fracture in comparison with women who did not fracture had a slightly higher BMI (P = .07), were less likely to report smoking (P = .04) and more likely to report diabetes (P = .08), corticosteroids (P = .08), and had a significantly lower vitamin D intake. Circulating 25(OH)D was marginally lower among those with a nontraumatic fracture compared with nonfracture women, (19.7 vs 21.8 ng/mL), (P = .06) respectively.
There was no association between 25(OH)D and traumatic fractures, Table 3. For every 10 ng/mL increase in 25(OH)D, the HR (HR) (95% confidence interval) of traumatic fractures was 1.02 (0.80, 1.28). Women with 25(OH)D at least 20 ng/mL had a similar risk of traumatic fracture as women with lower levels.
Table 3.
25(OH)D and Incident Traumatic and Non-Traumatic Fracture Over the Menopausal Transition
| Fractures | Base Model, HR (95% CI)a | Multivariate Model, HR (95% CI)b |
|---|---|---|
| Traumatic fractures | ||
| Per 10 ng/mL increase | 1.14 (0.94–1.37) | 1.02 (0.80–1.28) |
| ≥20 vs <20 ng/mL | 1.24 (0.83–1.87) | 1.11 (0.70–1.77) |
| Non-traumatic fractures | ||
| Per 10 ng/mL increase | 0.75 (0.58–0.96) | 0.72 (0.54–0.96) |
| ≥20 vs <20 ng/mL | 0.55 (0.35–0.86) | 0.54 (0.32–0.89) |
Abbreviation: 95% CI, 95% confidence interval.
Adjusted for age, site, and race.
Base model + fracture history, prior and current menopausal hormone therapy, BMI, physical activity, SF-36 Role–physical functioning score, education, lumbar spine BMD, calcium and vitamin D supplements, corticosteroids, diabetes, and dietary calcium.
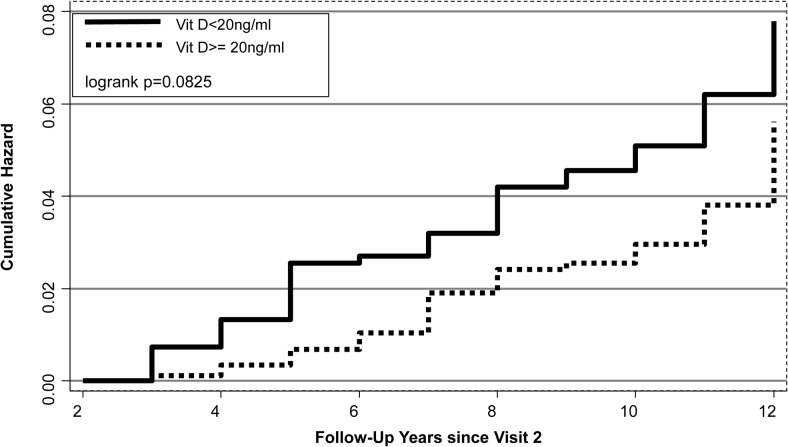
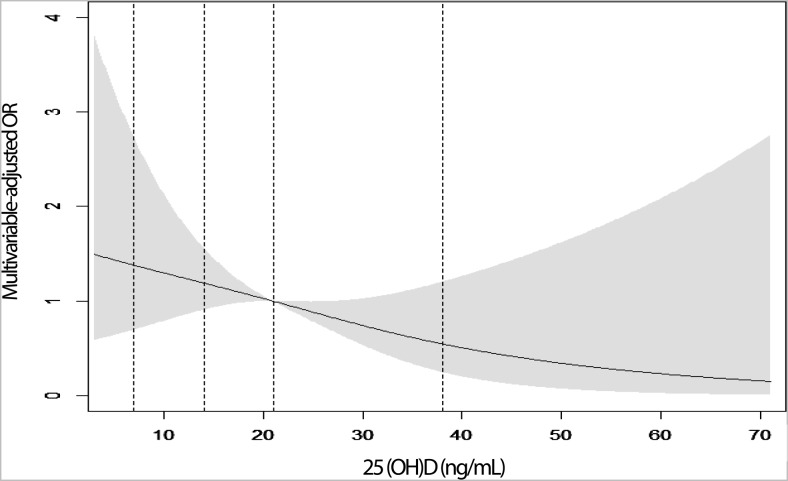
In contrast, the cumulative hazard of nontraumatic fracture was lower among women with 25(OH)D at least 20 ng/mL (Figure 1; P = .08). For every 10 ng/mL increase in 25(OH)D, the HR for nontraumatic fracture was 0.72 (0.54–0.96) (Table 3). Comparing women with 25(OH)D at least 20 ng/mL with those less than 20 ng/mL, the multivariable-adjusted HR was 0.54 (0.32–0.89). Over the full range of 25(OH)D, we found no significant threshold (Figure 2). The association between 25(OH)D and nontraumatic fracture seemed linear with decreasing risk with increasing 25(OH)D. We were not able to examine the 25(OH)D fracture association separately by race/ethinicity but in sensitivity analyses, we excluded Black participants from the nontraumatic fracture analysis and results were similar, multivariate-adjusted model at least 20 ng/mL vs less than 20 ng/mL; HR, 0.59 (95% CI, 0.34–1.02). We also excluded women who were already postmenopausal at visit 2 and results were similar: HR, 0.54 (95% CI, 0.32–0.89).
Figure 1.
Cumulative hazard of nontraumatic fractures by 25(OH)D: < 20 ng/mL vs ≥ 20 ng/mL.
Figure 2.
Cubic spline graph: 25(OH)D and nontraumatic fractures over the full range of 25(OH)D.
BMD across the menopausal transition and 25(OH)D
The annual percentage loss in LS and FN in each of the three periods (eg, pretransmenopause, transmenopause, and postmenopause) by 25(OH)D status is summarized in Table 4. There was no difference in the annualized slopes for those with 25(OH)D less than 20ng/mL or at least 20 ng/mL in any menopausal period. This was true in both non-Black and Black women. The annualized rates of BMD loss were greatest in the transmenopausal period with rates slightly lower among the Black participants. Results were similar at both the LS and FN.
Table 4.
Lumbar Spine and Femoral Neck BMD Annualized Percent Changes (%/y) Across the Menopausal Transition by 25(OH)D
| Spinal Changes | Non-Blacks |
Blacks |
||
|---|---|---|---|---|
| <20 ng/mL | ≥20 ng/mL | <20 ng/mL | ≥20 ng/mL | |
| Lumbar spine | ||||
| Pre-transmenopause (−5 FMP to −1) | −0.83 | −0.89 | −0.53 | −0.40 |
| Transmenopause (−1 FMP to +2) | −2.20 | −2.50 | −1.50 | −1.30 |
| Post-menopause (+2 to 8 yr) | −1.40 | −1.50 | −1.00 | −1.00 |
| Femoral Neck BMD | ||||
| Pre-transmenopause (−5 FMP to −1) | −0.50 | −0.46 | −0.53 | −0.31 |
| Transmenopause (−1 FMP to +2) | −1.80 | −1.70 | −1.30 | −0.70 |
| Post-menopause (+2 to +8 y) | −1.20 | −1.20 | −1.10 | −1.10 |
Bolded values indicate significant bone loss; P < .004.
No difference in rates of BMD loss by 25(OH)D levels.
Adjusted for age, site, and BMI.
Discussion
In our prospective study of women at midlife, we found that women with higher levels of 25(OH)D had a lower risk of nontraumatic fractures during the next 9.5 years. Compared with women with 25(OH)D less than 20ng/mL, those with higher values had an approximately 45% reduced risk of nontraumatic fractures. The overall trend between increasing 25(OH)D and lower risk of nontraumatic fracture was statistically significant with no evidence of a threshold. The association was independent of many factors including BMI and BMD. Levels of 25(OH)D were lower among the Black women but exclusion of Black women from the model led to similar HR. In contrast, we found no association between 25(OH)D and traumatic fractures. Traumatic fractures have been linked to low BMD (13). Indeed, we observed that women with traumatic fractures in our study had lower BMD than women with no fracture. To our knowledge, no previous study has examined the association between 25(OH)D and fractures separately by degree of trauma. The epidemiology and risk factors for traumatic fractures is less well developed. Women who experience traumatic fractures may engage in riskier activities. Our measure of physical activity did not differ across groups but our activity questionnaire may not be able to capture these riskier activities. Women with a traumatic fracture had a greater history of previous fracture suggesting that their lifestyle may predispose them to fractures.
We previously reported that faster rates of LS bone loss were associated with higher levels of FSH in the pretransmenopausal state, higher levels of FSH and SHBG in the transmenopausal period and lower levels of estradiol and sex hormone binding globulin (SHBG) in the later postmenopausal period (14). In the current analysis, 25(OH)D, measured for the most part during the premenopausal period was unrelated to changes in BMD across the MT. However, in contrast with our earlier report, 25(OH)D was only measured once; therefore, we could not assess whether change in 25(OH)D across menopause correlated with change in BMD. Serum 25(OH)D has been shown to track over time, at least after 3 years (r = 0.70), but examination of the correlation within individuals over longer periods was weaker, ranging from 0.42–0.52 (19–21). Nevertheless, it is currently unknown to what extent 25(OH)D changes across menopause. The early data from Heaney et al (1) suggested changes in calcium balance across menopause but this finding compared postmenopausal women on HT vs untreated women.
The optimal level of 25(OH)D needed for bone health is currently a major clinical controversy (22–24). Some groups such as the Endocrine Society propose an optimal level of 30 ng/mL or higher (20). The 2010 Institute of Medicine report concluded that a level of at least 20 ng/mL is needed for bone health in most individuals (12). Our findings are consistent with the IOM report as well as previous studies showing that it is only individuals with 25(OH)D < 20ng/mL who have an increased risk fracture (2, 3). However, we tested for a threshold but there was no evidence of one; the association between 25(OH)D and fracture risk seemed linear. Only approximately one third of the women had circulating levels at least 30 ng/mL so we had limited power to test for higher thresholds. In the National Health Examination Survey, the association between 25(OH)D and fracture was linear when major osteoporotic fractures was considered but quadratic when hip fractures were studied (25). We had too few hip fractures in these young women to test for nonlinearity in the fracture-vitamin D relationship.
We have previously found that the risk of clinical fracture increased with increasing 25(OH)D in Black women but decreased with increasing concentrations of 25(OH)D in White women (3). This study had a much larger sample size of Black women: 379 Black women with clinical fractures and a matched control for each. We were limited to a much smaller sample size of Black women and thus, we were unable to stratify by race/ethnicity separately. Of note, other studies have not supported a race/ethnic difference in the relationship between 25(OH)D and fractures (25–27).
Black women had lower 25(OH)D and experienced slower rate of annualized BMD loss across the MT. But in both non-Black and Black participants, there was no association between 25(OH)D and BMD changes across the MT. This result suggests that BMD does not mediate the association between 25(OH)D and fractures. Circulating 25(OH)D has been linked to lower fall rates (28) and improved muscle strength and physical performance (29). Hence, the lower fracture rates in SWAN women with higher levels of 25(OH)D may reflect greater muscle strength to perhaps break the impact of the fall and reduce the likelihood of fracture. We adjusted for self-reported physical function but we could not adjust for performance based measures of muscle strength or physical function because these data were not collected until SWAN visit 12 (2009–2011).
We were limited to self report of clinical fractures in the early years of the study but later we showed that our false-positive rate was less than 5%. In addition, the accuracy of self report of fractures has shown to be high in other studies (30, 31). We cannot provide annual information on radiographic vertebral fractures, which may be a common fracture in women as this would have required serial lateral lumbar and thoracic spine x-rays and we did not have these resources. Thus, the overall fracture rate may be underestimated.
In conclusion, women with higher (≥20 ng/mL) serum 25(OH)D at midlife have a lower risk of nontraumatic fractures across the MT. Our results are consistent with the IOM report. This association was independent of BMD, BMI, and other risk factors for fracture. There was no association between 25(OH)D and traumatic fractures or with changes in BMD across the MT. The lack of an association with transmenopausal bone loss suggests that other factors (eg, muscle strength) may contribute to the lower fracture risk with increasing 25(OH)D. Women with 25(OH)D (<20 ng/mL) compared with women with vitamin D levels at least 20 ng/mL were less likely to report vitamin D supplements (13% and 24%, respectively). Because few foods contain vitamin D, our results suggest that vitamin D supplementation is warranted in women at midlife with 25(OH)D less than 20 ng/mL.
Acknowledgments
The Study of Women's Health Across the Nation has grant support from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women's Health (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Disclosure Summary: J.A.C., G.A.G., K.R., Y.L., J.F.R., S.-A.B.-B., and J.S.F. have nothing to declare. J.C.L. has received research funding from Amgen and Sanofli unrelated to the current study.
Footnotes
- 25(OH)D
- serum 25 Hydroxyvitamin D
- BMD
- bone mineral density
- BMI
- body mass index
- FMP
- final menstrual period
- FN
- femoral neck
- FSH
- follicle stimulating hormone
- HR
- hazard ratio
- HT
- hormone therapy
- IOM
- Institute of Medicine
- LS
- lumbar spine
- MT
- menopausal transition
- SF-36
- 36-item short form
- SHBG
- sex hormone binding globulin
- SWAN
- Study of Women's Health Across the Nation.
References
- 1. Heaney RP, Recker RR, Saville PD. Menopausal changes in calcium balance performance. J Lab Clin Med. 1978;92:953–963. [PubMed] [Google Scholar]
- 2. Cauley JA, Parimi N, Ensrud KE, et al. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res. 2010;25:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: The Women's Health Initiative (WHI). J Bone Miner Res. 2011;26:2378–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Schoor NM, Visser M, Pluijm SM, et al. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone. 2008;42:260–266. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura K, Saito T, Oyama M, et al. Vitamin D sufficiency is associated with low incidence of limb and vertebral fractures in community-dwelling elderly Japanese women: The Muramatsu study. Osteoporos Int. 2011;22:97–103. [DOI] [PubMed] [Google Scholar]
- 6. Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adults. J Bone Miner Res. 2008;23:143–150. [DOI] [PubMed] [Google Scholar]
- 7. Gerdhem P, Ringsberg KA, Obrant KJ, et al. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. [DOI] [PubMed] [Google Scholar]
- 8. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–738. [DOI] [PubMed] [Google Scholar]
- 9. Garnero P, Munoz F, Sornay-Rendu E, et al. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716–722. [DOI] [PubMed] [Google Scholar]
- 10. Pramyothin P, Techasurungkul S, Lin J, et al. Vitamin D status and falls, frailty, and fractures among postmenopausal Japanese women living in Hawaii. Osteoporos Int. 2009;20:1955–1962. [DOI] [PubMed] [Google Scholar]
- 11. Roddam AW, Neale R, Appleby P, et al. Association between plasma 25-hydroxyvitamin D levels and fracture risk: The EPIC-Oxford study. Am J Epidemiol. 2007;166:1327–1336. [DOI] [PubMed] [Google Scholar]
- 12. IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 13. Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res. 2012;27:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crandall CJ, Tseng CH, Karlamangla AS, et al. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. J Clin Endocrinol Metab. 2013;98:E654–E663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sowers MR, Crawford SL, Sternfeld B, et al. 2000. SWAN: A multicenter, multiethnic, community based cohort study of women and the menopausal transition. In: Rogerio A, Lobo JKaRM, eds. In: Menopause: Biology and pathobiology. New York: Academic Press; 175–188. [Google Scholar]
- 16. Singh RJ. Quantitation of 25-OH-vitamin D (25OHD) using liquid tandem mass spectrometry (LC-MS-MS). Methods Mol Biol. 2010;603:509–517. [DOI] [PubMed] [Google Scholar]
- 17. Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 18. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 19. Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab. 2010;95:2637–2645. [DOI] [PubMed] [Google Scholar]
- 20. Jorde R, Sneve M, Hutchinson M, et al. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–908. [DOI] [PubMed] [Google Scholar]
- 21. Platz EA, Leitzmann MF, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. [DOI] [PubMed] [Google Scholar]
- 22. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 23. Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–457. [DOI] [PubMed] [Google Scholar]
- 24. Bouillon R, Van Schoor NM, Gielen E, et al. Optimal vitamin D status: A critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98:E1283–E1304. [DOI] [PubMed] [Google Scholar]
- 25. Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28:997–1006. [DOI] [PubMed] [Google Scholar]
- 26. Robinson-Cohen C, Katz R, Hoofnagle AN, et al. Mineral metabolism markers and the long-term risk of hip fracture: The cardiovascular health study. J Clin Endocrinol Metab. 2011;96:2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barbour KE, Houston DK, Cummings SR, et al. Calciotropic hormones and the risk of hip and nonspine fractures in older adults: The Health ABC study. J Bone Miner Res. 2012;27:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snijder MB, van Schoor NM, Pluijm SM, et al. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980–2985. [DOI] [PubMed] [Google Scholar]
- 29. Houston DK, Tooze JA, Neiberg RH, et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: The Health, Aging, and Body Composition Study. Am J Epidemiol. 2012;176:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: Results from the Women's Health Initiative observational study and clinical trials. Menopause. 2004;11:264–274. [DOI] [PubMed] [Google Scholar]
- 31. Nevitt MC, Cummings SR, Browner WS, et al. The accuracy of self-report of fractures in elderly women: Evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. [DOI] [PubMed] [Google Scholar]