Abstract
This case report illustrates and discusses the non-operative management of a complete anterior cruciate ligament (ACL) injury in an English Premier League football player, his return to play within 8 weeks and problem-free follow-up at 18 months post injury. When non-operative verses surgical ACL reconstruction is considered there are many fundamental gaps in our knowledge and currently, at elite level, there are no cases in cutting sports within the literature to guide these decisions. When the norm is for all professional footballers to be recommended surgery, it will be very challenging when circumstances and patient autonomy dictate a conservative approach, where prognosis, end points and risk are unclear and assumed to be high. This case challenges current dogma and provides a starting point for much needed debate about best practice, treatment options, research direction and not just at the elite level of sport.
Background
There are no case reports to follow of elite professional footballers in scientific literature who have chosen non-operative anterior cruciate ligament (ACL) injury treatment to guide patients, physicians and surgeons in the decision-making process when faced with an elite athlete with an ACL injury. There are examples reported in the media, but without any clinical details they remain shrouded in mystery. For this reason and to assist future research and medical care, we present the recent example of an elite footballer who sustained a non-impact ACL injury during a football match.
It is estimated that there are over 2 million ACL injuries annually1 and 98% of orthopaedic surgeons recommend ACL reconstruction (ACLR) to enable return to sports involving twisting, jumping and cutting.2 A recent prospective study of elite footballers over 9 years and 57 clubs, suggested 97% of ACL injured footballers (71/73) were treated surgically,3 which mirrors surgical opinions and suggests a strong influence on clinical decisions in practice. More than 90% of these ACLR football players returned to full elite level training within 10 months after surgery and 89% participated in elite match play within 12 months,3 whereas a systematic review and meta-analysis suggested only 44% had been able to return to competitive sport at final follow-up.4 In contrast, a recent systematic review and meta-analysis comparing ACLR versus non-operative treatment in the general population concluded that “based on poor evidence, the current evidence would indicate that people following ACL rupture should receive non-operative interventions before surgical intervention is considered.”5
In professional sport, hidden agendas, such as financial decisions, contracts, medicals, availability and performance pressure, can influence patient autonomy.6 In contrast, ethical considerations, informed consent and treatment decisions remain heavily influenced by treatments which have been more widely researched as prognosis, risks and return to play will be more clearly defined. Opinions about non-operative and surgical treatment for ACL injuries have even less justification or evidence base within the literature if the demands of elite professional sport are accepted to be greater than the general population, despite it being shown possible to return to lower level running, jumping and cutting sports with non-operative treatment.7
Return to play guidelines post ACLR are well defined8 but of no use if there has been no surgery. Fears of accelerated or early osteoarthritis are already justified following the initial injury.9 Higher risk of meniscal and chondral injury leading to accelerated arthritis also appear logical in an unstable and ACL absent knee10 but are high anyway after ACLR11 and unknown at an elite level where demands, medical input and expertise may vary greatly. Regardless of clinical laxity, a footballer who chooses the non-operative treatment route returning to training and competition, will by definition be a ‘coper’ and psychological resilience and physical stability through intensive rehabilitation concordance may also be protective against further injuries.12 13
Frobell et al14 have demonstrated with a randomised controlled trial in non-elite athletes that intensive rehabilitation may provide similar or better outcomes than ACLR, but applicability to elite athletes is not known. Opinions support the widely accepted notion that “not all ACL ruptures require reconstruction, but it should be offered to a specifically selected group of patients who we believe would benefit from surgery, including individuals active in cutting and jumping sport… Who have meniscus and/or cartilage injury, recurrent giving way, etc. The choice of surgery or no surgery should be individualised after a careful and open discussion between the patient and the surgeon.”15
Case presentation
The participant was a male 32-year-old international level professional footballer, who at the time of injury was playing football in the English Premier League. Imaging (figure 1) from a signing medical prior to the injury confirmed an intact ACL and there were no other knee injuries between this imaging and the injury reported in this paper. The ACL injury was sustained in the first half of a competitive match and following the injury the player was immediately substituted. The injury was non-contact, with the knee slightly flexed by about 30° with valgus knee force and external tibial rotation. Crutches were refused and he was able to walk immediately. Within 30 minutes a grade II effusion had developed with lateral joint line tenderness.
Figure 1.
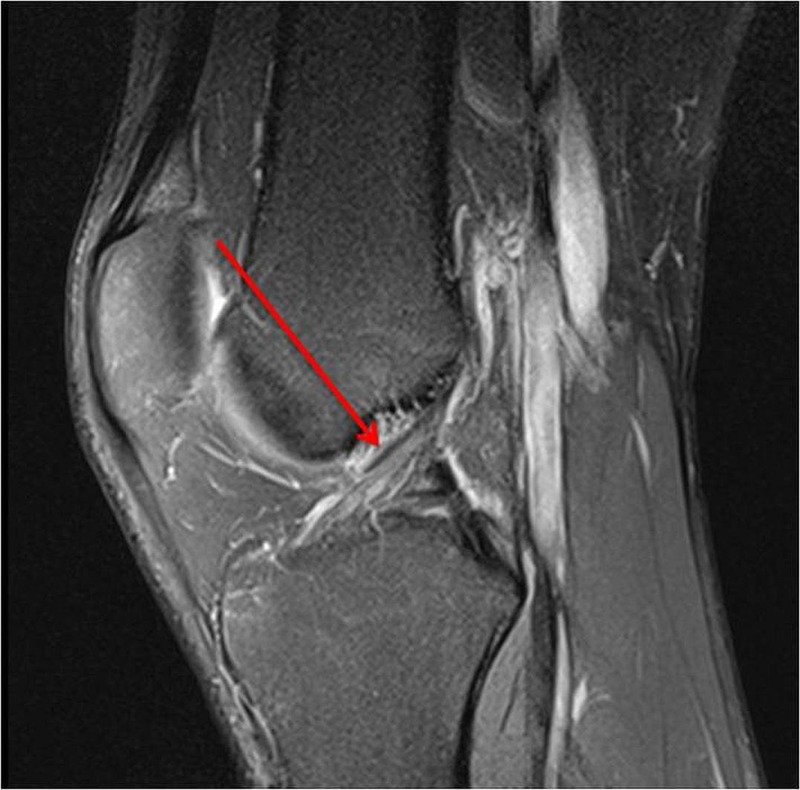
Anterior cruciate ligament intact 1 year prior to injury.
Following initial discussions, surgical opinions were sought by two independent orthopaedic knee surgeons; one in the UK and the other from the footballers home town in Europe, at the athletes request. Surgical reconstruction was offered by both surgeons using different techniques and both suggested that surgery would be inevitable due to the demands and level of football and suggested that ACLR surgery could take place at about 2 weeks postinjury if the knee effusion settled. When the pros and cons of treatment options were discussed, the footballer chose to begin the non-operative treatment route, re-evaluating progress at regular intervals and acknowledging an episode of instability at any time in the future may lead to surgical reconstruction and possibly further injury to meniscus and/or cartilage. The athlete chose to try a period of rehabilitation for 4 weeks, following an initial week of rest, ice, elevation and compression to reduce swelling. This 5-week period provided the footballer with opportunity for reflection, further discussion, rehabilitation to benefit surgery, if required, and regular review.
Investigations
Clinical examination was performed by the club specialist sport and exercise medicine physician, club physiotherapists and two independent orthopaedic knee surgeons. The orthopaedic knee surgeons confirmed the right knee had a grade II+ Lachman and left a stable Lachman test after injury. The right knee had a grade II+ anterior draw and the left grade I+. The right knee had a grade I pivot shift and the left was normal. The anterior draw test on the right corrected with internal rotation and the dial test of side to side difference was borderline at just under 10°, with 10° of varus valgus rock in both knees. Lateral collateral ligament and menisci were clinically unremarkable. Lachman, anterior draw and pivot shift test findings did not change throughout the rehabilitation process and were confirmed by one of the orthopaedic surgeons at review prior to return to play.
An MRI scan the day after injury confirmed a complete disruption of the right ACL (figure 2) with an osteochondral injury in the lateral femoral sulcus. There was also a grade 1 injury to the medial collateral ligament and low grade bone oedema in the posterolateral and posteromedial corners. An MRI at 10 months postinjury confirmed the persistent full rupture of his ACL (figure 3) and also how an osteochondral injury was not present prior to the injury (figure 4), was present in the lateral sulcus immediately after the injury (figure 5) and was not visible 10 months after the injury (figure 6). The radiologist report for figure 6 concluded: “The lateral femoral condyle osteochondral lesion has healed. The lateral tibial plateau chondral fibrillation adjacent to the posterior horn of the meniscus and femoral trochlea osteochondral lesions have not changed since post-injury MRI. No new osteochrondral lesions or new meniscal injury seen.”
Figure 2.
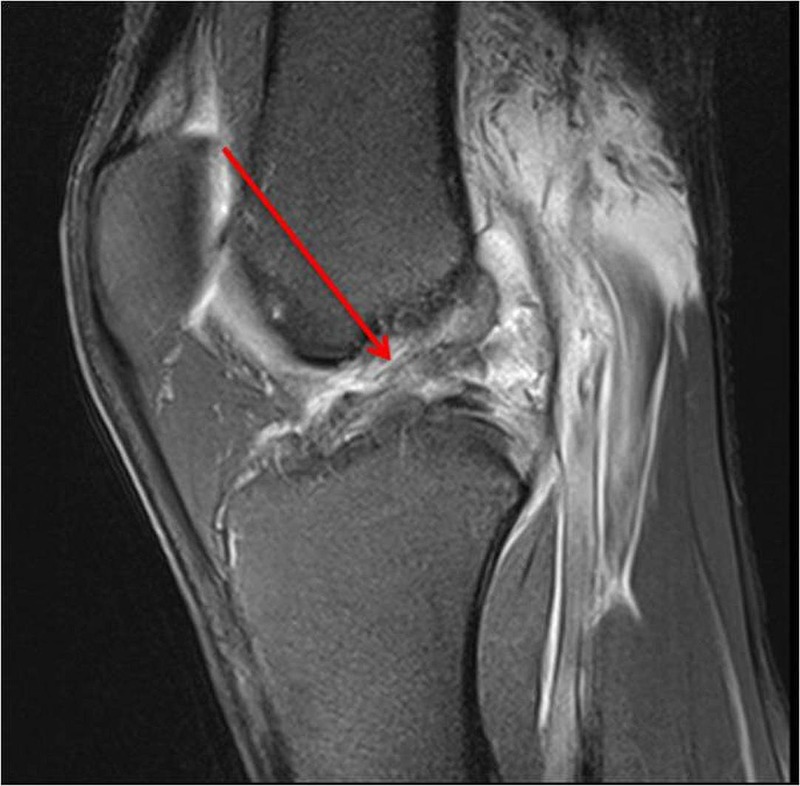
Anterior cruciate ligament complete tear 1 day after the injury.
Figure 3.
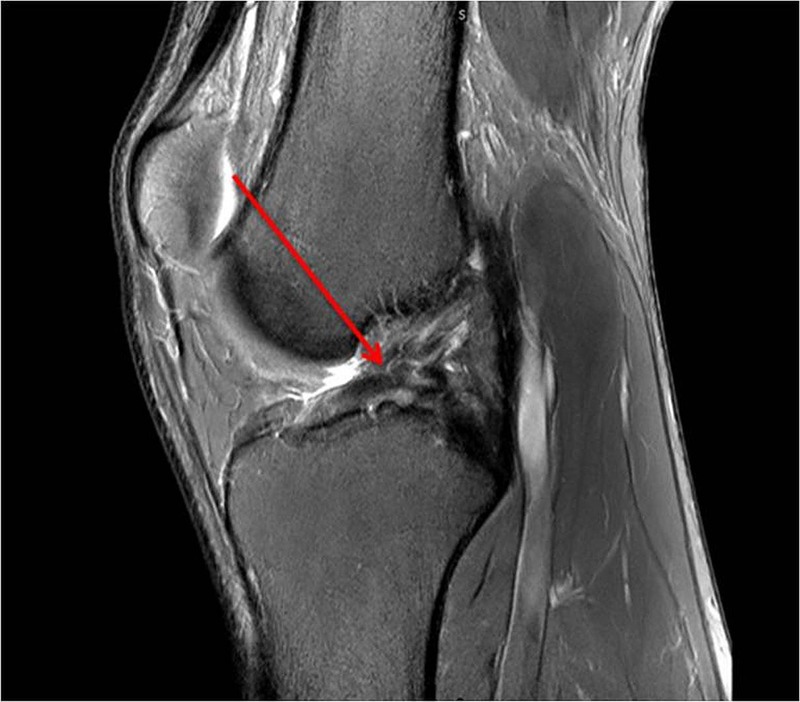
Anterior cruciate ligament still complete tear 10 months after injury.
Figure 4.
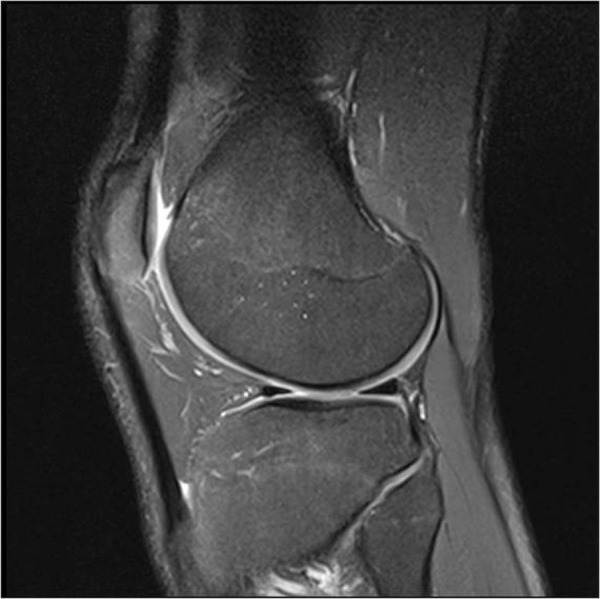
No osteochondral injury 1 year prior to injury.
Figure 5.
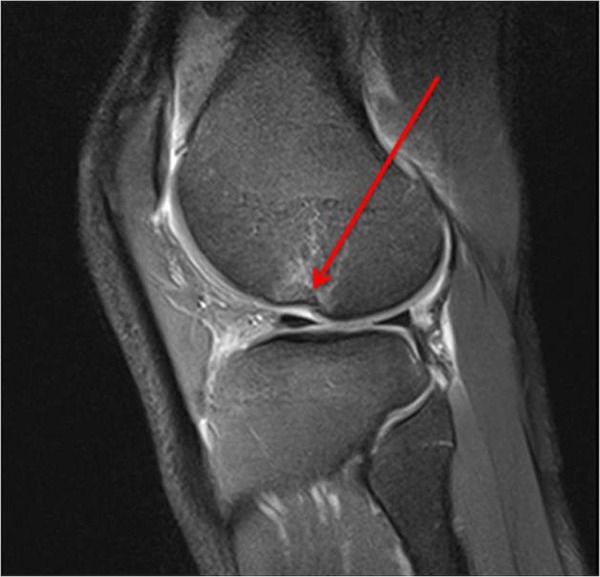
Osteochondral injury in lateral sulcus 1 day after Anterior cruciate ligamen injury.
Figure 6.
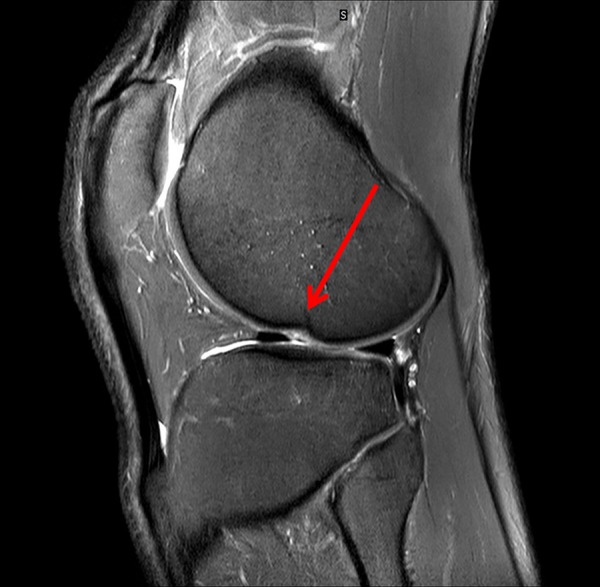
10 months after injury the radiology report concludes that “the lateral femoral condyle osteochondral lesion has healed. The lateral tibial plateau chondral fibrillation adjacent to the posterior horn of the meniscus and femoral trochlea osteochondral lesions have not changed since post-injury MRI (figure 5). No new osteochrondral lesions or new meniscal injury seen.”
Treatment
Testing
The tests chosen to determine knee stability, were the single leg hop and the single leg crossover hop test.13 16–18 The single leg hop test is a straight line hop for distance, looking at control of landing mechanics as well as distance. The crossover test is three maximal hops for distance, jumping left to right over a 30 cm gap as you hop forwards again looking at control and distance. These were used as a guide every 2–3 days for both the medical team and participant to score and determine knee stability and rehabilitation progress. He was also asked daily about knee ‘trust’ and during each rehabilitation session as functional stability is subjective and can exist in the presence of clinical instability (eg, a positive Lachman test, anterior draw and positive pivot shift test). As clinical tests do not determine subjective knee stability for the athlete, we decided this would be a key factor in rehabilitation progression. We devised a ‘trust’ Likert scale and asked a daily 1–10 scoring system, based on how he trusted his knee for stability, and this increased steadily from 1–10 over the rehabilitation period. Each day the medical team clinically assessed his knee before and after rehabilitation sessions, however, without an ACL the findings never changed and the effusion settled quickly both pre-exercise and postexercise over the rehabilitation period, which started after a week of strict rest, ice compression and elevation. There were no occasions when the effusion deteriorated following a gym or pitch-based session.
It was also decided that rest and recovery would be critical in the recovery process so it was agreed that the athlete would not rehabilitate for four consecutive days. He either worked for 2 days followed by a rest day off or 3 days of work followed by a rest day, which allowed recovery from the previous day’s work. His programme began with gym based sessions (sessions 1–9) before running and gym sessions were started and integrated (sessions 10 onwards).
Gym rehabilitation programme
An essential part of the rehabilitation process was to ensure the programme fitted the athlete and not the athlete fitting the programme.
The participant had no real training age in a gym. He did not enjoy load being placed across his shoulders (eg, traditional barbell exercises) and onto his spine. He also had strong opinions about certain exercises that he did not wish to perform and was therefore involved in the development of his own programme. Exercise selection was also experimental; as exercises were introduced the athlete provided feedback, which helped determine whether an exercise was kept in the programme. We hypothesise that this open teamwork, autonomy and adaptability may have helped ensure concordance, outcomes and laid the foundations for coping.
The progressive rehabilitation programme was planned to increase lower limb strength (with a focus on quadriceps) through high volume, made up of set repetitions and time at a low intensity (weight) before increasing the load and reducing the volume. When the athlete and medical team felt ready, with the benefit of stable uncomplicated progress, low-level plyometric exercises were introduced and gradually progressed in complexity, frequency and intensity. Double leg exercises to increase strength in both legs were started, with an early introduction of single leg exercises to develop strength and proprioception in the injured leg.
To increase rate of muscle hypertrophy and strength, electrical muscle stimulation was used for the first seven sessions, two small pads were placed on the vastus medialis obliqus and vastus lateralis and a large pad across the top of the quadriceps 3/4 down the femoral shaft from the femoral neck. Electrical muscle stimulus was intended to increase strength and overcome any muscular inhibition, from disuse, injury or the shrinking residual knee effusion.19 The athlete was in full control of the external stimulus he gave himself, following initial education on safe use.
Occlusion training was introduced in the first session and done every day through the rehabilitation process as his final exercise and remains part of his daily routine.20 21 The portable sphygmanometer cuff was placed around the top of his thigh and inflated to 150 mm Hg. The participant performed 4×12 reps with a 30 s rest between sets.
Time under tension was also included, with an eccentric time load of 3 s introduced on the sixth session and maintained throughout, for all exercises, allowing eccentric control and this remained in his programme following return to play.22
Range of motion (ROM) for all exercises was included18 23 and while risk of causing further soft tissue injury within the knee was discussed with the athlete, the decision was made that if the participant was to return to play conservatively, was able to manage early pain free range of movement exercises, that he would rehabilitate with exercises in full ROM from the start of the rehabilitation programme.
Running and pitch based return to fitness
During early and ongoing discussions the athlete expressed keenness to ‘test’ his knee by running as early as possible, as he felt that he would be able to determine stability and not delay surgery longer than was necessary. It was agreed running would commence on an antigravity running machine to reduce risk of injury and maintain gradual and controlled progression. This was started after the 7th session for three sessions starting at 70% of total bodyweight increasing to 80% then 90% in the following sessions. Running sessions outside then started with four sessions based on jogging below 50% of his max speed (controlled against pre injury data recorded using global positioning system data) around the pitches to build confidence and control.
Sessions were then designed around high-intensity running, sprinting, cutting, turning, jumping, accelerating, decelerating and individual football specific sessions. The intensity and rotational movements of each session were increased as the athlete completed the previous session without problems. The next step was to arrange specially designed football sessions to reintegrate full training (sessions 11 and 12).
Prior to return to full training surgical opinions were sought again and there was a preference to wait until 12 weeks had passed to allow further recuperation. The athlete and medical team were in unchartered territory and had not expected such quick progress, however, return to play goals had been met in accordance with guidelines (even though these were not designed for an ACL absent knee8). Surgical advice suggested ideally 12 weeks should pass, but following discussions with the athlete it was acknowledged that little would perceivably change between 7½ and 12 weeks postinjury.
On the 13th outside session he was reintroduced to complete his first full training session with the development squad (DS) without restrictions. This session was dictated by the coaches and included small sided games as well as fitness and technical drills. On his 14th session he was reintroduced to full 1st team training, which was 7 weeks and 6 days after the initial injury. After four full training sessions with the first team squad the participant was made available for 1st team match selection. He played 60 min in a DS match, which was his 23rd outside session. He then played in two more DS games 3 days apart a week later. Although named as a substitute in many first team games he was able to play in five first team matches in the 2014 season, including two full matches, with selection determined by coaching decisions rather than fitness and availability.
Outcome and follow-up
Table 1 compares mean performance data for three full matches completed in 2012/2013 season prior to ACL injury and two full matches in 2013/2014 season shortly following non-operative return to play, with few major differences visible. In the 2012/2013 season prior to the ACL injury he played six matches, of which three were complete matches. In the 2013/2014 season he played 14 and 34 min prior to the injury in two matches and then five matches after the injury, two of which were completed, the rest used as a second-half substitute.
Table 1.
Comparison of mean performance data for full competitive matches played before and after ACL injury (3 matches in season just prior to injury and 2 shortly after non-surgical return to play)
| Total distance (m) |
High-intensity distance (distance in m above 18.7 km/h) | Sprint distance (distance in m above 18.7 km/h) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90 min | 1st half | 2nd half | 90 min | 1st half | 2nd half | 90 min | 1st half | 2nd half | No of sprints (90 min) | |
| Preinjury mean 2012/2013 season | 10 416 | 5366 | 5050 | 848 | 393 | 455 | 254 | 105 | 148 | 30 |
| Postinjury mean 2013/2014 season | 10 344 | 5060 | 5284 | 801 | 353 | 448 | 205 | 78 | 127 | 26 |
At the time of submitting the paper, almost 18 months following the initial injury the participant remains fully available for selection and is now a regular starter for his current club.
Discussion
Case studies, such as this, challenge dogma, regardless of a limited 18-month follow-up. However, one arguably fortuitous case, limited to the short term, provides no evidential basis for others to necessarily base their clinical decisions. However, this case provides a starting point for much needed debate about best practice, treatment options, research direction and not just at the elite level of sport.
Risk is mitigated through informed consent, patient autonomy, continuous close monitoring, open communication and the application of common sense despite a limited evidence base. Our ability to select copers from non-copers remains limited, but psychological and physical attributes are likely to play key roles. Some may argue that this athlete made poor choices and will have accelerated osteoarthritis and further soft tissue damage, but if the evidence is explained and the risks understood then this risk is appropriately managed and informed consent followed. Furthermore, ACLR could take place at any time, when convenient, if indicated or desired should individual circumstances change in the future. Those few medical teams and medical professionals who have treated ACL injuries conservatively in professional football may all have felt vulnerable, working in undocumented territory and with so many unknowns against widely held norms. There are stories in the media and rumours of non-operative return to play and no doubt there are also cases that may have ‘failed’ the non-operative treatment route, but if one athlete can return to full training in less than 8 weeks and 18 months later have had no issues, then for all the research and surgical opinions, sports medicine and rehabilitation practitioners may have conclusive evidence that ACL surgery is truly “not for all patients and should not be for all surgeons”9 at the elite level of sport.
Publication bias must be acknowledged, in that this football players’ experiences, choice to explore relatively uncharted treatment territory at his level of competition, may not have been publishable or of interest in the event of an ‘unsuccessful’ non-operative approach within the limited 18-month follow-up period. We hope that this paper helps others faced by similar dilemmas, challenging circumstances and provides a starting point for further development of evidence base on which to determine conservative treatment decisions. In the absence of evidence, the outcomes of this athlete at 18 months, will hopefully provoke debate, a change in attitudes and questioning of accepted practice and opinions. Media reports provide no basis on which to make clinical decisions and without such peer reviewed examples, in the absence of evidence, sports medicine will struggle to progress.
Learning points.
Very little is known about non-operative management of complete anterior cruciate ligament (ACL) injuries in elite sport and professional football.
This case demonstrates, contrary to current practice, that it is possible to return to play with complete non-operative management, within 8 weeks, following a complete ACL injury in a professional football player, despite a dearth of evidence.
In the absence of evidence, (non-operative and surgical), the outcomes of this athlete at 18 months provide a starting point for the further development of research to provide an evidence base on which to determine treatment decisions and will hopefully lead to much needed debate, a change in attitudes and questioning of current opinions.
Acknowledgments
The authors wish to thank the professional footballer for choosing to share his story to help others in a similar situation and hopefully develop debate and much needed research in this important field. The authors also wish to thank all the medical and sports science staff at the football club, both past and present, for their support with this challenging case and paper.
Footnotes
Twitter: Follow Mathew Monte-Colombo at @Monte_the_bear
Contributors: RW conceived the paper. MM-C drafted the methods and RW edited this section and drafted the rest of the paper. AM drafted the radiology areas and AM and FH reviewed and provided feedback and amendments to the paper.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Samuelsson K. Anatomic ACL reconstruction—current evidence and future directions [PhD thesis]. Sweden: Goteberg University, 2012. https://gupea.ub.gu.se/bitstream/2077/28254/1/gupea_2077_28254_1.pdf [Google Scholar]
- 2.Marx RG, Jones EC, Angel M et al. . Beliefs and attitudes of members of the American Academy of Surgeons regarding the treatment of anterior cruciate ligament. Arthroscopy 2003;19:762–70. 10.1016/S0749-8063(03)00398-0 [DOI] [PubMed] [Google Scholar]
- 3.Waldén M, Hägglund M, Magnusson H et al. . Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc 2011;19:11–19. 10.1007/s00167-010-1170-9 [DOI] [PubMed] [Google Scholar]
- 4.Ardern CL, Webster KE, Taylor NF et al. . Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med 2011;45:596–606. 10.1136/bjsm.2010.076364 [DOI] [PubMed] [Google Scholar]
- 5.Smith TO, Postle K, Penny F et al. . Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta-analysis comparing anterior cruciate ligament reconstruction versus non-operative treatment. Knee 2014;21:462–70. 10.1016/j.knee.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 6.Devitt BM, McCarthy C. ‘I am in blood Stepp'd in so far…’: ethical dilemmas and the sports team doctor. Br J Sports Med 2010;44:175–8. 10.1136/bjsm.2009.068056 [DOI] [PubMed] [Google Scholar]
- 7.Grindem H, Eitzen I, Moksnes H et al. . A pair-matched comparison of return to pivoting sports at 1 year in anterior cruciate ligament-injured patients after a nonoperative versus an operative treatment course. Am J Sports Med 2012;40:2509–16. 10.1177/0363546512458424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med 2005;39:127–31. 10.1136/bjsm.2004.010900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engebretsen L. ACL surgery is not for all patients, nor for all surgeons. Knee Surg Sports Traumatol Arthrosc 2014;22:1–2. 10.1007/s00167-013-2748-9 [DOI] [PubMed] [Google Scholar]
- 10.Øiestad BE, Engebretsen L, Storheim K et al. . Knee osteoarthritis after anterior cruciate ligament injury; a systematic review. Am J Sports Med 2009;37:1434–43. 10.1177/0363546509338827 [DOI] [PubMed] [Google Scholar]
- 11.Waldén M, Hägglund M, Ekstrand J. High risk of new knee injury in elite footballers with previous anterior cruciate ligament injury. Br J Sports Med 2006;40:158–62. 10.1136/bjsm.2005.021055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan Y. Identifying individuals with an anterior cruciate ligament-deficient knee as copers and noncopers: a narrative literature review. J Orthop Sports Phys Ther 2011;41:758–66. 10.2519/jospt.2011.3384 [DOI] [PubMed] [Google Scholar]
- 13.Herrington L, Fowler E. A systematic literature review to investigate if we identify those patients who can cope with anterior cruciate ligament deficiency. Knee 2006;13:260–5. 10.1016/j.knee.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Frobell RB, Roos EM, Roos HP et al. . A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med 2010;363:331–42. 10.1056/NEJMoa0907797 [DOI] [PubMed] [Google Scholar]
- 15.Renstrom P. Eight clinical conundrums relating to anterior circulate ligament (ACL) injury in sport: recent evidence and a personal reflection. Br J Sports Med 2013;47:367–72. 10.1136/bjsports-2012-091623 [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald GK, Lephart SM, Hwang JH et al. . Hop tests as predictors of dynamic knee stability. J Orthop Sports Phys Ther 2001;31:588–97. 10.2519/jospt.2001.31.10.588 [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc 2000;8:76–82. [DOI] [PubMed] [Google Scholar]
- 18.Borsa PA, Lephart SM, Irrgang JJ et al. . The effects of joint position and direction of joint motion on proprioceptive sensibility in anterior cruciate ligament-deficient athletes. Am J Sports Med 1997;25:336–40. 10.1177/036354659702500311 [DOI] [PubMed] [Google Scholar]
- 19.Snyder-Mackler L, De Luca PF, Williams PR et al. . Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am 1994;76:555–60. [DOI] [PubMed] [Google Scholar]
- 20.Loenneke JP, Wilson GJ, Wilson JM. A mechanistic approach to blood flow occlusion. Int J Sports Med 2010;31:1–4. 10.1055/s-0029-1239499 [DOI] [PubMed] [Google Scholar]
- 21.Loenneke JP, Wilson JM, Marin PJ et al. . Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 2012;112:1849–59. 10.1007/s00421-011-2167-x [DOI] [PubMed] [Google Scholar]
- 22.Burd NA, Andrews RJ, West DW et al. . Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol 2012;590:351–62. 10.1113/jphysiol.2011.221200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ageberg E. Consequences of a ligament injury on neuromuscular function and relevance to rehabilitation—using the anterior cruciate ligament-injured knee as model. J Electromyogr Kinesiol 2002;12:205–12. 10.1016/S1050-6411(02)00022-6 [DOI] [PubMed] [Google Scholar]


