Abstract
Introduction
Data on clinical characteristics and outcomes of infective endocarditis (IE) in the Pacific are scarce.
Methods
Retrospective hospital-based study in New Caledonia, a high-income country, on patients aged over 18 years with definite IE according to the modified Duke criteria (2005–2010).
Results
51 patients were included: 31 (60.8%) men; median age of 52.4 years (IQR 33.0–70.0). Left-sided IE accounted for 47 (92.2%) patients: native valve IE in 34 (66.7%) and prosthetic valve IE in 13 (25.5%). The main underlying heart disease included: rheumatic valve disease in 19 (37.3%), degenerative heart valve disease in 12 (23.5%) and congenital heart disease in 6 (11.8%). Significant comorbidities (Charlson's score >3) were observed in 20 (38.7%) patients. Infection was community acquired in 43 (84.3%) patients. Leading pathogens included Staphylococcus aureus in 16 (31.4%) and Streptococcus spp in 15 (29.4%) patients. Complications were noted in 33 patients (64.7%) and 24 (47.1%) were admitted to the intensive care unit. Cardiac surgery was eventually performed in 22 of 40 (55.0%) patients with a theoretical indication. None underwent emergent cardiac surgery (ie, first 24 h); 2 (3.9%) were operated within 7 days; and 20 (39.2%) beyond 7 days. 11 (21.6%) patients died in hospital and 21 (42.9%) were dead after a median follow-up of 28.8 months (IQR 4.6–51.2). Two (3.9%) were lost to follow-up.
Conclusions
In New Caledonia, IE afflicts relatively young patients with rheumatic heart disease, and carries high complication and mortality rates. Access to heart surgery remains relatively limited in this remote archipelago.
Keywords: VALVULAR DISEASE
Key messages.
What is already known about this subject?
There have been major changes in the characteristics and epidemiology of infective endocarditis over the past three decades in North America and Europe.
Cardiac surgery is part of the management of infective endocarditis, with increasing data that favours early intervention from studies conducted in tertiary centres.
What does this study add?
We provide contemporary data on the characteristics, access to treatment and long-term outcomes of infective endocarditis in a remote Pacific Island.
Rheumatic heart disease remains the main underlying predisposing factor in a young Oceanic population.
Access to early heart valve surgery is limited to a fraction of patients in whom surgery was indicated.
Outcomes remain poor with over 40% mortality at approximately 2 years of follow-up.
How might this impact on clinical practice?
Prevention of underlying aetiologies such as rheumatic heart disease should lessen the burden of infective endocarditis in Oceanic populations.
Early diagnosis and early referral to overseas cardiac surgery facilities may improve outcomes.
Introduction
Infective endocarditis (IE) is a rare but severe infectious disease that has been extensively described in western countries. The epidemiology of IE has significantly changed across North America and Europe in recent years by affecting an increasing ageing population with comorbidities. Presentation is often acute nowadays, and characterised by a high rate of Staphylococcus aureus infection, cardiac complications and embolic events.1–3 Early surgery has become a mainstay in the treatment of IE.4–7 Series originate mainly from referral centres where access to urgent surgery is readily available,1 6 8 9 and expertise may significantly impact on outcomes.10 However, management and outcomes of IE in general hospitals located in remote settings may be drastically different, and have been scarcely explored so far.
We describe, by means of a general hospital-based retrospective study, the clinical characteristics, treatment and long-term outcomes of IE in New Caledonia, a high-income and remote South-Pacific archipelago.
Methods
Objectives
The main objective was to describe the clinical characteristics of patients admitted with IE in New Caledonia, their treatment and long-term outcomes.
Settings
New Caledonia, located in the southwest Pacific Ocean, is a special collectivity of France, with a population of approximately 250 000 inhabitants.11 Rheumatic heart disease remains prevalent among indigenous populations, with incidence in Oceanic populations, including Melanesians and Polynesians, being as high as 9.5 per 1000 schoolchildren, according to data obtained by means of a systematic echocardiography-based surveillance programme.12 The Centre Hospitalier Territorial de Nouvelle Calédonie is the only centre providing cardiology and infectious disease care in the archipelago. Air transport for urgent referral for remote communities is widely available within the archipelago. The New Caledonian social security system provides free-of-charge access to good-quality medicine, imaging and microbiological diagnostic testing. Nouméa, the capital city, can be reached by a 3 h flight from Sydney, Australia, the closest cardiac surgery centre. Patients in need of heart valve surgery are referred to neighbouring Australia by medical air transportation at no additional cost to the patient. Patient management is systematically discussed between the cardiologists and infectious disease specialists.13
Patients
Patients aged over 18 years, admitted from 1 January 2005 to 31 December 2010 to the Centre Hospitalier Territorial de Nouvelle Calédonie with definite IE according to the modified Duke criteria were included in the study.14
Data collection
Hospital records of individuals with a primary or secondary International Classification of Diseases 10th revision (ICD 10) separation diagnosis of IE (ICD I33.0) were selected. One hundred and thirty hospital charts were retrospectively reviewed, with a final definite IE diagnosis in 51 patients (figure 1). In case of IE reinfections or relapses, the first IE episode during the study period was considered for inclusion.
Figure 1.
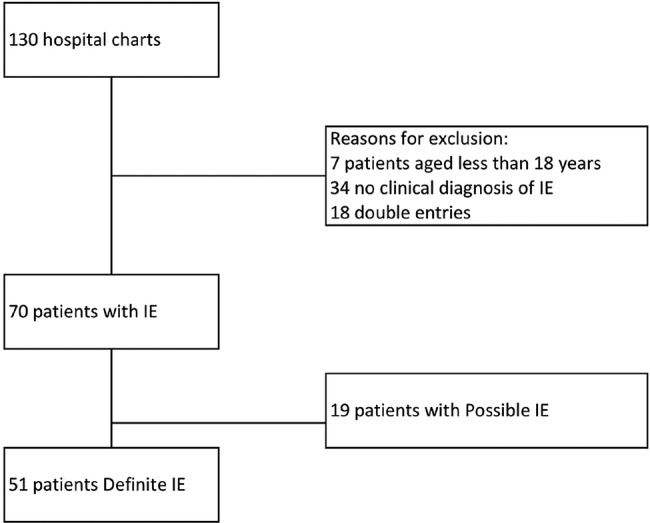
Flow chart of the study (IE, infective endocarditis).
The following data were collected: demographics; comorbidities assessed by the Charlson score15; underlying heart valve disease; history of cardiac surgery; IE characteristics (microbiological and echocardiographic findings); origin of bloodstream infection (as previously described);16 IE complications as per previously published guidelines5 and surgical treatment. Theoretical indication for surgery was also collected according to guidelines in use at the time of admission.17 Patients with left-sided and right-sided IE were assigned to the left-sided group. Those with native valve IE and prosthetic valve IE were assigned to the latter.
Outcome measures were: in-hospital mortality, relapse/reinfection and long-term mortality. Relapse referred to a repeat episode of IE caused by the same microorganism within the first 6 months, while reinfection included IE with a different microorganism or beyond 6 months after the first episode.5 Follow-up was undertaken as a cross-sectional study by contacting the patient or his/her next of kin in January 2013. Patients who could be joined were asked their New York Heart Association class (NYHA) functional status and occupation (employed or student, retired, unemployed), and whether they presented with fatigue or memory loss.
Statistical methods
The results are reported as median and IQR or as numbers and percentages. Categorical variables were compared using χ2 test or Fisher's exact test, and continuous variables using Student t test or Wilcoxon rank-sum test. Factors associated with long-term mortality were analysed using a Cox proportional hazard model, with binomial CIs. For the analysis of the overall population, time to death was calculated as time from hospital admission to death or last follow-up. Significance was defined as p values less than 0.05. Statistical analyses were performed using STATA software, version SE/12.1 (StataCorp LP, College Station, Texas, USA). Patients were asked to give oral consent to be enrolled in the study at the time of follow-up.
Results
Clinical characteristics at admission
Fifty-one patients with definite IE were included in the study (figure 1). Patient characteristics are depicted in table 1. Median age was 52.4 years (IQR 33.0–70.0). Thirty-one patients (60.8%) were men. Forty-three patients (84.3%) were Oceanic (including 33 indigenous Melanesians and 10 Polynesians) and 8 were non-Oceanic (11.8%) (6 European and 2 of other ethnic backgrounds). Overall, left-sided IE accounted for 47 (92.2%) cases: native valve IE in 34 (66.7%) and prosthetic valve IE in 13 (25.5%) patients. Four (7.8%) patients experienced pacemaker lead IE. Underlying heart disease included: rheumatic valve disease in 19 (37.3%), degenerative heart valve disease in 12 (23.5%), congenital heart disease in 6 (11.8%), functional in 2 (3.9%) and of unknown or unspecified origin in 12 (23.5%) patients. Four (7.8%) patients had a history of IE in keeping with reinfection, the first episode was before 2005. Significant comorbidities (Charlson's score >3) were observed in 20 patients (39.2%).
Table 1.
Characteristics by in-hospital mortality
| Factor | All patients N=51 |
Discharged alive N=40 |
Died in-hospital N=11 |
Univariate analysis p value* |
|---|---|---|---|---|
| Age ≥53 years, n (%) | 24 (47.1) | 16 (40.0) | 8 (72.7) | 0.05 |
| Male, n (%) | 31 (60.8) | 26 (65.0) | 5 (45.5) | 0.24 |
| Oceanic†, n (%) | 43 (84.3) | 34 (85.0) | 9 (81.8) | 0.80 |
| Charlson's score >3, n (%) | 20 (39.2) | 13 (32.5) | 7 (63.4) | 0.08 |
| Chronic renal replacement therapy, n (%) | 3 (5.9) | 2 (5.0) | 1 (9.1) | 0.61 |
| Left-sided IE, n (%) | 47 (92.2) | 37 (92.5) | 10 (90.9) | 0.86 |
| Native valve IE, n (%) | 34 (66.7) | 27 (67.5) | 7 (63.6) | 0.81 |
| Aetiology of valve disease, n (%) | 0.17 | |||
| Rheumatic heart disease | 19 (37.2) | 17 (42.5) | 2 (18.2) | |
| Degenerative | 12 (23.5) | 7 (17.5) | 5 (45.5) | |
| Congenital | 6 (11.8) | 5 (12.5) | 1 (9.1) | |
| Functional | 2 (3.9) | 2 (5.0) | 0 | |
| Healthcare-related IE, n (%) | 8 (15.7) | 6 (15.0) | 2 (18.2) | 0.80 |
| Microbiology, n (%) | ||||
| Streptococcus spp | 15 (29.4) | 12 (30.0) | 3 (27.3) | 0.86 |
| Staphylococcus aureus | 16 (31.4) | 12 (30.0) | 4 (36.4) | 0.69 |
| Acute heart failure, n (%) | 17 (33.3) | 13 (32.5) | 4 (36.4) | 0.81 |
| Severe valve regurgitation, n (%) | 15 (29.4) | 13 (32.5) | 2 (18.2) | 0.33 |
| Neurological complication, n (%) | 23 (45.1) | 18 (45.0) | 5 (45.5) | 0.87 |
| Shock, n (%) | 7 (13.7) | 2 (5.0) | 5 (45.5) | <0.01 |
| Vegetation length ≥15 mm, n (%) | 10 (19.6) | 7 (17.5) | 3 (27.3) | 0.39 |
| Perivalvular abscess‡, n (%) | 5 (9.8) | 4 (10.0) | 1 (9.1) | 0.94 |
| Cardiac surgery, n (%) | 22 (43.1) | 20 (50.0) | 2 (18.2) | 0.06 |
| Cardiac surgery denied§, n (%) | 19 (37.3) | 12 (30.0) | 7 (63.8) | 0.04 |
*Comparison of patients discharged alive versus patients who died during their first hospital admission.
†Includes indigenous Melanesians and Polynesians.
‡On echocardiography.
§In spite of at least one indication according to guidelines at the time of treatment.
IE, infective endocarditis.
IE was community acquired in 43 cases (84.3%), healthcare-related nosocomial in 7 cases (13.7%) and healthcare-related non-nosocomial in 1 case (2.0%).16 There were no intravenous drug users or patients with HIV infection. Pathogens identified by blood cultures were: S. aureus in 16 (31.4%) patients, of whom 1 had a methicillin-resistant strain and 15 had methicillin-susceptible strains; Streptococcus spp in 15 (29.4%) patients, of whom 8 had S. viridans (15.7%); coagulase-negative Staphylococcus in 7 (13.7%) patients; Enterococcus spp in 2 (3.9%) patients; Gram-negative bacteria (Citrobacter kosei, Actinobacillus) in 2 (3.9%) patients; S. pneumoniae in 1 (2.0%) patient; and Corynebacterium diphteriae in 1 (2.0%) patient. Microbiology was negative in seven patients (13.7%); three had received antibiotics before blood cultures were drawn. Primary site of infection in patients was dental in 7 (13.7%), cutaneous in 6 (11.8%), intravenous line/device related in 5 (9.8%), other in 5 (9.8%) and unknown in 27 (52.9%) patients. No patient with negative blood cultures who subsequently underwent surgery had PCR performed on the valve specimen.
Median time from first symptoms to admission was 3 days (IQR 2–29). Median time from hospital admission to IE diagnosis was 3 days (IQR 0–9).
Complications, investigations and treatment
Complications were noted in 33 patients (64.7%) and included: acute heart failure in 17 cases (33.3%), severe valve regurgitation in 15 cases (29.4%); neurological event (including symptomatic or asymptomatic stroke, haemorrhage, or encephalopathy) in 23 cases (45.1%); shock in 7 cases (13.7%); and conduction abnormalities in 2 cases (3.9%). Five patients (9.8%) presented with local complications (abscess, and/or fistula) on echocardiogram.
All patients underwent transthoracic echocardiography and blood cultures. Echocardiographic characteristics are depicted in table 2. Among 23 (45.1%) patients with neurological complications, 17 underwent a brain CT scan (of whom 13 had subsequent MRI) and 4 underwent brain MRI alone. Neurological complication was clinically silent in 3 of 23 patients.
Table 2.
Echocardiographic findings
| Echocardiography | All 51 IE cases |
|---|---|
| Transthoracic echocardiography, n (%) | 51 (100) |
| Transoesophageal echocardiography, n (%) | 34 (66.7) |
| Vegetation, n* (%) | 39 (76.5) |
| Vegetation size ≥10 mm† | 25 (49.0) |
| Vegetation size ≥15 mm† | 13 (25.5) |
| Mobile vegetation‡ | 39 (76.5) |
| Severe aortic regurgitation, n§ (%) | 7 (13.7) |
| Severe mitral regurgitation, n§ (%) | 8 (15.7) |
| Perivalvular abscess, n (%) | 5 (9.8) |
| Fistula, n§ (%) | 1 (2.0) |
| Valvular obstruction, n¶ (%) | 7 (13.7) |
| New abscess or fistula on serial echocardiography, n** (%) | 5 (9.8) |
| Increased severity in valvular regurgitation on serial echocardiography, n** (%) | 7 (13.7) |
*Missing data in two cases.
†Missing data in 11 cases.
‡Missing data in nine cases.
§Missing data in one case.
¶Missing data in three cases.
**Missing data in four cases; no pseudoaneurysm was noted.
IE, infective endocarditis.
Hospital length of stay was a median of 55 days (IQR 37–79). Median duration of intravenous antibiotic regimen was 42 days (IQR 30–45). Antibiotic regimens included: (1) a combination of amoxicillin and gentamycin in 12 of 15 cases with streptococcal infection, amoxicillin alone in 1 case, penicillin G and gentamycin in 1 case, and vancomycin and gentamycin in 1 case; (2) oxacillin and gemamycin in 9 of 14 cases with S. aureus methicillin-susceptible cases, oxacillin alone in 2 cases, oxacillin and amikacin in 1 case, oxacillin and rifampicin in 1 case, and cefotaxime and gentamycin in 1 case; (3) vancomycin and fosfomycin in 1 case of S. aureus methicillin-resistant case; and (4) a variety of regimens in negative blood cultures IE (amoxicillin and gentamycin in 2 cases, vancomycin and gentamycin in 1 case, ceftriaxone and gentamycin in 1 case, vancomcyin alone in 1 case, amoxicillin–clavulanate and gentamycin in 1 case, and ceftriaxone and gentamycin in 1 case). Twenty-four patients (47.1%) were admitted to the intensive care unit. Cardiac surgery was performed in 22 of 40 patients with a theoretical indication at a median 24 days (IQR 14–44) after IE diagnosis. None underwent emergent cardiac surgery (ie, first 24 h); 2 (3.9%) were operated within 7 days; and 11 (21.5%) between 8 and 30 days. The remaining nine (17.6%) patients were operated on after completion of antibiotic treatment. Eighteen (34.0%) patients did not undergo surgery in spite of theoretical indications. Reasons for denying surgery were available in 11 cases: underlying comorbidities in 4 (36.3%); neurological complications in 3 (27.3%); combination of comorbidities and neurological complications in 2 (18.2%); multiorgan failure including neurological complication in 1 (9.1%); and patient's refusal in 1 (9.1%) case.
Outcomes: in-hospital mortality and long-term outcomes
Eleven (21.6%) patients died in hospital after a median hospital stay of 25 days (IQR 16–43). Factors associated with in-hospital mortality on univariate analysis were: shock (p<0.001); and medical treatment alone in spite of surgical indications in 19 patients (p=0.04; table 1). Overall, 21 (42.9%) were dead at median 28.8-month follow-up (IQR 4.6–51.2); 2 (3.9%) were lost to follow-up (figure 2). Factors associated with long-term mortality on univariate analysis were: age (p=0.001); comorbidities by the Charlson score>3 (p<0.01); streptococcal IE (p<0.001); S. aureus IE (p=0.01); and cardiac surgery (p=0.02; table 3). The mortality rate was 57.1% (16/28) for patients treated medically compared to 23.8% (5/21) of those who underwent cardiac surgery (p=0.02). Among the 11 patients who died during the first hospital admission, the cause of death was documented in 10: 3 died from neurological complications of IE, 3 from heart failure or cardiogenic shock, 2 from septic shock at the acute phase of IE and 2 from underlying comorbidities. Among the remaining patients discharged alive and who subsequently died, cause of death could be collected in 3 of 10 cases: 1 died from heart failure, 1 from brain haemorrhage after the resolution of the IE episode under oral anticoagulant treatment and 1 from new sepsis. There were two (3.9%) relapses and two (3.9%) reinfections. Three patients (5.9%) needed redo surgery during the follow-up period.
Figure 2.
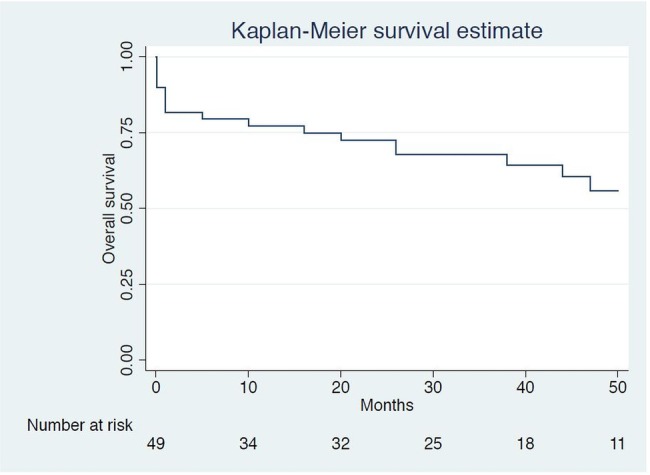
Kaplan-Meier survival estimates. Missing data for two patients.
Table 3.
Characteristics by long-term mortality (missing data for 2 patients)
| Factor | Alive at FU N=28 |
Dead at FU N=21 |
Univariate HR (95% CI) |
p Value |
|---|---|---|---|---|
| Age ≥53 years, n (%) | 9 (32.1) | 15 (71.4) | 1.22 (0.25 to 2.20) | 0.01 |
| Male, n (%) | 19 (67.9) | 11 (52.4) | −0.60 (−1.48 to 0.28) | 0.18 |
| Oceanic*, n (%) | 24 (85.7) | 17 (81.0) | −0.20 (−1.30 to 0.90) | 0.73 |
| Charlson's score >3, n (%) | 4 (14.3) | 16 (76.2) | 1.54 (0.52 to 2.55) | <0.01 |
| Chronic renal replacement therapy, n (%) | 0 | 3 (14.3) | −0.56 (−2.58 to 1.47) | 0.55 |
| Left-sided IE, n (%) | 25 (89.3) | 20 (95.2) | 0.15 (−1.88 to 2.18) | 0.89 |
| Native valve IE, n (%) | 20 (71.4) | 12 (57.1) | 0.57 (−0.53 to 1.63) | 0.31 |
| Aetiology of valve disease, n (%)† | −0.65 (−1.67 to 0.36) | 0.21 | ||
| Rheumatic heart disease | 12 (42.9) | 5 (28.8) | ||
| Degenerative | 3 (10.7) | 9 (42.9) | ||
| Congenital | 4 (14.3) | 2 (9.5) | ||
| Functional | 1 (3.6) | 1 (4.8) | ||
| Healthcare-related IE, n (%) | 1 (3.6) | 7 (33.3) | 0.61 (−0.35 to 1.58) | 0.21 |
| Microbiology, n (%) | ||||
| Streptococcus spp | 11 (39.3) | 2 (9.5) | −35.45 (−3.9×107 to 3.9×107) | 0.001 |
| Staphylococcus aureus | 4 (14.3) | 12 (57.1) | 1.11 (0.21 to 2.00) | 0.01 |
| Acute heart failure, n (%) | 9 (32.1) | 7 (33.3) | −0.28 (−1.24 to 0.69) | 0.58 |
| Severe valve regurgitation, n (%) | 10 (35.7) | 4 (19.0) | −0.82 (−1.93 to 0.29) | 0.15 |
| Neurological complication, n (%) | 13 (46.4) | 10 (47.6) | 0.04 (−0.83 to 0.92) | 0.92 |
| Shock, n (%) | 2 (7.1) | 5 (23.8) | – | – |
| Vegetation length ≥15 mm, n (%) | 5 (17.9) | 5 (23.8) | −0.12 (−1.94 to 0.85) | 0.81 |
| Perivalvular complications‡, n (%) | 2 (7.1) | 3 (14.3) | −0.91 (−2.94 to 1.11) | 0.38 |
| Cardiac surgery, n (%) | 16 (57.1) | 5 (23.8) | −1.29 (−2.33 to −0.25) | 0.02 |
*Indigenous Melanesians and Polynesians.
†Analysis of RHD versus other aetiologies.
‡Abscess or fistula on echocardiogram (no pseudoaneurysm noted). FU.
IE, infective endocarditis; FU, follow-up; RHD, rheumatic heart disease.
Among the 28 patients joined at follow-up (87.5% of survivors), 19 (67.9%) were in NYHA I, 9 (32.1%) in NYHA II and 2 (7.1%) in NYHA III. Thirteen (46.4%) reported about fatigue and 10 (35.7%) experienced concentration problems and memory loss. Sixteen patients (31.1%) were either employed or studying at follow-up.
Discussion
We present original data on IE, with long-term outcome measures, in a group of islands in the Pacific. The main underlying heart condition was rheumatic heart disease. The majority of patients presented with complicated IE. Barely half the surgical candidates were deemed fit for surgery. Access to emergent or urgent surgery is limited in this remote area. Mortality was extremely high (>40%) at long-term follow-up ∼2.5 years.
Clinical characteristics
Patterns of IE in New Caledonia, a high-income country, resemble those of many emerging market-economies with an on-going epidemiological transition.18 19 Although we focused our study on adults, patients were relatively young (mean age ∼53 years). Our patients are younger by 10 years when compared to a recent survey conducted in France.2 In the USA, patients with IE are increasingly older; 36% of patients are over 69 years of age.3
As in Europe and North America, the most frequent pathogen in IE was S. aureus.1–3 However, S. viridans remains the second leading pathogen in our series (15.7%), whereas it has almost disappeared in the USA.3 20 In our study, the microbiological profile reflects the emergence of healthcare-related bacteraemia and the persistence of poor dental health.18 Healthcare-associated IE was, however, present in a minority of our patients (13.7%). This finding differs from the epidemiology of IE in Western countries, where healthcare-associated IE accounts for over a third of cases.21
Rheumatic heart disease remains a major predisposing factor of IE, the condition being highly prevalent among Oceanic populations in New Caledonia.12 Although rheumatic heart disease still prevails in most of the developing countries and among indigenous populations,22 the importance of rheumatic heart disease as an underlying condition for IE is highly variable across other tropical or subtropical countries. The disease remains the main predisposing factor for IE in countries where a large proportion of the population lives in poverty,19 whereas its significance has diminished elsewhere over the past decade.18
Investigations, complications, treatment
Blood cultures and echocardiography were performed in all patients. However, advanced techniques for the diagnosis of blood culture negative endocarditis were not systematically performed.4 As in other series, approximately one-third of patients presented with heart failure and/or severe valve regurgitation,2 with neurological complications collected in almost half the cases,23 and a significant proportion (∼50%) being admitted to the intensive care unit.24
Management of IE is challenging,25 especially in non-surgical centres. Access to surgery, in our study, was relatively limited since barely 55% of patients with a theoretical indication for surgery were deemed fit for intervention.17 Of note, no patient underwent emergency surgery and only a minority of surgical candidates (3.9%) had surgery within 7 days after admission. Although comorbidities and neurological complications accounted for main reported reasons for denying surgery, no contraindications were identified in the remaining 7 of 18 surgical candidates not operated on. Remoteness may have contributed to favour medical treatment in patients with theoretical indications other than heart failure. Our study highlights the difficulties in applying current guidelines in remote locations,5 which may be even greater in poorly resourced settings.26 Of note, underuse of heart valve surgery is not restricted to IE or remote locations.27
Outcomes
Although the crude in-hospital case fatality rates observed are apparently similar to other reports, our patients were markedly younger.2 28 Early mortality was mostly IE related due to cardiogenic shock and/or neurological complications. Mortality almost doubled at long-term follow-up, reaching 42.9%. These findings highlight the severity of the condition and the need for longer follow-up periods to estimate the true impact of the disease.29 Factors associated with in-hospital and long-term mortality varied, as previously described by Bannay et al.29 Previously described factors, such as older age,30 31 comorbidities,32 microorganisms (S. aureus infection being of poor prognosis)31 33 and use of cardiac surgery,6 were associated with long-term outcomes in our study. The mortality rate is extremely high considering the young and relatively comorbid-free population affected by IE in our series. In addition, the rate of complications was similar to what has been described elsewhere.1 2 24 Reportedly, only patients admitted to intensive care experience higher case fatality rates after a similar follow-up period.34 Several hypotheses can be raised to explain our findings. Rapid diagnosis is key to early antibiotic treatment that impacts on the advent of complications, especially neurological emboli.35 Time from onset of symptoms to hospital admission varied, and there may still be room for improvement in the rapid diagnosis of IE through early referral from primary care centres. The higher than expected in-hospital mortality in our study may also be potentially and partly explained by the difficulties to access urgent cardiac surgery (ie, within the first 7 days), especially for those in cardiogenic shock.
Almost 1 of 10 patients experienced a relapse or reinfection during the follow-up period. This finding is a novelty in the field, seldom reported in the literature.10
In addition to high case fatality rates, only a third of survivors resumed their normal activities, albeit the majority did not experience cardiovascular symptoms. Of note, the mean unemployment rate in New Caledonia is 6%.11 Our results suggest that IE, a curable disease, may lead to long-term individual loss of income.
Ways of reducing the burden and mortality of IE
Prevention of rheumatic heart disease may diminish the incidence of IE in our population.22 Our findings should also promote periodontal health. Prophylaxis before oral procedures should be addressed in countries where the incidence of streptococcal IE remains high.36 Urgent referral to the hospital with echocardiography facilities may reduce the delay between the onset of symptoms and diagnosis, thereby preventing the advent of complications. Access to urgent heart surgery should be facilitated to prevent complications and decrease early mortality in patients with IE in countries with no on site surgical facilities.
Strengths and Limitations
This is, to the best of our knowledge, the first report on IE from a tropical Pacific Island. Our study suffers, however, from several limitations. It lacked power to assess factors associated with long-term outcomes given the sample size precluding multivariate analysis. Survivor selection bias prevents from drawing conclusions from the higher fatality rates among patients treated medically. Our results should foster further prospective studies to confirm our findings, find ways to increase access to surgery, and guide prevention programmes.
Conclusion
In New Caledonia, a high-income country located in the Pacific, IE still remains a disease of relatively young patients with underlying rheumatic heart disease. Almost half the surgical candidates are not deemed fit for cardiac surgery, and long-term mortality remains dramatically high. Ways to decrease the burden of IE and improve its management in remote locations need further assessment.
Footnotes
Contributors: MM designed and supervised the study, analysed the data and wrote the manuscript. RA, PBM and HC collected the data and provided critical review of the manuscript. FL, BN, JR, MN and CB provided critical review of the manuscript. SG provided the list of patients eligible to the study and provided critical review of the manuscript. EM, BI and XJ were involved in the design of the study, undertook critical review of the data analysis and provided critical review of the manuscript.
Competing interests: None declared.
Ethics approval: Ethical clearance was provided by the Ethical Review Committee of the Institut National de la Santé et de la Recherche Médicale (French Institute of Health and Medical Research), Paris, France (IRB 00003888FWA00005831).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Technical appendix is available by emailing MM (mariana.mirabel@inserm.fr).
References
- 1.Murdoch DR, Corey GR, Hoen B et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Int Med 2009;169:463–73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selton-Suty C, Celard M, Le Moing V et al. Preeminence of staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clinl Inf Dis 2012;54:1230–9. 10.1093/cid/cis199 [DOI] [PubMed] [Google Scholar]
- 3.Bor DH, Woolhandler S, Nardin R et al. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS ONE 2013;8:e60033 10.1371/journal.pone.0060033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuny F, Grisoli D, Collart F et al. Management of infective endocarditis: challenges and perspectives. Lancet 2012;379:965–75. 10.1016/S0140-6736(11)60755-1 [DOI] [PubMed] [Google Scholar]
- 5.Habib G, Hoen B, Tornos P et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for infection and cancer. Eur Heart J 2009;30:2369–413. 10.1093/eurheartj/ehp285 [DOI] [PubMed] [Google Scholar]
- 6.Kang DH, Kim YJ, Kim SH et al. Early surgery versus conventional treatment for infective endocarditis. NEJM 2012;366:2466–73. 10.1056/NEJMoa1112843 [DOI] [PubMed] [Google Scholar]
- 7.Erwin JP, Otto CM. Infective endocarditis: old problem, new guidelines and still much to learn. Heart 2014;100:996–8. 10.1136/heartjnl-2014-305836 [DOI] [PubMed] [Google Scholar]
- 8.Thuny F, Beurtheret S, Gariboldi V et al. Outcome after surgical treatment performed within the first week of antimicrobial therapy during infective endocarditis: a prospective study. Arch Cardiovasc Dis 2008;101:687–95. 10.1016/j.acvd.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Thuny F, Beurtheret S, Mancini J et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J 2011;32:2027–33. 10.1093/eurheartj/ehp089 [DOI] [PubMed] [Google Scholar]
- 10.Botelho-Nevers E, Thuny F, Casalta JP et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Int Med 2009;169:1290–8. 10.1001/archinternmed.2009.192 [DOI] [PubMed] [Google Scholar]
- 11.ISEE. http://www.isee.nc/population/telecharpdf/4page-rpnc09.pdf (accessed 1 Aug 2014).
- 12.Mirabel M, Fauchier B, Bacquelin R et al. Echocardiography screening to detect rheumatic heart disease A cohort study of schoolchildren in French Pacific Islands. Int J Cardiol 2015;188:89–95. 10.1111/jpc.12087 [DOI] [PubMed] [Google Scholar]
- 13.Chambers J, Sandoe J, Ray S et al. The infective endocarditis team: recommendations from an international working group. Heart 2014;100:524–7 10.1136/heartjnl-2013-304354. [DOI] [PubMed] [Google Scholar]
- 14.Li JS, Sexton DJ, Mick N et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 16.Friedman ND, Kaye KS, Stout JE et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Int Med 2002;137:791–7. 10.7326/0003-4819-137-10-200211190-00007 [DOI] [PubMed] [Google Scholar]
- 17.Baddour LM, Wilson WR, Bayer AS et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434. 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 18.Ferreiros E, Nacinovich F, Casabe JH et al. Epidemiologic, clinical, and microbiologic profile of infective endocarditis in Argentina: a national survey. The Endocarditis Infecciosa en la Republica Argentina-2 (EIRA-2) Study. Am Heart J 2006;151:545–52. 10.1016/j.ahj.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Gupta A, Kaul U et al. Infective endocarditis in an Indian setup: are we entering the ‘modern’ era? Ind J Crit Care Med 2013;17:140–7. 10.4103/0972-5229.117041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desimone DC, Tleyjeh IM, Correa de Sa DD et al. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association's endocarditis prevention guidelines. Circulation 2012;126:60–4. 10.1161/CIRCULATIONAHA.112.095281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benito N, Miro JM, de Lazzari E et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Int Med 2009;150:586–94. 10.7326/0003-4819-150-9-200905050-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marijon E, Mirabel M, Celermajer DS et al. Rheumatic heart disease. Lancet 2012;379:953–64. 10.1016/S0140-6736(11)61171-9 [DOI] [PubMed] [Google Scholar]
- 23.Klein I, Iung B, Labreuche J et al. Cerebral microbleeds are frequent in infective endocarditis: a case-control study. Stroke 2009;40:3461–5. 10.1161/STROKEAHA.109.562546 [DOI] [PubMed] [Google Scholar]
- 24.Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J 2010;31:1890–7. 10.1093/eurheartj/ehq110 [DOI] [PubMed] [Google Scholar]
- 25.Bolger AF. The special challenge of left-sided endocarditis. Heart 2011;97:1117–18. 10.1136/hrt.2010.218578 [DOI] [PubMed] [Google Scholar]
- 26.Zuhlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart 2013;99:1554–61. 10.1136/heartjnl-2013-303896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358–65. 10.1093/eurheartj/ehm001 [DOI] [PubMed] [Google Scholar]
- 28.Curlier E, Hoen B, Alla F et al. Relationships between sex, early valve surgery and mortality in patients with left-sided infective endocarditis analysed in a population-based cohort study. Heart 2014;100:1173–8. 10.1136/heartjnl-2013-304916 [DOI] [PubMed] [Google Scholar]
- 29.Bannay A, Hoen B, Duval X et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Eur Heart J 2011;32:2003–15. 10.1093/eurheartj/ehp008 [DOI] [PubMed] [Google Scholar]
- 30.Lopez J, Revilla A, Vilacosta I et al. Age-dependent profile of left-sided infective endocarditis: a 3-center experience. Circulation 2010;121:892–7. 10.1161/CIRCULATIONAHA.109.877365 [DOI] [PubMed] [Google Scholar]
- 31.Hill EE, Herijgers P, Claus P et al. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007;28:196–203. 10.1093/eurheartj/ehl427 [DOI] [PubMed] [Google Scholar]
- 32.Sy RW, Chawantanpipat C, Richmond DR et al. Development and validation of a time-dependent risk model for predicting mortality in infective endocarditis. Eur Heart J 2011;32:2016–26. 10.1093/eurheartj/ehp085 [DOI] [PubMed] [Google Scholar]
- 33.Lopez J, Fernandez-Hidalgo N, Revilla A et al. Internal and external validation of a model to predict adverse outcomes in patients with left-sided infective endocarditis. Heart 2011;97:1138–42. 10.1136/hrt.2010.200295 [DOI] [PubMed] [Google Scholar]
- 34.Mirabel M, Sonneville R, Hajage D et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur Heart J 2014;35:1195–204 10.1093/eurheartj/eht303. [DOI] [PubMed] [Google Scholar]
- 35.Dickerman SA, Abrutyn E, Barsic B et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J 2007;154:1086–94. 10.1016/j.ahj.2007.07.023 [DOI] [PubMed] [Google Scholar]
- 36.Parrish A, Maharaj B. Prevention of infective endocarditis in developing countries—justifiable caution? S Afr Med J 2012;102:652–4. [DOI] [PubMed] [Google Scholar]


