Abstract
Aim:
Blockade of EGFR by EGFR tyrosine kinase inhibitors such as erlotinib is insufficient for effective treatment of human pancreatic cancer due to independent activation of the Akt pathway, while amiloride, a potassium-sparing diuretic, has been found as a potential Akt inhibitor. The aim of this study was to investigate the anticancer effects of combined amiloride with erlotinib against human pancreatic cancer cells in vitro.
Methods:
Cell proliferation, colony formation, cell cycle and apoptosis were analyzed in 4 human pancreatic cancer cell lines Bxpc-3, PANC-1, Aspc-1 and CFPAC-1 treated with erlotinib or amiloride alone, or in their combination. The synergistic analysis for the effects of combinations of amiloride and erlotinib was performed using Chou-Talalay's combination index isobolographic method.
Results:
Amiloride (10, 30, and 100 μmol/L) concentration-dependently potentiated erlotinib-induced inhibition of cell proliferation and colony formation in the 4 pancreatic cancer cell lines. Isobolographic analysis confirmed that combinations of amiloride and erlotinib produced synergistic cytotoxic effects. Amiloride significantly potentiated erlotinib-induced G0/G1 cell-cycle arrest and apoptosis in Bxpc-3 and PANC-1 cells. Amiloride inhibited EGF-stimulated phorsphorylation of AKT, and significantly enhanced erlotinib-induced downregulation of phorsphorylation of EGFR, AKT, PI3K P85 and GSK 3β in Bxpc-3 and PANC-1 cells.
Conclusion:
Amiloride sensitizes human pancreatic cancer cells to erlotinib in vitro through inhibition of the PI3K/AKT signaling pathway. Treatment of pancreatic cancer patients with combination of erlotinib and amiloride merits further investigation.
Keywords: amiloride, erlotinib, pancreatic cancer, drug synergism, targeted therapy, PI3K/AKT signaling pathway
Introduction
Pancreatic cancer is one of the most refractory cancers, with a 5-year survival of less than 6%. In 2013, this disease was the fourth leading cause of cancer deaths overall, and it was responsible for approximately 38 500 deaths in the United States1. Although surgical resection is the only known cure for pancreatic cancer, 80% of patients are diagnosed with advanced-stage disease, which includes metastatic or locally advanced cancers2. Therefore, less than 20% of people with pancreatic cancer have disease that can be surgically removed at the time of diagnosis. Gemcitabine or gemcitabine-based regimens are the standard chemotherapy in patients with advanced metastatic disease3,4.
Erlotinib (OSI-774, Tarceva, OSI Pharmaceuticals/Genentech), an oral small-molecule epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), was the first FDA-approved targeting agent for use in combination with gemcitabine in the management of pancreatic cancer. Miyabayashi K et al demonstrated that erlotinib could increase gemcitabine efficacy by inhibiting the gemcitabine-induced up-regulation of the MAPK pathway in a pancreatic tumor model5. Patients with advanced pancreatic cancer who were treated with the combination of erlotinib and gemcitabine had a statistically significant survival advantage over patients treated with gemcitabine alone. However, the erlotinib plus gemcitabine regimen only slightly prolonged the one-year survival rate from 17% to 23% compared with gemcitabine alone6. Hence, there remains an urgent medical need to find more effective therapeutic approaches to treat this deadly disease.
Aberrant overactivation of the EGFR signaling pathway has been implicated in several cellular processes contributing to cancer progression, including cell proliferation, survival, angiogenesis, invasion and metastasis7. However, blockade of EGFR by EGFR TKIs is insufficient for effective treatment of human pancreatic cancer. Over activation of EGFR downstream signaling through the PI3K/AKT, Ras/Raf/MAPK, and Jak/STAT pathways can have similar functions that lead to cell growth and survival by bypassing normal EGFR regulation. Mutations in K-Ras are found in approximately 61% of pancreatic cancers, leading to EGFR-independent activation of the PI3K/AKT and MEK/ERK pathways8. Therefore, dual blockage of EGFR and its downstream signaling might be a rational strategy for pancreatic cancer chemotherapy.
In non-small-cell lung cancer (NSCLC), it has been well documented that overactivation of AKT is secondary to activation of the c-met pathway. Inhibition of the PI3K/AKT pathway by blocking Met or using a PI3K inhibitor restores HGF-induced mechanisms of EGFR-TKI resistance to NSCLC9,10. Similarly, inhibition of the PI3K/AKT pathway also potentiated the cytotoxicity of EGFR-TKIs in breast cancer cells11,12. These findings suggest that simultaneous inhibition of the EGFR and PI3K/AKT pathways may be an effective therapeutic strategy to enhance erlotinib sensitivity in pancreatic cancers with aberrant PI3K/AKT signaling.
Amiloride is one of the most prescribed oral potassium-sparing diuretics used in the management of hypertension and congestive heart failure. It acts on epithelial sodium channels and is usually well tolerated at normal doses. Several studies have suggested its potential anti-cancer role because of its ability to induce apoptosis in various cancer cells in association with inactivation of PI3K/AKT and its downstream survival pathway13,14. In addition, amiloride augmented TRAIL-induced tumor cell apoptosis through AKT inactivation15. These studies indicate that amiloride may have the potential to cure tumors with aberrant overactivation of the PI3K/AKT pathway.
Based on these findings, we attempted to exploit the anti-cancer interaction between erlotinib and amiloride in a panel of human pancreatic cancer cell lines, which may provide a novel therapeutic strategy for combination chemotherapy in pancreatic cancer. This is the first study to report that amiloride can sensitize pancreatic cancer cells to the EGFR TKI erlotinib through the inhibition of PI3K/AKT signaling.
Materials and methods
Drugs and reagents
Amiloride hydrochloride was obtained from the National Institutes for Food and Drug Control (Beijing, China) and prepared in DMSO to obtain a 50 mmol stock solution. Erlotinib hydrochloride, LY294002, and BKM120 were purchased from Selleck Chemicals (Houston, TX) and dissolved in DMSO as a stock solution at 10 mmol. Aliquots were stored at −20 °C, and a working solution was freshly diluted with PBS before use.
Anti-phosphorylated-Thr202/Tyr204 ERK1/2 (E10) mouse monoclonal antibody, anti-ERK1/2 (L34F12) mouse monoclonal antibody, anti-EGFR rabbit polyclonal antibody, anti-phosphorylated-AKT (Ser473) rabbit monoclonal antibody, anti-phosphorylated-AKT (Ser308) rabbit monoclonal antibody, anti-AKT rabbit monoclonal antibody, anti-GSK-3β monoclonal antibody, anti-phosphorylated GSK-3β (Ser9) monoclonal antibody, anti-PI3 kinase p85 monoclonal antibody, anti-phosphorylated PI3 kinase p85 (Tyr458) monoclonal antibody, anti-PTEN monoclonal antibody, and anti-cyclin D1 (M-20) were all purchased from Cell Signaling Technology Inc. Anti-phosphorylated-EGFR (Tyr1068) rabbit monoclonal antibody was purchased from Invitrogen. Anti-cleaved poly (ADP-ribose) polymerase (PARP) (p85) antibody was purchased from Epitomics (Burlingame, CA, USA). Anti-β-actin primary antibody, horseradish peroxidase conjugated anti-mouse, and anti-rabbit secondary antibody were obtained from Santa Cruz Biotechnology Inc (Dallas, TX, USA).
Cell culture
The human pancreatic cancer cell lines Bxpc-3, Aspc-1, and CFPAC-1 were obtained from the Cell Bank of the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). Bxpc-3 and Aspc-1 cells were maintained in RPMI-1640 culture medium (HyClone, Beijing, China) supplemented with 10% (v/v) fetal calf serum (FBS, Gibco BRL, Grand Island, New York, USA). CFPAC-1 cells were cultured in IMDM (HyClone, China) medium with 10% (v/v) FBS. PANC-1 cells were kindly provided by Prof Ke YU (Fudan University, Shanghai, China) and cultured in DMEM (HyClone, China) with 10% (v/v) FBS. All cells were incubated at 37 °C in 5% CO2 in a CO2 incubator. Cells were routinely checked for Mycoplasma contamination.
Cell viability assay
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in 96-well plates at a density of 5000–10 000 cells/well and allowed to grow for 24 h. They were then exposed to various concentrations of amiloride, erlotinib, or their combinations for 48 h. The IC50 values at which the proliferation was reduced by 50% compared with the untreated control were calculated using nonlinear regression by GraphPad Prism 3.0 (GraphPad software, San Diego, CA, USA). Synergism analysis for the combination effects of amiloride and erlotinib was performed using Chou-Talalay's combination index isobolographic method using CompuSyn Version 1.0 software (ComboSyn, Inc, Paramus, NJ, USA)16.
Colony formation assays
The long-term anti-cancer effect was assessed by determining the ability of pancreatic cancer cells to form colonies after drug treatment. Cells were plated on six-well plates (1000–2000 cells/well) containing 2 mL complete culture medium, incubated for 48 h, and then treated with 0.5% DMSO (control), amiloride, erlotinib, or their combinations. After 10–15 d, the medium was removed, and the colonies in each well were stained with Giemsa solution (Sigma Chemical Co, St Louis, CA, USA), photographed and counted.
Cell cycle analysis
Exponentially growing cells were seeded in six-well plates and treated with amiloride (30 μmol/L) and erlotinib (3 μmol/L) alone or in combination for 12, 24, or 48 h. Cells were collected and washed with cold PBS. After centrifugation at 1200 r/min for 5 min, cells were thoroughly suspended in 0.3 mL PBS and fixed in ice-cold 70% ethanol at −20 °C overnight. The fixed cells were washed twice with PBS and stained with a solution containing 50 μg/mL of propidium iodide (PI, Sigma) and 100 μg/mL of RNase A (Sigma) for 30 min at 37 °C. Cell cycle profiles were obtained using a BD FACSCaliber flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed by the ModFit software package (Verify Software House Inc, Topsham, ME, USA).
Apoptosis analysis
Cells were cultured in 6-well plates and treated with amiloride (100 μmol/L) and erlotinib (3 μmol/L for Bxpc-3 cells and 10 μmol/L for PANC-1 cells) alone or in combination for 48 h. Apoptotic cells were then identified by dual staining with FITC-conjugated Annexin V and PI following the manufacturer's protocol (BD Biosciences, San Jose, CA). Briefly, the collected cells were washed twice with PBS and incubated with Annexin V-FITC and then PI for 15 min in the dark at room temperature. Cells were then analyzed within 1 h by flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ, USA) and a computer station running Cell-Quest software (Becton Dickinson, Franklin Lakes, NJ, USA). Meanwhile, stained cells were captured by a cooled CCD camera (Leica DMI4000D, Wetzlar, Germany) coupled to the microscope.
Western blotting
After exposure to 0.5% DMSO, amiloride, erlotinib or the combination of amiloride and erlotinib, the cells were washed twice in ice-cold PBS and lysed in 1×NuPAGE® LDS sample buffer (Invitrogen, Carlsbad, CA, USA). The samples were then boiled for 10 min and centrifuged briefly. Samples were subjected to 10% SDS-PAGE and then transferred to a PVDF membrane. The membranes were then blocked with 5% non-fat milk and incubated with specific primary antibodies at 4 °C overnight. The membranes were washed and incubated with secondary HRP-conjugated antibodies. The membranes were washed again and detected using a chemiluminescence system according to the manufacturer's instructions (Immobilon™ western chemiluminescent HRP substrate, Millipore Corporation, Billerica, MA, USA).
Statistical analysis
All data were from at least three independent experiments and are expressed as means±SDs. One-way ANOVA followed by S-N-K multiple comparisons was used to determine the differences among groups. The level of significance was set at P<0.05.
Results
Amiloride enhanced the growth inhibition of erlotinib in a panel of human pancreatic cancer cell lines
The molecular profiles of the pancreatic cancer cell lines used in this study were obtained from the Sanger Institute (http://www.sanger.ac.uk/). Table 1 summarizes the histologic and molecular characteristics and the sensitivity of erlotinib and amiloride for the four pancreatic cancer cell lines. Because all the cell lines harbor wild-type EGFR, the anticancer effect of erlotinib was minor and resulted in IC50 values above 50 μmol/L. Amiloride mono-treatment also exerted minor anticancer activity against the tested cells, with IC50 values >100 μmol/L in all cases. Although previous studies reported the anti-cancer activity of amiloride in a panel of tumor cell lines, the results here indicated that pancreatic cancer cells had a relatively low in vitro sensitivity to amiloride.
Table 1. IC50 values of erlotinib or amiloride, and the mutation status of EGFR and K-Ras genes in human pancreatic cancer cell lines.
| Cell line | EGFR genotype | K-Ras genotype | IC50 of erlotinib (μmol/L, mean±SD) | IC50 of amiloride (μmol/L, mean±SD) |
|---|---|---|---|---|
| Bxpc-3 | WT | WT | 224.4±16.5 | 151.7±3.8 |
| PANC-1 | WT | G12D | 68.1±2.4 | 216.7±16.2 |
| Aspc-1 | WT | G12D | 103.7±8.8 | 283.9±26.1 |
| CFPAC-1 | WT | G12V | 125.8±7.3 | 188.3±17.9 |
WT: wild type; the mutation status of the EGFR or K-Ras genes was obtained from www.sanger.ac.uk; IC50 values were determined by MTT assay after 48 h treatment and calculated using nonlinear regression by GraphPad Prism 3.0, each value represents the mean±SD from at least three independent experiments.
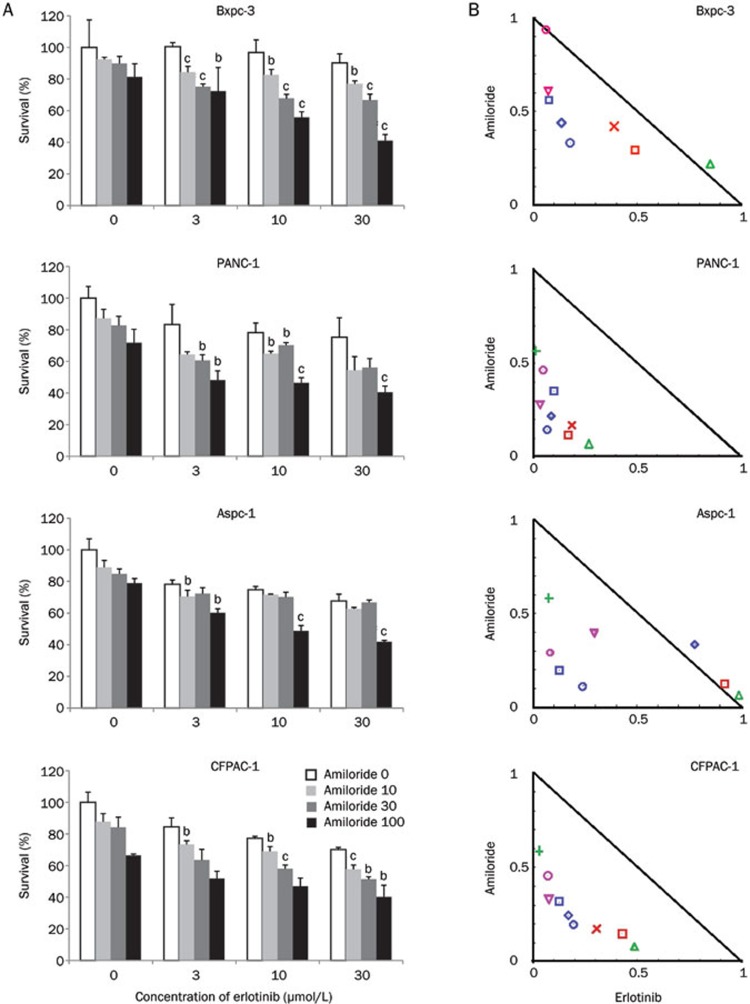
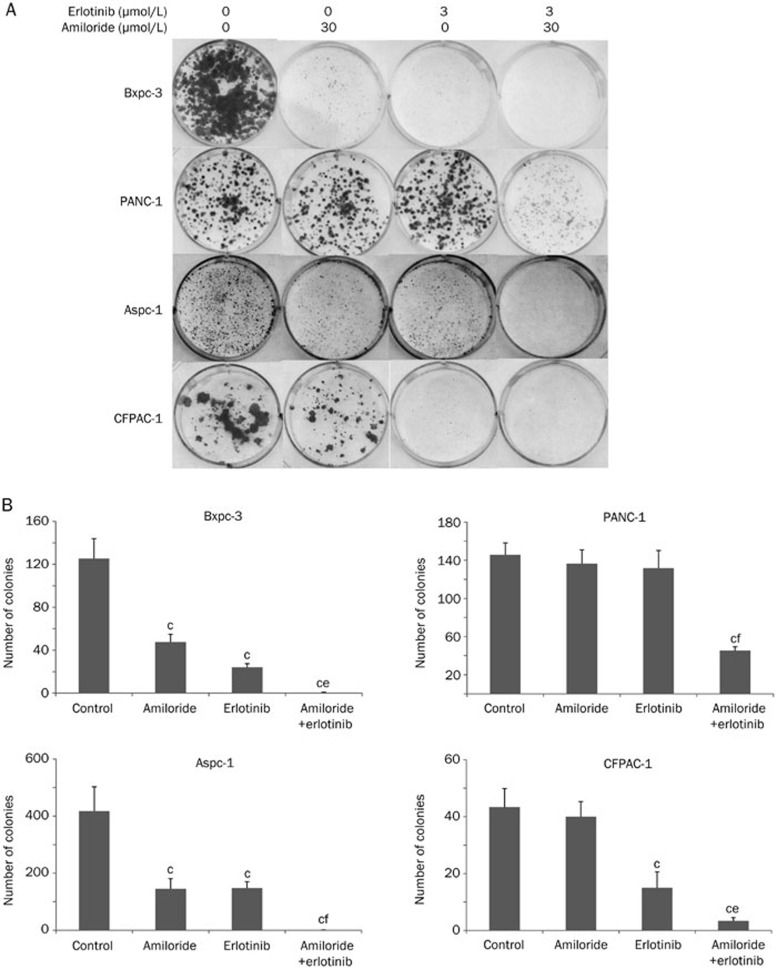
We therefore sought to determine whether the combination of erlotinib and amiloride would overcome the resistance observed with erlotinib treatment alone. The viability of Bxpc-3, PANC-1, Aspc-1, and CFPAC-1 pancreatic cancer cells treated with amiloride (10, 30, and 100 μmol/L), erlotinib (3, 10, and 30 μmol/L), or their combination was determined by the MTT assay (Figure 1A). Amiloride (10, 30, and 100 μmol/L) significantly enhanced the erlotinib-induced inhibition of tumor cell growth in a concentration-dependent manner in a heterogeneous group of pancreatic cancer cell lines, including one harboring a wild-type K-Ras (Bxpc-3), two lines with a K-Ras G12D mutation (PANC-1 and Aspc-1), and another cell line harboring a K-Ras G12V mutation (CFPAC-1). We also analyzed the interaction between amiloride and erlotinib using Chou-Talalay's synergism analysis, a widely used method to analyze the synergy interaction in combination therapies. Interestingly, synergistic drug activity was observed from the normalized isobolograms for drug combinations in all cell lines tested (Figure 1B). We also assessed the long-term anti-cancer effects of combination treatment on cell viability by clonogenic assays, as shown in Figure 2. The combination of amiloride and erlotinib resulted in a significant inhibition of colony formation in all the cell lines when compared with erlotinib alone. Taken together, these data revealed that amiloride enhanced the short-term and long-term growth inhibition of erlotinib in a panel of human pancreatic cancer cell lines independent of K-Ras status.
Figure 1.
Growth inhibition of human pancreatic cancer cell lines treated with amiloride, erlotinib, and their combination were evaluated by the MTT assay. Bxpc-3, PANC-1, Aspc-1, and CFPAC-1 cells were treated with amiloride (10, 30, and 100 μmol/L), erlotinib (3, 10, and 30 μmol/L), and their combination, as described in the Materials and methods. (A) Amiloride potentiated the growth inhibition of erlotinib in a dose-dependent manner. Data are expressed as the mean±SD (n=3). Significance is indicated by bP<0.05, cP<0.01 versus groups treated with erlotinib alone. (B) Assessment of the combination of amiloride and erlotinib by isobologram analysis. Values below the line are synergistic, whereas those close to the line are additive and those above the line are antagonistic.
Figure 2.
Amiloride enhanced the long-term growth inhibition of erlotinib in Bxpc-3, PANC-1, Aspc-1, and CFPAC-1 human pancreatic cancer cells. Cells were treated with 0.5% DMSO, amiloride (30 μmol/L), erlotinib (3 μmol/L), or their combination in triplicate. Colonies were stained with Giemsa and photographed. Experiments were repeated three times. (A) A representative photograph is shown. (B) Colonies consisting of greater than 20 cells were counted as shown. Mean±SD. n=3. bP<0.05, cP<0.01 compared with control. eP<0.05, fP<0.01 compared with groups treated with erlotinib alone.
Amiloride plus erlotinib exhibited significant effects on G1 cell cycle arrest and apoptosis
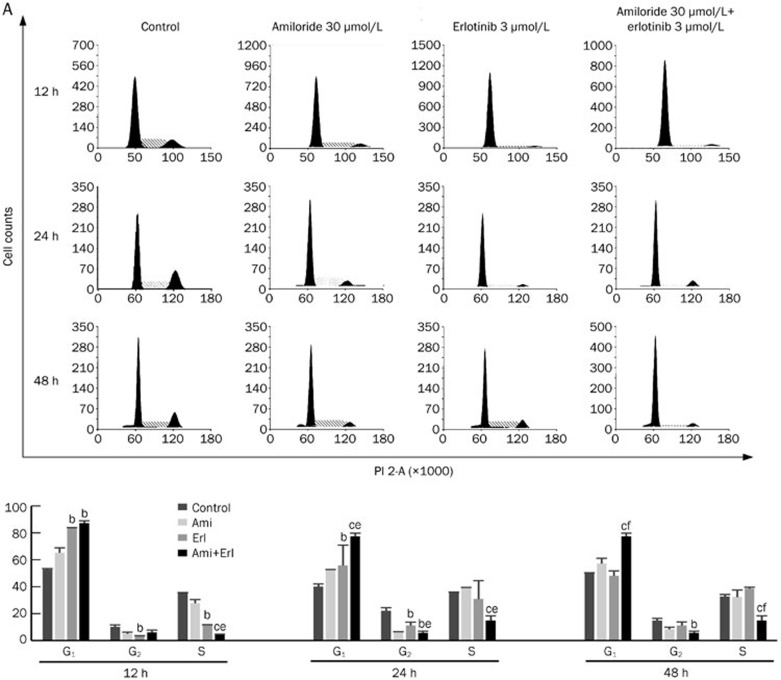
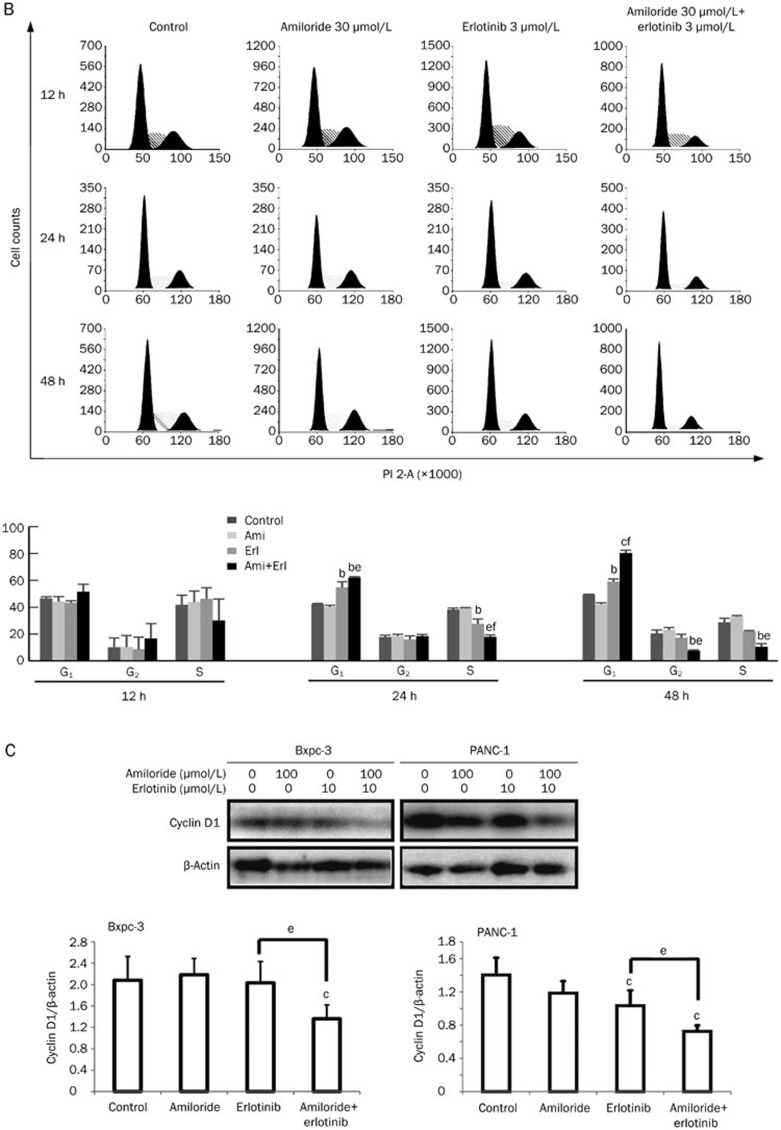
The cell cycle distribution was analyzed in cells exposed to amiloride, erlotinib, or their combination by flow cytometry. After single amiloride (30 μmol/L) or erlotinib (3 μmol/L) treatment for 24 or 48 h, the cell cycle profiles showed that Bxpc-3 and PANC-1 had a higher proportion of cells in G0/G1 phase and a lower proportion of cells in S phase (Figure 3A and 3B). This G1-phase arrest effect was more pronounced with concurrent exposure of cells to amiloride and erlotinib. Phase-specific cyclin D1 was also determined after 24 h of exposure by immunoblotting in parallel experiments. As shown in Figure 3C, concurrent administration of erlotinib and amiloride inhibited the expression of cyclin D1 to an increased extent compared with erlotinib treatment alone, consistent with G1-phase arrest. These data provide evidence that amiloride and erlotinib act synergistically to induce cell cycle arrest in G1 phase, which may explain the synergistic interaction of amiloride and erlotinib in pancreatic cancer.
Figure 3A.
Cell cycle effects of combination treatments with amiloride and erlotinib. Bxpc-3 and PANC-1 cells were incubated with amiloride (30 μmol/L) and erlotinib (3 μmol/L) alone or in combination for 12, 24, and 48 h. Cell cycle distribution was analyzed after PI staining by flow cytometry. Quantitative results are expressed as the mean of the percentage of cells in different phases of the cell cycle from three independent experiments. (A) Cell cycle distribution in Bxpc-3 cells. Mean±SD. n=3. bP<0.05, cP< 0.01 versus control; eP<0.05, fP< 0.01 vs groups treated with erlotinib alone.
Figure 3B, 3C.
(B) Cell cycle distribution in PANC-1 cells. (C) Western blot analysis of cyclin D1 expression confirms the phase-specific cell cycle effects of amiloride and erlotinib alone or in combination. Mean±SD. n=3. cP<0.01 compared with control. eP<0.05 compared with groups treated with erlotinib alone.
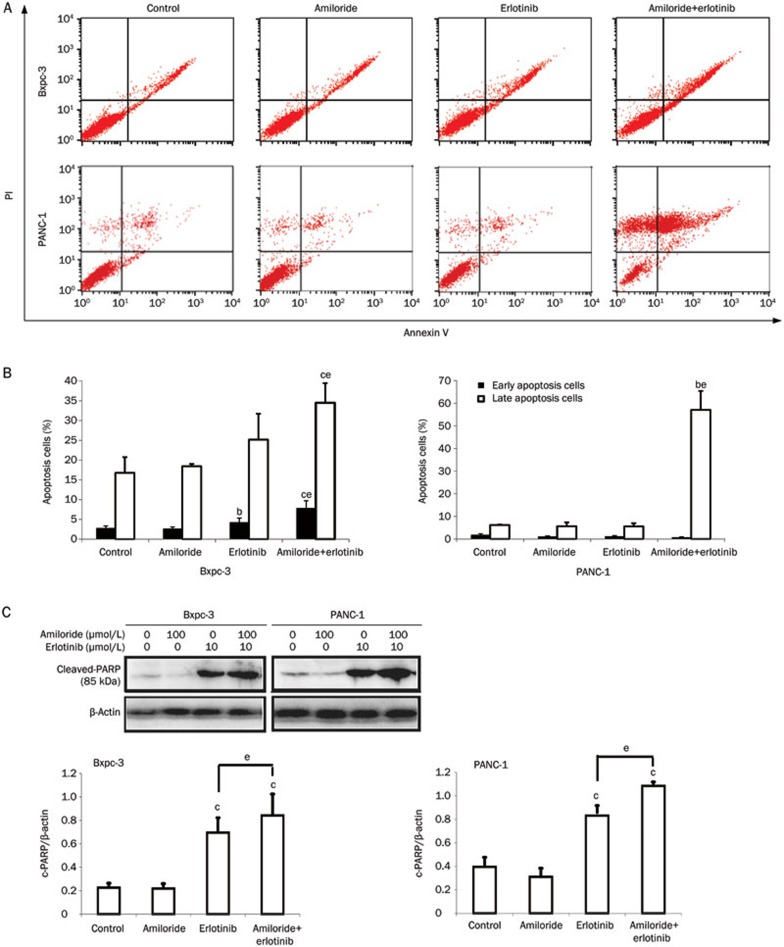
To determine whether apoptotic cell death was responsible for the synergistic cytotoxicity of amiloride and erlotinib, we performed flow cytometry analysis with Annexin V and PI staining. Cells were treated with amiloride, erlotinib, or their combination for 48 h. The results showed that the apoptosis rates of the amiloride plus erlotinib treatments in Bxpc-3 and PANC-1 cells were significantly increased compared with erlotinib treatment alone (Figure 4A and 4B). In addition, the combination of erlotinib and amiloride enhanced the expression of cleaved poly (ADP-ribose) polymerase (PARP) (p85) to a greater extent compared with erlotinib treatment alone (Figure 4C), thus confirming the synergistic apoptotic activity of the drug combination.
Figure 4.
Annexin V and PI staining for the apoptosis assay in Bxpc-3 and PANC-1 cells after 48 h of treatment with amiloride, erlotinib, or their combination. (A) Apoptosis was assayed by Annexin V and PI staining and fluorescence-activated cell sorting analysis. (B) The percentage of early and late apoptotic cells was indicated in the histogram. bP<0.05, cP<0.01 compared with control. eP<0.05 compared with groups treated with erlotinib alone. (C) Western blot analysis of cleaved PARP (p85) expression confirms the apoptotic effects of the drug combination.
Amiloride promoted the sensitivity of erlotinib through inhibition of the EGFR/PI3K/AKT signaling pathway
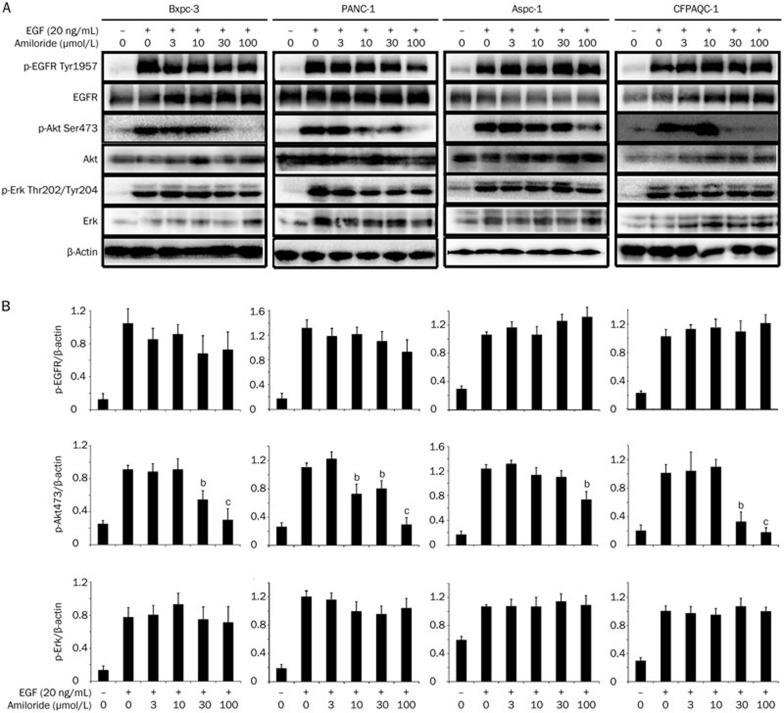
We further investigated the mechanism by which amiloride sensitized pancreatic cancer cells to erlotinib. First, we examined the impact of amiloride on the EGFR pathway. As indicated in Figure 5, amiloride caused an obvious inhibition of EGF-simulated phosphorylation of AKT in a dose-dependent manner, while it showed no effects on phospho-ERK in all four pancreatic cancer cells. Amiloride also showed slight inhibition of phospho-EGFR in Bxpc-3 and PANC-1 cells, but not in Aspc-1 and CFPAC-1 cells.
Figure 5.
Effects of amiloride on EGF (20 ng/mL) simulated phosphorylation of EGFR, Akt, and Erk in Bxpc-3, PANC-1, Aspc-1, and CFPAC-1 cells. Cells were pre-treated with different concentrations of amiloride for 2 h and then stimulated with 20 ng/mL EGF for 30 min, harvested and analyzed by Western blot analysis. (A) The experiment was independently repeated three times and representative results are shown in graphs. (B) Band intensities were quantified and the ratio of gray values for p-EGFR, p-Akt, and p-Erk was analyzed using β-actin as a control. Mean±SD. n=3. bP<0.05, cP<0.01 compared with control.
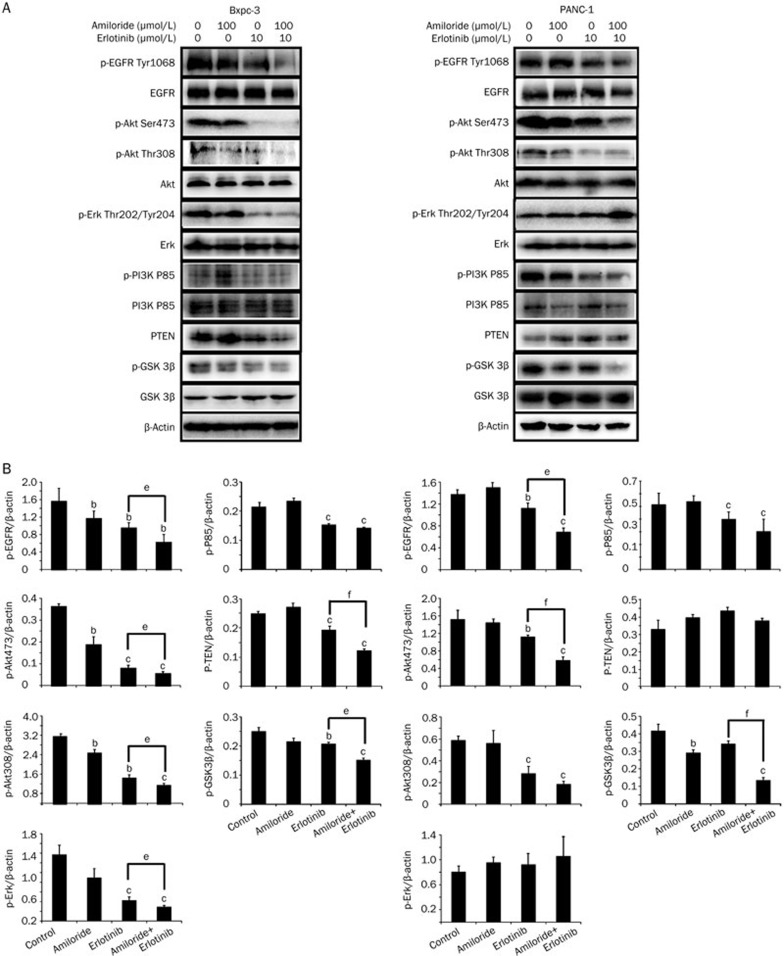
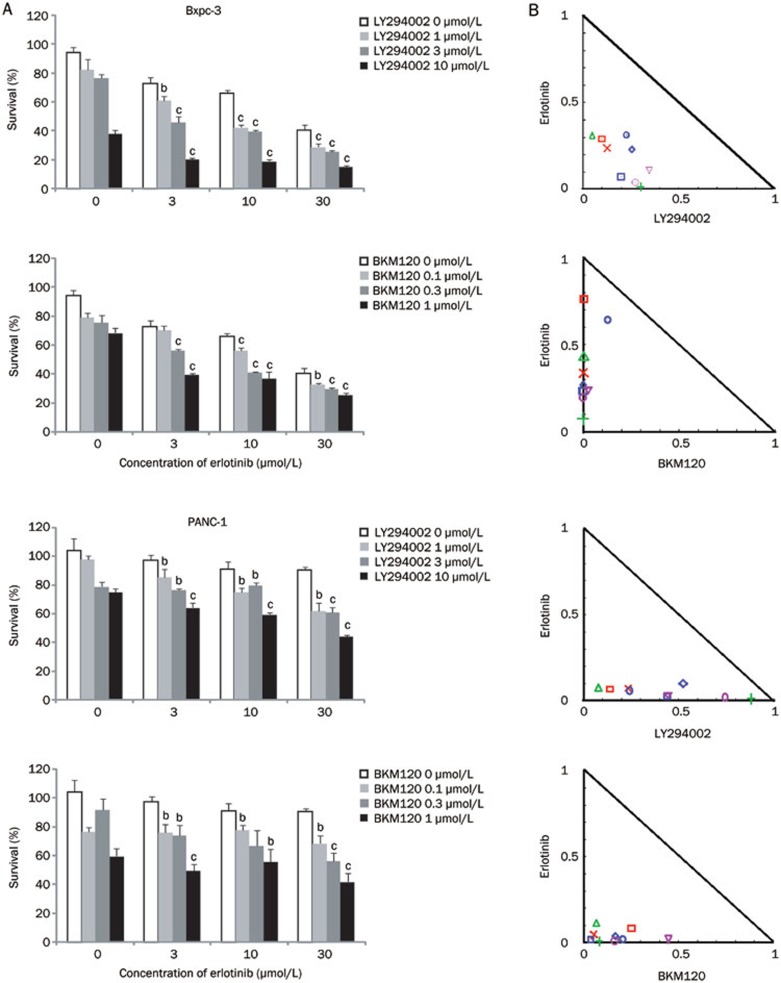
Next, we investigated the combination effects of erlotinib and amiloride on the EGFR signaling pathway after 12 h of drug exposure. In Bxpc-3 and PANC-1 cells, the down-regulation of phospho-EGFR, phospho-AKT (Ser 473), phospho-AKT (Thr 308), phospho-PI3K P85, and p-GSK 3β by erlotinib was significantly potentiated by co-administration with amiloride (Figure 6). However, combinational use of amiloride and erlotinib promoted down regulation of pho-ERK and PTEN in Bxpc-3 cells but not in PANC-1 cells. Collectively, these data indicate that at least one of the mechanisms of potentiation of erlotinib by amiloride is related to its inhibition of the EGFR/PI3K/AKT pathway, but not the MEK/MAPK pathway. In addition, we also investigated the effects of two PI3K inhibitors on the cell growth inhibition induced by erlotinib. As illustrated in Figure 7, both LY294002 and BKM120 showed obvious synergistic interaction with erlotinib, which indicates that the PI3K/AKT signaling pathway plays a critical role in the synergistic anti-cancer effects of amiloride and erlotinib.
Figure 6.
The effect of erlotinib and amiloride treatment on the EGFR signaling pathway in Bxpc-3 and PANC-1 cells. Cells were treated with erlotinib (10 μmol/L), amiloride (100 μmol/L), or their combination for 12 h and then harvested for western blot analysis (A). The experiment was independently repeated three times and representative results are shown in graphs (B) . Mean±SD. n=3. bP<0.05, cP<0.01 compared with control. eP<0.05, fP<0.01 compared with groups treated with erlotinib alone.
Figure 7.
Growth inhibition of human pancreatic cancer cell lines treated with amiloride, PI3K inhibitors (LY294002 and BKM120), or their combination was evaluated by the MTT assay. Bxpc-3 and PANC-1 cells were treated with erlotinib (3, 10, and 30 μmol/L), LY294002 (1, 3, and 10 μmol/L), BKM120 (0.1, 0.3, and 1 μmol/L), and the combinations for 72 h. (A) LY294002 and BKM120 potentiated the growth inhibition of erlotinib in a dose-dependent manner. Data are expressed as the mean±SD (n=3). Significance is indicated by bP<0.05, cP<0.01 vs groups treated with erlotinib alone. (B) Assessment of the combination of PI3K inhibitors and erlotinib by isobologram analysis. Values below the line are synergistic, whereas those close to the line are additive and those above the line are antagonistic.
Discussion
Amiloride has been widely used in the clinic as an oral potassium-sparing diuretic drug and is usually well tolerated at normal doses. Our results demonstrate for the first time that amiloride could synergize with erlotinib in various pancreatic cancer cell lines. These data provide a new promise for an old drug that may be used effectively in combination cancer therapies. Interestingly, the sensitization observed was independent of the mutation status of the K-Ras gene. Previous studies demonstrated that the K-Ras mutation can be regarded as a negative predictor of anti-EGFR agents in colorectal cancer17,18,19,20. In addition, K-Ras mutations were found to be present in approximately 61% of pancreatic cancers21. Thus, this combinational use of erlotinib and amiloride could particularly benefit pancreatic cancer patient subgroups that have little response to erlotinib monotherapy independent of K-Ras status.
The dependency of tumor cells on EGFR signaling for growth and survival is an important determinant of sensitivity to EGFR TKI monotherapy. A previous study revealed that amiloride may have the potential to inhibit EGFR phosphorylation22. Our studies supported that the combination of amiloride and erlotinib caused a significant inhibition of the EGFR/PI3K/AKT pathway compared with erlotinib alone. Up-regulation of phospho-AKT plays a pivotal role in tumor cell resistance to erlotinib23, and previous studies showed that inhibition of PI3K/AKT activity correlates with sensitivity to EGFR-TKIs in NSCLC cells24,25,26. Therefore, the addition of amiloride or agents that down-regulate EGFR/PI3K/AKT signaling may provide promise for improving the therapeutic efficacy of erlotinib in resistant tumor cells.
Many other studies have attempted to conquer pancreatic cancer treatment through dual blockage of EGFR and its downstream signaling. Diep et al reported that combination treatments with erlotinib and MEK inhibitors RDEA119 or AZD6244 had significant synergistic effects only in pancreatic cancer cells with wild-type K-Ras but not in cells with mutant K-Ras27. Amiloride was found to potentiate the growth inhibition of erlotinib in all the selected pancreatic cancer cell lines independent of K-Ras gene status. Considering the high incidence of K-Ras gene mutations in pancreatic cancer, dual blockage of EGFR and PI3K/AKT may be a better choice to treat this deadly disease.
Erlotinib causes cell growth arrest in G1 phase and subsequently induces apoptosis in a variety of tumor cells in vitro and in vivo28. We showed that amiloride also induced G1 phase arrest in Bxpc-3 cells, which was synergistic with erlotinib. In PANC-1 cells, amiloride and erlotinib alone failed to cause G1-phase arrest, but the cells were obviously arrested in G1 phase when treated with the combination of amiloride and erlotinib. Combination treatment of amiloride and erlotinib down regulated cyclin D1 in both Bxpc-3 and PANC-1 cells. Cyclin D1 was demonstrated to be a critical downstream effector of mutant EGFR signaling29. Previous studies indicated that gefitinib-sensitive cell lines showed G1 cell cycle arrest and inactivation of downstream signaling proteins, while resistant cell lines had no changes in NSCLC cells30. Therefore, reduction of cyclin D1 expression may be a sensitivity marker for the EGFR-TKI response. Our results indicate that cyclin D1 is down-regulated by amiloride through the suppression of GSK-3β signaling, resulting in G1 arrest and subsequent apoptosis. Collectively, these data suggest that the sensitizing effects of erlotinib by amiloride may be mediated via the cell cycle regulatory signaling pathway.
In conclusion, our study reported for the first time that amiloride can sensitize pancreatic cancer cells to erlotinib through down-regulation of the EGFR/PI3K/AKT pathway. Moreover, amiloride or other agents that modulate PI3K/AKT signaling may be useful therapeutic options for use in combination with erlotinib in pancreatic cancer patients. This new therapeutic strategy merits further investigation.
Author contribution
Yuan-ting ZHENG and Wei-min CAI designed the research; Yuan-ting ZHENG, Hui-ying YANG, Tao LI, Bei ZHAO, Teng-fei SHAO, and Xiao-qiang Xiang performed the research; and Yuan-ting ZHENG wrote the paper.
Acknowledgments
We sincerely appreciate Dr Li-xin Wu (Fudan-Zhangjiang New Drug R&D Joint Platform, Shanghai, China) for assistance with cell cycle analysis. The project was supported by the National Natural Science Foundation of China (81102459) and the Fundamental Research Funds for the Central Universities.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Werner J, Combs SE, Springfeld C, Harteig W, Hackert T, Büchler MW. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10:323–33. doi: 10.1038/nrclinonc.2013.66. [DOI] [PubMed] [Google Scholar]
- Burris H, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–52. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- Miyabayashi K, Ijichi H, Mohri D, Tada M, Yamamoto K, Asaoka Y, et al. Erlotinib prolongs survival in pancreatic cancer by blocking gemcitabine-induced MAPK signals. Cancer Res. 2013;73:2221–34. doi: 10.1158/0008-5472.CAN-12-1453. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. National cancer institute of canada clinical trials group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–67. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donev IS, Wang W, Yamada T, Li Q, Takeuchi S, Matsumoto K, et al. Transient PI3K inhibition induces apoptosis and overcomes HGF-mediated resistance to EGFR-TKIs in EGFR mutant lung cancer. Clin Cancer Res. 2011;17:2260–9. doi: 10.1158/1078-0432.CCR-10-1993. [DOI] [PubMed] [Google Scholar]
- Wang W, Li Q, Takeuchi S, Yamada T, Koizumi H, Nakamura T, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res. 2012;18:1663–71. doi: 10.1158/1078-0432.CCR-11-1171. [DOI] [PubMed] [Google Scholar]
- Li P, Torossian A, Zhang Q, Xu WC, Fu S. Inhibition of phosphoinositide 3-kinase enhances the cytotoxicity of AG1478, an epidermal growth factor receptor inhibitor, in breast cancer cells. Med Oncol. 2012;29:3258–64. doi: 10.1007/s12032-012-0279-8. [DOI] [PubMed] [Google Scholar]
- Yi YW, Hong W, Kang HJ, Kim HJ, Zhao W, Wang A, et al. Inhibition of the PI3K/AKT pathway potentiates cytotoxicity of EGFR kinase inhibitors in triple-negative breast cancer cells. J Cell Mol Med. 2013;17:648–56. doi: 10.1111/jcmm.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Baba M, Uehara H, Nakaizumi A. Chemoprevention by amiloride of experimental carcinogenesis in rat colon induced by azoxymethane. Carcinogenesis. 1995;16:941–2. doi: 10.1093/carcin/16.4.941. [DOI] [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Baba M, Yano H, Iseki K, Uehara H, et al. Inhibition by amiloride of experimental carcinogenesis induced by azaserine in rat pancreas. Cancer Lett. 1996;106:23–8. doi: 10.1016/0304-3835(96)04298-x. [DOI] [PubMed] [Google Scholar]
- Kim KM, Lee YJ. Amiloride augments TRAIL-induced apoptotic death by inhibiting phosphorylation of kinases and phosphatases associated with the PI3K-Akt pathway. Oncogene. 2005;24:355–66. doi: 10.1038/sj.onc.1208213. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Baynes RD, Gansert J. KRAS mutational status as a predictor of epidermal growth factor receptor inhibitor efficacy in colorectal cancer. Am J Ther. 2009;16:554–61. doi: 10.1097/MJT.0b013e318199fa17. [DOI] [PubMed] [Google Scholar]
- Ludovini V, Bianconi F, Pistola L, Chiari R, Minotti V, Colella R, et al. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:707–15. doi: 10.1097/JTO.0b013e31820a3a6b. [DOI] [PubMed] [Google Scholar]
- Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- Prior IA, Lewis PD, Mattos C. A comprehensive survey of ras mutations in cancer. Cancer Res. 2012;72:2457–67. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Czech MP. Amiloride directly inhibits growth factor receptor tyrosine kinase activity. J Biol Chem. 1985;260:2543–51. [PubMed] [Google Scholar]
- Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer. 2013;14:322–32. doi: 10.1016/j.cllc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–35. [PubMed] [Google Scholar]
- Tracy S, Mukohara T, Hansen M, Meyerson M, Johnson BE, Jänne PA. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 2004;64:7241–4. doi: 10.1158/0008-5472.CAN-04-1905. [DOI] [PubMed] [Google Scholar]
- Diep CH, Munoz RM, Choudhary A, VonHoff DD, Han H. Synergistic effects between erlotinib and MEK inhibitors in KRAS wild-type human pancreatic cancer cells. Clin Cancer Res. 2011;17:2744–56. doi: 10.1158/1078-0432.CCR-10-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. Clinical implications of the mechanism of epidermal growth factor receptor inhibitors. Cancer. 2006;107:1207–18. doi: 10.1002/cncr.22133. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Shimamura T, Monti S, Steidl U, Hetherington CJ, Lowell AM, et al. Transcriptional profiling identifies cyclin D1 as a critical downstream effector of mutant epidermal growth factor receptor signaling. Cancer Res. 2006;66:11389–98. doi: 10.1158/0008-5472.CAN-06-2318. [DOI] [PubMed] [Google Scholar]
- Helfrich BA, Raben D, Varella-Garcia M, Gustafson D, Chan DC, Bemis L, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–25. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]