Abstract
Ultraviolet (UV) radiation-induced loss of dermal extracellular matrix is associated with skin photoaging. Recent studies demonstrated that keratinocyte-releasable stratifin (SFN) plays a critical role in skin collagen metabolism by inducing matrix metalloproteinase 1 (MMP1) expression in target fibroblasts. In the present study, we examined whether SFN released from UVB-irradiated epidermal keratinocytes increases MMP1 release from dermal fibroblasts, and whether these events are affected by p-coumaric acid (p-CA), a natural phenolic compound with UVB-shielding and antioxidant properties. HaCaT cells were exposed to UVB in the absence and presence of p-CA, and the conditioned medium was used to stimulate fibroblasts in medium transfer experiments. The cells and media were analyzed to determine the expressions/releases of SFN and MMP1. UVB exposure increased SFN release from keratinocytes into the medium. The conditioned medium of UVB-irradiated keratinocytes increased MMP1 release from fibroblasts. The depletion of SFN using a siRNA rendered the conditioned medium of UVB-irradiated keratinocytes ineffective at stimulating fibroblasts to release MMP1. p-CA mitigated UVB-induced SFN expression in keratinocytes, and attenuated the MMP1 release by fibroblasts in medium transfer experiments. In conclusion, the present study demonstrated that the use of UV absorbers such as p-CA would reduce UV-induced SFN-centered signaling events involved in skin photoaging.
Keywords: Matrix metalloproteinase-1, Skin photoaging, Stratifin, UV
INTRODUCTION
Intrinsic aging of human skin due to genetic factors and the passage of time is inevitably exacerbated by extrinsic skin aging resulting from environmental factors such as solar radiation and pollutants [1]. Ultraviolet radiation (UV) is a major cause of photoaging, a type of extrinsic skin aging, which involves changes in the composition of the dermal extracellular matrix [2]. Matrix metalloproteinases (MMPs), a family of zinc endopeptidases, play a key role in the turnover of extracellular matrix macromolecules such as type I collagen [3,4]. Exposure to UV elevates the expression of MMPs in skin cells [5], which can impair the structural integrity of skin and trigger skin tissue remodeling, resulting in the formation of wrinkles and other phenotypes of skin aging [6,7].
Keratinocyte-releasable stratifin (SFN), also known as 14-3-3 sigma protein, is known to play a critical role in the induction of MMP1 expression in target fibroblasts by activating c-Fos and c-Jun through a p38 mitogen-activated kinase-dependent mechanism [8,9]. Consequently, SFN is an important signaling molecule involved in communication between keratinocytes and fibroblasts, especially concerning the regulation of collagen turnover in the dermis. A recent study demonstrated that increase of SFN due to UV radiation is potentially related to premature skin aging [10]. Thus, there is considerable interest in the development of cosmetic ingredients which can attenuate UV-induced, SFN-centered signaling events as a novel approach to retard skin photoaging processes.
In the present study, we examined whether SFN released from UVB-irradiated epidermal keratinocytes increases MMP1 release from dermal fibroblasts, and whether these events are affected by p-coumaric acid (p-CA), a natural metabolite produced in many plants such as Sasa quelpaertensis Nakai and Vaccinium bracteatum Thunberg [11,12]. p-CA was chosen as a test material because its UVB-shielding and anti-oxidant properties were previously demonstrated in cultured cells [13,14] and animal models [15,16,17]. The results from the current study showed that p-CA mitigated UVB-induced SFN expression in keratinocytes, and attenuated the MMP1 release by fibroblasts in medium transfer experiments, suggesting its utility as an active cosmetic ingredient.
METHODS
HaCaT cell culture
HaCaT cells were grown in Dulbecco's modified Eagle medium containing Nutrient Mixture F-12 (DMEM/F-12) medium (GIBCO-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U mL-1 penicillin, 0.1 mg mL-1 streptomycin, 0.25 µg mL-1 amphotericin B, and 10 µg mL-1 hydrocortisone. Cells were cultured at 37℃ in a humidified atmosphere containing 5% CO2.
UVB-exposure of HaCaT cells
HaCaT cells were seeded on a 6-well plate at a density of 3×105 cells per well and grown in the culture medium for 24 h. The cells were washed twice with PBS and exposed to UVB by using PBS as the irradiation medium. UVB irradiation was carried out under a cell culture hood using a UVB lamp (Model UVB-18; ULTRA*LUM Inc., Claremont, CA, USA) with maximum intensity at 300 nm. The intensity of UV radiation was determined using a UV light meter (Model UV 340A; Lutron Electronic Enterprise Co. Taipei, Taiwan). Cells were irradiated with UVB at a fixed intensity of 80 µW cm-2 for varied durations to provide different doses of UVB (5, 10, or 15 mJ cm-2) [18]. Following irradiation, the medium was changed to the growth medium, and the cells were incubated for 24 h. These cells were subjected to various assays as detailed below. The conditioned media from the control or the UVB-irradiated HaCaT cells were harvested and clarified by centrifugation at 1300×g for 5 min.
Transfection of HaCaT cells with a small interfering RNA (siRNA)
Human SFN siRNA (#1299001, HSS142243), human p53 siRNA (#1299001, HSS110905) and a negative control siRNA with scrambled sequences (#12935200) were purchased from Invitrogen (Grand Island, CA, USA). The nucleotide sequences of siRNAs were as follows: SFN (GenBank accession number NM_006142.3) 5'-UCU CAG UAG CCU AUA AGA ACG UGG U-3' (sense) and 5'-ACC ACG UUC UUA UAG GCU ACU GAG A-3' (antisense). For transfection, HaCaT cells were treated with a mixture of 20 nM siRNA and 1.25 µL mL-1 Lipofectamine RNAiMAX (Invitrogen) in Opti-MEM (Invitrogen) for 4 h followed by a 24 h recovery in the growth medium.
UVB irradiation of HaCaT cells in the presence of p-CA
p-CA was purchased from Sigma-Aldrich (St. Louis, MO, USA). HaCaT cells were exposed to UVB radiation at 15 mJ cm-2 or not, in PBS containing p-CA at the specified concentrations. After irradiation, cells were incubated in the growth medium without p-CA for 24 h.
Cell viability assay
Cell viability was assayed using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Briefly, cultured cells were washed with PBS and incubated in 1.0 mL culture media supplemented with 1.0 mg mL-1 MTT for 3 h. Following MTT incubation, the medium was discarded, and the accumulated formazan inside the cells was extracted with isopropanol and quantified by measuring the absorbance at 595 nm with a microplate reader (Model 550; Bio-Rad, Hercules, CA).
Culture of human dermal fibroblasts
Human dermal fibroblasts from adult skin were obtained from Cascade Biologics (Portland, OR, USA). The cells were cultured using Iscove's modified Dulbecco's medium (GIBCOBRL) containing 10% fetal bovine serum, 100 U mL-1 penicillin, 0.1 mg mL-1 streptomycin, and 0.25 µg mL-1 amphotericin B.
Treatment of fibroblasts with the conditioned medium of HaCaT cells
Human dermal fibroblasts were seeded on a 6-well plate at a density of 2×105 cells per well and grown in culture medium for 24 h. The fibroblasts were cultured for another 24 h after the growth medium was replaced with the conditioned medium of HaCaT cells.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total cellular RNA was extracted using the RNeasy Kit (Qiagen). To prepare the cDNA, 1 µg cellular mRNA was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). PCR amplification was conducted using the StepOnePlus™ Real-Time PCR System in reaction mixtures (20 µL) containing SYBR® Green PCR Master Mix, 60 ng cDNA, and 2 pmol gene-specific primer sets (Macrogen, Seoul, Korea). The primers used for PCR analysis are as follows: MMP1 (GenBank accession numbers NM_001145938.1) 5'-CAT ATA TGG ACG TTC CCA AAA TCC-3' (forward) and 5'-GTG CGC ATG TAG AAT CTG TCT TTA A-3' (reverse); SFN (NM_006142.3) 5'-AGA GAC ACA GAG TCC GGC AT-3' (forward) and 5'-ATG TCC TCA TAG CGT TCG GC-3' (reverse); glyceraldehyde 3-phosphate dehydrogenase (GAPDH; NM_0020-46.3) 5'-ATG GGG AAG GTG AAG GTC G-3' (forward) and 5'-GGG GTC ATT GAT GGC AAC AA-3' (reverse). Reactions were performed under the following conditions: 50℃ for 2 min, 95℃ for 10 min, 40 amplification cycles (95℃ for 15 s and 60℃ for 1 min), followed by a dissociation step. Melting curve analysis showed single peaks, which supported the homogeneity of amplicons. The mRNA expression level relative to the internal control GAPDH was calculated using the comparative threshold cycle method.
Western blot
Whole cell lysates of HaCaT cells and human dermal fibroblasts were prepared using a lysis buffer (10 mM Tris-Cl, pH 7.4, 120 mM NaCl, 25 mM KCl, 2 mM EGTA, 1 mM EDTA, 0.5% Triton X-100, and protease inhibitor cocktail). The total protein concentration was determined by the Lowry method. Aliquots of cell lysates (30 µg protein), or conditioned medium of HaCaT cells (40 µL) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions. Proteins separated on the gel were transferred to polyvinylidene fluoride membranes and stained with the Ponceau S reagent (Sigma-Aldrich). The membrane was incubated with an appropriate primary antibody overnight at 4℃, followed by a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. Immuno-reactive bands were detected using a picoEPD Western Reagent kit (ELPIS-Biotech, Daejeon, Korea) and subjected to densitometric analysis. The mouse monoclonal SFN antibody (ab14123) and the rabbit polyclonal MMP1 antibody (ab38929) were purchased from Abcam (Life Sciences-Biotech, Cambridge, UK). The rabbit polyclonal caspase 3 antibody (#9662) was purchased from Cell Signaling (Danvers, MA, USA). The mouse monoclonal β-actin antibody (#A5441) was purchased from Sigma-Aldrich.
Enzyme-linked immunosorbent assay (ELISA)
The human MMP1 ELISA Kit was purchased from Abcam. Human procollagen type I C-peptide (PIP) EIA Kit was purchased from Takara Bio, Inc. (Otsu, Japan). Human dermal fibroblasts were seeded in 48-well culture plates at a density of 5×104 cells per well and grown in the conditioned medium of HaCaT cells. After 24 h, the fibroblasts were washed with PBS and fed fresh fibroblast growth medium without serum, followed by an additional 24 h of incubation. The conditioned medium of the fibroblasts was collected, and the concentrations of MMP1 protein and PIP were measured using the aforementioned sandwich immunoassay kits according to the manufacturer's instructions. MMP1 protein samples (100 µL conditioned medium or 25~6000 pg mL-1 standard MMP1 protein) were transferred to microplate wells containing immobilized MMP1 antibody. The wells were washed and solutions of biotinylated MMP1 antibody and horseradish peroxidase-conjugated streptavidin were added. After washing the wells, the 3,3',5,5'-tetramethylbenzidine substrate solution was added to the wells to initiate enzymatic color development. After 30 min, the reaction was terminated using the stop solution, and the absorbance was measured at 450 nm. The PIP assay was used as a measure of collagen synthesis. In this assay, PIP antibody-peroxidase conjugate solution (100 µL) and samples (20 µL conditioned medium or 10~320 ng mL-1 of standard PIP) were added to the microplate well containing monoclonal PIP antibody. After a washing step, the substrate solution was added and the mixture was incubated for 15 min. Absorbance at 450 nm was measured after the addition of the stop solution.
Statistical analysis
Data are presented as the mean±the standard error of three or more independent experiments. The significance of differences between groups was determined using the Student's t-test, at a level of p<0.05.
RESULTS
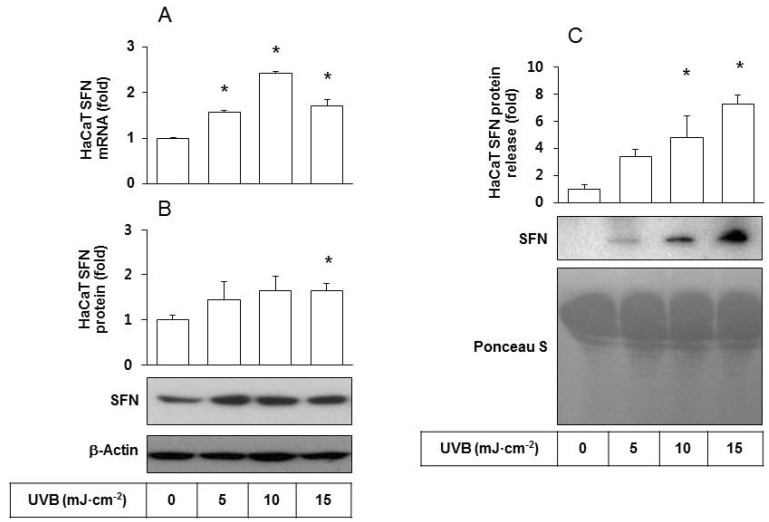
To examine cellular changes due to UVB irradiation, HaCaT cells were cultured and exposed to UVB at doses ranging from 5 to 15 mJ cm-2. The effects of UVB on SFN expression at the mRNA and protein levels were determined by qRT-PCR analysis and western blotting, respectively. The results showed that UVB exposure increased both mRNA and protein levels of SFN (Figs. 1A and 1B), indicating that SFN expression was stimulated by UVB at the transcription level. The level of SFN protein released was determined by western blot analysis of the conditioned medium derived from HaCaT cells. As shown in Fig. 1C, SFN protein release increased in a UVB dose-dependent manner. In contrast, the content of total soluble proteins stained with Ponceau S showed no significant changes due to UVB exposure.
Fig. 1. Effects of UVB irradiation SFN expression/release of HaCaT cells. HaCaT cells were irradiated with UVB at 5~15 mJ cm-2 and incubated for 24 h. (A) SFN mRNA levels were determined by qRT-PCR analysis using GAPDH as a control. (B) SFN protein levels were determined by Western blot analysis of whole cell lysates using β-actin as a control. (C) The released SFN protein levels were determined by Western blot analysis of the conditioned media derived from HaCaT cells exposed to different doses of UVB. For detection of total protein, the transferred membrane was stained with Ponceau S. Data are presented as fold changes versus the non-irradiated control (Mean±SE, n=3). *p<0.05 versus non-irradiated controls.
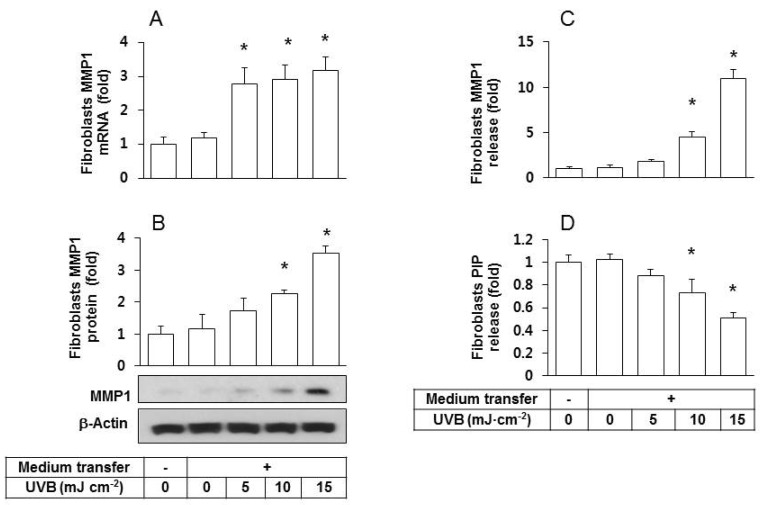
We previously showed that the conditioned medium of UVB irradiated HaCaT cells stimulated the MMP1 protein expression in fibroblasts [18]. In the current study, we repeated the identical medium transfer experiments and observed that fibroblasts grown in transferred condition medium had significantly augmented MMP1 mRNA (Fig. 2A) and protein expressions (Figs. 2A and 2B) compared to the control groups of fibroblasts without medium transfer and those treated with the conditioned medium of non-irradiated HaCaT cells. In addition, the release of MMP1 from fibroblasts into the extracellular space increased by the transfer with the conditioned medium of UVB-irradiated HaCaT cells (Fig. 2C).
Fig. 2. Effects of the conditioned medium derived from UVB-irradiated HaCaT cells on fibroblast expression/release of MMP1 and PIP. Human epidermal fibroblasts were seeded and cultured in growth medium for 24 h then the medium was replaced with the conditioned medium derived from HaCaT cells exposed to UVB at 5~15 mJ cm-2. After a 24 h incubation period, MMP1 expression was determined at the mRNA by qRT-PCR (A), and at the protein levels by western blots (B). The levels of MMP1 (C) and PIP (D) in the conditioned medium of fibroblasts were determined using sandwich immunoassay kits. Data are presented as fold changes versus the control cells without medium transfer (mean±SE, n=3). *p<0.05 versus controls.
Collagen molecules are synthesized from procollagen precursors, which contain additional N- and C-terminal propeptide sequences [19]. These propeptides are cleaved from collagen molecules towards the end of the collagen maturation process. Thus, the concentration of propeptides such as PIP directly correlates with collagen synthesis. This relationship was employed to measure the effects of the conditioned medium of UVB-irradiated HaCaT cells on fibroblast release of PIP into the extracellular space. The results showed a depressed release of PIP (Fig. 2D) from fibroblasts into the extracellular space.
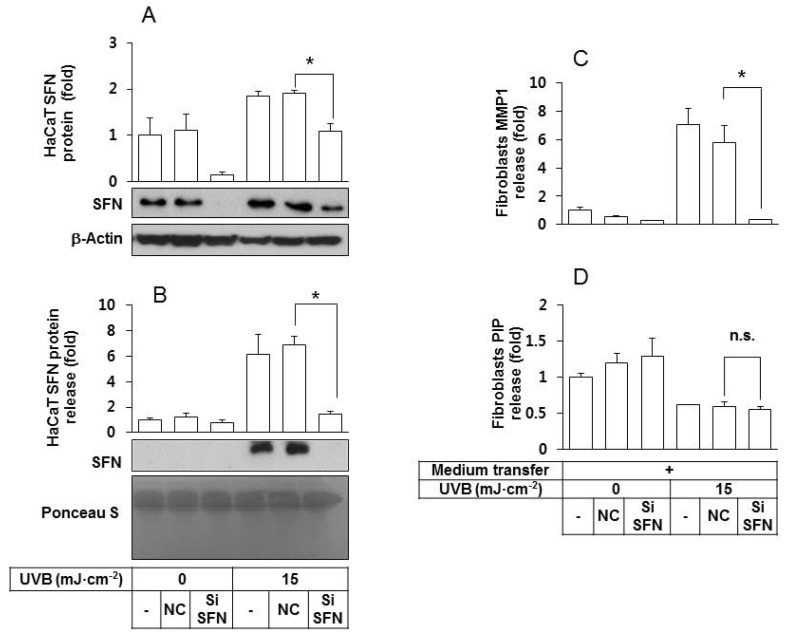
In order to determine whether SFN released from UVB-irradiated keratinocytes plays a critical role in stimulating fibroblasts to release MMP1, HaCaT cells were transfected with SFN siRNA under various conditions. As shown in Fig. 3A, SFN expression in the SFN siRNA-transfected HaCaT cells was much lower than in the control cells, under both non-irradiated and UVB-irradiated conditions. In addition, the quantity of SFN protein in the conditioned medium of UVB-irradiated HaCaT cells was lowered by SFN siRNA treatments (Fig. 3B). The depletion of SFN rendered the conditioned medium of UVB-irradiated HaCaT cells ineffective at stimulating fibroblasts to release MMP1 (Fig. 3C) without significantly affecting the levels of PIP (Fig. 3D).
Fig. 3. An essential role for SFN derived from UVB-irradiated HaCaT keratinocytes in stimulating fibroblasts to express/release MMP1 protein. (A, B) HaCaT cells were transfected with a SFN siRNA or negative control siRNA for 24 h, followed by exposure to UVB at 15 mJ cm-2. After a 24 h incubation period, the intracellular (A) and released SFN protein levels (B) were determined by western blots of whole cell lysates and the conditioned media, respectively. For detection of total protein, transferred membrane was stained with Ponceau S. (C, D) Fibroblasts were cultured for 24 h in their own growth medium or the conditioned medium derived from HaCaT cells. The medium was then replaced by the fibroblasts growth medium without serum. After 24 h of incubation, MMP1 (C) and PIP (D) in the conditioned medium of fibroblasts were quantified using sandwich immunoassay kits. Data are presented as fold changes versus the control cells without medium transfer (Mean±SE, n=3). *p<0.05; n.s., not significant.
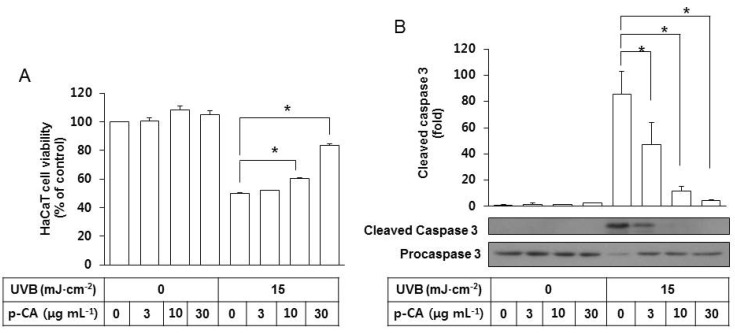
In additional experiments, the effects of p-CA on UVB-induced cytotoxicity and SFN-centered signaling events were examined. We reported in a previous study [18] that HaCaT cell viability was reduced by UVB exposure, and this change was accompanied by the activation of caspases involved in apoptosis [20,21]. In the present study, p-CA was included in the irradiation medium at 3~30 µg mL-1 during UVB exposure of HaCaT cells at 15 mJ cm-2 to examine its effects on apoptotic cell death induced by UVB. p-CA attenuated the UVB-induced cell death of HaCaT cells in a dose-dependent manner (Fig. 4A). As expected, UVB irradiation increased cleavage of procaspase-3 to its cleaved active form, and this change was attenuated by p-CA in a dose-dependent manner (Fig. 4B). In the absence of UVB irradiation, p-CA has no effects on cell viability and caspase 3 activation.
Fig. 4. Effects of p-CA on the UVB-induced cytotoxicity and caspase 3 activation in HaCaT cells. HaCaT cells were irradiated with UVB at 15 mJ cm-2 or not, in the absence or the presence of p-CA at the indicated concentrations and incubated for 24 h. (A) Cell viability was determined using an MTT assay. (B) Whole cell lysates of equal amounts of proteins were used for the western blot analysis of the active cleaved and inactive proforms of caspase 3. Data are presented as % of control or fold changes versus the control (Mean±SE, n=3). *p<0.05.
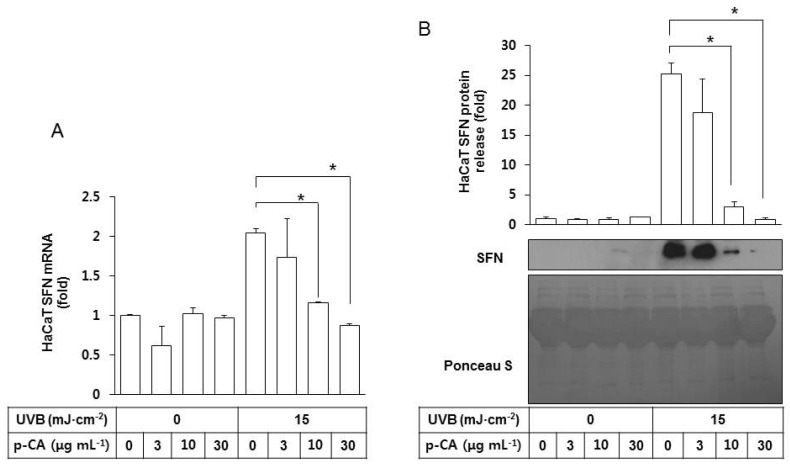
We then examined the effects of p-CA on the SFN expression and release by HaCaT cells irradiated with UVB. As shown in Fig. 5A, p-CA had no significant effects on basal SFN mRNA expression but it suppressed UVB-stimulated SFN expression in a dose-dependent manner. It also decreased SFN protein release from the UVB-irradiated HaCaT cells into the conditioned medium in a dose-dependent manner (Fig. 5B).
Fig. 5. Effects of p-CA on the UVB-induced SFN expression/release of HaCaT cells. HaCaT cells were irradiated with UVB at 15 mJ cm-2 or not, in the absence or the presence of p-CA at the indicated concentrations. The cells were then cultured in the fresh medium without p-CA for 24 h to harvest the cells and the conditioned medium. (A) SFN mRNA levels were determined by qRT-PCR analysis using GAPDH as a control. (B) The released SFN protein levels were determined by western blot analysis of the conditioned media derived from HaCaT cells. For detection of total protein, the transferred membrane was stained with Ponceau S. Data are presented as fold changes versus the control cells (mean±SE, n=3). *p<0.05.
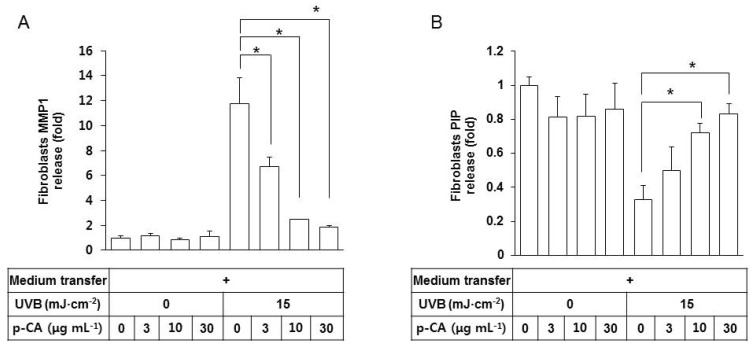
Because the conditioned medium of UVB-exposed HaCaT keratinocytes stimulated MMP-1 expression in fibroblasts via paracrine effects, we tested if this effect can be counteracted by p-CA in the medium transfer experiments. The conditioned medium from HaCaT cells, exposed to UVB in the absence or presence of p-CA, was transferred to fibroblasts cultures, and the releases of MMP-1 and PIP from fibroblasts were determined. As shown in Fig. 6A, the conditioned media of HaCaT cells, UVB-irradiated in the presence of increasing doses of p-CA, were less effective at stimulating fibroblasts to release MMP1 compared to the positive control medium of the cells UVB-irradiated in the absence of p-CA. The former medium was also less effective decreasing PIP release than the positive control medium (Fig. 6B). p-CA itself had no effects on the release of MMP1 or PIP from fibroblasts under non-irradiated conditions.
Fig. 6. Effects of the conditioned medium of HaCaT cells UVB-irradiated in the presence of p-CA on the releases of MMP1 and PIP from fibroblasts. HaCaT cells were irradiated with UVB at 15 mJ cm-2 or not, in the absence or the presence of p-CA at the indicated concentrations. The cells were then cultured in the fresh medium without p-CA for 24 h to harvest the conditioned medium. Fibroblasts were cultured for 24 h in the conditioned medium derived from HaCaT cells, and then the medium was replaced with fibroblast growth medium without serum. After incubating for 24 h, MMP1 (A) and PIP (B) in the fibroblasts conditioned medium were quantified using sandwich immunoassay kits. Data are presented as fold changes versus the control cells (mean±SE, n=3). *p<0.05.
DISCUSSION
The present study clearly reveals that UVB irradiation of cultured keratinocytes increased SFN release into extracellular medium. The stimulatory effects of the conditioned medium of HaCaT cells on the MMP1 expression in fibroblasts were largely dependent on the SFN level.
We observed that the conditioned medium from UVB-irradiated HaCaT cells increased the MMP1 release while decreasing PIP release from fibroblasts. This indicates that UVB not only stimulates collagen degradation but also inhibits collagen synthesis in fibroblasts through mechanisms mediated by the released factors from the UVB-irradiated keratinocytes. Initially, we assumed that SFN released from the UVB-irradiated keratinocytes might regulate both collagen degradation and collagen synthesis in target fibroblasts. However, both depletion and over-expression of SFN in HaCaT cells only changed the MMP1, but not PIP, release from fibroblasts. These results suggest that SFN in the conditioned medium of the UVB-irradiated HaCaT cells is a key regulator of MMP1 expression by fibroblasts, and there may be other factors responsible for the inhibition of collagen synthesis. Another research group has proposed that degraded collagen fragments inhibit collagen synthesis [22].
The loss of components from the dermal extracellular matrix due to over-activation of MMPs is considered an important mechanism in the tissue remodeling associated with phenotypic changes during skin photoaging [6,7]. Thus, the pharmacological targeting of MMPs, including MMP1, is a promising strategy to retard the skin aging process. Because SFN derived from UVB-irradiated keratinocytes plays a role in the regulation of MMP1 expression in fibroblasts, intervention of SFN expression or SFN-mediated signaling pathways may be useful to control expression and release of MMP1 from fibroblasts.
In the present study, p-CA was tested as a model compound of naturally occurring UV absorbers. As expected from its UV-absorbing property, p-CA mitigated UVB-induced cytotoxicity and caspase 3 activation, in a dose-dependent manner. This compound also suppressed SFN expression/release by UVB-irradiated HaCaT cells and indirectly prevented MMP1 expression/release from fibroblasts, in a dose-dependent manner. Collagen biosynthesis in fibroblasts, as monitored by PIP content, was also restored by the inclusion of p-CA in the medium during UVB exposure of HaCaT cells. Thus, the UVB-induced various biochemical events including SFN-mediated signal transduction leading to MMP1 activation can be avoided by using effective UVB absorbers such as p-CA.
We have previously reported that p-CA formulated in a form of cosmetic cream attenuated UVB-induced erythema and subsequent pigmentation in hairless mouse and human skin [15,23]. Other studies reported that SFN expression level was higher in sun exposed human skin than in sun-shielded skin, supporting a role of SFN as a contributor to in skin photoaging [10]. It would be of interest to examine whether p-CA attenuates SFN expression and consequent MMP1 expression in the skin in vivo, in the future study.
CONCLUSION
In conclusion, the present study demonstrated that the use of UV absorbers such as p-CA would be a promising strategy to reduce UV-induced, SFN-centered signaling events associated with dermal collagen loss during skin photoaging.
ACKNOWLEDGMENTS
This research was supported by Kyungpook National University Research Fund 2014.
Footnotes
CONFLICT OF INTEREST: The authors report no conflicts of interest.
References
- 1.Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 3.Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 4.Bode W, Fernandez-Catalan C, Tschesche H, Grams F, Nagase H, Maskos K. Structural properties of matrix metalloproteinases. Cell Mol Life Sci. 1999;55:639–652. doi: 10.1007/s000180050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan M, Bhatti H, Nerusu KC, Bhagavathula N, Kang S, Fisher GJ, Varani J, Voorhees JJ. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43–48. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Scharffetter K, Wlaschek M, Hogg A, Bolsen K, Schothorst A, Goerz G, Krieg T, Plewig G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res. 1991;283:506–511. doi: 10.1007/BF00371923. [DOI] [PubMed] [Google Scholar]
- 7.Brenneisen P, Oh J, Wlaschek M, Wenk J, Briviba K, Hommel C, Herrmann G, Sies H, Scharffetter-Kochanek K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem Photobiol. 1996;64:649–657. doi: 10.1111/j.1751-1097.1996.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 8.Ghahary A, Marcoux Y, Karimi-Busheri F, Li Y, Tredget EE, Kilani RT, Lam E, Weinfeld M. Differentiated keratinocyte-releasable stratifin (14-3-3 sigma) stimulates MMP-1 expression in dermal fibroblasts. J Invest Dermatol. 2005;124:170–177. doi: 10.1111/j.0022-202X.2004.23521.x. [DOI] [PubMed] [Google Scholar]
- 9.Lam E, Kilani RT, Li Y, Tredget EE, Ghahary A. Stratifin-induced matrix metalloproteinase-1 in fibroblast is mediated by c-fos and p38 mitogen-activated protein kinase activation. J Invest Dermatol. 2005;125:230–238. doi: 10.1111/j.0022-202X.2005.23765.x. [DOI] [PubMed] [Google Scholar]
- 10.Adachi H, Murakami Y, Tanaka H, Nakata S. Increase of stratifin triggered by ultraviolet irradiation is possibly related to premature aging of human skin. Exp Dermatol. 2014;23(Suppl 1):32–36. doi: 10.1111/exd.12390. [DOI] [PubMed] [Google Scholar]
- 11.An SM, Lee SI, Choi SW, Moon SW, Boo YC. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by alpha-melanocyte stimulating hormone. Br J Dermatol. 2008;159:292–299. doi: 10.1111/j.1365-2133.2008.08653.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Park J, Song K, Kim HG, Koh JS, Boo YC. Screening of plant extracts for human tyrosinase inhibiting effects. Int J Cosmet Sci. 2012;34:202–208. doi: 10.1111/j.1468-2494.2012.00704.x. [DOI] [PubMed] [Google Scholar]
- 13.Song K, Boo YC. UVB shielding effects of para-Coumaric acid. J Soc Cosmet Scientists Korea. 2012;38:263–273. [Google Scholar]
- 14.Lee SJ, Mun GI, An SM, Boo YC. Evidence for the association of peroxidases with the antioxidant effect of p-coumaric acid in endothelial cells exposed to high glucose plus arachidonic acid. BMB Rep. 2009;42:561–567. doi: 10.5483/bmbrep.2009.42.9.561. [DOI] [PubMed] [Google Scholar]
- 15.Song K, An SM, Kim M, Koh JS, Boo YC. Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J Dermatol Sci. 2011;63:17–22. doi: 10.1016/j.jdermsci.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Zang LY, Cosma G, Gardner H, Shi X, Castranova V, Vallyathan V. Effect of antioxidant protection by p-coumaric acid on lowdensity lipoprotein cholesterol oxidation. Am J Physiol Cell Physiol. 2000;279:C954–C960. doi: 10.1152/ajpcell.2000.279.4.C954. [DOI] [PubMed] [Google Scholar]
- 17.Lee SI, An SM, Mun GI, Lee SJ, Park KM, Park SH, Boo YC. Protective effect of Sasa quelpaertensis and p-coumaric acid on ethanol-induced hepatotoxicity in mice. J Appl Biol Chem. 2008;51:148–154. [Google Scholar]
- 18.Park J, Seok JK, Suh HJ, Boo YC. Gardenia jasminoides extract attenuates the UVB-induced expressions of cytokines in keratinocytes and indirectly inhibits matrix metalloproteinase-1 expression in human dermal fibroblasts. Evid Based Complement Alternat Med. 2014;2014:429246. doi: 10.1155/2014/429246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canty-Laird EG, Lu Y, Kadler KE. Stepwise proteolytic activation of type I procollagen to collagen within the secretory pathway of tendon fibroblasts in situ. Biochem J. 2012;441:707–717. doi: 10.1042/BJ20111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitailo LA, Tibudan SS, Denning MF. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Wu SB, Hong CH, Yu HS, Wei YH. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci. 2013;14:6414–6435. doi: 10.3390/ijms14036414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo YK, Kim SJ, Boo YC, Baek JH, Lee SH, Koh JS. Effects of p-coumaric acid on erythema and pigmentation of human skin exposed to ultraviolet radiation. Clin Exp Dermatol. 2011;36:260–266. doi: 10.1111/j.1365-2230.2010.03983.x. [DOI] [PubMed] [Google Scholar]