Abstract
An oral environment is constantly exposed to environmental factors and microorganisms. The periodontal ligament (PDL) fibroblasts within this environment are subject to bacterial infection and allergic reaction. However, how these condition affect PDL fibroblasts has yet to be elucidated. PDL fibroblasts were isolated from healthy donors. We examined using reverse transcription-polymerase chain reaction and measuring the intracellular Ca2+ concentration ([Ca2+]i). This study investigated the receptors activated by exogenous bacterial pathogens (Lipopolysaccharide and peptidoglycan) and allergens (German cockroach extract and house dust mite) as well as these pathogenic mediators-induced effects on the intracellular Ca2+ signaling in human PDL fibroblasts. Moreover, we evaluated the expression of pro-inflammatory cytokines (interleukin (IL)-1β, IL-6, and IL-8) and bone remodeling mediators (receptor activator of NF-κB ligand and osteoprotegerin) and intracellular Ca2+-involved effect. Bacterial pathogens and allergic mediators induced increased expression of pro-inflammatory cytokines, and these results are dependent on intracellular Ca2+. However, bacterial pathogens and allergic mediators did not lead to increased expression of bone remodeling mediators, except lipopolysaccharide-induced effect on receptor activator of NF-κB ligand expression. These experiments provide evidence that a pathogens and allergens-induced increase in [Ca2+]i affects the inflammatory response in human PDL fibroblasts.
Keywords: Calcium signaling, Cytokines, Inflammation, Interleukins, Periodontal ligament
INTRODUCTION
Periodontal tissue is constantly exposed to environmental factors and microorganisms during breathing, speaking, eating, and dental hygiene [1]. Extracellular components including bacterial toxins, allergens, thermal changes, and osmotic molecules are potential risks and stress factors that threaten periodontal health [2,3]. Periodontal disease is often caused by a bacterial infection, which commonly results from irritation, mediated by dental calculus formation, deposits, and poor oral hygiene. However, it is still unknown how these risk factors act at the cellular level to destroy the periodontal tissue and progress inflammatory condition, and there is not a definitive causative link between bacterial infection and periodontal disease.
Periodontal ligament (PDL) fibroblasts play a critical role to support teeth and maintain the homeostasis of the periodontium [4]. The biological properties of the PDL fibroblasts are important for both tooth eruption and alveolar bone metabolism [5]. Deficiencies in the PDL and disorder of the periodontal tissues by inflammation result in formation of periodontal pocket, loss of alveolar bone, and subsequent mobility of tooth [6]. These events make the chance of contact with various pathogenic mediators, directly. The destruction of PDL tissue might be encountered extracellular risk factors including pathogens and allergens through the saliva followed by the inhalation and intake foods. Thus PDL metabolism is likely challenged by thousands of pathogens, its associated patterns, allergens, and systemic conditions.
PDL fibroblasts respond to bacterial lipopolysaccharide (LPS) by producing several cytokines and chemokines, although the exact mechanisms have not been elucidated [7]. Bacterial pathogens such as LPS and peptidoglycan (PGN) recognized by cells as pathogen-associated molecular patterns (PAMPs). And they activate innate immune responses via toll-like receptor (TLR)-2 and/or TLR-4. As a characteristic component of the cell wall of gram-negative bacteria, LPS primarily acts on the innate immune system through TLR-4, and some bacterial species also activate cells via TLR-2 [8]. PGN, found in the outer structure of gram-positive bacteria, is recognized by TLR-2 [9,10] and, like LPS, is considered a potent bacterial component which trigger infectious disease.
A variety of extracellular stimuli, such as exogenous allergens including proteases, are considered significant modulators of epithelial function [11]. German cockroach extract (GCE), chitinase, and house dust mite (HDM) allergens Der p3 and Der p9 induced Ca2+ increase through the protease-activated receptor (PAR)-2 in airway epithelial cells [11,12,13,14,15]. A variety of antigens, including those from bacterial [16] and fungal [17] extracts, stimulate the release of cytokines and inflammatory mediators from airway epithelia [11]. As these are likely the allergic mediators, it is notable that the response to allergens and its pro-inflammatory factors in PDL fibroblasts is not well understood.
PDL fibroblasts may affect osteoclast differentiation in alveolar bone by expressing the receptor activator of NF-κ B ligand (RANKL) and osteoprotegerin (OPG). Porphyromonas (P.) gingivalis-derived LPS and several cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, were proposed as the extracellular stimuli that induce inflammatory mediators during chronic periodontitis [18] and LPS-induced inflammatory bone loss in cultured mandible [19]. However, it remains uncertain whether the expression levels of RANKL and OPG are influenced by bacterial PAMPs and allergens.
LPS- and PGN-induced activation of Ca2+ signaling entails secretion of the pro-inflammatory cytokine IL-8 [20,21,22,23]. It is not known whether exogenous allergens are involved in the regulation of cytokine expression through intracellular Ca2+ in PDL fibroblasts.
In this work, we address a potential effect, mediated by Ca2+ signaling, of bacterial PAMPs, such as LPS and PGN, and allergens, such as GCE and HDM, on primarily cultured human PDL fibroblasts and investigate the expression levels of pro-inflammatory and bone remodeling cytokines.
METHODS
Reagents
Fura-2/AM and Pluronic F-127 were purchased from Teflabs (Austin, TX). α-minimal essential medium (α-MEM), Penicillin-Streptomycin, fetal bovine serum (FBS), and Superscript III were purchased from Invitrogen (Carlsbad, CA). LPS (from Escherichia coli), PGN (from Staphylococcus aureus), thrombin, trypsin, and all other chemicals not mentioned were obtained from Sigma (St. Louis, MO). GCE (from Blattella germanica) and HDM (from Dermatophagoides pteronyssinus) were provided by the Arthropods of Medical Importance Resource Bank (Yonsei University College of Medicine, Seoul, Korea).
Cell culture
All experimental protocols were reviewed and approved by the Research Ethics Committee of Yonsei University College of Dentistry and Dental Hospital. Informed consent was acquired from all volunteers according to the requirements of the Institutional Review Board. The human PDL fibroblasts were isolated from 16 different healthy (systemically and periodontally) donors as previously described [24]. We used premolar extracted for orthodontic reasons. PDL fibroblasts were obtained from the tissues located in the middle of the tooth root by scraping, mincing, and incubating for 40 min at 37℃ in a cell culture incubator containing 5% CO2 and 95% air. The PDL fibroblasts were maintained in α-MEM containing 10% FBS with 100 U/ml penicillin and 100 µg/ml streptomycin. When cells were 80% confluent, they were washed with PBS, treated with Trypsin/EDTA for 1 min, and then transferred to new culture dishes. Primary cultured human PDL fibroblasts, which had undergone 4~7 passages, were used for all experiments.
Semi-quantitative reverse transcription-polymerase chain reaction (semi-quantitative RT-PCR)
Total RNA was extracted from human PDL fibroblasts using Trizol reagent extraction system (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. The concentration and purity of RNA was determined by measuring the absorbance at 260 and 280 nm. The mRNA was amplified according to the manufacturer's protocol using AccuPower® RT PreMix (BIONEER, Seoul, Korea). The cDNA was amplified by PCR with HiPiTM Thermo-stable DNA polymerase (Elpis, Seoul, Korea) and nested primers. The PCR program included a denaturation step at 95℃ for 5 min, followed by 35 cycles of 95℃ for 30 sec, annealing for 30 sec, an extension step of 72℃ for 1 min, and a final extension step of 72℃ for 10 min. Sequence and optimal annealing temperature (At) of each primer are presented in Table 1. The PCR products were electrophoresed same volume from each sample on 1.2% agarose gels and the image of the band on agarose gel was acquired with a CCD camera and scanned by using CoreImager MC 2000 (Sam Learmdro, CA, USA). Intensities of PCR bands were analyzed by the densitometry program ImageJ (National Institutes of Health, Bethesda, MD) and statistics were performed by using Graphpad Prism 4.0 (Graphpad Software. San Diego, CA). Mean and standard deviation in all samples were calculated after normalization to level of GAPDH as a loading control. The mRNA expression levels of the pro-inflammatory cytokines were represented as the ratio of IL-1β, IL-6, or IL-8 intensity/GAPDH intensity [25,26].
Table 1. Primers used for semi-quantitative RT-PCR.
Measurement of intracellular Ca2+ concentration ([Ca2+]i)
The human PDL fibroblasts were cultured on cover glasses. Cells were loaded with 5 µM fura-2, AM in the presence of 0.05% Pluronic F-127 for 30 min in physiological salt solution (PSS; 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM HEPES, 10 mM D-glucose, titrated to pH 7.4 with NaOH and 310 mOsm with NaCl). Changes in [Ca2+]i were measured by fluorescence intensities of with excitation wavelengths of 340 and 380 nm, respectively, and an emission wavelength of 510 nm. The emitted fluorescence was monitored with a CCD camera attached to an inverted microscope (Nikon, Japan) and analyzed with a MetaFlour system (Molecular Devices, PA). Fluorescence images were obtained at 2 sec intervals. The fluorescence ratio was calculated as follows: Ratio=F340/F380
Statistical analysis
Data from more than three independent experiments were expressed as mean±SE. Statistically significant differences between experimental groups were determined using the paired Student's t-test.
RESULTS
Characterization of the human PDL fibroblasts in primary culture
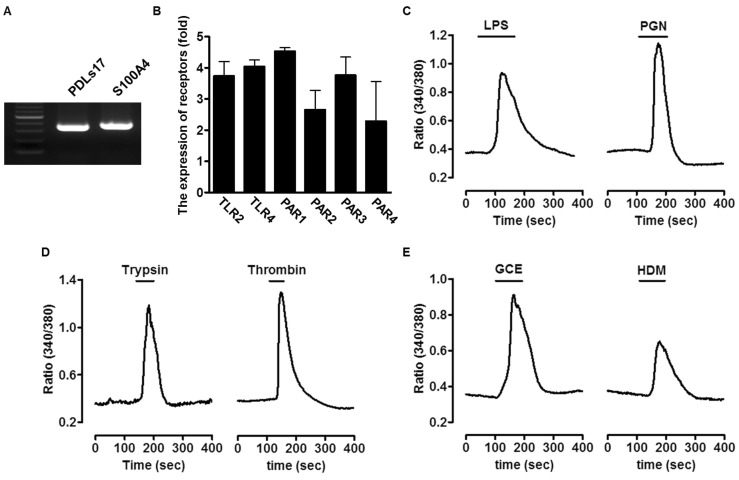
To characterize the cell types of PDL fibroblasts, we confirmed marker genes using reverse RT-PCR. As shown in Fig. 1A, PDL fibroblast marker genes (PDLs17 and S100A4) were strongly expressed in primary human PDL fibroblasts.
Fig. 1. Expression of bacterial pathogens-associated molecular patterns (PAMPs) or allergic mediators-associated receptors and Ca2+-induced signaling in primary cultured human periodontal ligament (PDL) fibroblasts. (A) The marker genes to characterize the PDL cell population were detected in the primary culture using reverse transcription-polymerase chain reaction (RT-PCR). The following marker genes were used PDLs17 and S100A4 for PDL fibroblast. (B) The total mRNA of human PDL fibroblasts was extracted and mRNA for toll-like receptor (TLR)-2, TLR-4, and protease-activated receptors (PARs: PAR-1, -2, -3, -4) was amplified. The expression of these receptors was quantified after the value was normalized to expression level of GAPDH. (C~E) The human PDL fibroblasts were directly stimulated with 20 µg/ml lipopolysaccharide (LPS), 50 µg/ml peptidoglycan (PGN), 1 µM trypsin, 1 Unit thrombin, 50 µg/ml German cockroach extract (GCE), or 50 µg/ml house dust mite (HDM) to measure intracellular Ca2+ concentration.
Expression of bacterial PAMPs or allergens-associated receptors and intracellular Ca2+ signaling in human PDL fibroblasts
To determine whether the receptors of bacterial PAMPs (LPS and PGN) and allergic mediators (Trypsin, thrombin, GCE, and HDM), we performed RT-PCR and verified the presence of TLR-2, TLR-4, and PAR-1 through -4. As shown in Fig. 1B, mRNA expression of receptors was detected. Next, human PDL fibroblasts were treated with either 20 µg/ml LPS, 50 µg/ml PGN as bacterial PAMPs, and 1 µM trypsin, 1 Unit thrombin, 50 µg/ml GCE, or 50 µg/ml HDM as allergic mediators of PAR-2 and the other PARs, respectively, and the resulting Ca2+ signaling was measured (Fig. 1C~1E). All stimuli directly increased Ca2+ levels in human PDL fibroblasts.
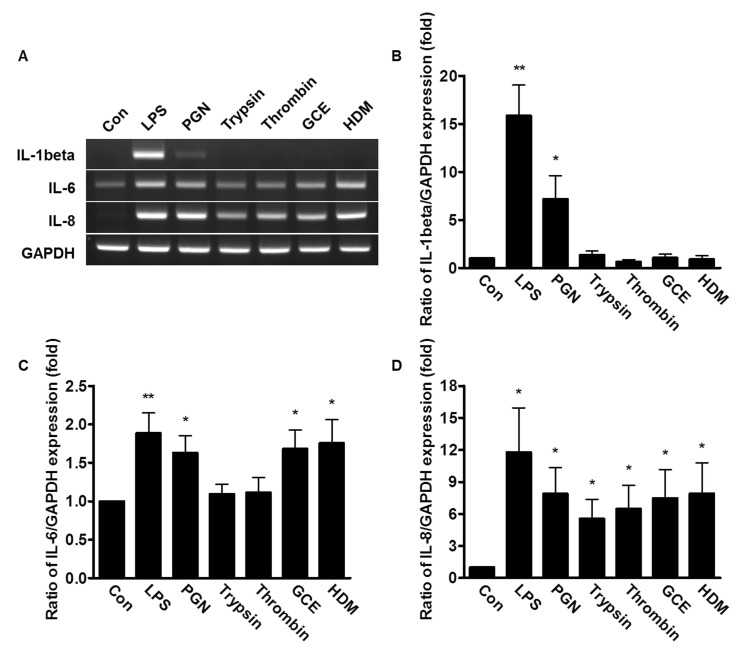
Effect of bacterial PAMPs and allergic mediators on the expression of IL-1β, IL-6 and IL-8
To examine whether the stimuli from bacterial PAMPs and allergic mediators affect the expression of pro-inflammatory cytokines, the PDL fibroblasts were treated with either 20 µg/ml LPS, 50 µg/ml PGN, 1 µM trypsin, 1 Unit thrombin, 50 µg/ml GCE, or 50 µg/ml HDM, and then the mRNA expression of IL-1β, IL-6, and IL-8 were measured. The mRNA expression of IL-1β was highly up-regulated with the treatment of LPS or PGN (16 and 7-fold, respectively) compared to cells treated with the other allergic mediators (Fig. 2A and 2B). The mRNA expression of IL-6 increased with the treatment of all pathogenic mediators described (Fig. 2A and 2C). The mRNA levels of IL-8 were significantly stimulated in human PDL fibroblasts by all allergic mediators tested, as well as LPS and PGN (Fig. 2A and 2D). Treatment with LPS led to more dramatic increases of the IL-1β and IL-8 mRNA expression, compared to IL-6 in these cells.
Fig. 2. Effect of bacterial PAMPs and allergic mediators on mRNA expression of interleukin (IL)-1β, IL-6 and IL-8 in human PDL fibroblasts. (A) The human PDL fibroblasts were stimulated with 20 µg/ml LPS, 50 µg/ml PGN, 1 µM trypsin, 1 Unit thrombin, 50 µg/ml GCE, or 50 µg/ml HDM for 3 h. The total mRNA of the stimulated human PDL fibroblasts was extracted and amplified with IL-1β, IL-6, IL-8, and GAPDH specific primers. (B~D) The expression of IL-1β, IL-6, and IL-8 was quantified after the value was normalized to expression level of GAPDH. Data are a mean±SE of values from more than five independent experiments. *p<0.05, **p<0.01 versus control.
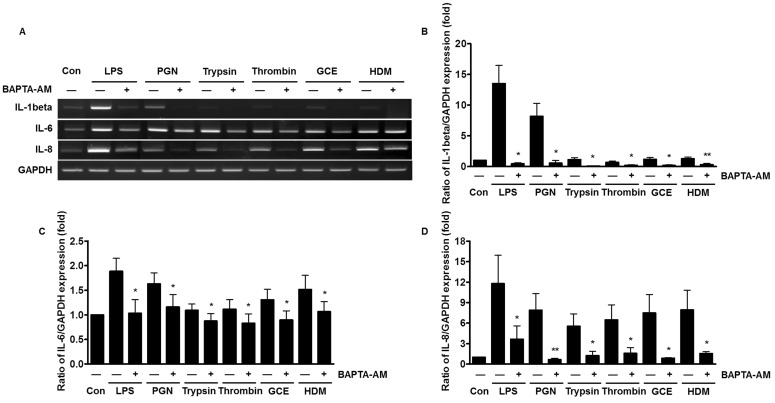
Reduced expression of pro-inflammatory cytokines by chelating intracellular Ca2+
Receptor-mediated intracellular Ca2+ signaling is involved in the production of cytokines and chemokines [22]. To examine whether the Ca2+ mobilization leads to the increased expression of pro-inflammatory cytokines, the human PDL fibroblasts were pre-treated with the selective intracellular Ca2+ chelator, 1,2-bis (o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid (BAPTA)-AM for 30 min. The mRNA expression levels of IL-1β, IL-8, and IL-6 were suppressed by BAPTA-AM, which sequesters intracellular Ca2+ (Fig. 3A). The inhibitory effect of chelating Ca2+ is more significant for IL-1β and IL-8 mRNA expression, compared to the effect on IL-6 (Fig. 3B~3D).
Fig. 3. Reduced expression of pro-inflammatory cytokines by chelating intracellular Ca2+ in human PDL fibroblasts exposed to bacterial PAMPs and allergic mediators. (A) The human PDL fibroblasts were pretreated with 5 µM 1,2-bis (o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid (BAPTA)-AM for 30 min and then stimulated with either 20 µg/ml LPS, 50 µg/ml PGN, 1 µM trypsin, 1 Unit thrombin, 50 µg/ml GCE, or 50 µg/ml HDM for 3 h. The total mRNA of the stimulated human PDL fibroblasts was extracted and amplified with IL-1β, IL-6, IL-8, and GAPDH specific primers. (B~D) The expression levels of IL-1β, IL-6, and IL-8 were quantified after the value was normalized to the expression level of GAPDH. Data are a mean±SE of values from more than five independent experiments. *p<0.05, **p<0.01 versus control.
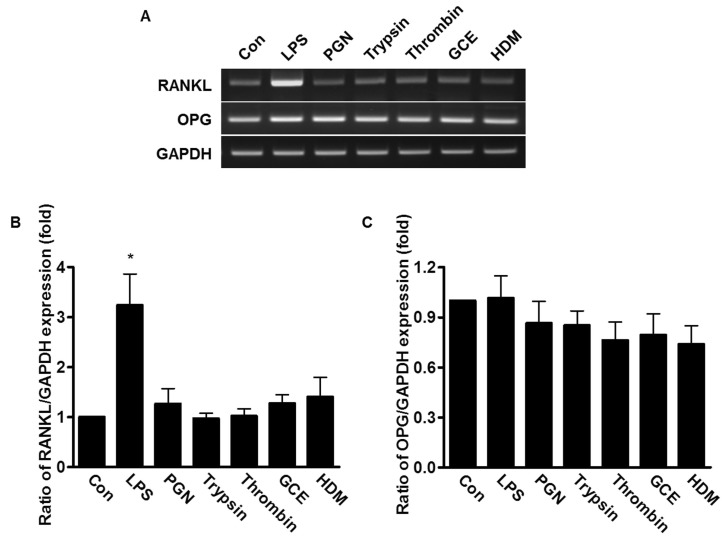
Effect of bacterial PAMPs and allergic mediators on the expression of RANKL and OPG
PDL fibroblasts are known to play important roles in metabolism of alveolar bones, which requires expression of RANKL and OPG. To determine whether mRNA expression of RANKL and OPG was affected by bacterial PAMPs and allergic mediators, the human PDL fibroblasts were treated with 20 µg/ml LPS, 50 µg/ml PGN, 1 µM trypsin, 1 Unit thrombin, 50 µg/ml GCE, or 50 µg/ml HDM for 24 h. Only LPS-treated cells induced 3 fold-higher RANKL mRNA expression compared to untreated control cells; whereas, the mRNA level of OPG was not changed in any sample, suggesting that LPS, but not other pathogenic mediators, induced RANKL mRNA expression, which is involved in bone metabolism (Fig. 4).
Fig. 4. Effect of bacterial PAMPs and allergic mediators on expression of RANKL and OPG mRNA in human PDL fibroblasts. (A) The human PDL fibroblasts were stimulated with either 20 µg/ml LPS, 50 µg/ml PGN, 1 µM trypsin, 1 Unit thrombin, 50 µg/ml GCE, or 50 µg/ml HDM for 24 h. The total mRNA of the stimulated human PDL fibroblasts was extracted and amplified with RANKL, OPG, and GAPDH specific primers. (B, C) The expression levels of RANKL and OPG were quantified after the value was normalized to the expression level of GAPDH. Data are a mean±SE of values from three independent experiments. *p<0.05 versus control.
DISCUSSION
The aim of this study was to examine the effect of bacterial PAMPs and allergens on cytokine expression through Ca2+ signaling in primary cultured human PDL fibroblasts. An immune response caused by bacterial inflammation, as well as direct activation of bacterial PAMPs or allergens-associated receptors, can trigger Ca2+ signaling and the subsequent expression of pro-inflammatory cytokines (Figs. 2 and 3). However, the activation by allergic mediators caused no significant change in expression levels of bone remodeling mediators, RANKL and OPG. Only the presence of LPS led to an increase in RANKL mRNA expression (Fig. 4).
Up-regulated TLR expression and responses to TLR agonists of microbial origin result in inflammatory reactions [27]. The TLR-activating virulence factors may be extracellular components such as bacterial toxins and allergens, suggesting that these detrimental immunoreactive factors can threaten the periodontal tissue. Many allergens from cockroach, dust mite, and fungi are also significant modulators of epithelial cell function [28], including PDL fibroblasts. PARs are positioned between innate immunity and coagulation, making them ideal pharmacological targets for the treatment of several airway epithelial pathologies [13,29]. PARs are family of G protein-coupled receptors (GPCRs) containing the domain which is cleaved and activated by a protease [13]. Activated G protein stimulates phospholipase C (PLC), which hydrolyzes inositol phosphates to generate inositol 1, 4, 5-trisphosphate (IP3). Released IP3 binds to IP3 receptor (IP3R) and stimulates the release of Ca2+ from the endoplasmic reticulum (ER) into the cytosol [21]. An increased Ca2+ level is critically involved in cell pathology [30,31]. Thus, our data suggest that cytokines induced by their specific pathological agonist are dependent on Ca2+ mobilization.
Pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α play significant roles in the inflammatory responses in the periodontium [32]. IL-1β is elevated in patients suffering from chronic inflammatory disorders such as rheumatoid arthritis and periodontitis [33]. Multiple studies indicate that IL-6 levels were increased in the gingival crevicular fluid and serum in patients with periodontitis compared to healthy subjects [34,35]. However, no differences in IL-6 or other cytokine levels were detected in salivary samples from chronic periodontitis patients compared to healthy subjects [36]. Our data indicate that bacterial PAMPs and allergens lead to increased IL-6 production (Figs. 3 and 4). IL-1β and IL-8 expression are more sensitive to an increase in intracellular Ca2+ than IL-6 expression.
In summary, we provide novel insights into the inflammatory process, investigating the result of increased Ca2+ signaling by analyzing the expression levels of pro-inflammatory cytokines induced by bacterial PAMPs and allergens in human PDL fibroblasts. We found that the mRNA expression levels of RANKL and OPG was not altered by the stimulation of allergens, but was changed by the bacterial LPS. The regulatory role played by LPS in PDL fibroblasts will be the subject of future investigation in the coming years. Here we suggested that bacterial pathological and allergic modulation of periodontal cell function has the potential to be a new therapeutic phase in restoring periodontal tissue. Further studies are required to clarify the intracellular mechanisms of bacterial PAMPs and allergens described in this report. For example, the bacterial PAMPs, LPS was shown here to participate in cytokine release, and thus is associated with the progression of periodontitis after destruction of the PDL tissue. Identification of the periodontal response and the inflammatory mechanism will be an important future step to develop a prime target for periodontal therapy.
ACKNOWLEDGEMENTS
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A-2006269) and by the Gachon University research fund of 2014 (GCU 2014-5096).
ABBREVIATIONS
- PDL
periodontal ligament
- [Ca2+]i
intracellular Ca2+ concentration
- IL
interleukin
- LPS
lipopolysaccharide
- PGN
peptidoglycan
- PAMPs
pathogen-associated molecular patterns
- TLR
toll-like receptor
- GCE
German cockroach extract
- HDM
house dust mite
- PAR
protease-activated receptor
- RANKL
receptor activator of NF-κB ligand
- OPG
osteoprotegerin
Footnotes
CONFLICT OF INTERESTS: The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Nogueira AV, Nokhbehsaim M, Eick S, Bourauel C, Jäger A, Jepsen S, Cirelli JA, Deschner J. Regulation of visfatin by microbial and biomechanical signals in PDL cells. Clin Oral Investig. 2014;18:171–178. doi: 10.1007/s00784-013-0935-1. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghutaimel H, Riba H, Al-Kahtani S, Al-Duhaimi S. Common periodontal diseases of children and adolescents. Int J Dent. 2014;2014:850674. doi: 10.1155/2014/850674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung UW, Kim CS, Choi SH, Kim S. Gingival coverage of iatrogenically denuded labial bone resulting from thermal trauma. Int J Periodontics Restorative Dent. 2013;33:635–639. doi: 10.11607/prd.1024. [DOI] [PubMed] [Google Scholar]
- 4.Kanjanamekanant K, Luckprom P, Pavasant P. Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J Periodontal Res. 2013;48:169–176. doi: 10.1111/j.1600-0765.2012.01517.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakao A, Kajiya H, Fukushima H, Fukushima A, Anan H, Ozeki S, Okabe K. PTHrP induces Notch signaling in periodontal ligament cells. J Dent Res. 2009;88:551–556. doi: 10.1177/0022034509337899. [DOI] [PubMed] [Google Scholar]
- 6.Gher ME. Changing concepts. The effects of occlusion on periodontitis. Dent Clin North Am. 1998;42:285–299. [PubMed] [Google Scholar]
- 7.Jönsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. J Periodontal Res. 2011;46:153–157. doi: 10.1111/j.1600-0765.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 8.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Hong JH, Hong JY, Park B, Lee SI, Seo JT, Kim KE, Sohn MH, Shin DM. Chitinase activates protease-activated receptor-2 in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:530–535. doi: 10.1165/rcmb.2007-0410OC. [DOI] [PubMed] [Google Scholar]
- 12.Cho HJ, Choi JY, Yang YM, Hong JH, Kim CH, Gee HY, Lee HJ, Shin DM, Yoon JH. House dust mite extract activates apical Cl(-) channels through protease-activated receptor 2 in human airway epithelia. J Cell Biochem. 2010;109:1254–1263. doi: 10.1002/jcb.22511. [DOI] [PubMed] [Google Scholar]
- 13.Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, Shin DM. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004;113:315–319. doi: 10.1016/j.jaci.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008;128:1930–1939. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 15.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- 16.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–1193. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wu Z, Zhang X, Ni J, Yu W, Zhou Y, Nakanishi H. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS. Mediators Inflamm. 2013;2013:407562. doi: 10.1155/2013/407562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloan AJ, Taylor SY, Smith EL, Roberts JL, Chen L, Wei XQ, Waddington RJ. A novel ex vivo culture model for inflammatory bone destruction. J Dent Res. 2013;92:728–734. doi: 10.1177/0022034513495240. [DOI] [PubMed] [Google Scholar]
- 20.Aki D, Minoda Y, Yoshida H, Watanabe S, Yoshida R, Takaesu G, Chinen T, Inaba T, Hikida M, Kurosaki T, Saeki K, Yoshimura A. Peptidoglycan and lipopolysaccharide activate PLCgamma2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells. 2008;13:199–208. doi: 10.1111/j.1365-2443.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 21.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 22.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son A, Shin DM, Hong JH. Peptidoglycan induces the production of interleukin-8 via calcium signaling in human gingival epithelium. Korean J Physiol Pharmacol. 2015;19:51–57. doi: 10.4196/kjpp.2015.19.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards D, Rutherford RB. The effects of interleukin 1 on collagenolytic activity and prostaglandin-E secretion by human periodontal-ligament and gingival fibroblast. Arch Oral Biol. 1988;33:237–243. doi: 10.1016/0003-9969(88)90184-7. [DOI] [PubMed] [Google Scholar]
- 25.Chamizo C, Moreno J, Alvar J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Vet Immunol Immunopathol. 2005;103:67–75. doi: 10.1016/j.vetimm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online. 2001;3:19–25. doi: 10.1251/bpo20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol. 2002;7:72–78. doi: 10.1902/annals.2002.7.1.72. [DOI] [PubMed] [Google Scholar]
- 28.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 29.Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003;33:35–42. doi: 10.1046/j.1365-2222.2002.01481.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son A, Kim MS, Jo H, Byun HM, Shin DM. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in RANKL-induced osteoclastogenesis. Korean J Physiol Pharmacol. 2012;16:31–36. doi: 10.4196/kjpp.2012.16.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nibali L, Fedele S, D'Aiuto F, Donos N. Interleukin-6 in oral diseases: a review. Oral Dis. 2012;18:236–243. doi: 10.1111/j.1601-0825.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013;92:399–408. doi: 10.1177/0022034513483142. [DOI] [PubMed] [Google Scholar]
- 34.Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB, Jr, Taba M., Jr Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010;81:384–391. doi: 10.1902/jop.2009.090510. [DOI] [PubMed] [Google Scholar]
- 35.Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita N, Yoshie H. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010;81:1118–1123. doi: 10.1902/jop.2010.090741. [DOI] [PubMed] [Google Scholar]
- 36.Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44:411–417. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]