Abstract
Pregnancy is associated with marked physiological changes challenging the cardiovascular system. Among the more severe pregnancy associated cardiovascular complications, peripartum cardiomyopathy (PPCM) is a potentially life-threatening heart disease emerging towards the end of pregnancy or in the first postpartal months in previously healthy women. A major challenge is to distinguish the peripartum discomforts in healthy women (fatigue, shortness of breath, and oedema) from the pathological symptoms of PPCM. Moreover, pregnancy-related pathologies such as preeclampsia, myocarditis, or underlying genetic disease show overlapping symptoms with PPCM. Difficulties in diagnosis and the discrimination from other pathological conditions in pregnancy may explain why PPCM is still underestimated. Additionally, underlying pathophysiologies are poorly understood, biomarkers are scarce and treatment options in general limited. Experience in long-term prognosis and management including subsequent pregnancies is just beginning to emerge. This review focuses on novel aspects of physiological and pathophysiological changes of the maternal cardiovascular system by comparing normal conditions, hypertensive complications, genetic aspects, and infectious disease in PPCM-pregnancies. It also presents clinical and basic science data on the current state of knowledge on PPCM and brings them in context thereby highlighting promising new insights in diagnostic tools and therapeutic approaches and management.
Keywords: Peripartum cardiomyopathy, Heart failure, Pregnancy
Introduction
Cardiovascular disease affects around 4% of all pregnancies in Western industrialized countries with increasing tendencies.1 Even in the absence of pre-existing cardiovascular disease pregnancy complications such as hypertensive complications, i.e. gestational hypertension and its more severe forms preeclampsia, the HELLP syndrome (H: haemolysis, EL: elevated liver enzymes, LP: low platelet count), and peripartum cardiomyopathy (PPCM) may induce cardiovascular disease.2–4 In addition, previously unrecognized genetic forms of cardiomyopathies can be demasked by pregnancy stress5–7 and latent cardiac viral infection can be activated in pregnancy as a cause for myocarditis.8
Pregnancy, delivery, and the peripartum period provide a challenge to the entire maternal organism, as they are associated with weight gain, oedema, physical discomfort, fatigue, and breathlessness. Therefore, understanding normal pregnancy is important for the timely recognition of cardiovascular pathologies to distinguish the peripartum discomfort in healthy women from signs of cardiovascular disease. This is a major challenge for physicians, midwives, and patients and may explain why diagnosis of peripartum heart failure is frequently delayed and still underestimated.
Here we summarize the current knowledge on aetiology, risk factors, and pathophysiology of PPCM in comparison with normal physiological changes in pregnancy and with related pathologies, specifically preeclampsia, myocarditis, and genetic forms of cardiomyopathies. Furthermore, we discuss state-of-the-art treatment options, prognosis, and management of PPCM.
Normal physiological changes during pregnancy
Haemodynamic changes in the maternal circulation start already in the first trimester with a profound decline in systemic vascular resistance and a reciprocal increase in cardiac output.9 Activation of the renin–angiotensin–aldosterone system maintains blood pressure and helps retaining salt and water as maternal systemic and renal arterial dilation creates an ‘underfilled’ cardiovascular system. At the same time, pregnancy hormones such as oestrogen, progesterone, and relaxin promote vascular relaxation.10,11 Cardiac remodelling leads to substantial increase in left-ventricular mass and enhanced angiogenesis. Labour during delivery is associated with the maximum cardiac output related to increased heart rate and preload caused by the uterine contractions, increased circulating catecholamines, and autotransfusion of 300–500 mL blood from the uterus into the maternal circulation.9,10,12 The risk for thrombosis is 4–10-fold higher around pregnancy and maximal around term as the factors VII, X, VIII, fibrinogen, and von Willebrand factor rise throughout gestation.13 Reprogramming the maternal metabolism in pregnancy optimizes shunting of glucose to the foetus by developing some degree of insulin resistance in the mother shifting her metabolism more towards fatty acids.14 Oxidative stress increases during pregnancy and is paralleled by a slightly delayed increase in antioxidant capacity.15
Thus, pregnancy represents one of the most profound mechanisms of system-wide hormonal, haemodynamic, and metabolic reprogramming that should be further explored.
Peripartum cardiomyopathy: definition and incidence
Peripartum cardiomyopathy is defined as a non-familial form of peripartum heart failure characterized as an ‘idiopathic cardiomyopathy presenting with heart failure secondary to left-ventricular (LV) systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found’ as proposed by the Working Group on PPCM of the Heart Failure Association of the European Society of Cardiology (ESC).4 Clinically, PPCM resembles a dilated cardiomyopathy (DCM) but the left ventricle may not always be dilated. The ejection fraction is nearly always reduced below 45%.4 PPCM is considered an independent disease, whose diagnosis relies on its relation to pregnancy and the exclusion of other cardiomyopathies.4,16
Reported incidences for PPCM vary among different geographic regions with potential hotspots in Africa (1:100 to 1: 1000) and Haiti (1:299).17–21 In Western societies, the incidence of PPCM is rising (in the USA from 1 in 4350 in 1993 to 1 in 2229 in 2002) possibly because of socio-economic changes (rising maternal age, fertility-assisted treatments, and multifoetal pregnancies), better diagnostic tools and increasing awareness created by large prospective international registry, i.e. the ESC EURObservational Research Programme (http://www.eorp.org).22–25
Diagnostic challenge in peripartum cardiomyopathy due to a wide variety of cardiac phenotypes
Establishing the diagnosis of PPCM relies on a high index of suspicion as it can present dramatically with acute heart failure necessitating intensive care or it may develop subtly over several weeks. Especially, the slow developing form with unspecific symptoms of cardiac congestion (abdominal discomfort, pleuritic chest pain, and palpitations) is sometimes difficult to be distinguished from peripartum discomfort in healthy women.3,4,8 Therefore, the choice and value of diagnostic tools is critical.
Electrocardiogram
Specific electrocardiogram (ECG) patterns are not known for PPCM but ECG abnormalities seem to be frequently present in PPCM patients.26 In a small minority of patients, intraventricular conductance abnormalities, such as a left bundle branch block, have been reported.27 Typically, ECGs are not routinely performed in normal pregnancies and a comparison with previous ECGs is impossible in most cases since ECGs prior to pregnancy are missing. However, an ECG should be performed if symptoms of heart failure are suspected or present, as it is an important tool in the differential diagnostic procedure and may help to rule out or point to pulmonary embolism or an acute ischaemic event.
Echocardiography
Transthoracic echocardiography is the most important tool for diagnostic confirmation or exclusion of PPCM and should be performed in every suspected case.3,4
Chest X-ray
Chest X-ray at the acute presentation may depict signs of decompensated heart failure with pulmonary congestion or oedema that may by complicated by pneumonia and pleural effusion.
Cardiac magnetic resonance imaging
The role of cardiac magnetic resonance imaging (CMR) in the assessment of patients with PPCM needs further evaluation. CMR provides valuable information about myocardial structure and right-ventricular function and should be considered in more severe forms of PPCM. Observations from a German multicentre CMR study on patients with PPCM (unpublished data) point to the presence of myocardial scars in a considerable portion of the patients. A transient LV hypertrabeculation mimicking the myocardial structure of non-compaction cardiomyopathy is sometimes observed but hypertrabeculation seems to resolve frequently after functional recovery.28 A recent report demonstrated reversible hypertrabeculations in normal pregnancies suggesting that hypertrabeculation may to some degree be part of normal cardiac remodelling of pregnancy.29
Cardiac catheterization/myocardial biopsies
Although in most cases thorough non-invasive testing obviates the need for invasive diagnostics, in some rare cases, cardiac catheterization and myocardial biopsies may be needed to obtain information on an ischaemic cause of heart failure or infection.
How to distinguish peripartum cardiomyopathy from other forms of pregnancy-induced heart failure
With regard to the broad range of cardiac phenotypes and time point of onset, a frequently posed question concerns how sure the diagnosis of PPCM is and if other possible forms of cardiomyopathies can be excluded.
Differences and overlaps with hypertensive complications in pregnancy
Hypertensive disorders (either new onset or superimposed on chronic hypertension) in pregnancy with the most severe forms preeclampsia and HELLP-syndrome affect up to 8% of pregnant women worldwide.30 Preeclampsia is a leading cause for premature delivery with high risk for maternal, foetal, and neonatal morbidity and mortality.11 Cardiac involvement in preeclampsia is frequently present mostly as diastolic dysfunction with raised filling pressures but normal systolic function and cardiac output.31 Systolic heart failure has been reported in pre-term preeclampsia often associated with proteinuria, oedema, and abnormalities of hepatic, haematological, and cerebral function.32 Preeclampsia and PPCM share several pathomechanisms including endothelial damage and increased serum levels of soluble fms-like tyrosine kinase-1 (sFlt1).2,33 Some cohorts of PPCM patients display a high prevalence of hypertensive disorders and preeclampsia during pregnancy23 suggesting that preeclampsia may predispose to PPCM. While timely delivery by an interdisciplinary team is recommended for both, severe preeclampsia and PPCM, postpartum management is quite different. For PPCM heart failure therapy for several months and years with close monitoring by a cardiologist is strongly recommended. In contrast, as symptoms resolve quickly after delivery in patients with preeclampsia, blood pressure control by antihypertensive medication is the only therapy suggested.1,34 Thus, since PPCM patients need full heart failure therapy for several months or years and since clear diagnostic discrimination between PPCM and severe gestational hypertensive complications is difficult, it would be important to monitor the postpartum course of both patient groups for optimal management.
Genetic forms of pregnancy-associated cardiomyopathies
The higher incidence observed in patients with African ancestry and reports of about 16% of PPCM patients with positive familial history for heart failure suggest genetic aspects in PPCM.3,4,23 Indeed, subsets of PPCM patients are carriers of mutations associated with familial DCM forms.5,6,35 While PPCM has been defined as a non-familial and non-genetic form of heart failure, the reality shows that it is not easy to distinguish non-genetic PPCM from genetic forms of peripartum heart failure and therefore careful familial history should be taken in all PPCM patients. As genetic forms may a have a lower chance for recovery23 such information is important for risk stratification and management as well as for counselling affected families.
Peripartum heart failure triggered by pathogens
Several studies pointed out the potential importance of viral infection for inducing or driving PPCM but results are controversial as reviewed by Selle et al.8 in the present paragraph. However, the highly variable prevalence of myocarditis in PPCM patients ranging from 8.8 to 78% suggest that in some cases viral infection may be among the trigger for PPCM.8 In this regard, it is interesting to note that primary viral infection, usually in childhood, can lead to a latent life-long viral persistence, which is by definition of no pathogenetic significance.8 Since pregnancy is a condition with a lowered immune defence, latent viral infections may become reactivated in the peripartum phase and may lead to peripartum myocarditis. This idea is supported by experimental data in mice showing that encephalomyocarditis viral infection increases the severity of myocardial damage in postpartum mice compared with non-pregnant control mice.8 Thus, it is likely that such a scenario may explain viral myocarditis as the cause of peripartum heart failure in some patients. However, to date, the impact of infectious diseases and their role on the development and the prognosis of patients with PPCM is not well understood and should be investigated in larger prospective study collectives and registries.
In conclusion, it may be difficult to clearly distinguish PPCM from the above-mentioned conditions as no specific diagnostic marker profiles exist and presentation and pathophysiologies may be overlapping. However, these conditions may trigger similar downstream pathomechanisms, which drive disease progression and may therefore respond to similar treatment strategies.
Pathomechanisms of peripartum cardiomyopathy
We only briefly summarize current advances in knowledge on pathomechanisms of PPCM and refer to a recent review for more detail.2 Among potential factors leading to PPCM, low selenium levels, various viral infections, stress-activated cytokines, inflammation, autoimmune reaction, a pathological response to haemodynamic stress, and unbalanced oxidative stress have been mentioned.3,33,36–38 A novel finding is the discovery that oxidative stress-mediated cleavage of the nursing hormone prolactin into a smaller biologically active subfragment, 16-kDa prolactin, may be a major factor initiating and driving PPCM.33,37,38 This 16-kDa prolactin up-regulates miR-146a, which mediates most of the adverse effects of 16-kDa prolactin in endothelial cells and is released in microparticles (exosomes) into the circulation from where it also affects cardiomyocytes.38 Along this line, multiple anti-angiogenic factors such as 16-kDa prolactin and sFlt1 disturb the angiogenic balance in the peripartum phase thereby causing vascular impairment and subsequently heart failure.2,33,37
Thus, PPCM appears as a disease caused by unbalanced oxidative stress, impaired cardioprotective and pro-angiogenic signalling, and high expression of anti-angiogenic factors. As outlined earlier, these mechanisms may already be initiated by comorbidities during pregnancy such as severe gestational hypertension and infectious disease.2
Biomarkers for diagnosis and risk stratification of peripartum cardiomyopathy
Diagnosis of PPCM is often delayed and complicated by the fact that symptoms of heart failure to some degree overlap with normal pregnancy-associated discomfort. In addition, most PPCM patients are initially not seen by cardiologists. Biomarkers would help to identify PPCM patients early and refer them to expert physicians for further diagnostic assessment. Of note, biomarker-screens around pregnancy need controls exactly matched to pregnancy stage since many hormones, growth factors, and enzymes display highly specific kinetic pattern throughout pregnancy and postpartum (Table 1). So far, NT-proBNP, an unspecific marker for pregnancy complications such as preeclampsia as well as for heart failure and other diseases, is markedly elevated in most PPCM patients with little overlap to healthy peripartum women (Table 1).23,39,40 MiR-146a is specifically increased in the serum of PPCM patients compared with healthy postpartum women and patients with DCM (Table 1).23,38 sFlt1, a biomarker for preeclampsia that is supposed to clear rapidly after delivery, is significantly increased in PPCM patients as are asymmetric dimethyl arginine and Cathepsin D activity (Table 1).23,33 Failure to normalize a biomarker profile including NT-proBNP, oxLDL, interferon-g, and prolactin is associated with adverse outcome in PPCM patients.39 In turn, creatine-kinase and C-reactive protein are increased by delivery stress in healthy postpartum women and troponin T, a marker for cardiac injury, is often within normal ranges in PPCM patients (Table 1) and therefore less suited as biomarkers for PPCM.11
Table 1.
Overview of biomarkers analysed in peripartum cardiomyopathy patients
| Biomarker | Relevance for PPCM |
|---|---|
| NT-proBNP | Not specific for PPCM, but good sensitivity for heart failure.23,39 |
| 16-kDa Prolactin | Pathophysiological factor of PPCM, high technical effort for measurement, diagnostic accuracy needs to be evaluated.37,42 |
| Interferon-γ | Elevated plasma levels in PPCM patients, diagnostic accuracy needs to be evaluated.39,49 |
| Asymmetric Dimethylarginine (ADMA) | Marker for endothelial dysfunction and cardiovascular risk, diagnostic accuracy needs to be evaluated.23 |
| Cathepsin D | Activity elevated in plasma of PPCM patients, diagnostic accuracy needs to be further evaluated.23,37 |
| Soluble fms-like tyrosine kinase-1 (sFlt-1) | Elevated plasma levels in PPCM patients, diagnostic accuracy needs to be further evaluated.33 |
| microRNA-146a | Pathophysiological factor of PPCM, high technical effort for measurement, diagnostic accuracy needs to be further evaluated.23,38 |
PPCM, peripartum cardiomyopathy.
Taken together, currently NT-proBNP is the only commercially available marker for efficient screening of peripartum heart failure (Table 1). However, it is not specific for PPCM and may be elevated in other conditions such as preeclampsia and pulmonary embolism. MiR-146a is a disease specific potential candidate, but overall there is an urgent need for better diagnostic and prognostic biomarkers for PPCM.
Therapeutic concepts and management for peripartum cardiomyopathy
Heart failure around pregnancy provides a challenge for the treating physician especially due to a lack of evidence-based clinical data.
Currently, PPCM is treated according to the ESC guidelines for heart failure in pregnancy.1 In brief, late in pregnancy, therapeutic interventions need to consider the health of the mother and the foetus. Beta-blocker, thiazide diuretics, or furosemide treatment can be necessary in some patients with PPCM before delivery, however, as diuretic therapy may impair perfusion of the placenta with potential harm to the foetus, the lowest dosages possible of diuretic therapy have to be used. After delivery, standard therapy for heart failure is recommended in PPCM including beta-blockers, ACE-inhibitors/AT1-blockers, mineralocorticoid receptor antagonists (MRA), and diuretics. As no data on the use of inotropes exist in patients with PPCM and catecholamines may aggravate myocardial damage, an i.v. infusion of an inotrope (e.g. dobutamine) should only be considered in patients with severe hypotension and/or signs of cardiogenic shock. As soon as haemodynamic stability is achieved, inotropes should be tapered and general recommendations for patients with heart failure in pregnancy should be followed.1 Early treatment with beta-blockers started at very low dosages appears to be protective even in patients with severely depressed ejection fraction. In patients with persistent sinus tachycardia, in whom uptitration of beta-blocker dose was not feasible due to hypotension or heart failure, we also have good experience with the use of ivabradine.
A South African study reports improved outcome in PPCM patients with elevated serum markers of inflammation (C-reactive protein and tumour necrosis factor-α, TNF-α) after treatment with pentoxifylline on top of conventional heart failure therapy suggesting a potential benefit of anti-TNF-α treatment.41
A pilot study in South Africa reported highly beneficial effect of the prolactin-blocker bromocriptine on top of heart failure medication in patients with acute onset PPCM.3,42 Likewise, the majority of patients in the German PPCM registry (96%) who had obtained this therapy concept improved their condition.23 Currently, the efficacy of bromocriptine on top of heart failure medication is investigated in a larger randomized multicentre trial in Germany (ClinicalTrials.gov, study number: NCT00998556). Importantly, a recent statement from the European Medicines Agency (EMA) recommends restricted use of bromocriptine for women to stop breast milk production due to adverse effects on blood pressure and thrombosis. To our knowledge, no adverse effects were observed with low dose bromocriptine (2–5 mg/day) in 57 PPCM patients from the German PPCM registry or in the 44 patients currently enrolled in the German bromocriptine trial. Moreover, no adverse event related to bromocriptine was reported from the 75 PPCM patients listed in the international PPCM registry of the ESC EURObservational Research Programme. Results from larger studies are awaited for to be more conclusive on this therapy concept.
PPCM patients have an increased risk for sudden death and seem to benefit from implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT).39,43,44 As many PPCM patients recover from the disease or display at least marked improvement of systolic LV function, a wearable cardioverter/defibrillator would be an alternative option vs. operative ICD or CRT-D implantation for primary prophylaxis. We recommend the use of a wearable cardioverter/defibrillator as a bridge-to-decision for 3–6 months with serial echocardiographic assessments of LV function. After a follow-up period of 6 months, the indication for ICD and CRT implantation should be evaluated according to the current guidelines.45 A recent observational study on a small PPCM collective with severely depressed cardiac function and/or ventricular arrhythmias seems to confirm the value of the wearable cardioverter/defibrillator in PPCM patients.46
In patients with refractory acute heart failure, an extra corporal life support system may be used for stabilization and/or transport in a tertiary centre. The implantation of an LV assist device may be a therapeutic option in critically ill patients with no signs of recovery over several weeks. Heart transplantation is only an ‘ultimo ratio’ given the rather good prognosis for recovery within the first 6–12 months of many PPCM patients and invasive therapeutic modalities should not be installed over actively.
A general agreement among experts (study group of PPCM of the HFA/ESC) suggests continued therapy with standard heart failure medications for a minimum of 12 months. As some PPCM patients showed continued improvement in cardiac function up to 5 years after diagnosis,47 standard heart failure therapy may be continued in patients with persistently reduced LV ejection fraction for several years or even lifelong. Full recovery of LV structure and function is not always associated with stable condition. Therefore, we continue heart failure drug therapy including β-blockade, ACE-inhibition, and MRA for at least 6 months after full recovery and recommend drug tapering according to the weaning protocol provided in Table 2.
Table 2.
Proposed strategy for heart failure drug therapy in peripartum cardiomyopathy patients after delivery before and after complete recovery of left-ventricular structure and function
| Drug | Safety during lactationa | Absence of complete recovery | Complete and sustained recovery of left-ventricular structure and function (echocardiographic follow-up every 6 months) |
|||
|---|---|---|---|---|---|---|
| 6 months | 6–12 months | >12 months | >18 months | |||
| β-Blocker | Bradycardia of the newborn reported in rare cases. Metoprolol is the best-studied β-blocker during lactation. | Essential for all patients. Up-titration to standard or maximally tolerated dosages. | Continue all drugs for at least 6 months after full recovery to avoid relapse | Continue β-blocker and ACE-inhibitor/ARB for at least 6 months after stopping MRA | Continue β-blocker for at least 6 months after stopping ACE-inhibitor/ARB | Discontinue β-blockade, ensure echocardiographic follow-up |
| ACE-inhibitor | Low transfer of enalapril and captopril into the breast milk. | Essential for all patients. Up-titration to standard or maximally tolerated dosages. | Reduce dosage and then discontinue ACE-inhibitor/ARB | |||
| ARB | Very limited data on ARB during lactation and should be avoided. | Recommended for patients who cannot tolerate ACE-inhibition. Up-titration to standard or maximally tolerated dosages. | ||||
| MRA | Very limited data on MRA during lactation and should be avoided | Recommended for all patients with LVEF < 40%. Eplerenone may be considered due to less hormonal side effects. | Discontinue only if complete and sustained recovery of left-ventricular structure and function | |||
| Ivabradine | No data on ivabradine during lactation available and should be avoided. | For patients with heart rate >75/min, when β-blocker up-titration is not possible. Should be tapered when β-blocker up-titration is possible and/or heart rate is < 60/min |
Continue when heart rate is >75/min despite β-blocker up-titration | Discontinue only if complete and sustained recovery of left-ventricular structure and function | ||
| Diuretics | Thiazides are the best-studied diuretics during lactation and well tolerated. They may decrease milk production. Very limited data on furosemide and torasemide during lactation. | Only when oedema/congestion is present. Early tapering of dose according to symptoms, even before full recovery of left-ventricular function | Continue only when symptoms (congestion/oedema) are present without diuretic therapy as part of an antihypertensive drug therapy | |||
aAccording to the ESC guidelines, the manufacturers' instructions are mainly based on the fact that drugs are not tested sufficiently during pregnancy and breastfeeding. For this and for legal reasons, drugs are frequently considered prohibited during pregnancy and breastfeeding.1
According to the guidelines of the ESC on cardiovascular disease in pregnancy, breastfeeding bears a low risk for bacterial or fungal infection and therefore in highly symptomatic/unwell patients bottle feeding should be considered.1 In addition, since many drugs including heart failure medication are not tested sufficiently during pregnancy and breastfeeding, these drugs are frequently not recommended during pregnancy and breastfeeding.1
Subsequent pregnancy in peripartum cardiomyopathy patients
Peripartum cardiomyopathy patients should be informed about contraceptive options since cardiac dysfunction re-emerges frequently in the peri- and postpartum phase often with worse outcome especially when LV structure and function did not completely recover.2–4,37 The use of an intrauterine device is recommended for PPCM patients since hormonal contraceptives may interact with heart failure medication.4
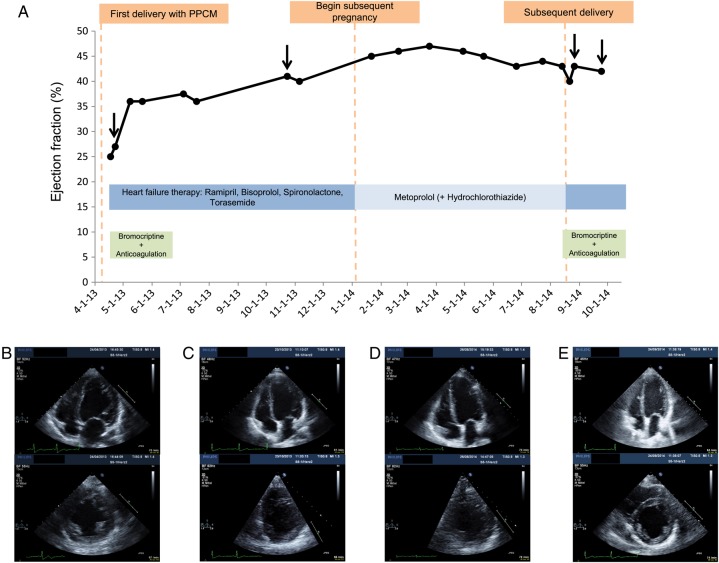
If PPCM patients get pregnant again it should be noted that the pregnancy state is frequently tolerated quite well, especially if the subsequent pregnancy is entered with fully recovered cardiac function.4,48 However, cardiac dysfunction re-emerges frequently in the peri- and postpartum phase.4,37,48 Thus, it seems that the stage of pregnancy may exert protection even for damaged hearts. To illustrate this phenomenon, we present the case of a 37-year-old PPCM patient whose LV ejection fraction was impaired when entering the subsequent pregnancy 8 months after diagnosis of the index PPCM. We observed an improvement of the LV function during the first two trimesters and a moderate decline in the third trimester (Figure 1). Therefore, delivery by Caesarean section in week 36 was induced and full standard heart failure therapy and bromocriptine were introduced. Thereafter, the patient showed subsequent improvement of cardiac function (Figure 1). A potential beneficial effect of heart failure therapy and bromocriptine in PPCM patients with subsequent pregnancies immediately after delivery is also supported by a small pilot study with 12 PPCM patients. All 12 patients displayed a moderate impaired LV function immediately after delivery of the subsequent pregnancy (control group, n = 6, EF: 45 ± 7%, bromocriptine group, n = 6, EF 40 ± 14). Three to four months postpartum, the control group displayed heart failure (EF: 23 ± 3%) and high mortality (n = 3 or 50% of patients had died) while all patients in the bromocriptine group survived and maintained or even improved cardiac function (EF: 52 ± 6%).37 In conclusion, PPCM patients should be advised not to get pregnant again. In case of a subsequent pregnancy in a PPCM patient, appropriate therapy during pregnancy and postpartum as well as management by an interdisciplinary team of cardiologists, obstetricians, anaesthetists, and neonatologists are recommended. First data are carefully optimistic with regard that heart failure therapy in combination with bromocriptine may be effective in preventing relapse of PPCM but further larger studies are required to validate these observations.
Figure 1.
(A) Time course of LVEF in a peripartum cardiomyopathy patient (37 years, 3 Gravida, 3 Para) with severe peripartum cardiomyopathy after delivery of her second child and her subsequent pregnancy. Fast improvement of cardiac function and symptoms on immediate treatment with standard heart failure therapy and bromocriptine on index peripartum cardiomyopathy (BR: 5 mg/day for 2 months). The LVEF was still impaired when entering the subsequent pregnancy 8 months later. During pregnancy, the patient was treated with β-blocker occasionally supported by diuretics and further improvement of her condition in the first two trimesters was observed. A moderate decline of cardiac function in the third trimester occurred promoting Cesarean-section in week 36 followed by full standard heart failure therapy and bromocriptine (BR: 5 mg/day for 2 months) and subsequent improvement of cardiac function. Images of echocardiographic examinations shortly after onset of index peripartum cardiomyopathy (B), before subsequent pregnancy (C), shortly after subsequent delivery (D), and at 1-month-follow-up (E).
Conclusion
In recent years, the awareness for PPCM has increased for the benefit of these patients. Larger clinical data sets are collected and analysed allowing more insight into the pathophysiology of the disease and providing important information for diagnosis and management. Large clinical registries on peripartum heart failure (ESC EUROOBS program, www.escardio.org)25 together with experimental research are needed to further broaden our understanding of PPCM with regard to its aetiology, risk factors, diagnosis, and, most importantly, optimized treatment strategies and management.
Funding
This review was supported by the DFG (excellence cluster Rebirth) and the BMBF. Funding to pay the Open Access publication charges for this article was provided by Research funds of the Medical School Hannover.
Conflict of interest: none declared.
References
- 1.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L, Bax J, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Aguiar C, Al-Attar N, Garcia AA, Antoniou A, Coman I, Elkayam U, Gomez-Sanchez MA, Gotcheva N, Hilfiker-Kleiner D, Kiss RG, Kitsiou A, Konings KT, Lip GY, Manolis A, Mebaaza A, Mintale I, Morice MC, Mulder BJ, Pasquet A, Price S, Priori SG, Salvador MJ, Shotan A, Silversides CK, Skouby SO, Stein JI, Tornos P, Vejlstrup N, Walker F, Warnes C. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147–3197. [DOI] [PubMed] [Google Scholar]
- 2.Hilfiker-Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 2014;11:364–370. [DOI] [PubMed] [Google Scholar]
- 3.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet 2006;368:687–693. [DOI] [PubMed] [Google Scholar]
- 4.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010;12:767–778. [DOI] [PubMed] [Google Scholar]
- 5.van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IA, Sliwa K, Alders M, Almomani R, van Langen IM, van der Meer P, Sinke RJ, van der Velden J, Van Veldhuisen DJ, van Tintelen JP, Jongbloed JD. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J 2014;35:2165–2173. [DOI] [PubMed] [Google Scholar]
- 6.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, Paulus WJ, Dooijes D, van den Berg MP. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 2010;121:2169–2175. [DOI] [PubMed] [Google Scholar]
- 7.van Tintelen JP, Pieper PG, van Spaendonck-Zwarts KY, van den Berg MP. Pregnancy, cardiomyopathies and genetics. Cardiovasc Res 2014;101:571–578. [DOI] [PubMed] [Google Scholar]
- 8.Selle T, Renger I, Labidi S, Bultmann I, Hilfiker-Kleiner D. Reviewing peripartum cardiomyopathy: current state of knowledge. Future Cardiol 2009;5:175–189. [DOI] [PubMed] [Google Scholar]
- 9.Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–1008. [DOI] [PubMed] [Google Scholar]
- 11.Conrad KP, Davison JM. The renal circulation in normal pregnancy and preeclampsia: is there a place for relaxin? Am J Physiol Renal Physiol 2014;306:F1121–F1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med 2006;16:285–291. [DOI] [PubMed] [Google Scholar]
- 13.Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409–414. [DOI] [PubMed] [Google Scholar]
- 14.Liu LX, Arany Z. Maternal cardiac metabolism in pregnancy. Cardiovasc Res 2014;101:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 2002;57:609–613. [DOI] [PubMed] [Google Scholar]
- 16.Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000;283:1183–1188. [DOI] [PubMed] [Google Scholar]
- 17.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol 2011;58:659–670. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya CA, Kitakaze M, Ishibashi-Ueda H, Nakatani S, Murohara T, Tomoike H, Ikeda T. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ J 2011;75:1975–1981. [DOI] [PubMed] [Google Scholar]
- 19.Karaye KM. Right ventricular systolic function in peripartum and dilated cardiomyopathies. Eur J Echocardiogr 2011;12:372–374. [DOI] [PubMed] [Google Scholar]
- 20.Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, Vacek J, Weiner CP, Ellerbeck E, Schreiber T, Lakkireddy D. Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol 2014;63(25 Pt A):2831–2839. [DOI] [PubMed] [Google Scholar]
- 21.Sliwa K, Mayosi BM. Recent advances in the epidemiology, pathogenesis and prognosis of acute heart failure and cardiomyopathy in Africa. Heart 2013;99:1317–1322. [DOI] [PubMed] [Google Scholar]
- 22.Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, Green MS, Gollob MH, Haddad H, Birnie DH. Frequency of peripartum cardiomyopathy. Am J Cardiol 2006;97:1765–1768. [DOI] [PubMed] [Google Scholar]
- 23.Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, Tsikas D, Jordan J, Lichtinghagen R, von Kaisenberg CS, Struman I, Bovy N, Sliwa K, Bauersachs J, Hilfiker-Kleiner D. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013;108:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol 2012;154:27–31. [DOI] [PubMed] [Google Scholar]
- 25.Sliwa K, Hilfiker-Kleiner D, Mebazaa A, Petrie MC, Maggioni AP, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Roos-Hesslink JW, Shah AJ, Seferovic PM, Elkayam U, van Spaendonck-Zwarts K, Bachelier-Walenta K, Mouquet F, Kraigher-Krainer E, Hall R, Ponikowski P, McMurray JJ, Pieske B. EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail 2014;16:583–591. [DOI] [PubMed] [Google Scholar]
- 26.Tibazarwa K, Lee G, Mayosi B, Carrington M, Stewart S, Sliwa K. The 12-lead ECG in peripartum cardiomyopathy. Cardiovasc J Afr 2012;23:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labidi S, Hilfiker-Kleiner D, Klein G. Left bundle branch block during pregnancy as a sign of imminent peripartum cardiomyopathy. Eur Heart J 2011;9:1076. [DOI] [PubMed] [Google Scholar]
- 28.Hilfiker-Kleiner D, Struman I, Hoch M, Podewski E, Sliwa K. 16-kDa prolactin and bromocriptine in postpartum cardiomyopathy. Curr Heart Fail Rep 2012;9:174–182. [DOI] [PubMed] [Google Scholar]
- 29.Gati S, Papadakis M, Papamichael ND, Zaidi A, Sheikh N, Reed M, Sharma R, Thilaganathan B, Sharma S. Reversible de novo left ventricular trabeculations in pregnant women: implications for the diagnosis of left ventricular noncompaction in low-risk populations. Circulation 2014;130:475–483. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 2014;63:1815–1822. [DOI] [PubMed] [Google Scholar]
- 31.Dennis AT, Castro JM. Echocardiographic differences between preeclampsia and peripartum cardiomyopathy. Int J Obstet Anesth 2014;23:260–266. [DOI] [PubMed] [Google Scholar]
- 32.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy 2012;31:454–471. [DOI] [PubMed] [Google Scholar]
- 33.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firoz T, Magee L, MacDonell K, Payne B, Gordon R, Vidler M, von Dadelszen P, the Community Level Interventions for Pre-eclampsia Working G. Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum: a systematic review. BJOG 2014;121:1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales A, Painter T, Li R, Siegfried JD, Li D, Norton N, Hershberger RE. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation 2010;121:2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sliwa K, Forster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker-Kleiner D, Ansari AA. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 2006;27:441–446. [DOI] [PubMed] [Google Scholar]
- 37.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600. [DOI] [PubMed] [Google Scholar]
- 38.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 2013;123:2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster O, Hilfiker-Kleiner D, Ansari AA, Sundstrom JB, Libhaber E, Tshani W, Becker A, Yip A, Klein G, Sliwa K. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail 2008;10:861–868. [DOI] [PubMed] [Google Scholar]
- 40.Junus K, Wikstrom AK, Larsson A, Olovsson M. Placental expression of proBNP/NT-proBNP and plasma levels of NT-proBNP in early- and late-onset preeclampsia. Am J Hypertens 2014;27:1225–1230. [DOI] [PubMed] [Google Scholar]
- 41.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail 2002;4:305–309. [DOI] [PubMed] [Google Scholar]
- 42.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, McMurray J, Yamac H, Labidi S, Struman I, Hilfiker-Kleiner D. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation 2010;121:1465–1473. [DOI] [PubMed] [Google Scholar]
- 43.Sliwa K, Forster O, Tibazarwa K, Libhaber E, Becker A, Yip A, Hilfiker-Kleiner D. Long-term outcome of peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol 2011;147:202–208. [DOI] [PubMed] [Google Scholar]
- 44.Mouquet F, Mostefa Kara M, Lamblin N, Coulon C, Langlois S, Marquie C, de Groote P. Unexpected and rapid recovery of left ventricular function in patients with peripartum cardiomyopathy: impact of cardiac resynchronization therapy. Eur J Heart Fail 2012;14:526–529. [DOI] [PubMed] [Google Scholar]
- 45.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. Task Force for the D, Treatment of A, Chronic Heart Failure of the European Society of C, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. Guidelines ESCCfP. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 46.Duncker D, Haghikia A, Konig T, Hohmann S, Gutleben KJ, Westenfeld R, Oswald H, Klein H, Bauersachs J, Hilfiker-Kleiner D, Veltmann C. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur J Heart Fail 2014;16:1331–1336. [DOI] [PubMed] [Google Scholar]
- 47.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc 2005;80:1602–1606. [DOI] [PubMed] [Google Scholar]
- 48.Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, Hameed A, Gviazda I, Shotan A. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 2001;344:1567–1571. [DOI] [PubMed] [Google Scholar]
- 49.Ricke-Hoch M, Bultmann I, Stapel B, Condorelli G, Rinas U, Sliwa K, Scherr M, Hilfiker-Kleiner D. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc Res 2014;101:587–596. [DOI] [PubMed] [Google Scholar]