Abstract
The in utero environment is a key determinant of long-term health outcomes; poor maternal metabolic state and placental insufficiency are strongly associated with these long-term health risks. Human epidemiological studies link maternal obesity and offspring cardiovascular disease in later life, but mechanistic studies in animal models are limited. Here, we review the literature pertaining to maternal consequences of obesity during pregnancy and the subsequent impact on fetal cardiovascular development.
The Obesity Epidemic
The prevalence of obesity has reached epic proportions, with rates reported from 24 to 36% in developed nations (28a, 56) and a global estimate of adult obesity at 11% (83). Despite more recent awareness and efforts to address this epidemic, a large proportion of the population has been impacted by obesity and is now affected by the associated health consequences. Of particular concern is maternal obesity during pregnancy, which confers numerous risks to the developing offspring that may have a significant impact in later life. The concept of developmental programming, as it was first introduced by Dr. David Barker and colleagues almost 30 years ago, originated from data demonstrating the impact of maternal under-nutrition on fetal development and long-term health outcomes. However, with the obesity epidemic now faced by the Western world, attention in both human and animal model studies has turned to the current issue of maternal over-nutrition on fetal programming. Maternal obesity is associated with increased incidence of morbidity and mortality in both the mother and fetus and is one of the most prevalent preventable causes of pregnancy complications. This review will focus on the current understanding of the role of maternal obesity and over-nutrition in altering maternal metabolic health and in programming of cardiovascular disease risk in the offspring.
Maternal Vascular Adaptations to Pregnancy: Impact of Obesity
Due to the physiological requirements of pregnancy, the maternal cardiovascular system must undergo significant adaptations to accommodate and adequately meet the oxygen and nutritional demands of the developing fetus (60), including a 40% increase in blood volume and cardiac enlargement (24, 40). The greater blood volume serves to supply the uterus in response to the needs of the utero-placental circulation, which functions as a low-resistance circuit. Vascular adaptation to pregnancy is characterized by marked vasodilation, a process that is dependent on endothelial cell and smooth muscle cell changes, and is reflected by a reduction in systemic vascular resistance (79). In obese individuals, there are structural alterations in blood vessels, which include increases in basement membrane thickness and vessel stiffness (87). These vascular changes likely occur in response to elevation in blood pressure, as suggested by a study in hypertensive human subjects where non-invasive imaging of the carotid artery demonstrated increased arterial stiffening corresponding to increased diastolic pressure (78). With progression of obesity, microvascular walls undergo atrophy, which leads to narrowing of the microvessels (14, 67) and a risk of local tissue ischemia (10). Using wire myography studies, it has been demonstrated that vasoconstriction and vasodilatation are impaired in myometrial arteries from obese women with otherwise uncomplicated pregnancies (28, 27).
Endothelial cells play a crucial role in the regulation of vascular tone (30), and obese women enter pregnancy with chronic preexisting endothelial activation resulting in endothelial dysfunction (10), which is a central pathological status in the early development of atherosclerosis (33). Endothelial dysfunction refers to the altered capacity of the endothelium to uptake and metabolize L-arginine, the substrate for nitric oxide (NO) synthesis (50). Obesity is associated with altered L-arginine transport and NO production resulting from altered uptake and metabolism of the endogenous vasodilator adenosine (82). In addition to vasodilators, vascular tone is also maintained by vasoconstrictors such as endothelin-1 (ET-1) (86). Levels of endogenous ET-1 are elevated in the setting of obesity, and this likely contributes to endothelial dysfunction in obese individuals (reviewed in Ref. 6).
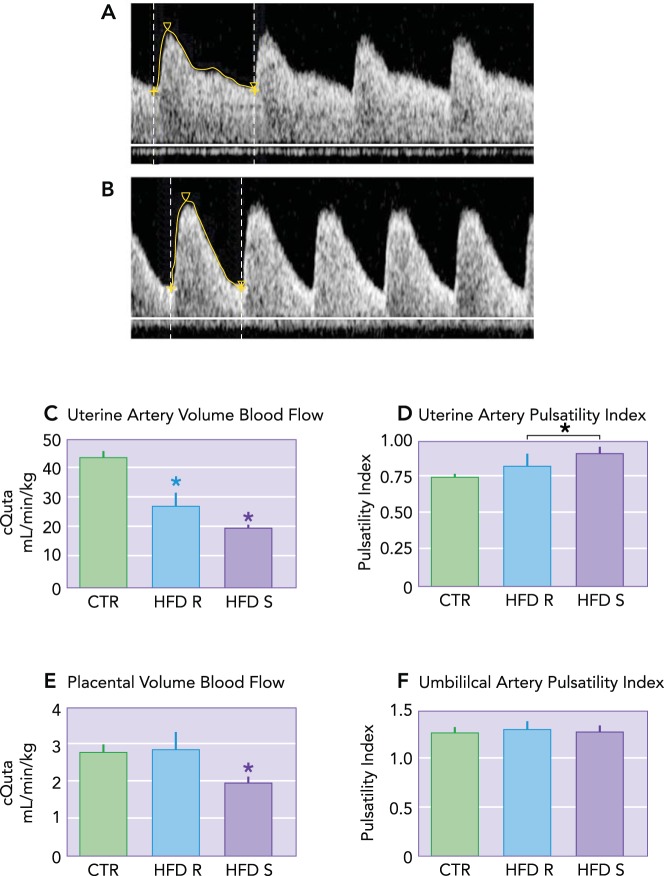
The mechanisms regulating the vascular adaptations of pregnancy are governed in part by sex steroids (23) but in animal models have also been shown to be influenced by environmental factors such as maternal nutrition (41). Our group utilizes a nonhuman primate (NHP) model of high-fat diet (HFD) consumption in which we have the advantage of being able to distinguish between diet-alone effects (in animals who are consuming the HFD but are resistant to weight gain and have normal insulin sensitivity) and HFD in combination with maternal obesity (animals who have excessive weight gain and become insulin resistant). We have previously demonstrated that uterine blood flow is decreased in both lean and obese HFD-fed dams compared with chow-fed dams; however, this impaired flow is exacerbated in the obese HFD animals, demonstrating an adverse influence of maternal obesity in addition to the effect of diet-alone (13) (FIGURE 1). Since the fetal side circulation of the placenta does not receive neuronal innervation, local vascular tone is regulated and maintained by the synthesis and release of vasoactive modulators from the endothelium (52), rendering this organ particularly susceptible to maternal obesity and endothelial dysregulation given the increased levels of circulating inflammatory mediators. Importantly, endothelial dysfunction in obese women is likely to impact spiral artery remodeling at the time of trophoblast invasion, leading to inadequate establishment of the placenta and ultimately to placental insufficiency. However, correlating in vivo blood flow with placental nutrient transporter function is not currently possible in humans, which limits our understanding of the placental contribution to the observed increased perinatal risks associated with maternal obesity.
FIGURE 1.
Decreased uteroplacental perfusion in Japanese macaques fed a high-fat diet
Maternal high-fat diet (HFD) leads to increased uterine artery pulsatility Index (PI). A: uterine artery (Uta) PI is 0.74 in a representative control animal. B: the Uta PI is 1.17 in a representative HFD-sensitive (HFD-S) animal with a Doppler waveform that demonstrates decreased diastolic flow consistent with increased vascular impedance compared with A. C: the calculated Uta blood flow (cQUta) normalized to maternal weight was significantly reduced in HFD-R and HFD-S animals compared with CTR. D: Uta PI is increased in HFD-S animals compared with CTR. As a group, HFD (HFD-R + HFD-S) had a significant increase in Uta PI compared with CTR. E: the calculated umbilical vein blood flow (cQUV) normalized to fetal abdominal circumference was reduced in HFD-S animals compared with controls. There was no difference in HFD-R animals compared with controls. F: the umbilical artery (UA) PI was unaffected by diet group. *Significant difference (P < 0.0): CTR, n = 9; HFD-R, n = 6; HFD-S, n = 9. Reproduced from Ref. 13 with permission.
Maternal Obesity and Placental Phenotype
As the mediator of maternal and fetal interactions, the placenta has a central role in fetal organ growth and development, thus the impact of maternal obesity and nutrition on placental function in the context of fetal cardiovascular development is an important research focus. However, the mechanisms that regulate and alter placental development in response to an adverse in utero environment are incompletely understood, in part due to the current limitations of in vivo imaging methods, timing of tissue collection, and animal models that do not recapitulate human placental structure (21). During implantation in humans, the high-resistance maternal spiral arteries of the endometrium must adapt to become low-resistance vessels, which are invaded by rapidly proliferating endovascular trophoblast cells to form the maternal decidua and establish a functional placenta (57). Cytotrophoblast cells replace the endothelial and muscular linings of the uterine arterioles, a process that enlarges vessel diameter and initiates maternal blood flow to the intervillous space by the end of the first trimester (17). Thus, within the hemochorial placenta of primates, two separate vascular networks develop in tandem: the maternal compartment as the source of nutrients and the fetal compartment structured in a complex arborized pattern of villi intended to maximize the surface area available for the exchange of nutrients with the maternal vasculature. Recently, in a human study from obese mothers, an association was found between a lipid-rich placental environment where obese women had 50% greater placental lipid droplet accumulation than lean women, decreased regulators of angiogenesis (e.g., angiopoietin, vascular endothelial growth factor A, and hypoxia inducible factor 1), and increased expression of interleukins and chemokine receptors (66), demonstrating a pathological placental phenotype with maternal obesity. Although the rodent placenta differs structurally from the human placenta, in particular with less extensive endovascular invasion, these lower-order species still provide a useful animal model to gain insight into developmental abnormalities. Data from a rat model of maternal obesity achieved by chronic HFD consumption suggest that the uterine adaptations to pregnancy are impaired, which leads to inadequate trophoblast invasion and poor establishment of the placenta (26). Specifically, this model has been used to demonstrate altered vascular development and increased hypoxia (25), elevated expression of the tissue remodeling modulator matrix metalloproteinase 9, and increased smooth muscle actin surrounding the spiral arteries (26). The NHP has a developmental ontogeny more closely related to humans, the animals can be maintained in a controlled environment, and their use permits timed tissue collection that is not possible for ethical reasons in human studies. In our NHP model of HFD consumption, we have demonstrated reduced uterine blood flow, increased expression of inflammatory cytokines, and pathological evidence of infarction (13, 62). Importantly, animal studies have shown that placental vascular insufficiencies alter fetal hemodynamics in an attempt to preserve growth of vital fetal organs (84, 63); such vascular adaptations likely cause structural and physiological changes that influence cardiovascular development.
The In Utero Environment of the Obese Mother
What distinguishes obese women from their lean counterparts is an excess of adipose tissue. Rather than simply being a storage depot for fat, adipose is a metabolically active tissue that makes a significant contribution to endocrine function through the production of a variety of adipokines, including leptin (77), which is important in the regulation of multiple aspects of pregnancy (1, 71). It has been demonstrated in in vitro studies of arterial vascular smooth muscle cells that leptin inhibits cell growth (5). We could postulate that, in vivo, leptin may impede angiogenesis and subsequently impact maternal-fetal exchange through regulation of placental nutrient transport (e.g., amino acid uptake), although this remains to be proven.
Obesity is considered a physiological state of chronic, low-grade inflammation that is further exacerbated by the metabolic changes of pregnancy. Obese individuals have elevated levels of inflammatory markers including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) (42), and elevated levels of CRP correlate with endothelial dysfunction in obese women (59). Maternal obesity also leads to insulin resistance, elevated serum triglycerides, and increased blood pressure (22). Higher levels of circulating free fatty acids serve as ligands for the toll-like receptors, which activate an inflammatory signaling cascade (38), thus enhancing chronic inflammation in obese individuals. Hyperlipidemia decreases prostacyclin secretion and enhances local peroxidase production, resulting in vasoconstriction and platelet aggregation (9) (Table 1). These local vascular changes in pre-eclamptic patients, who share many abnormal vascular characteristics with obese mothers, have been shown to result in atherosclerotic-like plaques in placental arterioles (85). In addition to systemic metabolic disturbances, tissue-specific effects, such as increased reactive oxygen species production and subsequently elevated oxidative stress levels, are associated with obesity (15). Obese women have been found to have significant placental pro-inflammatory macrophage accumulation and high levels of inflammatory cytokines (7), as well as nitrative stress (64), compared with lean subjects. Nutrient transporters are known to be responsive to inflammatory cytokines in vitro (72, 31), which may provide a plausible explanation for fetal growth discrepancies seen in children of obese mothers; specifically, in macrosomic infants, there may be upregulation of placental nutrient transfer or downregulation in fetuses that are growth restricted. A comprehensive understanding of placental perfusion, its effects on placental nutrient transport and how the fetus regulates its metabolic demand through the placenta is not known and is an area of keen interest.
Table 1.
Maternal and fetal consequences of obesity and poor nutrition during pregnancy
| Maternal | Fetal |
|---|---|
| Increased morbidity and mortality | Increased morbidity and mortality |
| Impaired vasoconstriction and vasodilation | Systemic inflammation |
| Endothelial dysfunction | Altered nutrient transport |
| Increased placental pathology and impaired function | Hyperlipidemia |
| Hyperlipidemia | Hypertension |
| Hypertension | Altered growth trajectory |
| Increased oxidative stress | Altered cardiovascular development |
| Increased circulating leptin |
Cardiomyocyte Development
Regulation of cardiomyocyte growth in utero is a key determinant of heart health in later life as the number of cells at birth determines the number of myocytes for life (73). The myocardium of the fetal heart expands by replication of cardiomyocytes under the regulation of mediators such as insulin-like growth factor (69), angiotensin II (70), and cortisol (20), as well as mechanical stimuli such as arterial pressure load (19). Suppressors of myocyte proliferation include tri-iodo-l-thyronine (T3) (8), atrial natriuretic peptide (ANP) (55), and reduced cardiac systolic load (54). Additionally, increased ventricular wall stress negatively impacts cardiomyocyte proliferation, which can result in restricted cardiac expansion and regeneration of myocytes as required for normal heart function throughout life. If total myocyte number is reduced, the burden of mechanical stresses and contractile force generation is greater than normal, resulting in myocyte enlargement accompanied by hypertrophy of the chamber wall as a compensatory mechanism to normalize wall stress (73).
Data from sheep models of placental insufficiency demonstrate reduced, absent or reversed diastolic flow in the umbilical artery (75, 51), which are flow patterns that are typical of placentas with high impedance to pulsatile flow. Doppler ultrasound studies in human subjects have shown that, with placental insufficiency, fetuses with a normal umbilical vein blood flow pattern have increased ANP secretion (44), whereas abnormal umbilical vein flow patterns are associated with fetal myocardial cell damage (43). In addition, myocardial damage is associated with increased systemic venous pressure, a redistribution of cardiac output to favor the left ventricle (primarily responsible for blood flow supply to the coronary and cerebral circulations), and a rise in right ventricular afterload (45). Since fetal cardiac myocytes are sensitive to sustained loading conditions, it has been postulated that placental vascular insufficiency would increase systolic load to the fetal heart and cause myocyte hypertrophy (74). Furthermore, we suggest that endothelial dysfunction in obese women may lead to suboptimal trophoblast invasion, yielding placental vascular insufficiency, and that this may provide a link between maternal obesity and fetal cardiovascular disease.
Fetal Cardiac Development in Obese Pregnancy
Although the connection between maternal nutritional status and offspring health has been well established, the mechanisms underlying fetal cardiovascular programming are in the early stages of investigation. There have been relatively few studies of the association between obesity in pregnant women and the cardiovascular risk in their children. The relationship between gestational weight gain and elevated blood pressure in the offspring has been demonstrated both in young children (37) and at age 21 yr (47), with close correlations to offspring BMI demonstrated in these studies. Recently, the Amsterdam Born Children Development study found that a higher prepregnancy maternal BMI is associated with higher blood pressure in children aged 5-6 yr (16), although, when adjusted for birth weight and child BMI, this effect is reduced by 50%. In a recent longer-term follow-up study using a Cox proportional hazard regression model to correlate maternal BMI with cardiovascular events in adults aged 34–61 yr, those born to obese mothers were found to have an increased incidence of adverse cardiovascular outcomes and premature death (<55 yr of age) (61). Studies have also demonstrated that children of obese women are at a greater risk for congenital heart defects (49) and myocardial hypertrophy (88).
At birth, infants born to obese mothers have a greater atherogenic lipid profile (48), which is the precursor to cardiovascular disease. Children born to women with a higher prepregnancy BMI, independent of maternal diabetes, have significantly elevated levels of the circulating cellular and vascular adhesion molecules, E-selectin and vascular adhesion molecule-1, which are biomarkers of adverse endothelium perturbation (81). Stimulated by adhesion molecules, monocytes and lymphocytes attach to endothelial cells and initiate the atherosclerotic process (65). Additionally, in utero programming not only influences systemic risk factors for cardiovascular disease but can also affect the cardiac muscle directly through mechanical stimulation (58).
Evidence of the link between maternal nutritional status and offspring cardiovascular disease risk in human cohorts is mostly indirect and based on associations. Animal models are advantageous in that they allow the underlying links to be investigated through diet and experimental manipulation. Mechanistic studies are limited, but the importance of the placenta and utero-placental hemodynamics in cardiac development is clear. A suboptimal in utero environment in which the developing placenta is functionally compromised is the foundation for aberrant fetal development (2). Indeed, in a mouse model of obesogenic diet during pregnancy, pup body weight at term was appropriate for gestational age, but the placentas were smaller and morphologically compromised. Importantly, cardiovascular and metabolic dysfunction was observed in the adult offspring despite the appropriate birth weight (68). Underlying those observed changes were altered development of the three placental layers with reduced placental weight, evidence of an altered fetal growth trajectory and perturbed signaling via three major metabolic pathways (i.e., mitogen-activated protein kinase, phosphoinositide 3-kinase, and mammalian target of rapamycin) in the placenta (68). In offspring follow-up studies from rodent models of maternal obesity, increased risk of myocardial dysfunction, including ventricular hypertrophy (12) and myocardial fibrosis (29), have been demonstrated. Cardiac hypertrophy has been attributed to activation of the protein kinase B, extracellular signal-regulated kinase, and mammalian target of rapamycin pathway (12). In addition, blood pressure monitoring in conscious, unrestrained offspring from HFD-fed rats demonstrated elevated systolic pressure compared with control-fed dams accompanied by reduced endothelium-dependent relaxation in small mesenteric arteries (35, 34). Most recently, Blackmore et al. (4) used in vivo and in vitro manipulation to address the mechanistic cause linking maternal diet-induced obesity with cardiovascular function in C57BL/6J mice offspring studied up to 12 wk of age (4). Their data demonstrate increased heart weight due to cardiac hypertrophy accompanied by increased left ventricular volume at 3 and 8 wk, which is normalized by 12 wk. Interestingly, functional studies utilizing the Langendorff in vitro perfusion system at 12 wk demonstrated both systolic and diastolic dysfunction and a shift in the sympathetic-to-parasympathetic ratio with maternal obesity, indicating sympathetic dominance in this model. A corresponding increase in the β1-adrenergic receptor, the primary norepinephrine mediator in the mouse heart, and reduced expression of the sarcoplasmic reticulum ATPase (SERCA2a) protein, which is important for both Ca2+ extrusion (required for cardiomyocyte relaxation) and Ca2+ accumulation, was found (4). Taken together, these data suggest that cardiomyocyte hypertrophy is a protective mechanism that cannot be sustained long term, as demonstrated by the normal cell size at 12 wk, but that cardiac dysfunction persists; this may lead to premature cardiac failure in adulthood.
In a sheep model of overnutrition, fetal ventricular tissue was demonstrated to have notable lipid droplet accumulation and irregular myofiber orientation in addition to elevated toll-like receptor 4, interleukin-1α, interleukin-1β, and IL-6 levels, along with inflammatory cell infiltration. The authors suggest that maternal overfeeding before and throughout pregnancy promotes cardiac growth and morphometric changes (32). In our NHP model of HFD consumption, we have observed alterations in the heart/weight index (HWI) in juvenile offspring at 13 mo of age. Specifically, offspring born to HFD-fed dams maintained on a HFD postnatally have an increased HWI compared with control-fed offspring from control-fed dams. Offspring with increased HWI have abnormal expression of cardiac hypertrophy and contractility related genes (Fan L, Grove KL, unpublished observations).
The majority of data pertaining to lipotoxicity in the heart has been demonstrated in models of maternal undernutrition, but a recent study in mice showed that maternal HFD exposure followed by HFD in the postnatal period leads to decreased mitochondrial integrity in the heart and increased cardiac lipid content. In addition, that study reported decreased insulin sensitivity of the heart with diminished cardiac function and an increase in hypertrophy, apoptosis, and fibrosis (76). The myocardial fibrosis observed in the rodent supports earlier data from a sheep model of maternal diet-induced obesity, which demonstrated transforming growth factor beta as the underlying signaling pathway resulting in cardiac fibrosis in the fetal offspring (29).
Interestingly, in a sheep study of offspring born to obese mothers, cardiac function was normal at baseline but impaired with increasing cardiac workload (80). Juvenile offspring from our NHP model of diet-induced obesity during pregnancy demonstrated a significant reduction in endothelium-dependent vasorelaxation in the abdominal aorta, a 90% increase in intima thickness, and increased proliferation of smooth muscle cells deposited underneath the endothelium (11). These studies indicate that maternal obesity contributes to the detrimental effect of insulin resistance on vasorelaxant and cardiac function in offspring.
Maternal nutrition is likely to affect epigenetic modification of gene expression within the placenta (18), with subsequent effects on fetal development. Indeed, an adverse in utero environment can modify how the fetal genome is expressed with outcomes from a recent study using a baboon model of high-fat/high-sucrose diet consumption demonstrating increased myocardial fibrosis and proliferation in the fetal heart (46). Prior evidence demonstrates a role of mirco-RNAs (miRNAs), small, noncoding RNA molecules that regulate numerous cellular processes, in fetal cardiac development and etiology of cardiac pathology (3, 36) but not in the context of maternal obesity. The study by Maloyan and colleagues revealed significant alterations in cardiac miRNA expression in the fetus of obese baboons, which correlated with gene targets involved in developmental disorders. The authors suggest a role for epigenetic modifications in the fetal programming of cardiovascular disease (46). Additionally, one study has focused on epigenetic effects in isogenic mice with natural onset obesity (39). In this rodent model, offspring of obese mothers have a latent metabolic phenotype that is unmasked by later exposure to a HFD, with observed defects in glucose and lipid metabolism. The authors demonstrate widespread methylation changes with maternal obesity and report from their data that, despite the maladaptive fetal response to maternal obesity, by maintaining offspring on a control chow diet, the development of metabolic disease is prevented (39). They postulate that, if this finding can be translated to the human population, it may be possible to intervene and break the perpetual cycle of obesity. This may present one solution to address the longer-term issue of obesity, but aberrant fetal cardiovascular development and the long-term consequences of fetal exposure to an obese in utero environment remains to be resolved.
Conclusion
The alarming rise in maternal obesity rates warrants increased research efforts to understand and address the in utero relationships between maternal body composition, placental adaptations, and fetal cardiovascular development. Offspring vulnerability to later life disease results from a mismatch between the in utero environment in which developmental cues and signals adapt to adverse stimuli, yielding a phenotype that may not be ideally suited to lifestyle changes in future generations. The mechanisms associating maternal obesity and offspring cardiovascular disease are multifactorial and complex. This review has summarized the alterations of pregnancy complicated by maternal obesity and the current knowledge of the mechanistic links underlying fetal cardiovascular development and later life disease, but much remains to be understood in this field.
Footnotes
The authors of this review received funding support from the National Institutes of Health Grants (R24-DK-090964, (R21-HD-076265, and (P51-OD-011092.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: V.H.J.R. drafted manuscript; V.H.J.R., A.E.F., and K.L.G. edited and revised manuscript; V.H.J.R., A.E.F., and K.L.G. approved final version of manuscript.
References
- 1.Adam CL, Findlay PA, Aitken RP, Milne JS, Wallace JM. In vivo changes in central and peripheral insulin sensitivity in a large animal model of obesity. Endocrinology 153: 3147–3157, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clin Obstet Gynecol 56: 511–519, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J. Regulation of myocardial fibrosis by micrornas. J Cardiovasc Pharmacol 56: 454–459, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Blackmore HL, Niu Y, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 155: 3970–3980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohlen F, Kratzsch J, Mueller M, Seidel B, Friedman-Einat M, Witzigmann H, Teupser D, Koerner A, Storck M, Thiery J. Leptin inhibits cell growth of human vascular smooth muscle cells. Vascul Pharmacol 46: 67–71, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Campia U, Tesauro M, Di Daniele N, Cardillo C. The vascular endothelin system in obesity and Type 2 diabetes: pathophysiology and therapeutic implications. Life Sci 118: 149–155, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29: 274–281, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattergoon NN, Giraud GD, Thornburg KL. Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. J Endocrinol 192: 1–8, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 338: 147–152, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction 140: 373–385, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Fan L, Lindsley SR, Comstock SM, Takahashi DL, Evans AE, He GW, Thornburg KL, Grove KL. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes (Lond) 37: 254–262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Twinn DS, Blackmore HL, Siggens L, Giussani DA, Cross CM, Foo R, Ozanne SE. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, akt, erk, and mtor activation. Endocrinology 153: 5961–5971, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152: 2456–2464, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese zucker rat model of the metabolic syndrome. Microcirculation 12: 383–392, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gademan MG, van Eijsden M, Roseboom TJ, van der Post JA, Stronks K, Vrijkotte TG. Maternal prepregnancy body mass index and their children's blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension 62: 641–647, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Genbacev O, Miller RK. Post-implantation differentiation and proliferation of cytotrophoblast cells: in vitro models: a review. Placenta 21, Suppl A: S45–S49, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta 30: 411–417, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraud GD, Faber JJ, Jonker S, Davis L, Anderson DF. Intravascular infusions of plasma into fetal sheep cause arterial and venous hypertension. J Appl Physiol 99: 884–889, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology 147: 3643–3649, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Guttmacher AE, Maddox YT, Spong CY. The human placenta project: placental structure, development, and function in real time. Placenta 35: 303–304, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29: 2959–2971, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276: 36869–36872, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hart MV, Morton MJ, Hosenpud JD, Metcalfe J. Aortic function during normal human pregnancy. Am J Obstet Gynecol 154: 887–891, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Hayes EK, Lechowicz A, Petrik JJ, Storozhuk Y, Paez-Parent S, Dai Q, Samjoo IA, Mansell M, Gruslin A, Holloway AC, Raha S. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: Role of altered development of the placental vasculature. PLos One 7: e33370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes EK, Tessier DR, Percival ME, Holloway AC, Petrik JJ, Gruslin A, Raha S. Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod Sci 21: 648–657, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayward CE, Cowley EJ, Mills TA, Sibley CP, Wareing M. Maternal obesity impairs specific regulatory pathways in human myometrial arteries. Biol Reprod 90: 65, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Hayward CE, Higgins L, Cowley EJ, Greenwood SL, Mills TA, Sibley CP, Wareing M. Chorionic plate arterial function is altered in maternal obesity. Placenta 34: 281–287, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Health and Social Care Information Centre. Statistics on Obesity, Physical Activity and Diet: England, 2013; National Statistics [Online]. HSCIC http://www.hscic.gov.uk/catalogue/PUB10364. [Google Scholar]
- 29.Huang Y, Yan X, Zhao JX, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Ren J, Du M. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J Physiol Endocrinol Metab 299: E968–E975, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignarro LJ, Napoli C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Curr Diab Rep 5: 17–23, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Jones HN, Jansson T, Powell TL. Il-6 stimulates system a amino acid transporter activity in trophoblast cells through stat3 and increased expression of snat2. Am J Physiol Cell Physiol 297: C1228–C1235, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Kandadi MR, Hua Y, Zhu M, Turdi S, Nathanielsz PW, Ford SP, Nair S, Ren J. Influence of gestational overfeeding on myocardial proinflammatory mediators in fetal sheep heart. J Nutr Biochem 24: 1982–1990, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Kelishadi R, Poursafa P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care 44: 54–72, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 110: 1097–1102, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Kumarswamy R, Volkmann I, Thum T. Regulation and function of mirna-21 in health and disease. RNA Biol 8: 706–713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Davey Smith G. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the mater-university study of pregnancy and its outcomes. Circulation 110: 2417–2423, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res 44: 479–486, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Li CC, Young PE, Maloney CA, Eaton SA, Cowley MJ, Buckland ME, Preiss T, Henstridge DC, Cooney GJ, Febbraio MA, Martin DI, Cropley JE, Suter CM. Maternal obesity and diabetes induces latent metabolic defects and widespread epigenetic changes in isogenic mice. Epigenetics 8: 602–611, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol Regul Integr Comp Physiol 245: R720–R729, 1983. [DOI] [PubMed] [Google Scholar]
- 41.Ma Y, Zhu MJ, Zhang L, Hein SM, Nathanielsz PW, Ford SP. Maternal obesity and overnutrition alter fetal growth rate and cotyledonary vascularity and angiogenic factor expression in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R249–R258, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, Dammann O. Maternal obesity and markers of inflammation in pregnancy. Cytokine 47: 61–64, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Association of severe placental insufficiency and systemic venous pressure rise in the fetus with increased neonatal cardiac troponin t levels. Am J Obstet Gynecol 183: 726–731, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Umbilical artery n-terminal peptide of proatrial natriuretic peptide in hypertensive pregnancies and fetal acidemia during labor. Obstet Gynecol 97: 23–28, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation 105: 2058–2063, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, Nijland MJ. Identification and comparative analyses of myocardial mirnas involved in the fetal response to maternal obesity. Physiol Genomics 45: 889–900, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 119: 1720–1727, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Merzouk H, Meghelli-Bouchenak M, Loukidi B, Prost J, Belleville J. Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biol Neonate 77: 17–24, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population-based study. Am J Clin Nutr 91: 1543–1549, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147, Suppl 1: S193–S201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 161: 1055–1060, 1989. [DOI] [PubMed] [Google Scholar]
- 52.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 31, Suppl: S66–S69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Tierney PF, Anderson DF, Faber JJ, Louey S, Thornburg KL, Giraud GD. Reduced systolic pressure load decreases cell-cycle activity in the fetal sheep heart. Am J Physiol Regul Integr Comp Physiol 299: R573–R578, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Tierney PF, Chattergoon NN, Louey S, Giraud GD, Thornburg KL. Atrial natriuretic peptide inhibits angiotensin II-stimulated proliferation in fetal cardiomyocytes. J Physiol 588: 2879–2889, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the united states, 2009–2010. NCHS Data Brief 82: 1–8, 2012. [PubMed] [Google Scholar]
- 57.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 2: 71–91, 1981. [DOI] [PubMed] [Google Scholar]
- 58.Pruis MG, van Ewijk PA, Schrauwen-Hinderling VB, Plosch T. Lipotoxicity and the role of maternal nutrition. Acta Physiol (Oxf). In press. [DOI] [PubMed] [Google Scholar]
- 59.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 87: 4231–4237, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci 73: 1839–1851, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, Sarwar N, Lee AJ, Bhattacharya S, Norman JE. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347: f4539, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, Frias AE, Grove KL. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J 28: 2466–2477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts VH, Rasanen JP, Novy MJ, Frias A, Louey S, Morgan TK, Thornburg KL, Spindel ER, Grigsby PL. Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta 33: 73–76, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta 30: 169–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 35: 171–177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seifalian AM, Filippatos TD, Joshi J, Mikhailidis DP. Obesity and arterial compliance alterations. Curr Vasc Pharmacol 8: 155–168, 2010. [DOI] [PubMed] [Google Scholar]
- 68.Sferruzzi-Perri AN, Vaughan OR, Haro M, Cooper WN, Musial B, Charalambous M, Pestana D, Ayyar S, Ferguson-Smith AC, Burton GJ, Constancia M, Fowden AL. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J 27: 3928–3937, 2013. [DOI] [PubMed] [Google Scholar]
- 69.Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate igf-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol 285: R1481–R1489, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin ii stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol 548: 881–891, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta 34: 205–211, 2013. [DOI] [PubMed] [Google Scholar]
- 72.Thongsong B, Subramanian RK, Ganapathy V, Prasad PD. Inhibition of amino acid transport system a by interleukin-1beta in trophoblasts. J Soc Gynecol Investig 12: 495–503, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Thornburg K, Jonker S, O'Tierney P, Chattergoon N, Louey S, Faber J, Giraud G. Regulation of the cardiomyocyte population in the developing heart. Prog Biophys Mol Biol 106: 289–299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thornburg KL, O'Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta 31, Suppl: S54–S59, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trudinger BJ, Giles WB, Cook CM, Bombardieri J, Collins L. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. Br J Obstet Gynaecol 92: 23–30, 1985. [DOI] [PubMed] [Google Scholar]
- 76.Turdi S, Ge W, Hu N, Bradley KM, Wang X, Ren J. Interaction between maternal and postnatal high fat diet leads to a greater risk of myocardial dysfunction in offspring via enhanced lipotoxicity, irs-1 serine phosphorylation and mitochondrial defects. J Mol Cell Cardiol 55: 117–129, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Valsamakis G, Kumar S, Creatsas G, Mastorakos G. The effects of adipose tissue and adipocytokines in human pregnancy. Ann NY Acad Sci 1205: 76–81, 2010. [DOI] [PubMed] [Google Scholar]
- 78.van den Berkmortel FW, van der Steen M, Hoogenboom H, Wollersheim H, van Langen H, Thien T. Progressive arterial wall stiffening in patients with increasing diastolic blood pressure. J Hum Hypertens 15: 685–691, 2001. [DOI] [PubMed] [Google Scholar]
- 79.van Drongelen J, Hooijmans CR, Lotgering FK, Smits P, Spaanderman ME. Adaptive changes of mesenteric arteries in pregnancy: a meta-analysis. Am J Physiol Heart Circ Physiol 303: H639–H657, 2012. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Ma H, Tong C, Zhang H, Lawlis GB, Li Y, Zang M, Ren J, Nijland MJ, Ford SP, Nathanielsz PW, Li J. Overnutrition and maternal obesity in sheep pregnancy alter the jnk-irs-1 signaling cascades and cardiac function in the fetal heart. FASEB J 24: 2066–2076, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 54: 504–507, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Westermeier F, Salomon C, Gonzalez M, Puebla C, Guzman-Gutierrez E, Cifuentes F, Leiva A, Casanello P, Sobrevia L. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes 60: 1677–1687, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.WHO. Obesity and Overweight Fact sheet. N°311 [Online] World Health Organization; http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- 84.Wladimiroff JW, vd Wijngaard JA, Degani S, Noordam MJ, van Eyck J, Tonge HM. Cerebral and umbilical arterial blood flow velocity waveforms in normal and growth-retarded pregnancies. Obstet Gynecol 69: 705–709, 1987. [PubMed] [Google Scholar]
- 85.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol 98: 757–762, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl 6: S188–S191, 1988. [DOI] [PubMed] [Google Scholar]
- 87.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 23: 1839–1846, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Zielinsky P, Piccoli AL Jr. Myocardial hypertrophy and dysfunction in maternal diabetes. Early Hum Dev 88: 273–278, 2012. [DOI] [PubMed] [Google Scholar]